Abstract
Objective
Increasing evidence shows a close relationship between gut microbiota and major depressive disorder (MDD), but the specific mechanisms remain unknown. This study was conducted to explore differential gut microbiota compositions related to the severity of MDD.
Methods
Healthy controls (HC) (n = 131) and MDD patients (n = 130) were included. MDD patients with Hamilton Depression Rating Scale (HDRS) score <25 and ≥25 were assigned into moderate (n = 72) and severe (n = 58) MDD groups, respectively. Univariate and multivariate analyses were used to analyze the gut microbiota compositions at the genus level.
Results
Thirty-six and 27 differential genera were identified in moderate and severe MDD patients, respectively. The differential genera in moderate and severe MDD patients mainly belonged to three (Firmicutes, Actinobacteriota, and Bacteroidota) and two phyla (Firmicutes and Bacteroidota), respectively. One specific covarying network from phylum Actinobacteriota was identified in moderate MDD patients. In addition, five genera (Collinsella, Eggerthella, Alistipes, Faecalibacterium, and Flavonifractor) from the shared differential genera by two MDD groups had a fair efficacy in diagnosing MDD from HC (AUC = 0.786).
Conclusions
Our results were helpful for further exploring the role of gut microbiota in the pathogenesis of depression and developing objective diagnostic methods for MDD.
Keywords: major depressive disorder, gut microbiota, Firmicutes, Actinobacteriota, Bacteroidota
Background
Major depressive disorder (MDD) is a common but serious neuropsychiatric disorder that can greatly affect the patients’ quality of life (Martins-de-Souza, 2014; Zhu et al., 2020; Liu et al., 2021; Tian et al., 2021). It is mainly characterized by emptiness or hopelessness, loss of interest, and sleep disturbances (Rana et al., 2021). Previous studies reported that MDD was closely related to hippocampal atrophy, disorder of the hypothalamic–pituitary–adrenal (HPA) axis, and reduction of glial cells in the prefrontal cortex (Ongür et al., 1998; Campbell and Macqueen, 2004; Pariante and Lightman, 2008). However, commonly accepted theories about the pathogenesis of MDD are still not available. Meanwhile, the first-line treatment according to these theories can only alleviate symptoms in about half of MDD patients (Al-Harbi, 2012), and there are no validated biomarkers for objective diagnosis of MDD nowadays. Thus, it is urgently needed to further study the pathogenesis of MDD from new perspectives.
Gut microbiota plays an important role in maintaining the host’s health, and many researchers pay attention to the cross talk between the gut and brain (Han et al., 2020; Qiao et al., 2020; Kovács et al., 2021; Khoshkam et al., 2021). Mounting evidence shows that gut microbiota can affect the host’s brain functions and behaviors via the “microbiota–gut–brain” axis (Chen et al., 2021; Rajput et al., 2021; Ding et al., 2021; Dong et al., 2021). In our previous work, we found significant differences in gut microbiota compositions between MDD patients and healthy controls (HC) (Chen et al., 2020; Bai et al., 2021), and these differences were specifically relative to bipolar disorder and schizophrenia (Zheng et al., 2019; Zheng et al., 2020). Other researchers also found that some bacterial taxa, such as Flavonifractor and Faecalibacterium, changed in patients with depression (Coello et al., 2019; Zhou et al., 2020; Coello et al., 2021). Using an animal depression model, we reported that gut microbiota could induce depression-like behaviors by regulating the host’s metabolism (Zheng et al., 2016) and that glycerophospholipid metabolism might be the vital node between microbiota and depression (Tian et al., 2022). These findings suggested that further exploring the role of gut microbiota in the onset of depression may be helpful for revealing the pathogenesis of MDD.
Many metabolites produced by gut microbiota are closely related to health (Lu et al., 2021; Zhong et al., 2021; Xie et al., 2021). Short-chain fatty acids (SCFAs), as the main products of gut microbiota, have been found to change in many diseases such as cardiovascular disease and autism (Chambers et al., 2018; Tran and Mohajeri, 2021). Our previous studies found some differential microbial metabolites in the urine and plasma of MDD patients (Zheng et al., 2013; Zheng et al., 2013; Chen et al., 2015). Moreover, we found differential urinary and plasma metabolites related to the severity of MDD (Liu et al., 2015; Chen et al., 2017). Considering the close relationships between metabolites and gut microbiota in MDD, we conducted this study to explore whether the differences in gut microbiota compositions were also related with the severity of MDD.
