Abstract
The effect of irradiance in the range of 400 to 700 nm or photosynthetically active radiation (PAR) on bacterial heterotrophic production estimated by the incorporation of 3H-leucine (referred to herein as Leu) was investigated in the northwestern Mediterranean Sea and in a coastal North Atlantic site, with Leu uptake rates ranging over 3 orders of magnitude. We performed in situ incubations under natural irradiance levels of Mediterranean samples taken from five depths around solar noon and compared them to incubations in the dark. In two of the three stations large differences were found between light and dark uptake rates for the surfacemost samples, with dark values being on average 133 and 109% higher than in situ ones. Data obtained in coastal North Atlantic waters confirmed that dark enclosure may increase Leu uptake rates more than threefold. To explain these differences, on-board experiments of Leu uptake versus irradiance were performed with Mediterranean samples from depths of 5 and 40 m. Incubations under a gradient of 12 to 1,731 μmol of photons m−2 s−1 evidenced a significant increase in incorporation rates with increasing PAR in most of the experiments, with dark-incubated samples departing from this pattern. These results were not attributed to inhibition of Leu uptake in the light but to enhanced bacterial response when transferred to dark conditions. The ratio of dark to light uptake rates increased as dissolved inorganic nitrogen concentrations decreased, suggesting that bacterial nutrient deficiency was overcome by some process occurring only in the dark bottles.
A precise estimation of the fraction of organic matter being incorporated, assimilated and respired by heterotrophic bacterioplankton is crucial for the full understanding of the oceanic part of the global carbon cycle. Bacterial heterotrophic production (BHP) in aquatic systems is commonly estimated after conversion of the uptake rates of radiolabeled leucine (Leu) or thymidine (TdR) to carbon units. TdR and Leu are assumed to be taken up only—or to a larger extent—by heterotrophic bacterioplankton (11, 19). The papers published in the last decade show that common procedures have been adopted among aquatic microbiologists. Thus, although some standard protocols for TdR explicitly state the need for in situ or in situ-simulated light levels during the incubation (e.g., see reference 5) researchers usually perform both Leu and TdR uptake experiments in the dark (e.g., see reference 32). The problem of reproducing ambient light levels is obviously circumvented by incubating in the dark. Yet, another implicit justification for carrying out dark incubations is the avoidance of the possible stimulatory effect of primary production on bacterial activity (1). But the evidence of diel cycles of bacterial activity closely following the peaks of activity of primary producers (9, 12, 13) suggests that a limitation in the supply of dissolved compounds by dark enclosure of the autotrophic plankton community could result in lower estimates of BHP. Two possible hypotheses arise from the above-mentioned observations: either light has no effect on Leu or TdR uptake measurements, or, if there is an effect, light incubations should yield higher estimates than incubations undertaken in the dark. A third possibility, hinted at by results reported by Aas et al. (1) and Sommaruga et al. (32), is that incubating in the light could suppress labeled substrate incorporation by some not fully known photodynamic process (32).
The question of how solar radiation influences the abundance and activity of heterotrophic bacterioplankton has recently called the attention of aquatic ecologists in the framework of stratospheric ozone depletion. Most of the published work has, thus, concentrated on the effect of the UV range of the sunlight spectrum (290 to 400 nm) on natural communities of bacteria, reporting either the direct (e.g., see references 4, 16, 17, and 30) or the indirect effect through dissolved organic matter (DOM) photochemical reactions in surface waters (27). Few studies have addressed the specific effect of visible light or photosynthetically active radiation (PAR) (400 to 700 nm) on bacterial activity and/or abundance. Those that have report inhibition (1, 32), stimulation (1), or no effect (15) of PAR on bacteria, indicating that the interaction is far from simple.
We report here the results of three types of experiments aimed at understanding the effect of PAR on heterotrophic bacterial activity, as measured by Leu uptake rates. Knowledge of this process is important, methodologically (whether the most correct estimate of bacterial production is obtained with dark incubations or not) but also conceptually, as we will be able to obtain insight into the mechanisms driving the often-observed algal-bacterioplankton coupling in the ocean (13, 23). We first compare the estimates of Leu uptake rates of samples incubated both at in situ PAR irradiance and in the dark at three stations in the northwestern Mediterranean Sea and report results of similar experiments under in situ-simulated conditions conducted during two cruises in the North Atlantic. More insight into the relationship between PAR and BHP estimates was attempted at the Mediterranean stations by means of on-board incubations under a gradient of irradiance covering 2 orders of magnitude, starting at ∼10 μmol of photons m−2 s−1. The results obtained allow us to discuss the planktonic processes affected by light that ultimately determine the total amount of leucine incorporated.
MATERIALS AND METHODS
Experiments were performed at three stations in the Catalano-Balearic Sea (northwestern Mediterranean Sea) and in a transect over the Galician Atlantic shelf (northwestern Spain), on board of R/Vs García del Cid and Cornide de Saavedra, respectively. The Mediterranean stations D (open sea), F (shelf break), and C (shelf) (Table 1) were sampled on consecutive days in February 2000 between 8 h and 10 h Greenwich mean time during the Hivern cruise. In addition, station F was sampled in the night (2 h Greenwich mean time) two days before diurnal sampling. The Atlantic experiments were performed during the Incocéano cruises, in April to May 1998 and September 1999. Whole water samples were taken from Niskin bottles mounted on a hydrographic cable or from bottles in a rosette sampler attached to a conductivity, temperature, and depth probe. All experiments started within 1 h after water collection.
