Abstract
Adjuvant radiation therapy is a critical component of breast cancer management. However, when breast cancer patients receive incidental radiation to the heart, there is an increased risk of cardiac disease and mortality. This is most common for patients with left-sided breast cancers and those receiving nodal irradiation as part of treatment. The overall risk of cardiac toxicity increases 4–16% with each Gray increase in mean heart radiation dose, with data suggesting that no lower limit exists which would eliminate cardiac risk entirely. Radiation techniques have improved over time, leading to lower cardiac radiation exposure than in the past. This decline is expected to reduce the incidence of radiation-induced heart dysfunction in patients. Deep inspiration breath hold (DIBH) is one such technique that was developed to reduce the risk of cardiac death and coronary events. DIBH is a non-invasive approach that capitalizes on the natural physiology of the respiratory cycle to increase the distance between the heart and the therapeutic target throughout the course of radiation therapy. DIBH has been shown to decrease the mean incidental radiation doses to the heart and left anterior descending coronary artery by approximately 20–70%. In this review, we summarize different techniques for DIBH and discuss recent data on this technique.
Keywords: deep inspiration breath hold, breast cancer, radiation, active breathing control, real-time position management, heart
Introduction and Background
While adjuvant radiation therapy (RT) has been shown to improve local control and breast cancer-specific survival, previous studies have demonstrated that RT using older techniques resulted in increased all-cause mortality rates.1–3 Specifically, several studies demonstrated that exposure of cardiac tissue to radiation increases the risk of cardiovascular disease and cardiac mortality.4,5 In fact, there was a 4–16% increase in risk of major coronary events per Gray (Gy) of mean heart dose (MHD).6–9 For patients with left-sided breast cancer or those receiving regional nodal irradiation this is of special concern due to the proximity of the heart to the target(s).8–11 More recent data suggests that dose to the left anterior descending (LAD) artery is a much better predictor than MHD alone.12–15 Minimizing both the LAD artery dose and the MHD is particularly important when including the regional lymph nodes (in particular the internal mammary chain (IMC) nodes). When the IMC nodes are included as part of the target, the dose to the coronary artery branches on the left-anterior surface of the heart can increase significantly when compared to whole breast or chest wall radiation alone.16 Given the recent data to support the inclusion of regional lymph nodes, it is essential to incorporate treatment methods that reduce the cardiac dose and associated toxicities in standard practice.17,18
Much of the radiation-induced cardiac toxicity data is from studies prior to routine use of three-dimensional conformal RT (3DCRT).3,11,12,19,20 However, since 3DCRT has been adopted, there have also been further advances in radiation practices leading to more precise, conformal treatments. As the risk of cardiac toxicity is linearly associated with heart doses received, it is logical to hypothesize that treatments that highly conform to targets are important in limiting high doses to the left-anterior surface of the heart, which may be at the expense of added low-dose regions.13,15,21 Indeed, studies have illustrated decreases in the incidence of major cardiac events based upon the era of radiation treatment, with recent time periods having significantly less cardiac toxicity.9,21–25 Forward planned intensity-modulated radiation therapy (FP-IMRT) for whole breast radiotherapy has been shown to improve dose homogeneity and reduce acute radiation dermatitis with improved cosmetic outcomes.26,27 Inverse planned IMRT (IP-IMRT) for tangent whole breast radiation provides little to no benefit when compared to FP-IMRT, given the simple geometry and ability to achieve similar plan quality with either technique.28 However, the inclusion of loco-regional lymph node treatment, and most notably IMC lymph nodes, makes IP-IMRT a potentially more attractive option given the complex shape and the ability of IP-IMRT to create concave dose distributions.29–34 Volumetric modulated arc therapy (VMAT) is an IP-IMRT technique that can be delivered in much less time and has shown similar benefits as static gantry IP-IMRT for locoregional left-sided breast radiotherapy.35–37
The use of modern photon techniques, like IP-IMRT and VMAT, should be considered carefully, as their high-dose conformality and organ at risk sparing comes at the cost of low-dose scatter. Previously reported data suggests that there is no minimum dose below which no RT induced cardiac toxicity exists.6–9,38 However, the inclusion of IMC nodes in the radiation target increases the risk of high doses to portions of the heart, making IP-IMRT and VMAT techniques more favorable in these situations. Thus, utilizing techniques that produce a decrease in heart and coronary artery doses, particularly the left-anterior aspect of the heart, are critical to minimizing the risk of radiation-induced cardiac dysfunction. In addition to different RT delivery techniques, treating patients in the prone position,39,40 lateral decubitus position,41 with deep inspiration breath hold (DIBH),42–44 or a combination of these techniques,45 can also aid in decreasing cardiac doses. Another example of advanced radiation techniques is proton therapy, which has unique physical properties which drastically decrease the exit dose when compared to photon-based radiation. This type of radiation comes with a tradeoff, as it is very sensitive to the density of the substances it passes through making the dose distribution less predictable.46
In this review we will summarize the use of DIBH in more detail. DIBH can be used for tangent-only breast plans or for plans that also include treatment of the regional lymph nodes. DIBH has been demonstrated to decrease doses to the heart and LAD by 25–67% and 20–73%, respectively.32,43–45,47–60 DIBH can also be used with techniques such as prone positioning and IMRT/VMAT. The use of DIBH with 3DCRT or with the highly conformal and sharp dose gradients of IP-IMRT can be important techniques to decrease high cardiac doses in patients receiving loco-regional nodal irradiation with left breast/chest wall treatment.36,61–63 DIBH with IP-IMRT is preferentially delivered with VMAT or an aperture-based approach given the reduced treatment times and improved target coverage, dose homogeneity, and sparing of the heart and LAD when compared to 3DCRT.63
DIBH Methods
DIBH capitalizes on the physiology of the heart and chest wall during the natural respiratory cycle to decrease cardiac dose. During the inspiratory phase, the flattening of the diaphragm and the expansion of the lungs pulls the heart away from the chest wall (Figure 1). This action increases the distance between the heart and chest wall, which decreases the volume of the heart being irradiated, and often yields significant decreases in LAD doses.42 When treating a patient with DIBH, the patient takes a deep breath and holds the deep inspiration while radiation therapy is delivered. For a typical breast cancer treatment session, a patient would have a number of deep inspiration breath holds to complete the full treatment. Inspiratory breath hold techniques can be broken down into voluntary DIBH (vDIBH) and involuntary DIBH (iDIBH). vDIBH does not necessarily require additional equipment and can be performed using the light field or the distance between lateral skin marks or anterior chest displacement from room lasers or other devices to monitor DIBH. However, computer monitoring systems are often used, which include optical surface monitoring devices to monitor lung expansion or spirometers to monitor air flow throughout the respiratory cycle. For example, a spirometer can be used to provide feedback using an open airway audio-visual system, whereby a “target zone” is projected on a screen or through video goggles and the patient is instructed to inhale to reach a certain signal position on the screen (SDX System; Dyn’R Medical Systems, Provence-Alpes-Cote dAzur, France).64,65 In contrast, involuntary DIBH relies on active breathing control (ABC) devices that stop airflow at preset threshold volumes, thereby resulting in an involuntary breath hold66–68 (Active Breathing Coordinator System; Elekta, Stockholm, Sweden). Studies have shown that ABC devices are consistently reproducible, with low inter- and intrafraction variability, and that these systems can reduce heart and LAD artery dose.32,43–45,47–60,69
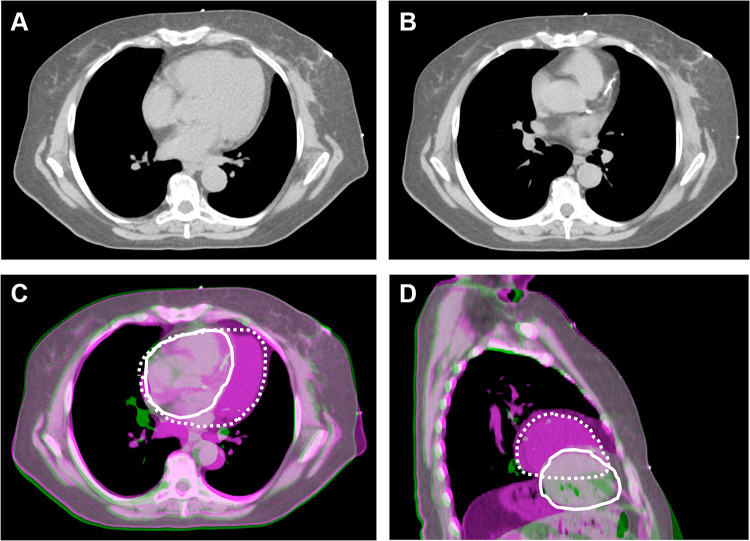
Figure 1.