Methods
Subject Recruitments
This study was approved by the Ethical Committee of Chongqing Medical University (No. 20200320), and all the included subjects provided written informed consent. Subjects meeting the fourth Diagnostic and Statistical Manual of Mental Disorders criteria for MDD (DSM-IV) were included as MDD patients. HC were from the Medical Examination Center. In total, 131 HC and 130 MDD patients were included from our previous studies (Chen et al., 2020; Bai et al., 2021). There were 21 MDD patients receiving antidepressants (mainly citalopram, fluoxetine, paroxetine, sertraline, and venlafaxine) for 1 month prior to sample collections. In the MDD group, 78 patients with Hamilton Depression Rating Scale (HDRS) score <25 were assigned into the moderate MDD group, and the other 52 patients with HDRS score ≥25 were assigned into the severe MDD group (Kriston and von Wolff, 2011; Liu et al., 2015; Chen et al., 2017). The age, body mass index (BMI), and sex ratio were matched among the three groups. The detailed information of these subjects is found in Table 1.
Table 1.
Characteristics of the included subjects.
| HC | Moderate MDD | p-valuea | Severe MDD | p-valueb | Total MDD | p-valuec | |
|---|---|---|---|---|---|---|---|
| Number | 131 | 78 | – | 52 | – | 130 | – |
| Age | 37.07 (14.22) | 35.77 (13.92) | 0.80 | 37.88 (15.5) | 0.93 | 36.61 (14.57) | 0.79 |
| Sex (F/M) | 89/42 | 53/25 | 0.99 | 35/17 | 0.93 | 88/42 | 0.96 |
| BMI | 21.95 (3.48) | 21.76 (2.42) | 0.96 | 21.70 (2.70) | 0.94 | 21.74 (2.52) | 0.57 |
| HDRS | 0.48 (0.83) | 20.47 (2.26) | <0.00001 | 29.27 (3.70) | <0.00001 | 23.99 (5.21) | <0.00001 |
| Medication | 0/131 | 13/65 | <0.00001 | 8/44 | <0.00001 | 21/109 | <0.00001 |
ap-value was from HC vs. moderate MDD; bp-value was from HC vs. severe MDD; cp-value was from HC vs. total MDD.
HC, healthy controls; MDD, major depressive disorder; F, female; M, male; BMI, body mass index; HDRS, Hamilton Depression Rating Scale.
Gut Microbiota Compositions
The procedures for the measurement of gut microbiota compositions were identical to our previous studies (Chen et al., 2020; Bai et al., 2021). Briefly, after the raw 16S rRNA gene sequencing reads were obtained using the Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, USA), they were then demultiplexed, quality-filtered by FASTP (version 0.20.0), and merged by FLASH (version 1.2.7) with the following criteria. (i) The 300-bp reads were truncated at any site receiving an average quality score of <20 over a 50-bp sliding window, and the truncated reads shorter than 50 bp were discarded. (ii) Only overlapping sequences longer than 10 bp were assembled according to their overlapped sequence. The maximum mismatch ratio of the overlap region was 0.2. Reads that could not be assembled were discarded. (iii) Exact barcode matching, two-nucleotide mismatch in primer matching, and reads containing ambiguous characters were removed. The operational taxonomic units (OTUs) with 97% similarity cutoff were clustered using UPARSE (version 7.1), and chimeric sequences were removed. The taxonomy of each OTU representative sequence was analyzed by RDP Classifier (version 2.2) against the 16S rRNA database using a confidence threshold of 0.7. At last, we obtained the relative abundances of gut microbiota at different levels. In this study, we analyzed the abundance score for each genus in the three groups.