TABLE 1.
Selected characteristics of the Mediterranean stations where in situ and dark incubations of Leu uptake were compareda
| Station | Location | Depth (m) | Chl a (mg m−3) | BN (cells ml−1) | Syne (cells ml−1) | Proc (cells ml−1) | PAR (μmol of photons m−2 s−1) | DIN (μM) |
|---|---|---|---|---|---|---|---|---|
| D | 40°43.1′N, 2°50.8′E | 5 | 0.72 | 5.5 × 105 | 4.7 × 104 | 2.6 × 103 | 588 | 0.69 |
| 10 | 0.79 | 4.5 × 105 | 5.0 × 104 | 1.4 × 103 | 321 | 0.43 | ||
| 20 | 0.80 | 5.1 × 105 | 4.6 × 104 | 2.7 × 103 | 95 | 0.17 | ||
| 40 | 0.64 | 3.4 × 105 | 1.3 × 104 | 6.1 × 102 | 15 | 1.37 | ||
| 60 | 0.28 | 3.8 × 105 | 9.9 × 103 | 5.8 × 102 | 4 | 1.99 | ||
| F | 41°7.7′N, 2°26.8′E | 5 | 0.89 | 2.4 × 105 | 6.0 × 103 | 2.1 × 103 | 861 | 4.67 |
| 10 | 0.84 | 2.4 × 105 | 4.5 × 103 | 2.0 × 102 | 611 | 4.67 | ||
| 20 | 0.64 | 2.8 × 105 | 2.4 × 103 | 4.5 × 102 | 308 | 4.66 | ||
| 40 | 0.18 | 3.0 × 105 | 1.0 × 103 | 2.1 × 102 | 79 | 3.93 | ||
| 60 | 0.20 | 3.0 × 105 | 7.9 × 102 | 1.8 × 102 | 20 | 4.13 | ||
| C | 41°19.9′N, 2°15.3′E | 5 | 2.27 | 1.9 × 105 | 5.4 × 102 | 5.4 × 102 | 609 | 2.50 |
| 10 | 2.02 | 2.2 × 105 | 3.4 × 102 | 2.1 × 102 | 419 | 2.65 | ||
| 20 | 2.34 | 2.7 × 105 | — | — | 199 | 2.61 | ||
| 40 | 2.34 | 3.6 × 105 | 4.2 × 102 | 2.0 × 102 | 45 | 2.79 | ||
| 60 | 0.47 | 3.2 × 105 | 1.0 × 103 | 2.9 × 102 | 10 | 4.08 | ||
| Fb | 41°7.7′N, 2°26.8′E | 5 | 2.05 | 8.5 × 105 | 3.1 × 104 | 1.5 × 103 | 0 | 1.74 |
| 40 | 0.53 | 3.6 × 105 | 6.2 × 103 | 2.0 × 103 | 0 | 2.81 |
The average PAR irradiance received during in situ experiments and concentration of dissolved inorganic nitrogen (DIN) are also shown. —, not available. Abbreviations: BN, bacterial abundance; Syne, Synechococcus sp. abundance; Proc, Prochlorococcus sp. abundance.
Sampled at night.
The first experiment was a comparison between in situ and dark incubations. Water samples were taken at stations D, F, and C from five depths of the water column between 5 and 60 m. The activity of heterotrophic bacteria was measured as total leucine incorporation rates by the 3H-Leu method (18) in 1.2-ml samples incubated in Eppendorf vials as described by Smith and Azam (31). We added 40 nM leucine to the vials; then, they were introduced into transparent 125-ml Nalgene bottles filled with seawater and the bottles were subsequently attached to a rope hanging from a buoy and suspended at the same sampling depths. While the bottles and the Eppendorf vials were transparent, the exact PAR experienced by bacteria was slightly less than the value measured in the water. Four vials plus two trichloroacetic acid (TCA)-killed controls were incubated per in situ depth, and the same quantity was incubated on deck in the dark, after being wrapped in black plastic bags and put in an incubator with flowing surface seawater. Temperature did not vary more than 1.5% (standard deviation, 1.6%) from the surface to a depth of 60 m. Incubations lasted ∼2 h around midday and were stopped with 50% TCA and vortexed. On land, upon centrifugation and aspiration of the supernatant, pellets were rinsed with 1 ml of 5% TCA. The samples were centrifuged and aspirated again, and 0.5 ml of scintillation cocktail was added to the vial. Radioactivity was measured in a Beckman LD6000 LL liquid scintillation counter and disintegrations per minute were calculated by the external-standard method. No conversion of leucine incorporation rates (in picomoles of Leu liter−1 hour−1) to carbon units was attempted.
In the Atlantic cruises, duplicate 70-ml surface water samples (9 in the first cruise and 11 in the second one, taken at different stations and/or on different days) were placed in sterile polystyrene bottles and preconditioned on deck for 3 to 6 h under different treatments. One bottle was kept with in situ-simulated PAR, and the other one was kept in the dark. Posttreatment incubations lasted for ca. 2 h and were carried out in Eppendorf vials kept in the dark as described above. This design was similar to that described by Sommaruga et al. (33) and allowed us to compare the effect that preincubating the sample in PAR-transparent or dark containers could have on Leu uptake.