Cardiac position and proximity to the left breast target volume for a patient simulated in both free-breathing and DIBH positions. Position of the heart in free-breathing (A), demonstrating the proximity of the heart to the left breast and nodal target volume. Position of the heart in DIBH (B), demonstrating that the heart is positioned further away from the target volume. When the free-breathing (purple) and DIBH (green) images are fused based on the left breast target volume, the significant difference in cardiac position in the axial (C) and sagittal (D) planes can be appreciated. The position of the heart is contoured in white (solid white, DIBH; dashed white, FB). With DIBH, the heart is displaced inferiorly and medially, further away from the left lateral chest wall.
Monitoring the respiratory cycle during vDIBH can be accomplished via many different methods. For example, the Varian real-time position management (RPM) system (Varian Medical Systems, Palo Alto, CA, USA) involves placing a device on the patient’s chest which measures vertical displacement throughout the respiratory cycle, creating a tracing of the patient’s breathing. Using the tracing as a guide, the patient is instructed when to hold their breath, which they do voluntarily. The radiation beam can also be programmed to stop when the breathing signal falls outside a predefined threshold. In contrast to traditional respiratory gating, in which the patient is breathing freely, and the beam is repeatedly turned off during a predetermined portion of the respiratory cycle, the treatment beam during respiratory gated vDIBH only turns off when the breath hold is outside of the target range. Respiratory gating during free breathing is an ineffective method for cardiac sparing because the peak inspiratory volume of the natural respiratory cycle does not move the heart as far away from the target as deep inspiration. Studies comparing vDIBH to iDIBH demonstrate that both methods provide comparable improvements in mean heart dose.70 However, vDIBH has shorter daily treatment times, higher patient and therapist satisfaction, and a better cost profile when compared to iDIBH.71,72
There are also more advanced methods of tracking a patient’s respiratory cycle during vDIBH, such as optical tracking. Developed due to concerns that previous methods such as RPM did not correlate well with the actual patient position,73,74 optical tracking systems rely on stereovision to reconstruct the 3D surface of the patient allowing medical providers to visualize the alignment of the reference surface to the reconstructed surface in real-time.75,76 This method of RPM during vDIBH is associated with high patient setup accuracy and reliable chest wall geometry.65,77 It is also possible to improve upon the consistency of the optical tracking system by using it in conjunction with other manual checkpoints (ie, tattoo markings and image guidance).56,63,71 Despite studies of multiple DIBH techniques, there is not a gold standard for DIBH procedures or other cardiac-sparing techniques.72,78 Within the United States, one survey of practicing radiation oncologists found that DIBH is one of the most utilized cardiac-sparing technique with 83% of physicians treating with prone positioning and/or DIBH.78 It is also important to note that heart sparing techniques utilized by physicians depends upon what is available at individual radiation treatment centers. Most physicians who do not use DIBH cite lack of facilities as the reason.78
Patient Selection
DIBH is a relatively simple and non-invasive approach to reduce radiation-induced cardiac toxicities associated with adjuvant breast RT. However, not every patient is able to tolerate DIBH, and not every patient necessarily benefits dosimetrically from DIBH.79 In one study, 29% of 72 women could not adequately complete a DIBH CT scan despite meeting pre-selection criteria and undergoing training.79 Among the patients that underwent successful CT simulation, only two were unable to tolerate DIBH treatment. While this suggests that completion of DIBH at the time of simulation is a positive predictor of treatment completion with DIBH, it also emphasizes the point that women must continue to perform DIBH for 1 to 6 weeks of breast or chestwall radiation therapy. Supporting this, another study found that 20 of 112 patients receiving adjuvant RT using the DIBH technique were unable to tolerate the ABC system.48 Therefore, it is important to deploy DIBH techniques on a case-by-case basis, keeping in mind a patient’s ability to tolerate the technique, the cost, patient convenience, tumor characteristics (ie, laterality, size, location, histology), and whether the patient may benefit from in-advance respiratory training.80–82 Regardless of the technique used, DIBH results in a longer simulation and treatment time compared to free breathing, and patients should be able to lie comfortably in the supine or prone position for the duration of the therapy delivery.
Though evidence suggests that patients with left-sided malignancies derive the most benefit from cardiac sparing techniques due to the proximity of the heart to the left chest wall, patients with right-sided disease may also benefit, especially those receiving regional nodal irradiation.49,50 Regardless of laterality, including the IMC lymph nodes in a patient’s treatment plan is associated with worse cardiac toxicities, largely due to the increased heart dose when compared to breast or chest wall alone treatment.16 In addition to the cardiac benefits of DIBH in right-sided breast cancer patients, there can also be a decline in the dose to the ipsilateral lung and liver.79–83 Therefore, patients with right-sided disease who have pre-existing conditions necessitating the lowest feasible dose to either the lungs or liver, and those whose plan include the IMC lymph nodes, should be considered for DIBH in addition to those with left-sided disease.