Statistical Analysis
Firstly, the Student’s t-test, non-parametric test, chi-square test, or one-way analysis was used to check whether there were significant differences on the demographic data among the three groups (Ma et al., 2021). Secondly, the orthogonal partial least-square discriminant analysis (OPLS-DA) was used to identify the differential genera responsible for the discrimination between MDD patients and HC. Here, the default seven-round cross-validation in OPLS-DA was applied. The genus with important variables on the projection (VIP) > 1.0 (equivalent to a p-value of less than 0.05) was identified as the differential genus. Thirdly, the co-occurrence network was built using the identified differential genera to reflect the microbial changes in HC, moderate MDD patients, and severe MDD patients (Abdullaeva et al., 2021). Fourth, to identify the genera with the promise as the potential biomarkers for diagnosing MDD, the stepwise logistic-regression analysis based on Akaike’s information criterion (AIC) rule was used to analyze the shared differential genera in moderate and severe MDD patients (Ferlizza et al., 2020; Fuchs-Leitner et al., 2021). By dealing with the trade-off between simplicity and goodness of fit of the built model, the AIC rule was often applied to conduct model selection during stepwise logistic-regression analysis (Chen et al., 2020). The model with the minimum AIC value was the preferred model, and genera in this model were viewed as the potential biomarkers. Receiver operating characteristic (ROC) curve analysis was conducted to evaluate the diagnostic performance of the identified potential biomarkers (Kumstel et al., 2020; Wang et al., 2021; Fang et al., 2021). The area under the curve (AUC) was used to assess the diagnostic performance: 0.9–1, excellent; 0.8–0.9, good; 0.7–0.8, fair; 0.6–0.7, poor; and 0.5–0.6, failed. Moreover, sensitivity analysis in the logistic regression model was conducted by excluding these 21 MDD patients receiving antidepressants in 1 month prior to sample collection. Finally, the correlation between HDRS score and the abundance score of all differential genera was investigated. SPSS 19.0 and R software 3.6 were used to do all the analyses, and P-value <0.05 was viewed as significant difference.
Results
Differential Genera in MDD Patients
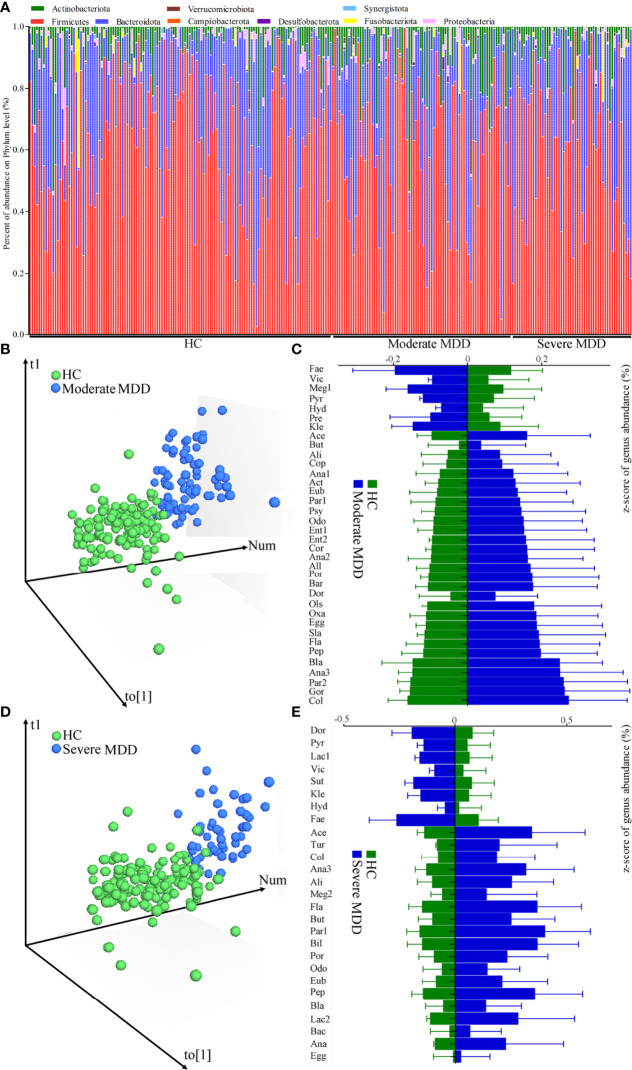
The relative abundances of gut microbiota in MDD patients and HC are described in Figure 1A. Firmicutes, Bacteroidota, Actinobacteriota, and Proteobacteria were the main phyla in both MDD patients and HC. Here, two parameters (Shannon and Simpson) were calculated to assess α-diversity, and principal-coordinate analysis was used to assess β-diversity. There was no significant difference on α-diversity between HC and moderate MDD patients (Shannon, p = 0.32; Simpson, p = 0.16), but principal-coordinate analysis showed that there were significant differences on β-diversity between the two groups (p = 0.012). Meanwhile, non-significant differences on α-diversity (Shannon, p = 0.44; Simpson, p = 0.25) and significant differences on β-diversity (p = 0.031) were observed between HC and severe MDD patients.