The experiments on leucine uptake versus irradiance relationships, aimed at determining the response of Leu uptake rates to different PAR levels, were conducted inside linear incubators kept at a constant temperature with running surface seawater, of the type commonly used for assessing photosynthesis-irradiance relationships. Samples from depths of 5 and 40 m of the three Mediterranean stations were placed in Eppendorf vials and processed in the same way and at the same time as those incubated in situ. Three replicate vials plus one control were introduced into a Nalgene bottle per irradiance level. Six bottles (light bottles) were exposed to different irradiance levels, ranging from 12 to 1,731 μmol photons m−2 s−1, inside the linear incubator, and an additional bottle (dark bottle) was wrapped with aluminum foil. Illumination was provided by a 150-W UV-free halogen lamp.
Chlorophyll a (Chl a) was estimated fluorometrically with a Turner Designs fluorometer after acetone extraction of pigments in Whatman GF/F filters, and concentrations of nutrients including total dissolved inorganic nitrogen (DIN) (NO3− and NO2− plus NH4+), phosphate, and silicate were measured with a Technicon Autoanalyzer using the standard protocols of Hansen and Grasshoff (14) with some minor modifications (2, 25). PAR in the water column was measured immediately after water sampling with a spherical quantum sensor (LiCor LI-193SA). Values at 1-m depth intervals were used to calculate the diffuse vertical attenuation coefficient of PAR. Total incident irradiance was also continuously measured on deck with a pyranometer connected to a LiCor Li-1000 Data Logger, allowing for the calculation of the average PAR to which samples were exposed during the in situ incubation. In the Leu uptake versus irradiance experiments, PAR was measured at each bottle position with a cosine LI-190SZ quantum sensor. An intercalibration with the LI-193SA sensor was made in order to avoid biases due to the use of different sensors. The abundance of heterotrophic bacteria (BN) was determined by flow cytometry as described by del Giorgio et al. (10). Subsamples (1.2 ml) were taken from the same water samples used to estimate Leu uptake and immediately fixed with 1% paraformaldehyde–0.05% glutaraldehyde. Samples were stored frozen in liquid N2 prior to analysis on land, where they were thawed, stained with SYTO-13 (Molecular Probes) at 2.5 μM and analyzed in a FACScalibur (Becton & Dickinson) flow cytometer, equipped with a laser emitting at 488 nm. An aliquot of a known concentration of fluorescent 0.92-μm-diameter Polysciences latex beads was added as an internal standard. Autotrophic picoplankton (Synechococcus and Prochlorococcus spp.) abundance was determined with the flow cytometer in aliquots of 600 μl of the same samples. Both groups were identified by their differential signature in plots of light scatter versus orange and red fluorescence.
RESULTS AND DISCUSSION
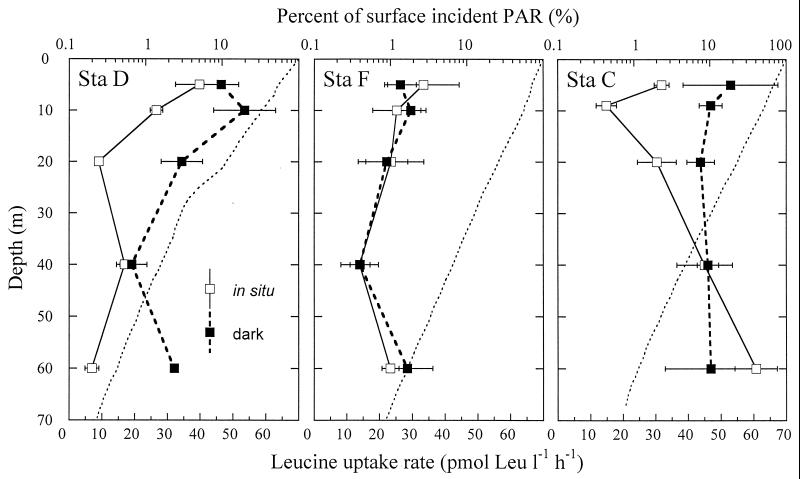
The three stations in the Mediterranean had rather similar light fields (Fig. 1); the vertical light attenuation coefficient was highest at station D for the 0- to 30-m-depth range (0.12 m−1), but from 30 m downwards it was similar to that of the other two stations (0.06 to 0.07 m−1). The abundance (BN) of heterotrophic bacteria and autotrophic picoplankton generally increased as we moved offshore (Table 1), with Synechococcus and Prochlorococcus also more abundant towards the surface. The leucine uptake rates in the upper 60 m of the water column ranged from 7 to 61 pmol of Leu liter−1 h−1, with higher values at station C, which also showed the highest values of Chl a (Table 1) and primary productivity (data not shown). Differences in bacterial activity were observed both with depth and among stations. In situ and dark vertical profiles were virtually identical for station F, but remarkably different at stations D and C, where not only was the absolute rate of Leu uptake generally higher for the samples incubated in the dark than for those incubated in situ but the shape of the depth profiles changed too (Fig. 1). These differences translated into integrated rates, with dark values being virtually the same as in situ ones (only 1% higher) at station F, but 89 and 21% higher at stations D and C, respectively. At these two stations, dark rates were coherently higher than in situ ones at depths receiving irradiances higher than ∼10% of the surface value (Fig. 1). Within the 5- to 20-m depth range of stations D and C, dark rates were 133% and 109% higher, respectively, than light ones. In contrast, no clear trend appeared deeper in the water column, indirectly suggesting that this discrepancy could be related to some variable that changes vertically in the water column, with PAR as the most obvious candidate. Likewise, in the Atlantic experiments, with uptake rates ranging from 10 to 1,465 pmol of Leu liter−1 h−1, most often greater bacterial activity was measured after dark preconditioning than after in situ-simulated PAR conditions (Table 2). In the first cruise, dark Leu uptake rates were higher than their in situ-simulated counterparts in all experiments (127% higher on average). This difference was significant (t tests, P < 0.05) in seven out of nine experiments (Table 2). During the second cruise, although values in dark bottles were on average 22% higher than those in light bottles, differences were significant only in 4 out of 11 experiments (dark values greater than light values in three experiments; light values greater than dark values in one experiment [Table 2]).