In addition to the factors mentioned above, anatomical variations can also be used determine which patients who would receive a benefit from DIBH. Specifically, studies have shown that the maximum heart distance (defined as the maximum distance within the radiation field between the anterior border of the heart contour and the posterior edge of the tangent field), parasagittal cardiac contact distance, and axial contact distance can be used as predictive factors for DIBH selection.10,51,52,84 Regardless of what the estimated benefit may be, it is estimated that 75% of patients who undergo DIBH derive some benefit, defined as a decrease in mean heart dose compared to free breathing.51 Other anatomical differences that are independent of the heart’s distance from the target and that increase the likelihood a patient would benefit from DIBH include pendulous breasts and a more posteriorly positioned tumor bed.44 For patients with pendulous breasts treated in the supine position, the tangential fields tend to extend more inferiorly than they do for patients without this characteristic, thus increasing the radiation dose to the heart. The cardiac dose may also rise for patients with posterior tumor beds, as the boost fields can contribute to cardiac doses due to their proximity to the heart. It is also possible to utilize a rapid planning method to generate a free breathing plan that can calculate the dose to the heart and categorize patients as having favorable or unfavorable cardiac anatomy.32
Because cardiac toxicity is a late sequelae of radiation therapy in patients with breast cancer, there is no direct clinical evidence that quantifies the effect DIBH has on cardiac morbidity and mortality. However, dosimetric analysis of DIBH plans have shown significant improvements in both mean cardiac (25–67%) and mean LAD doses (20–73%) when compared to traditional free breathing plans.32,43–45,47–60 There have been studies that demonstrate that perfusion defects are seen in breast cancer patients who receive RT, and these defects also correlate with the radiation fields used during treatment.12,85 Additionally, these defects were not seen in patients who were treated with fields that excluded the heart entirely.21,86 In contrast, Zellars et al analyzed the perfusion defects seen in patients treated with DIBH and free breathing techniques and found no difference in the rate of perfusion defects.87 Despite conflicting data, it is not unjustified to suggest that DIBH has the potential to reduce radiation-induced cardiac toxicity.
DIBH Treatment Planning
Much like the free breathing technique, DIBH can be used in 3DCRT, IMRT, and arc therapy.36,88,89 The key to selecting the appropriate modality is determining how to best minimize dosimetric variability when intrafraction alterations occur. A more robust plan can lead to a more stable, predictable dose distribution.26 By creating robust DIBH plans, confidence that prescribed and delivered doses correlate increases regardless of changes in patient setup or breast anatomy throughout treatment.26 Examples of free breathing (FB) and DIBH plans for a single patient are shown in Figure 2, with values for the organs at risk for these studies shown in Figure 3. In this patient, significant decreases in doses to the heart and lungs were obtained using the DIBH VMAT plan (Figure 3F). The ability of VMAT to create more robust plans is an attribute noted in prior studies.26
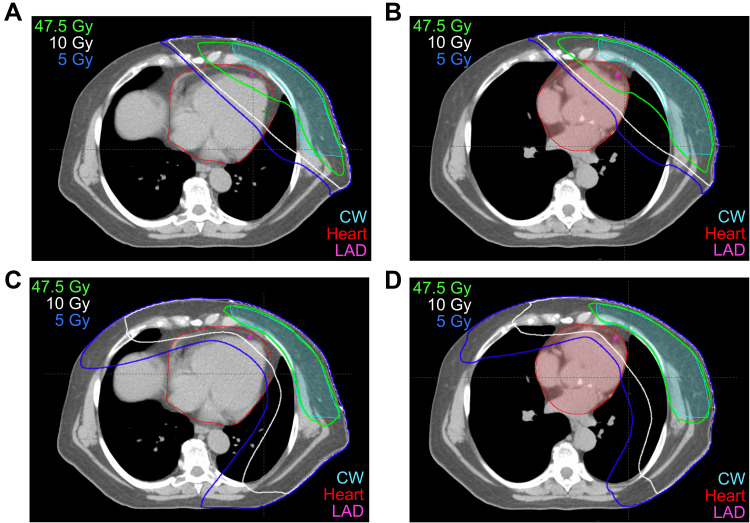
Figure 2.
Axial sections with isodose lines for a patient planned for left chestwall and regional nodal treatment (including the internal mammary chain lymph nodes) using four different techniques. (A) Free breathing (FB) 3DCRT, (B) DIBH 3DCRT, (C) FB VMAT, (D) DIBH VMAT.
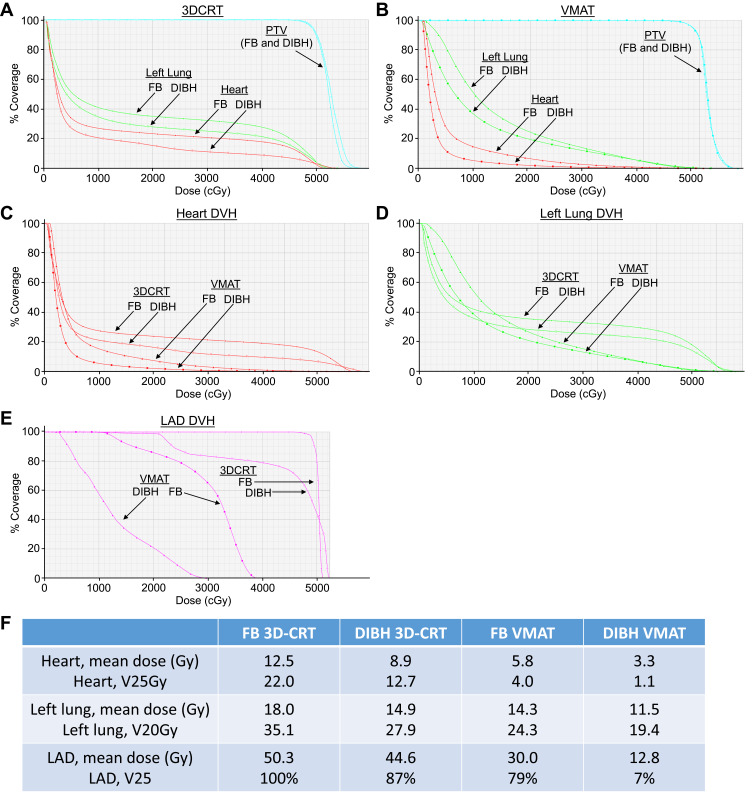
Figure 3.
Dosimetric comparison for the heart and left lung, using free-breathing (FB) 3DCRT, DIBH 3DCRT, FB VMAT and DIBH VMAT for the patient shown in Figure 2. (A) Comparison of dose to PTV (cyan), left lung (green), and heart (red) for FB 3DCRT (closed circles) and DIBH 3DCRT (open circles). (B) Comparison of dose to PTV (cyan), left lung (green), and heart (red) for FB VMAT (triangles) and DIBH VMAT (squares). (C) DVH for the heart with all four planning techniques: FB 3DCRT (closed circles), DIBH 3DCRT (open circles), FB VMAT (triangles), and DIBH VMAT (squares). (D) DVH for the left lung with all four planning techniques: FB 3DCRT (closed circles), DIBH 3DCRT (open circles), FB VMAT (triangles), and DIBH VMAT (squares). (E) DVH for the left anterior descending coronary artery (LAD) with all four planning techniques: FB 3DCRT (closed circles), DIBH 3DCRT (open circles), FB VMAT (squares), and DIBH VMAT (triangles). (F) Comparison of doses to the heart, left lung, and LAD with FB 3DCRT, DIBH 3DCRT, FB VMAT, and DIBH VMAT plans. Note in this patient that DIBH vs FB reduced the percentage of higher doses received by the left lung, heart, and LAD, and that DIBH VMAT showed greater reductions than DIBH 3DCRT and FB VMAT.