Figure 1.
Changes of gut microbiota compositions in HC and moderate and severe MDD patients. (A) Relative abundances of gut microbiota at the genus level in MDD patients and HC. (B) OPLS-DA model showed that there was only a small overlap between HC and moderate MDD patients, suggesting the divergent microbial changes between the two groups; (C) differential genera responsible for discriminating moderate MDD patients from HC; (D) OPLS-DA model showed that there was only a small overlap between HC and severe MDD patients, suggesting the divergent microbial changes between the two groups; (E) differential genera responsible for discriminating severe MDD patients from HC. HC, healthy controls; MDD, major depressive disorder; Fae, Faecalibacterium; Vic, Victivallis; Meg1, Megamonas; Pyr, Pyramidobacter; Hyd, Hydrogenoanaerobacterium; Pre, Prevotella; Kle, Klebsiella; Ace, Acetanaerobacterium; But, Butyricimonas; Ali, Alistipes; Cop, Coprobacillus; Ana1, Anaerococcus; Act, Actinomyces; Eub, Eubacterium; Par1, Parabacteroides; Psy, Psychrobacter; Odo, Odoribacter; Ent1, Enterococcus; Ent2, Enterorhabdus; Cor, Corynebacterium; Ana2, Anaerofustis; All, Allisonella; Por, Porphyromonas; Bar, Barnesiella; Ols, Olsenella; Dor, Dorea; Oxa, Oxalobacter; Egg, Eggerthella; Sla, Slackia; Fla, Flavonifractor; Pep, Peptoniphilus; Bla, Blautia; Ana3, Anaerotruncus; Par2, Parvimonas; Gor, Gordonibacter; Col, Collinsella; Lac1, Lactococcus; Sut, Sutterella; Tur, Turicibacter; Meg2, Megasphaera; Bil, Bilophila; Lac2, Lactobacillus; Bac, Bacteroides; Ana, Anaeroglobus.
After adjusting for age, sex, and BMI, the OPLS-DA model displayed that the HC and moderate MDD patients could be obviously separated by the microbiota genera, which suggested the divergent microbial changes between HC and moderate MDD patients (Figure 1B). By analyzing the loading plot of the model, 36 genera with VIP > 1.0 were identified as the differential genera in moderate MDD patients. Compared with HC, the abundance scores of Victivallis, Pyramidobacter, Hydrogenoanaerobacterium, Megamonas, Klebsiella, Prevotella, and Faecalibacterium were decreased, while those of Dorea, Butyricimonas, Alistipes, Parabacteroides, Blautia, Coprobacillus, Flavonifractor, Odoribacter, Actinomyces, Collinsella, Barnesiella, Eubacterium, Anaerococcus, Allisonella, Anaerofustis, Oxalobacter, Anaerotruncus, Acetanaerobacterium, Eggerthella, Peptoniphilus, Enterococcus, Gordonibacter, Porphyromonas, Parvimonas, Slackia, Psychrobacter, Corynebacterium, Olsenella, and Enterorhabdus were increased in moderate MDD patients (Figure 1C). These differential genera mainly belonged to phyla Firmicutes (n = 16, 44.44%), Actinobacteriota (n = 8, 22.22%), and Bacteroidota (n = 7, 19.44%). The detailed information of these differential genera is described in Table 2.
Table 2.