FIG. 1.
Vertical profiles of the incorporation rates of leucine at the three Mediterranean stations estimated both in situ and in dark containers. The horizontal bars represent the standard error of replicates. The percent of surface irradiance received in the upper meters of the water column is also given (dotted line).
TABLE 2.
Average leucine uptake rates measured in the dark in the Atlantic experiments after two preconditioning treatments
| Cruise | Preconditioning treatment
|
Dark:light ratioa (%) | |
|---|---|---|---|
| In situ-simulated PAR (pmol Leu liter−1 h−1) | Dark (pmol Leu liter−1 h−1) | ||
| Incocéano-1 | 19.4 | 31.5 | 162** |
| 15.7 | 20.6 | 131** | |
| 30.2 | 117.6 | 389 | |
| 20.8 | 52.7 | 253*** | |
| 9.5 | 13.6 | 143 | |
| 35.4 | 104.0 | 294** | |
| 48.2 | 90.4 | 188** | |
| 154.7 | 280.8 | 181** | |
| 15.1 | 45.7 | 304** | |
| Mean ± SE | 38.8 ± 15.0 | 84.1 ± 27.5 | 227 ± 29 |
| Incocéano-2 | 27.8 | 13.6 | 49 |
| 27.1 | 21.2 | 78 | |
| 48.6 | 51.5 | 106 | |
| 262.9 | 731.5 | 278*** | |
| 1464.6 | 1112.2 | 76** | |
| 401.2 | 338.0 | 84 | |
| 10.2 | 25.3 | 247 | |
| 32.0 | 47.2 | 147* | |
| 44.6 | 62.6 | 140* | |
| 164.1 | 109.0 | 66 | |
| 51.7 | 35.2 | 68 | |
| Mean ± SE | 230.4 ± 128.9 | 231.6 ± 109.5 | 122 ± 23 |
The average ratio of the value in dark bottle to that in the light bottle (expressed as a percentage) is given, with an indication of significant differences between in situ and dark data (t test) as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Given the assumption that during short-term incubations heterotrophic bacteria incorporate 3H-Leu at a rate proportional to their growth rate, incorporation in samples incubated in the dark should not be higher than that in samples incubated in situ, if the well-known detrimental effects on bacterial activity of UV radiation (4, 16, 29) are avoided, as was the case in our experiments. However, differences in shallow water Leu and/or TdR uptake rates like those presented here have also been found in previous studies (1, 32). Higher values in the dark can be interpreted on two fully opposed grounds: (i) solar radiation in the PAR range inhibits Leu uptake or (ii) darkness enhances Leu uptake. The first interpretation, which could be related to the proved damage in bacterial metabolism directly caused by UV parts of the solar spectrum (e.g., see references 17 and 26), has always been favored (1, 32). Sommaruga et al. (32) argued that a photodynamic process involving reduced transport of Leu into the cell caused a decrease in the measured rate at ambient PAR levels in their experiments. Suppressed bacterial incorporation of leucine could be due to a lower availability of substrate in the light bottles by phototransformation of DOM (e.g., see references 6, 20, 21, and 34) or a photoreaction causing precipitation of 3H-leucine (G. J. Herndl, personal communication). This photoinhibition hypothesis, however, is not supported by our results. First, according to such a hypothesis, increased irradiance towards the water surface would imply a greater relative decrease in light bottles, but neither depth nor total incident PAR during the Mediterranean experiments bore any significant relationship with the difference between in situ and dark values (Table 1 and Fig. 1). Second and more relevantly, if the photoinhibition effect were important, the Leu uptake versus irradiance experiments would have showed a negative relationship between uptake rate and experimental irradiance (dark values not considered) rather than the positive or nonsignificant one observed in all experiments (Fig. 2 and 3; also see below).