Using a wide tangent method in conjunction with DIBH has been shown to reduce cardiac dose even further than other methods and achieves superior IMC dose coverage compared to the traditional photon-electron technique. These factors have made the wide tangent in conjunction with DIBH technique the preferred approach when creating plans that include the IMC lymph nodes.89–91 It is important to note that if using the wide tangent with DIBH technique for a patient where therapeutic dose to the level 1 axillae is desired, delineating the axillary region as a separate target and designing fields that appropriately cover this target is recommended. Not doing so can result in up to a 10% reduction in mean dose to the axillary nodes because the movement of the axillary nodes differs significantly from the tumor bed and breast.92
It has been recommended that a planning organ at risk volume (PRV) with a margin of 0.5 cm be applied to the LAD due to the potential implications of dose to the structure.93 While the inter- and intrafraction variability of the breast during DIBH is minimal when aligning to bony anatomy,65,94 LAD displacement can be more variable during DIBH when compared to free breathing.62 Incorrect prediction of the LAD position can lead to a discrepancy between the predicted and actual cardiac doses. For example, if treatment fields were set up too posteriorly there would be a significant increase in the heart dose compared to what was projected. Conversely, if treatment fields are too anterior there would be a small decrease in the anticipated cardiac dose.
Lastly, creating a PRV for the left ventricle (LV) may also be beneficial. Prior studies have shown that if >5% of the LV were within the radiation field, the incidence of cardiac perfusion defects rises from 10–20% to 50–60%95–98 and that reducing the mean dose to this structure leads to preservation of LV function.85 These perfusion defects correlate with abnormalities in regional wall motion but not with changes in ejection fraction even after long-term follow-up.95–98
An important consideration is selecting plans that minimize dosimetric variability when intrafraction alterations occur, ie, selecting a more “robust” plan, which can lead to a more stable and predictable dose distribution. By optimizing DIBH plans in this manner, confidence that prescribed and delivered doses correlate increases regardless of changes in patient setup or breast anatomy throughout treatment.26
Additional Heart Sparing Techniques
As noted above, multiple techniques exist for sparing cardiac dose, including prone positioning, proton therapy, and lateral decubitus positioning.41 Each of these methods can be used independently or in conjunction with DIBH. When selecting the appropriate cardiac sparing procedure for a patient, it is important to know the differences between each option. Treating a patient in the prone position uses gravity to pull the breast away from the chest wall allowing treatment with shallow tangent beams. In most cases, the heart dose is lower in the prone position than free breathing patients treated in the supine position;39,99,100 however, for a select group of patients, the heart can also be pulled forward by gravity, minimizing the cardiac-sparing impact desired from treating in the prone position.101 Because of this variation treating regional lymph nodes, including the IMC nodes can at times cause increased heart doses using prone positioning.39 However, if treating the regional lymph nodes without inclusion of the IMC, cardiac sparing can still be seen using the prone technique.102,103 When comparing patients treated with free breathing in the prone position to DIBH and supine positioning, the lung dose was noted to be lower in the prone position and heart dose was lower in the supine position.43,71 There is early evidence from a group in Belgium to suggest that combining these two techniques and treating a patient in a prone position with DIBH yields lower lung and heart doses.45
Left-sided breast cancer patients receiving IMC nodal radiation are typically at the highest risk of developing late radiation-induced cardiotoxicity compared to other breast cancer patients due to the increased cardiac doses received. This is especially relevant to young patients who have the highest lifetime cumulative risk of cardiac disease. In younger women, proton therapy may provide additional dosimetric advantages for cardiac radiation exposure, both with and without DIBH.104 In prior studies comparing free breathing passive scatter46 and free breathing intensity modulated105 proton plans to DIBH photon plans, the proton plans yielded lower heart and LAD doses. In a recent study comparing VMAT and IMPT in women with left-sided breast cancer requiring complex planning (IMC nodal radiation, boost) that did not meet normal organ at risk constraints based on 3DCRT planning, IMPT reduced LAD and LV dose relative to photons when combined with and without DIBH.106 For women receiving whole breast irradiation and regional lymph node irradiation, dosimetric analysis demonstrated that DIBH significantly decreased cardiac dose for protons and photons.104 While DIBH reduced the lung dose in photon plans compared to proton free breathing treatment, DIBH increased lung dose in IMPT plans versus free breathing IMPT because the retracted heart was displaced by low-density lung tissue. However, the lung doses with IMPT were still significantly less than those using photons.104 For other organs-at-risk, DIBH resulted in significant dose reductions versus free breathing when using photons, but only led to minor differences in dose deposition between DIBH and shallow breathing with protons. In women with high risk for cardiac and lung cancer mortality, the average thirty-year mortality rates from radiotherapy-related cardiac injury and lung cancer were estimated at 3.1% (photon DIBH), 4.0% (photon shallow breathing), 1.8% (proton DIBH), and 1.7% (proton shallow breathing).104 Other studies have reported similar benefits when combining DIBH and IMPT in left-sided cancers requiring supraclavicular and IMC nodal irradiation.107,108 Other factors should be taken into consideration when combining DIBH with protons. For example, a major advantage of combining DIBH with IMPT is that DIBH will minimize the dose variation from the interplay effect, which is defined as the difference between the absorbed dose volume during motion and when stationary.104 While protons may reduce cardiac dose, is also important to note that due to the physical properties of protons, range uncertainty and variations of RBE and LTE remain a challenge that can lead to increased toxicity.109 Robust planning techniques help reduce uncertainties, but it may limit plan quality and clinical implementation can vary significantly across institutions. Lastly, the availability of proton therapy remains limited and socioeconomic factors play a significant role in proton therapy use.110,111
Conclusions
RT has been proven to be an important part of the breast cancer treatment paradigm due to the improvements in local control and survival radiation provides. However, radiation-induced cardiac toxicity from cardiac exposure is a known side effect of RT that can potentially diminish the positive impact radiation has in this population. Modern improvements in technology and delivery have led to significant decreases in the cardiac doses received by breast cancer patients over time. An important mainstay for cardiac sparing is DIBH, which very often reduces the mean heart and LAD doses in breast cancer patients being treated with RT, especially in patients with left-sided disease and those whose treatment plan includes the IMC lymph nodes. In addition to the cardiac benefits of DIBH, this technique can also result in a decline in ipsilateral lung exposure. Ongoing trials exist that seek to further delineate patient selection and improve upon the current DIBH technique.