Differential genera responsible for discriminating moderate MDD patients from HC.
| Genus | VIP | FC | Phylum |
|---|---|---|---|
| Enterorhabdus | 1.24 | 0.06 | Actinobacteriota |
| Olsenella | 1.34 | 0.1 | Actinobacteriota |
| Corynebacterium | 1.01 | 0.14 | Actinobacteriota |
| Psychrobacter | 1.04 | 0.15 | Proteobacteria |
| Slackia | 1.43 | 0.17 | Actinobacteriota |
| Parvimonas | 1.54 | 0.19 | Firmicutes |
| Porphyromonas | 1.31 | 0.2 | Bacteroidota |
| Gordonibacter | 1.95 | 0.22 | Actinobacteriota |
| Enterococcus | 1.02 | 0.22 | Firmicutes |
| Peptoniphilus | 1.51 | 0.26 | Firmicutes |
| Eggerthella | 1.26 | 0.28 | Actinobacteriota |
| Acetanaerobacterium | 1.07 | 0.3 | Firmicutes |
| Anaerotruncus | 2.11 | 0.31 | Firmicutes |
| Oxalobacter | 1.66 | 0.31 | Proteobacteria |
| Anaerofustis | 1.37 | 0.32 | Firmicutes |
| Allisonella | 1.21 | 0.32 | Firmicutes |
| Anaerococcus | 1.06 | 0.41 | Firmicutes |
| Eubacterium | 1.45 | 0.42 | Firmicutes |
| Barnesiella | 1.37 | 0.42 | Bacteroidota |
| Collinsella | 2.22 | 0.45 | Actinobacteriota |
| Actinomyces | 1.08 | 0.49 | Actinobacteriota |
| Odoribacter | 1.34 | 0.5 | Bacteroidota |
| Flavonifractor | 1.46 | 0.5 | Firmicutes |
| Coprobacillus | 1 | 0.53 | Firmicutes |
| Blautia | 1.83 | 0.66 | Firmicutes |
| Parabacteroides | 1.74 | 0.67 | Bacteroidota |
| Alistipes | 1.47 | 0.8 | Bacteroidota |
| Butyricimonas | 1.53 | 0.85 | Bacteroidota |
| Dorea | 1.02 | 0.85 | Firmicutes |
| Faecalibacterium | 1.19 | 1.32 | Firmicutes |
| Prevotella | 1.24 | 1.46 | Bacteroidota |
| Klebsiella | 1.14 | 2.39 | Proteobacteria |
| Megamonas | 1.19 | 2.51 | Firmicutes |
| Hydrogenoanaerobacterium | 1.39 | 6.81 | Firmicutes |
| Pyramidobacter | 1.46 | 8.84 | Synergistota |
| Victivallis | 1.34 | 14.11 | Verrucomicrobiota |
HC, healthy controls; MDD, major depressive disorder; VIP, important variables on the projection; FC, fold change, compared to HC. >1.0 and <1.0 indicated significantly lower and higher levels, respectively, in MDD patients.
Similarly, after adjusting for age, sex, and BMI, using OPLS-DA (Figure 1D), we identified 27 differential genera with VIP >1.0 in severe MDD patients. Compared with HC, the abundance scores of Lactococcus, Pyramidobacter, Victivallis, Sutterella, Klebsiella, Hydrogenoanaerobacterium, Dorea, and Faecalibacterium were decreased, while those of Eggerthella, Bacteroides, Blautia, Collinsella, Odoribacter, Alistipes, Megasphaera, Flavonifractor, Butyricimonas, Parabacteroides, Bilophila, Porphyromonas, Anaerotruncus, Eubacterium, Peptoniphilus, Acetanaerobacterium, Lactobacillus, Turicibacter, and Anaeroglobus were increased in severe MDD patients (Figure 1E). These differential genera mainly belonged to phyla Firmicutes (n = 14, 51.85%) and Bacteroidota (n = 6, 22.22%). The detailed information of these differential genera is described in Table 3.
Table 3.