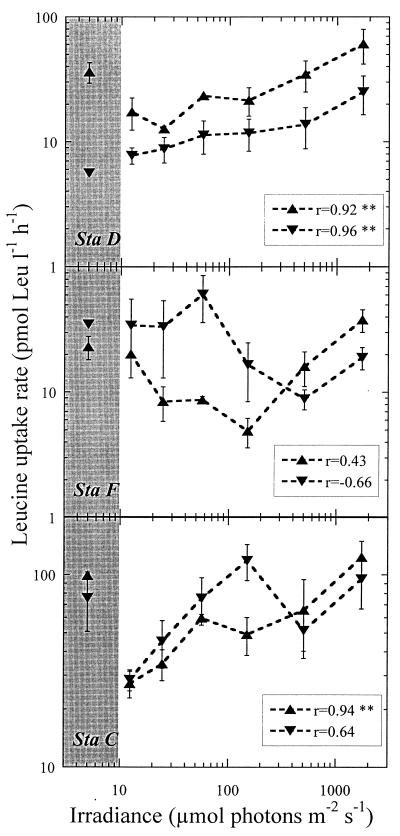
FIG. 2.
Plots of leucine uptake versus irradiance for the three stations sampled during the day. Water was taken from depths of 5 m (▴) and 40 m (▾). The points included in the shaded area correspond to dark bottles. Error bars represent standard errors. The coefficients of correlation between both variables are given, with the level of significance indicated as follows: no asterisk, P > 0.05; ∗, P < 0.05; ∗∗, P < 0.01.
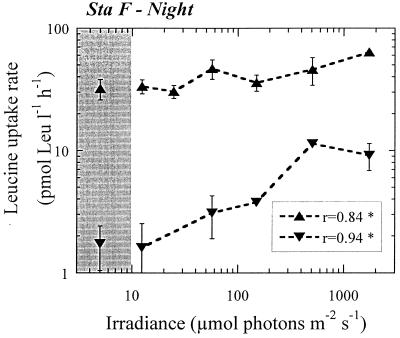
FIG. 3.
Plot of leucine uptake versus irradiance for the experiment carried out at night at station F. Symbols and significance levels are explained in the legend to Fig. 2.
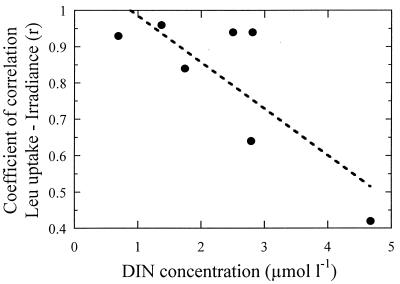
As far as we know, the results shown in Fig. 2 and 3 are the first reported on the response of Leu incorporation rates to a gradient of PAR that comprises values usually met in the water column. The positive relationship between PAR irradiance and Leu uptake found in most of the experiments suggests that at the time scale of the experiments bacterioplankton assemblages were responding to a photosynthesis-related process. The total supply of dissolved organic compounds amenable to bacterial uptake is expected to increase with higher photosynthetic rates, as suggested by Aas et al. (1) to explain their higher rates of Leu and TdR incorporation in PAR-exposed samples compared to dark ones in the Adriatic. Nevertheless, stimulation of heterotrophic bacteria by the activity of primary producers is not the only possible explanation to the observed relationship. Some authors have described that under particular conditions, such as an insufficient supply of N inorganic forms, uptake of dissolved organic nitrogen compounds—including amino acids such as leucine—by phytoplankton may occur (3, 7). Paerl (28) demonstrated that picoplankton incorporated more amino acids under illuminated conditions than in the dark. With the experimental design used here we were not able to determine the relative contribution of the heterotrophic and autotrophic compartments to the measured rates, but some rough calculations can be made at this point. Even if we consider that at the lowest experimental irradiance all heterotrophic and autotrophic picoplankton cells were incorporating Leu at the same rate per cell, a 75% increase in the rates of autotrophic picoplankton under fully illuminated conditions (the maximum increase over dark-measured amino acid incorporation rates reported by Paerl [28]) would make Synechococcus plus Prochlorococcus account for only 1.9% (standard deviation, 1.5%) of the total amount of leucine incorporated by the planktonic community at the highest irradiance. This argument strongly suggests that heterotrophic bacteria were responsible for the bulk Leu incorporation throughout the experimental gradient in irradiance. Nitrogen concentrations seemed to affect the strength of the Leu uptake-irradiance relationship, as a significant negative relationship (r = −0.80; N = 7; P = 0.031) (Fig. 4) existed between the value of the correlation coefficients shown in Fig. 2 and 3 (the negative correlation coefficient −0.66 not considered) and DIN (Table 1), indicating that the response of leucine uptake to PAR was stronger when N approached limiting values.
FIG. 4.
Relationship between the coefficient of correlation between Leu uptake and experimental irradiance (see Fig. 2 and 3) and the concentration of DIN in the water sample. The fitted line is the linear regression.
A result similar to the in situ versus dark incubations was found when the uptake rates at the different experimental irradiances were compared with the corresponding dark bottle value. The incorporation in the dark bottle was higher than at least one of the light bottles at irradiances lower than 100 μmol of photons m−2 s−1 in all the diurnal experiments, except at station D at a depth of 40 m (Fig. 2). Light bottle uptake rates were split into two groups according to the irradiance received, lower or higher than 100 μmol of photons m−2 s−1, and averages were calculated for each experiment. These values, termed “low” and “high” irradiance, are presented in Table 3 together with the dark values. As a general pattern, higher Leu incorporation rates were found at high than at low irradiance (61% more on average), whereas a similar increase over the low irradiance values was obtained in dark incubations (54% more on average) (Table 3).