Funding Statement
CB is funded by the NIH, R01HL147884.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Favourable and unfavourable effects on long-term survival of radiotherapy for early breast cancer: an overview of the randomised trials. Lancet. 2000;355(9217):1757–1770. doi: 10.1016/S0140-6736(00)02263-7 [DOI] [PubMed] [Google Scholar]
- 3.Hooning MJ, Aleman BMP, van Rosmalen AJM, Kuenen MA, Klijn JGM, van Leeuwen FE. Cause-specific mortality in long-term survivors of breast cancer: a 25-year follow-up study. Int J Radiat Oncol Biol Phys. 2006;64(4):1081–1091. doi: 10.1016/j.ijrobp.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 4.Rutqvist LE, Johansson H. Mortality by laterality of the primary tumour among 55,000 breast cancer patients from the Swedish Cancer Registry. Br J Cancer. 1990;61(6):866–868. doi: 10.1038/bjc.1990.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer. 2013;108(1):179–182. doi: 10.1038/bjc.2012.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlson LE, Watt GP, Tonorezos ES, et al. Coronary artery disease in young women after radiation therapy for breast cancer. JACC. 2021;3(3):381–392. doi: 10.1016/j.jaccao.2021.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Bogaard VAB, Ta BDP, van der Schaaf A, et al. Validation and modification of a prediction model for acute cardiac events in patients with breast cancer treated with radiotherapy based on three-dimensional dose distributions to cardiac substructures. J Clin Oncol. 2017;35(11):1171–1178. doi: 10.1200/JCO.2016.69.8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. New Engl J Med. 2013;368(11):987–998. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6(8):557–565. doi: 10.1016/S1470-2045(05)70251-5 [DOI] [PubMed] [Google Scholar]
- 10.Taylor CW, McGale P, Povall JM, et al. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys. 2009;73(4):1061–1068. doi: 10.1016/j.ijrobp.2008.05.066 [DOI] [PubMed] [Google Scholar]
- 11.Gyenes G, Rutqvist LE, Liedberg A, Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol. 1998;48(2):185–190. doi: 10.1016/S0167-8140(98)00062-0 [DOI] [PubMed] [Google Scholar]
- 12.Gyenes G. Detection of radiation-induced myocardial damage by technetium-99m sestamibi scintigraphy. Eur J Nucl Med. 1997;24(3):7. [DOI] [PubMed] [Google Scholar]
- 13.Jacob S, Camilleri J, Derreumaux S, et al. Is mean heart dose a relevant surrogate parameter of left ventricle and coronary arteries exposure during breast cancer radiotherapy: a dosimetric evaluation based on individually-determined radiation dose (BACCARAT study). Radiother Oncol. 2019;14(1):29. doi: 10.1186/s13014-019-1234-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson G, Holmberg L, Garmo H, et al. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30(4):380–386. doi: 10.1200/JCO.2011.34.5900 [DOI] [PubMed] [Google Scholar]
- 15.Moignier A, Broggio D, Derreumaux S, et al. Coronary stenosis risk analysis following Hodgkin lymphoma radiotherapy: a study based on patient specific artery segments dose calculation. Radiother Oncol. 2015;117(3):467–472. doi: 10.1016/j.radonc.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 16.Chargari C, Castadot P, MacDermed D, et al. Internal mammary lymph node irradiation contributes to heart dose in breast cancer. Med Dosimet. 2010;35(3):163–168. doi: 10.1016/j.meddos.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 17.Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. New Engl J Med. 2015;373(4):317–327. doi: 10.1056/NEJMoa1415369 [DOI] [PubMed] [Google Scholar]
- 18.Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. New Engl J Med. 2015;373(4):307–316. doi: 10.1056/NEJMoa1415340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12(3):447–453. doi: 10.1200/JCO.1994.12.3.447 [DOI] [PubMed] [Google Scholar]
- 20.Bouillon K, Haddy N, Delaloge S, et al. Long-term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57(4):445–452. doi: 10.1016/j.jacc.2010.08.638 [DOI] [PubMed] [Google Scholar]
- 21.Zagar TM, Marks LB. Breast cancer radiotherapy and coronary artery stenosis: location, location, location. J Clin Oncol. 2012;30(4):350–352. doi: 10.1200/JCO.2011.38.9304 [DOI] [PubMed] [Google Scholar]
- 22.Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419–424. doi: 10.1093/jnci/dji067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paszat F, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol. 1998;16(8):2625–2631. doi: 10.1200/JCO.1998.16.8.2625 [DOI] [PubMed] [Google Scholar]
- 24.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose–volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76(3):S77–S85. doi: 10.1016/j.ijrobp.2009.04.093 [DOI] [PubMed] [Google Scholar]
- 25.Patt DA, Goodwin JS, Kuo YF, et al. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23(30):7475–7482. doi: 10.1200/JCO.2005.13.755 [DOI] [PubMed] [Google Scholar]
- 26.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488 [DOI] [PubMed] [Google Scholar]
- 27.Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008 [DOI] [PubMed] [Google Scholar]
- 28.Mihai A, Rakovitch E, Sixel K, et al. Inverse vs. forward breast IMRT planning. Med Dosimet. 2005;30(3):149–154. doi: 10.1016/j.meddos.2005.03.004 [DOI] [PubMed] [Google Scholar]
- 29.Beckham WA, Popescu CC, Patenaude VV, Wai ES, Olivotto IA. Is multibeam IMRT better than standard treatment for patients with left-sided breast cancer? Int J Radiat Oncol Biol Phys. 2007;69(3):918–924. doi: 10.1016/j.ijrobp.2007.06.060 [DOI] [PubMed] [Google Scholar]
- 30.Krueger EA, Fraass BA, McShan DL, Marsh R, Pierce LJ. Potential gains for irradiation of chest wall and regional nodes with intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56(4):1023–1037. doi: 10.1016/S0360-3016(03)00183-4 [DOI] [PubMed] [Google Scholar]
- 31.Ho AY, Ballangrud A, Li G, et al. Long-term pulmonary outcomes of a feasibility study of inverse-planned, multibeam intensity modulated radiation therapy in node-positive breast cancer patients receiving regional nodal irradiation. Int J Radiat Oncol Biol Phys. 2019;103(5):1100–1108. doi: 10.1016/j.ijrobp.2018.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Jie W, Liang Z, Wu H, Cheng J. Postmastectomy intensity modulation radiated therapy of chest wall and regional nodes: retrospective analysis of the performance and complications up for 5 years. Medicine. 2017;96(39). doi: 10.1097/md.0000000000007956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick B, Hunt M. Intensity-modulated radiation therapy for breast: is it for everyone? Semin Radiat Oncol. 2011;21(1):51–54. doi: 10.1016/j.semradonc.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 34.Bazan JG, Healy E, Beyer S, et al. Clinical effectiveness of an adaptive treatment planning algorithm for intensity modulated radiation therapy versus 3D conformal radiation therapy for node-positive breast cancer patients undergoing regional nodal irradiation/postmastectomy radiation therapy. Int J Radiat Oncol Biol Phys. 2020;108(5):1159–1171. doi: 10.1016/j.ijrobp.2020.07.027 [DOI] [PubMed] [Google Scholar]