Differential genera responsible for discriminating severe MDD patients from HC.
| Genus | VIP | FC | Phylum |
|---|---|---|---|
| Anaeroglobus | 1.91 | 0.04 | Firmicutes |
| Turicibacter | 1.40 | 0.09 | Firmicutes |
| Lactobacillus | 1.68 | 0.11 | Firmicutes |
| Acetanaerobacterium | 1.61 | 0.16 | Firmicutes |
| Peptoniphilus | 1.66 | 0.16 | Firmicutes |
| Eubacterium | 1.75 | 0.30 | Firmicutes |
| Anaerotruncus | 1.71 | 0.35 | Firmicutes |
| Porphyromonas | 1.37 | 0.39 | Bacteroidota |
| Bilophila | 2.55 | 0.40 | Desulfobacterota |
| Parabacteroides | 2.14 | 0.41 | Bacteroidota |
| Butyricimonas | 1.92 | 0.43 | Bacteroidota |
| Flavonifractor | 2.42 | 0.43 | Firmicutes |
| Megasphaera | 1.00 | 0.45 | Firmicutes |
| Alistipes | 1.83 | 0.60 | Bacteroidota |
| Odoribacter | 1.49 | 0.65 | Bacteroidota |
| Collinsella | 1.47 | 0.66 | Actinobacteriota |
| Blautia | 1.58 | 0.80 | Firmicutes |
| Bacteroides | 1.55 | 0.90 | Bacteroidota |
| Eggerthella | 1.23 | 0.92 | Actinobacteriota |
| Faecalibacterium | 1.41 | 1.37 | Firmicutes |
| Dorea | 1.53 | 1.55 | Firmicutes |
| Hydrogenoanaerobacterium | 1.15 | 1.98 | Firmicutes |
| Klebsiella | 1.37 | 2.28 | Proteobacteria |
| Sutterella | 1.24 | 2.97 | Proteobacteria |
| Victivallis | 1.14 | 6.39 | Verrucomicrobiota |
| Pyramidobacter | 1.27 | 36.23 | Synergistota |
| Lactococcus | 1.03 | 37.70 | Firmicutes |
HC, healthy controls; MDD, major depressive disorder; VIP, important variables on the projection; FC, fold change, compared to HC. >1.0 and <1.0 indicated significantly lower and higher levels, respectively, in MDD patients.
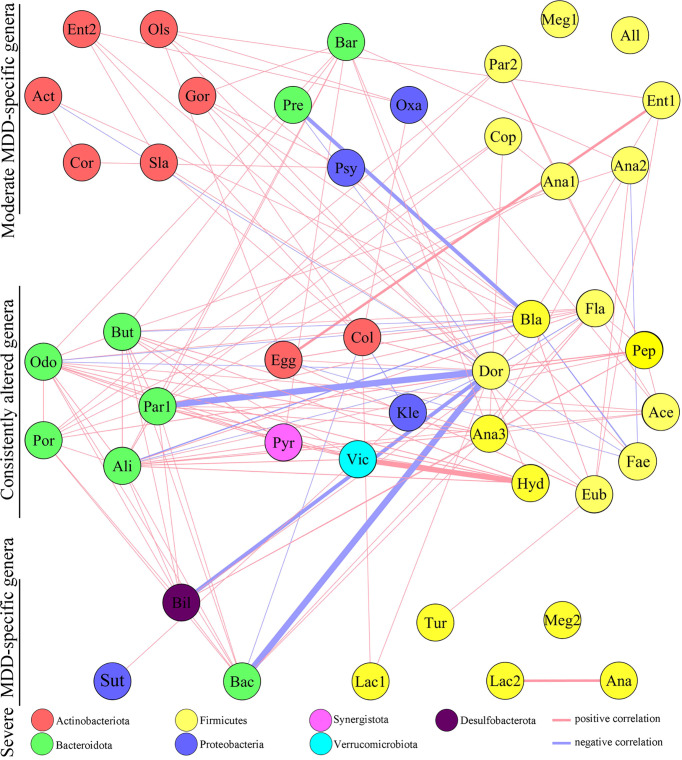
Co-occurrence Network of Differential Genera
The co-occurrence networks deduced from the relative abundance of moderately or severely related genera were generated using Spearman’s correlation coefficient, which was used to reflect microbial changes in HC and moderate and severe MDD patients. As shown in Figure 2, the majority of altered genera belonging to phylum Actinobacteriota (n = 6, 75%) were specific to moderate MDD patients. The co-occurrence network showed that in moderate MDD patients, six genera from phylum Actinobacteriota and seven genera from phylum Firmicutes significantly covaried with one another, which generated two characteristic covarying networks from phyla Actinobacteriota and Firmicutes. No such specific covarying network was found in severe MDD patients. Meanwhile, among the identified differential genera, 19 (nine belonging to phylum Firmicutes and five belonging to phylum Bacteroidota) were consistently changed in MDD patients compared with HC (Figure 2). The co-occurrence network showed that there were two characteristic covarying networks from phyla Bacteroidota and Firmicutes in MDD groups.