TABLE 3.
Dark leucine uptake rates and means at low and high irradiance in the uptake versus irradiance experimentsa
| Station | Depth (m) | Uptake rate (pmol of Leu liter−1 h−1)
|
||
|---|---|---|---|---|
| Dark | Irradiance
|
|||
| Low | High | |||
| D | 5 | 36.1 (+102) | 17.9 | 39.0 (+118) |
| 40 | 5.6 (−40) | 9.3 | 16.9 (+81) | |
| F | 5 | 23.1 (+86) | 12.4 | 19.7 (+58) |
| 40 | 35.0 (−19) | 42.9 | 14.8 (−65) | |
| C | 5 | 98.7 (+144) | 40.5 | 79.2 (+96) |
| 40 | 75.8 (+52) | 49.8 | 88.3 (+77) | |
| Fb | 5 | 31.9 (−13) | 36.9 | 48.4 (+31) |
| 40 | 1.7 (−26) | 2.4 | 8.1 (+245) | |
| Mean (%) | +54 | +61 | ||
| SE (%) | 29 | 27 | ||
Low and high irradiance values are the respective averages of the light bottles incubated at <100 and >100 μmol of photons m−2 s−1 (n = 3 each) (see Fig. 2 and 3). The value in parentheses is the percent difference of dark and high irradiance values relative to the low irradiance value. Mean percent differences do not include the night experiment.
Sampled at night.
In view of these results, we believe that dark enhancement of the amino acid uptake rates is more likely an explanation for the differences shown in Fig. 1 and Table 2. Apparently, by dark enclosing a planktonic community naturally exposed to solar radiation prior to the experimental uptake rate determinations, some process within the microbial food web becomes altered to the point of inducing artificially high Leu uptake rates. Changes in the photosynthetic response of primary producers due to abrupt changes in irradiance (22, 24, 35) could be expected to induce erratic or artificial measurements of bacterial activity. A possible explanation would be the enhanced release of DOM by algae due to light stress (22, 35). Light stress has been suggested as the cause for high dissolved organic nitrogen release rates during incubations (8). This hypothesis implicitly assumes a rapid bacterial response to a higher concentration of labile compounds (33). Interestingly, for the two samples collected in the night at station F, bacterial activity measured in the dark bottle was equal or lower than in the rest of the light bottles (Fig. 3), strongly suggesting that whichever the process responsible for the high dark uptake in the diurnal experiments, it was related to sudden changes in the natural irradiance cycle.
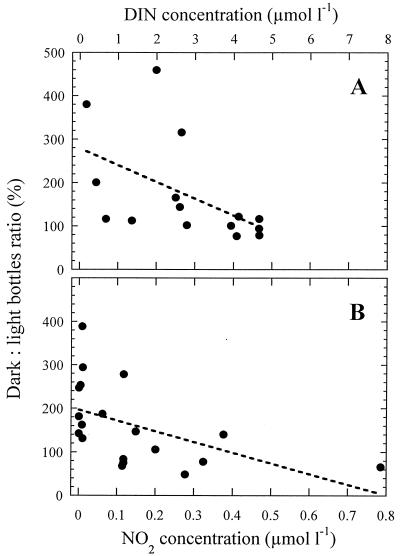
If a higher availability of bacterial substrate under dark conditions was the explanation for the observed results, then the difference between dark and light Leu uptake values could somehow reflect the nutritional status of the heterotrophic bacterial assemblages, as suggested by Aas et al. (1). It was surprising to find in our experiments that the ratio of the rate measured in the dark to that measured in the light correlated with nitrogen content in the water. For all Mediterranean data pooled, the dark:light bottle ratio decreased significantly (P < 0.05) with increasing concentration of DIN (Fig. 5A) (r = −0.52; N = 15), and a significant negative relationship was also found between this ratio and nitrite concentration for pooled data from the two Atlantic cruises (Fig. 5B) (r = −0.51; N = 19). In the latter experiments, the correlation with DIN was close to significant (r = −0.41; P = 0.08). These inverse relationships between inorganic nitrogen concentration and the ratio of dark to light measurements indicate that nutrient-replete bacteria did not show appreciable responses to a possibly higher concentration of DOM in the dark bottles. At station F, where we found no differences between light and dark incubations (Fig. 1), DIN concentrations exceeded 3 μM at all depths (Table 1). The relationship of the difference between dark and light bottles with nutrient concentration is further supported by experiments carried out in the Southern Ocean, where nitrate and phosphate are known not to be limiting. Helbling et al. (16) did not find differences in bacterial abundance between dark and PAR exposed samples after 5 to 7 h, and results of experiments analogous to the Atlantic ones (Table 2) undertaken in the Weddell and Scotia Seas showed no evidence of increased bacterial activity in the dark bottles (24).
FIG. 5.