- 35.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys. 2010;76(1):287–295. doi: 10.1016/j.ijrobp.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 36.Osman SOS, Hol S, Poortmans PM, Essers M. Volumetric modulated arc therapy and breath-hold in image-guided locoregional left-sided breast irradiation. Radiother Oncol. 2014;112(1):17–22. doi: 10.1016/j.radonc.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Huang Y, Ding S, et al. A dosimetric and radiobiological evaluation of VMAT following mastectomy for patients with left-sided breast cancer. Radiother Oncol. 2021;16(1):171. doi: 10.1186/s13014-021-01895-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sardaro A, Petruzzelli MF, D’Errico MP, Grimaldi L, Pili G, Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 2012;103(2):133–142. doi: 10.1016/j.radonc.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 39.Formenti SC, DeWyngaert JK, Jozsef G, Goldberg JD. Prone vs supine positioning for breast cancer radiotherapy. JAMA. 2012;308(9):861–863. doi: 10.1001/2012.jama.10759 [DOI] [PubMed] [Google Scholar]
- 40.Bergom C, Kelly T, Morrow N, et al. Prone whole-breast irradiation using three-dimensional conformal radiotherapy in women undergoing breast conservation for early disease yields high rates of excellent to good cosmetic outcomes in patients with large and/or pendulous breasts. Int J Radiat Oncol Biol Phys. 2012;83(3):821–828. doi: 10.1016/j.ijrobp.2011.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campana F, Kirova YM, Rosenwald JC, et al. Breast radiotherapy in the lateral decubitus position: a technique to prevent lung and heart irradiation. Int J Radiat Oncol Biol Phys. 2005;61(5):1348–1354. doi: 10.1016/j.ijrobp.2004.08.051 [DOI] [PubMed] [Google Scholar]
- 42.Latty D, Stuart KE, Wang W, Ahern V. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci. 2015;62(1):74–81. doi: 10.1002/jmrs.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verhoeven K, Sweldens C, Petillion S, et al. Breathing adapted radiation therapy in comparison with prone position to reduce the doses to the heart, left anterior descending coronary artery, and contralateral breast in whole breast radiation therapy. Pract Radiat Oncol. 2014;4(2):123–129. doi: 10.1016/j.prro.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 44.Bergom C, Currey A, Desai N, Tai A, Strauss JB. Deep inspiration breath hold: techniques and advantages for cardiac sparing during breast cancer irradiation. Front Oncol. 2018;8:87. doi: 10.3389/fonc.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mulliez T, Veldeman L, Speleers B, et al. Heart dose reduction by prone deep inspiration breath hold in left-sided breast irradiation. Radiother Oncol. 2015;114(1):79–84. doi: 10.1016/j.radonc.2014.11.038 [DOI] [PubMed] [Google Scholar]
- 46.Bradley JA, Dagan R, Ho MW, et al. Initial report of a prospective dosimetric and clinical feasibility trial demonstrates the potential of protons to increase the therapeutic ratio in breast cancer compared with photons. Int J Radiat Oncol Biol Phys. 2016;95(1):411–421. doi: 10.1016/j.ijrobp.2015.09.018 [DOI] [PubMed] [Google Scholar]
- 47.Vikström J, Hjelstuen MHB, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncologica. 2011;50(1):42–50. doi: 10.3109/0284186X.2010.512923 [DOI] [PubMed] [Google Scholar]
- 48.Eldredge-Hindy HB, Duffy D, Yamoah K, et al. Modeled risk of ischemic heart disease following left breast irradiation with deep inspiration breath hold. Pract Radiat Oncol. 2015;5(3):162–168. doi: 10.1016/j.prro.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 49.Mohamad O, Shiao J, Zhao B, et al. Deep inspiration breathhold for left-sided breast cancer patients with unfavorable cardiac anatomy requiring internal mammary nodal irradiation. Pract Radiat Oncol. 2017;7(6):e361–e367. doi: 10.1016/j.prro.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 50.Yeung R, Conroy L, Long K, et al. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiother Oncol. 2015;10(1):200. doi: 10.1186/s13014-015-0511-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rochet N, Drake JI, Harrington K, et al. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract Radiat Oncol. 2015;5(3):e127–e134. doi: 10.1016/j.prro.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 52.Lee G, Rosewall T, Fyles A, Harnett N, Dinniwell RE. Anatomic features of interest in women at risk of cardiac exposure from whole breast radiotherapy. Radiother Oncol. 2015;115(3):355–360. doi: 10.1016/j.radonc.2015.05.002 [DOI] [PubMed] [Google Scholar]
- 53.Bruzzaniti V, Abate A, Pinnarò P, et al. Dosimetric and clinical advantages of deep inspiration breath-hold (DIBH) during radiotherapy of breast. J Exp Clin Cancer Res. 2013;32(1):88. doi: 10.1186/1756-9966-32-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mast ME, van Kempen-Harteveld L, Heijenbrok MW, et al. Left-sided breast cancer radiotherapy with and without breath-hold: does IMRT reduce the cardiac dose even further? Radiother Oncol. 2013;108(2):248–253. doi: 10.1016/j.radonc.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 55.Swanson T, Grills I, Ye H, et al. Six-year experience routinely utilizing moderate deep inspiration breath-hold (mDIBH) for the reduction of cardiac dose in left-sided breast irradiation for patients with early stage or locally advanced breast cancer. Am J Clin Oncol. 2013;36(1):24–30. doi: 10.1097/COC.0b013e31823fe481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borst GR, Sonke JJ, den Hollander S, et al. Clinical results of image-guided deep inspiration breath hold breast irradiation. Int J Radiat Oncol Biol Phys. 2010;78(5):1345–1351. doi: 10.1016/j.ijrobp.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 57.Hayden AJ, Rains M, Tiver K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J Med Imaging Radiat Oncol. 2012;56(4):464–472. doi: 10.1111/j.1754-9485.2012.02405.x [DOI] [PubMed] [Google Scholar]
- 58.Tanguturi SK, Lyatskaya Y, Chen Y, et al. Prospective assessment of deep inspiration breath-hold using 3-dimensional surface tracking for irradiation of left-sided breast cancer. Pract Radiat Oncol. 2015;5(6):358–365. doi: 10.1016/j.prro.2015.06.002 [DOI] [PubMed] [Google Scholar]