Difference between Leu uptake rates measured in dark and light bottles, expressed as the percent ratio of dark to light incorporation, in relationship with the concentration of inorganic nitrogen in the water. (A) Mediterranean in situ experiments; (B) North Atlantic in situ-simulated experiments. Fitted lines are linear regressions. More details are given in the text.
Considering all types of experiments, our work provides evidence of two effects: a positive relationship between the level of PAR during the incubation and Leu uptake rate (Fig. 2 and 3) and a clear effect of dark enclosure that may result in considerably greater measured rates (Fig. 1 to 3; Tables 1 and 2). Dark incubations of radiolabeled substrates could help eliminate the possible enhancement of bacterial uptake rates due to algal stimulation. But bacteria are naturally exposed to solar radiation in the surfacemost layers of the ocean, and it is not straightforward to justify why bacterium-phytoplankton interactions occurring during daylight hours should not be considered in methodological procedures. The use of darkened containers had already been questioned by Aas et al. (1), who claimed that overestimation of true rates caused by not taking into account the detrimental effects of UV radiation could be avoided with UV-transparent containers. Here, it is shown that exclusion of PAR can lead to substantially different estimates of bacterial production and that the likely cause for the differences is some artifact related to a higher availability of bacterial substrate within the dark containers rather than to photoinhibition. Until further research determines under which circumstances differences are expected to be important (such as nutrient availability, as we suggest here), routine comparisons of dark versus in situ incubations of Leu and TdR uptake should be recommended in order to better constrain the flux of carbon through heterotrophic bacterioplankton in the ocean.
ACKNOWLEDGMENTS
We are very grateful to M. Estrada for her valuable comments on an earlier version of the manuscript and to two anonymous reviewers. Nutrient data were kindly provided by M. D. Doval and X. A. Álvarez-Salgado. Thanks are also given to M. M. Sala and B. Díez for their help in the Hivern experiments and to C. Pedrós-Alió and D. Vaqué for assistance during the Incocéano cruises. S. Canut helped with his usual guidance. X.A.G.M. wants to thank the people at the Departments of Applied Physics and of Ecology and Animal Biology of the University of Vigo (Spain), especially E. Marañón, for providing the ideal environment for writing the first version of this manuscript.
This work was supported by research grants from the Spanish CICYT for the Hivern project (MAR98-0932) and the Incocéano project (MAR95-1901-C03).
REFERENCES
- 1.Aas P, Lyons M M, Pledger R, Mitchell D L, Jeffrey W H. Inhibition of bacterial activities by solar radiation in nearshore waters and the Gulf of Mexico. Aquat Microb Ecol. 1996;11:229–238. [Google Scholar]
- 2.Álvarez-Salgado X A, Pérez F F, Fraga F. Determination of nutrient salts by automatic methods both in seawater and brackish water: the phosphate blank. Mar Chem. 1992;39:311–319. [Google Scholar]
- 3.Antia N J, Berland B R, Bonin D J, Maestrini S Y. Comparative evaluation of certain organic and inorganic sources of nitrogen for phototrophic growth of marine microalgae. J Mar Biol Assoc U K. 1991;55:519–539. [Google Scholar]
- 4.Bailey C A, Niehof R A, Tabor P S. Inhibitory effect of solar radiation on amino acid uptake in Chesapeake Bay bacteria. Appl Environ Microbiol. 1983;46:44–49. doi: 10.1128/aem.46.1.44-49.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell R T. Estimating production of heterotrophic bacterioplankton via incorporation of tritiated thymidine. In: Kemp P F, et al., editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 495–503. [Google Scholar]
- 6.Benner R, Biddanda B. Photochemical transformations of surface and deep marine dissolved organic matter: effects on bacterial growth. Limnol Oceanogr. 1998;43:1373–1378. [Google Scholar]
- 7.Bronk D A, Glibert P M. Application of a 15N tracer method to the study of dissolved organic nitrogen uptake during spring and summer in Chesapeake Bay. Mar Biol. 1993;115:501–508. [Google Scholar]
- 8.Bronk D A, Ward B B. Magnitude of dissolved organic nitrogen release relative to gross nitrogen uptake in marine systems. Limnol Oceanogr. 2000;45:1879–1883. [Google Scholar]
- 9.Coffin R B, Connolly J P, Harris P S. Availability of dissolved organic carbon to bacterioplankton examined by oxygen utilization. Mar Ecol Prog Ser. 1993;101:9–22. [Google Scholar]
- 10.del Giorgio P A, Bird D F, Prairie Y T, Planas D. The flow cytometric determination of lake bacterioplankton abundance using the nucleic acid stain SYTO-13. Limnol Oceanogr. 1996;41:783–789. [Google Scholar]
- 11.Fuhrman J A, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Biol. 1982;66:109–120. [Google Scholar]