- 59.Walston S, Quick AM, Kuhn K, Rong Y. Dosimetric Considerations in respiratory-gated deep inspiration breath-hold for left breast irradiation. Technol Cancer Res Treat. 2017;16(1):22–32. doi: 10.1177/1533034615624311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hong JC, Rahimy E, Gross CP, et al. Radiation dose and cardiac risk in breast cancer treatment: an analysis of modern radiation therapy including community settings. Pract Radiat Oncol. 2018;8(3):e79–e86. doi: 10.1016/j.prro.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 61.Dumane VA, Saksornchai K, Zhou Y, Hong L, Powell S, Ho AY. Reduction in low-dose to normal tissue with the addition of deep inspiration breath hold (DIBH) to volumetric modulated arc therapy (VMAT) in breast cancer patients with implant reconstruction receiving regional nodal irradiation. Radiother Oncol. 2018;13(1):187. doi: 10.1186/s13014-018-1132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagsi R, Griffith KA, Moran JM, et al. A randomized comparison of radiation therapy techniques in the management of node-positive breast cancer: primary outcomes analysis. Int J Radiat Oncol Biol Phys. 2018;101(5):1149–1158. doi: 10.1016/j.ijrobp.2018.04.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jensen CA, Roa AMA, Johansen M, Jå L, Frengen J. Robustness of VMAT and 3DCRT plans toward setup errors in radiation therapy of locally advanced left- sided breast cancer with DIBH. Physica Medica. 2018;45:12–18. doi: 10.1016/j.ejmp.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 64.Kimura T, Murakami Y, Kenjo M, et al. Interbreath-hold reproducibility of lung tumour position and reduction of the internal target volume using a voluntary breath-hold method with spirometer during stereotactic radiotherapy for lung tumours. Br J Radiol. 2007;80(953):355–361. doi: 10.1259/bjr/31008031 [DOI] [PubMed] [Google Scholar]
- 65.Betgen A, Alderliesten T, Sonke JJ, van Vliet-Vroegindeweij C, Bartelink H, Remeijer P. Assessment of set-up variability during deep inspiration breath hold radiotherapy for breast cancer patients by 3D-surface imaging. Radiother Oncol. 2013;106(2):225–230. doi: 10.1016/j.radonc.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 66.McConnell K, Kirby N, Rasmussen K, Gutierrez AN, Papanikolaou N, Stanley D. Variability of breast surface positioning using an active breathing coordinator for a deep inspiration breath hold technique. Cureus. 2021;13(6). doi: 10.7759/cureus.15649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44(4):911–919. doi: 10.1016/S0360-3016(99)00056-5 [DOI] [PubMed] [Google Scholar]
- 68.Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55(2):392–406. doi: 10.1016/s0360-3016(02)04143-3 [DOI] [PubMed] [Google Scholar]
- 69.Nissen HD, Appelt AL. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106(1):28–32. doi: 10.1016/j.radonc.2012.10.016 [DOI] [PubMed] [Google Scholar]
- 70.Ranger A, Dunlop A, Grimwood A, et al. Voluntary versus ABC breath-hold in the context of VMAT for breast and locoregional lymph node radiotherapy including the internal mammary chain. Clin Transl Radiat Oncol. 2021;27:164–168. doi: 10.1016/j.ctro.2021.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartlett FR, Colgan RM, Carr K, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol. 2013;108(2):242–247. doi: 10.1016/j.radonc.2013.04.021 [DOI] [PubMed] [Google Scholar]
- 72.Macrie BD, Donnelly ED, Hayes JP, et al. A cost-effective technique for cardiac sparing with deep inspiration-breath hold (DIBH). Physica Medica. 2015;31(7):733–737. doi: 10.1016/j.ejmp.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 73.Rong Y, Walston S, Welliver MX, Chakravarti A, Quick AM. Improving intra-fractional target position accuracy using a 3d surface surrogate for left breast irradiation using the respiratory-gated deep-inspiration breath-hold technique. PLoS One. 2014;9(5):e97933. doi: 10.1371/journal.pone.0097933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kubo HD, Len PM, Sichi M, Mostafavi H. Breathing-synchronized radiotherapy program at the University of California Davis Cancer Center. Med Phys. 2000;27(2):346–353. doi: 10.1118/1.598837 [DOI] [PubMed] [Google Scholar]
- 75.Peng JL, Kahler D, Li JG, et al. Characterization of a real-time surface image-guided stereotactic positioning system. Med Phys. 2010;37(10):5421–5433. doi: 10.1118/1.3483783 [DOI] [PubMed] [Google Scholar]
- 76.Alderliesten T, Sonke JJ, Betgen A, Honnef J, van Vliet-Vroegindeweij C, Remeijer P. Accuracy evaluation of a 3-dimensional surface imaging system for guidance in deep-inspiration breath-hold radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(2):536–542. doi: 10.1016/j.ijrobp.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 77.Tang X, Zagar TM, Bair E, et al. Clinical experience with 3-dimensional surface matching-based deep inspiration breath hold for left-sided breast cancer radiation therapy. Pract Radiat Oncol. 2014;4(3):e151–e158. doi: 10.1016/j.prro.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 78.Desai N, Currey A, Kelly T, Bergom C. Nationwide trends in heart-sparing techniques utilized in radiation therapy for breast cancer. Adv Radiat Oncol. 2019;4(2):246–252. doi: 10.1016/j.adro.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schröder C, Kirschke S, Blank E, Rohrberg S, Förster R, Buchali A. Deep inspiration breath-hold for patients with left-sided breast cancer - A one-fits-all approach? A prospective analysis of patient selection using dosimetrical and practical aspects. Br J Radiol. 2021;20210295. doi: 10.1259/bjr.20210295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalet AM, Kim A, Hippe DS, et al. The dosimetric benefit of in-advance respiratory training for deep inspiration breath holding is realized during daily treatment in left breast radiotherapy: a comparative retrospective study of serial surface motion tracking. J Med Imaging Radiat Oncol. 2021;65(3):354–364. doi: 10.1111/1754-9485.13181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mayr NA, Borm KJ, Kalet AM, et al. Reducing cardiac radiation dose from breast cancer radiation therapy with breath hold training and cognitive behavioral therapy. Top Magn Reson Imaging. 2020;29(3):135–148. doi: 10.1097/RMR.0000000000000241 [DOI] [PubMed] [Google Scholar]
- 82.Kim A, Kalet AM, Cao N, et al. Effects of preparatory coaching and home practice for deep inspiration breath hold on cardiac dose for left breast radiation therapy. Clin Oncol. 2018;30(9):571–577. doi: 10.1016/j.clon.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 83.Conway JL, Conroy L, Harper L, et al. Deep inspiration breath-hold produces a clinically meaningful reduction in ipsilateral lung dose during locoregional radiation therapy for some women with right-sided breast cancer. Pract Radiat Oncol. 2017;7(3):147–153. doi: 10.1016/j.prro.2016.10.011 [DOI] [PubMed] [Google Scholar]
- 84.Kong FM, Klein EE, Bradley JD, et al. The impact of central lung distance, maximal heart distance, and radiation technique on the volumetric dose of the lung and heart for intact breast radiation. Int J Radiat Oncol Biol Phys. 2002;54(3):963–971. doi: 10.1016/S0360-3016(02)03741-0 [DOI] [PubMed] [Google Scholar]
- 85.Evans ES, Prosnitz RG, Yu X, et al. Impact of patient-specific factors, irradiated left ventricular volume, and treatment set-up errors on the development of myocardial perfusion defects after radiation therapy for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2006;66(4):1125–1134. doi: 10.1016/j.ijrobp.2006.06.025 [DOI] [PubMed] [Google Scholar]