- 12.Fuhrman J A, Eppley R W, Hagström Å, Azam F. Diel variations in bacterioplankton, phytoplankton, and related parameters in the Southern California Bight. Mar Ecol Prog Ser. 1985;27:9–20. [Google Scholar]
- 13.Gasol J M, Doval M D, Pinhassi J, Calderón-Paz J I, Guixa-Boixareu N, Vaqué D, Pedrós-Alió C. Diel variations in bacterial heterotrophic activity and growth in the northwestern Mediterranean Sea. Mar Ecol Prog Ser. 1998;164:107–124. [Google Scholar]
- 14.Hansen H P, Grasshoff K. Automated chemical analysis. In: Grasshoff K, Ehrhardt M, Kermling K, editors. Methods of seawater analysis. 2nd ed. Weinheim, Germany: Verlag Chemie; 1983. pp. 347–395. [Google Scholar]
- 15.Helbling E W, Marguet E R, Villafañe V E, Holm-Hansen O. Bacterioplankton viability in Antarctic waters as affected by solar ultraviolet radiation Mar. Ecol Prog Ser. 1995;126:293–298. [Google Scholar]
- 16.Herndl G J, Müller-Niklas G, Frick J. Major role of ultraviolet-B in controlling bacterioplankton growth in the surface layer of the ocean. Nature. 1993;361:717–719. [Google Scholar]
- 17.Jeffrey W H, Paul J H, Aas P, Hager S, Coffin R B, von Haven R, Mitchell D L. Diel and depth profiles of DNA photodamage in bacterioplankton exposed to ambient solar ultraviolet radiation. Mar Ecol Prog Ser. 1996;137:283–291. [Google Scholar]
- 18.Kirchman D L. Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp P F, et al., editors. Handbook of methods in aquatic microbial ecology. Boca Raton, Fla: Lewis Publishers; 1993. pp. 509–512. [Google Scholar]
- 19.Kirchman D L, K'nees E, Hodson R. Leucine incorporation and its potential as a measure of protein synthesis by bacteria in natural aquatic systems. Appl Environ Microbiol. 1985;49:599–607. doi: 10.1128/aem.49.3.599-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindell M J, Granéli W, Tranvik L J. Enhanced bacterial growth in response to photochemical transformation of dissolved organic matter. Limnol Oceanogr. 1995;40:195–199. [Google Scholar]
- 21.Lindell M J, Graneli H W, Tranvik L J. Effects of sunlight on bacterial growth in lakes of different humic content. Aquat Microb Ecol. 1996;11:135–141. [Google Scholar]
- 22.Mague T H, Friberg E, Hughes D J, Morris I. Extracellular release of carbon by marine phytoplankton; a physiological approach. Limnol Oceanogr. 1980;25:262–279. [Google Scholar]
- 23.Morán, X. A. G., J. M. Gasol, C. Pedrós-Alió, and M. Estrada. Dissolved and particulate primary production and bacterial production in offshore Antarctic waters during austral summer: coupled or uncoupled? Mar. Ecol. Prog. Ser., in press.
- 24.Morán X A G, Estrada M. Short-term variability of photosynthetic parameters and particulate and dissolved primary production in the Alboran Sea (SW Mediterranean) Mar Ecol Prog Ser. 2001;212:53–67. [Google Scholar]
- 25.Mouriño C, Fraga F. Determinación de nitratos en agua de mar. Investig Pesq. 1985;49:81–96. [Google Scholar]
- 26.Müller-Niklas G, Heissenberger A, Pukaric S, Herndl G J. Ultraviolet-B radiation and bacterial metabolism in coastal waters. Aquat Microb Ecol. 1995;9:111–116. [Google Scholar]
- 27.Obernosterer I, Reitner B, Herndl G J. Contrasting effects of solar radiation on dissolved organic matter and its bioavailability to marine bacterioplankton. Limnol Oceanogr. 1999;44:1645–1654. [Google Scholar]
- 28.Paerl H W. Ecophysiological and trophic implications of light stimulated amino-acid utilization in marine picoplankton. Appl Environ Microbiol. 1991;57:473–479. doi: 10.1128/aem.57.2.473-479.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakulski J D, Aas P, Jeffrey W, Lyons M, Von Waasenbergen L, Mitchell D, Coffin R. Influence of light on bacterioplankton production and respiration in a subtropical coral reef. Aquat Microb Ecol. 1998;14:137–148. [Google Scholar]
- 30.Sieracki M E, Sieburth J M. Sunlight-induced growth delay of planktonic marine bacteria in filtered seawater. Mar Ecol Prog Ser. 1986;33:19–27. [Google Scholar]
- 31.Smith D C, Azam F. A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-leucine. Mar Microb Food Webs. 1992;6:107–114. [Google Scholar]
- 32.Sommaruga R, Obernosterer I, Herndl G J, Psenner R. Inhibitory effect of solar radiation on thymidine and leucine incorporation by freshwater and marine bacterioplankton. Appl Environ Microbiol. 1997;63:4178–4184. doi: 10.1128/aem.63.11.4178-4184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Søndergaard M, Riemann B, Jørgensen N O G. Extracellular organic carbon (EOC) released by phytoplankton and bacterial production. Oikos. 1985;45:323–332. [Google Scholar]
- 34.Wetzel R G, Hatcher P G, Binachi T S. Natural photolysis by ultraviolet irradiance of recalcitrant dissolved organic matter to simple substrates for rapid bacterial growth. Limnol Oceanogr. 1995;40:1369–1380. [Google Scholar]
- 35.Wood A M, Rai H, Garnier J, Kairesalo T, Gresens S, Orive E, Ravail B. Practical approaches to algal excretion. Mar Microb Food Webs. 1992;6:21–38. [Google Scholar]