- 86.Chung E, Corbett JR, Moran JM, et al. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys. 2013;85(4):959–964. doi: 10.1016/j.ijrobp.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zellars R, Bravo PE, Tryggestad E, et al. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: results of a randomized Phase 3 trial. Int J Radiat Oncol Biol Phys. 2014;88(4):778–785. doi: 10.1016/j.ijrobp.2013.12.035 [DOI] [PubMed] [Google Scholar]
- 88.Bolukbasi Y, Saglam Y, Selek U, et al. Reproducible deep-inspiration breath-hold irradiation with forward intensity-modulated radiotherapy for left-sided breast cancer significantly reduces cardiac radiation exposure compared to inverse intensity-modulated radiotherapy. Tumori J. 2014;100(2):169–178. doi: 10.1177/030089161410000209 [DOI] [PubMed] [Google Scholar]
- 89.Stranzl H, Zurl B, Langsenlehner T, Kapp KS. Wide tangential fields including the internal mammary lymph nodes in patients with left-sided breast cancer. Strahlenther Onkol. 2009;185(3):155–160. doi: 10.1007/s00066-009-1939-2 [DOI] [PubMed] [Google Scholar]
- 90.Pierce LJ, Butler JB, Martel MK, et al. Postmastectomy radiotherapy of the chest wall: dosimetric comparison of common techniques. Int J Radiat Oncol Biol Phy. 2002;52(5):1220–1230. doi: 10.1016/S0360-3016(01)02760-2 [DOI] [PubMed] [Google Scholar]
- 91.Thomsen MS, Berg M, Nielsen HM, et al. Post-mastectomy radiotherapy in Denmark: from 2D to 3D treatment planning guidelines of The Danish Breast Cancer Cooperative Group. Acta Oncologica. 2008;47(4):654–661. doi: 10.1080/02841860801975000 [DOI] [PubMed] [Google Scholar]
- 92.Borm KJ, Oechsner M, Combs SE, Duma MN. Deep-inspiration breath-hold radiation therapy in breast cancer: a word of caution on the dose to the axillary lymph node levels. Int J Radiat Oncol Biol Phys. 2018;100(1):263–269. doi: 10.1016/j.ijrobp.2017.09.026 [DOI] [PubMed] [Google Scholar]
- 93.Wang W, Purdie TG, Rahman M, Marshall A, Liu FF, Fyles A. Rapid automated treatment planning process to select breast cancer patients for active breathing control to achieve cardiac dose reduction. Int J Radiat Oncol Biol Phys. 2012;82(1):386–393. doi: 10.1016/j.ijrobp.2010.09.026 [DOI] [PubMed] [Google Scholar]
- 94.Gierga DP, Turcotte JC, Sharp GC, Sedlacek DE, Cotter CR, Taghian AG. A voluntary breath-hold treatment technique for the left breast with unfavorable cardiac anatomy using surface imaging. Int J Radiat Oncol Biol Phys. 2012;84(5):e663–e668. doi: 10.1016/j.ijrobp.2012.07.2379 [DOI] [PubMed] [Google Scholar]
- 95.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63(1):214–223. doi: 10.1016/j.ijrobp.2005.01.029 [DOI] [PubMed] [Google Scholar]
- 96.Lind PA, Pagnanelli R, Marks LB, et al. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys. 2003;55(4):914–920. doi: 10.1016/S0360-3016(02)04156-1 [DOI] [PubMed] [Google Scholar]
- 97.Gyenes G, Fornander T, Carlens P, Glas U, Rutqvist LE. Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: a prospective study. Int J Radiat Oncol Biol Phys. 1996;36(4):899–905. doi: 10.1016/S0360-3016(96)00125-3 [DOI] [PubMed] [Google Scholar]
- 98.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64(1):53–63. doi: 10.1016/S0167-8140(02)00133-0 [DOI] [PubMed] [Google Scholar]
- 99.Lymberis SC, deWyngaert JK, Parhar P, et al. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: volumetric and dosimetric correlations in 100 patients. Int J Radiat Oncol Biol Phys. 2012;84(4):902–909. doi: 10.1016/j.ijrobp.2012.01.040 [DOI] [PubMed] [Google Scholar]
- 100.Griem KL, Fetherston P, Kuznetsova M, Foster GS, Shott S, Chu J. Three-dimensional photon dosimetry: a comparison of treatment of the intact breast in the supine and prone position. Int J Radiat Oncol Biol Phys. 2003;57(3):891–899. doi: 10.1016/S0360-3016(03)00723-5 [DOI] [PubMed] [Google Scholar]
- 101.Chino JP, Marks LB. Prone positioning causes the heart to be displaced anteriorly within the thorax: implications for breast cancer treatment. Int J Radiat Oncol Biol Phys. 2008;70(3):916–920. doi: 10.1016/j.ijrobp.2007.11.001 [DOI] [PubMed] [Google Scholar]
- 102.Deseyne P, Speleers B, De Neve W, et al. Whole breast and regional nodal irradiation in prone versus supine position in left sided breast cancer. Radiother Oncol. 2017;12(1):89. doi: 10.1186/s13014-017-0828-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gielda BT, Strauss JB, Marsh JC, et al. A dosimetric comparison between the supine and prone positions for three-field intact breast radiotherapy. Am J Clin Oncol. 2011;34:223–230. doi: 10.1097/COC.0b013e3181dbb9c1 [DOI] [PubMed] [Google Scholar]
- 104.Speleers B, Schoepen M, Belosi F, et al. Effects of deep inspiration breath hold on prone photon or proton irradiation of breast and regional lymph nodes. Sci Rep. 2021;11(1):6085. doi: 10.1038/s41598-021-85401-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jie AW, Marignol L. Pro-con of proton: dosimetric advantages of intensity-modulation over passive scatter for thoracic malignancies. Tech Innov Patient Support Radiat Oncol. 2020;15:37–46. doi: 10.1016/j.tipsro.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loap P, Goudjil F, Dendale R, Kirova Y. Clinical and technical considerations for mediastinal Hodgkin lymphoma proton therapy based on a single-center early experience. Cancer Radiother. 2021;25(8):779–785. doi: 10.1016/j.canrad.2021.06.016 [DOI] [PubMed] [Google Scholar]
- 107.Mondal D, Jhawar SR, Millevoi R, Haffty BG, Parikh RR. Proton versus photon breath-hold radiation for left-sided breast cancer after breast-conserving surgery: a dosimetric comparison. Int J Part Ther. 2021;7(3):24–33. doi: 10.14338/IJPT-20-00026.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cartechini G, Fracchiolla F, Menegotti L, et al. Proton pencil beam scanning reduces secondary cancer risk in breast cancer patients with internal mammary chain involvement compared to photon radiotherapy. Radiother Oncol. 2020;15(1):228. doi: 10.1186/s13014-020-01671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang CC, McNamara AL, Shin J, et al. End-of-range radiobiological effect on rib fractures in patients receiving proton therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2020;107(3):449–454. doi: 10.1016/j.ijrobp.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lawell MP, Bajaj BVM, Gallotto SL, et al. Increased distance from a treating proton center is associated with diminished ability to follow patients enrolled on a multicenter radiation oncology registry. Radiother Oncol. 2019;134:25–29. doi: 10.1016/j.radonc.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 111.Shen CJ, Hu C, Ladra MM, Narang AK, Pollack CE, Terezakis SA. Socioeconomic factors affect the selection of proton radiation therapy for children. Cancer. 2017;123(20):4048–4056. doi: 10.1002/cncr.30849 [DOI] [PubMed] [Google Scholar]