Figure 2.
Co-occurrence network showing microbial changes in moderate and severe MDD patients. The microbial genera changed in moderate or severe MDD were identified by OPLS-DA. In total, 63 differential genera were identified in the two groups. Nineteen of 63 genera were consistently altered in both moderate and severe MDD patients relative to HC, and 17 and 8 genera were specific to moderate MDD alone and severe MDD alone, respectively. Compared to HC, moderate MDD was mainly characterized by altered covarying genera assigned to phylum Firmicutes, Actinobacteriota, and Bacteroidota, while severe MDD was mainly characterized by altered covarying genera assigned to phyla Firmicutes and Bacteroidota. Lines between nodes indicate Spearman’s correlation > +0.30 (light red) or < −0.30 (light blue)); line thickness indicates p value (p < 0.05).
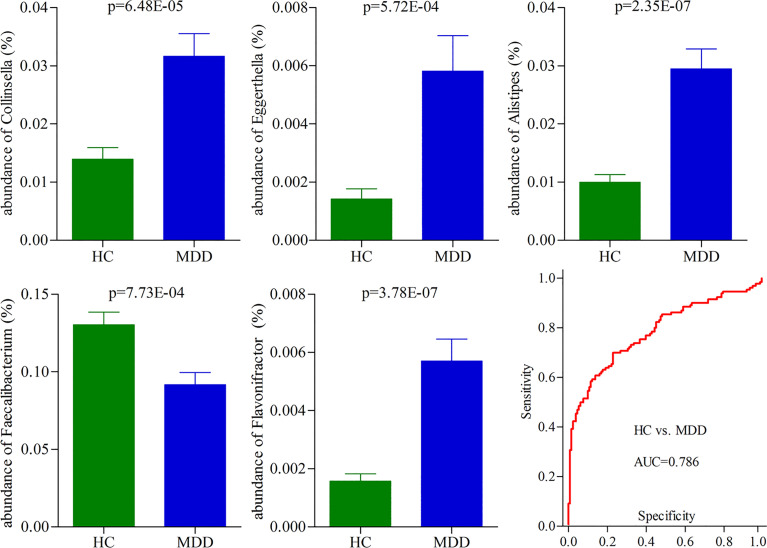
Potential Biomarkers for Diagnosing MDD
To identify the potential biomarkers for diagnosing MDD, we used the logistic regression analysis to further analyze the consistently changed genera in both moderate and severe MDD patients when compared with HC. After adjusting for age, sex, and BMI, the results showed that the most significant deviations between HC and MDD patients were explained by five differential genera (Collinsella, Eggerthella, Alistipes, Faecalibacterium, and Flavonifractor) (Figure 3). The ROC curve analysis was then used to evaluate the diagnostic performance of these differential genera. The results showed that the panel consisting of these five genera could yield an AUC of 0.786 for classifying MDD patients from HC (Figure 3). The sensitivity analysis by excluding the medicated MDD patients showed a similar diagnostic performance of this panel in diagnosing MDD. These results suggested that these five differential genera might hold promise as potential biomarkers for diagnosing MDD.
Figure 3.
Five differential genera as potential biomarkers for diagnosing MDD. The model consisting of these five genera had the minimum AIC value; thus, they were viewed as the potential biomarkers. The panel consisting of these five genera could yield an AUC of 0.786 for classifying MDD patients from HC, suggesting fair diagnostic performance in diagnosing MDD. HC, healthy controls; MDD, major depressive disorder; AUC, area under the curve.
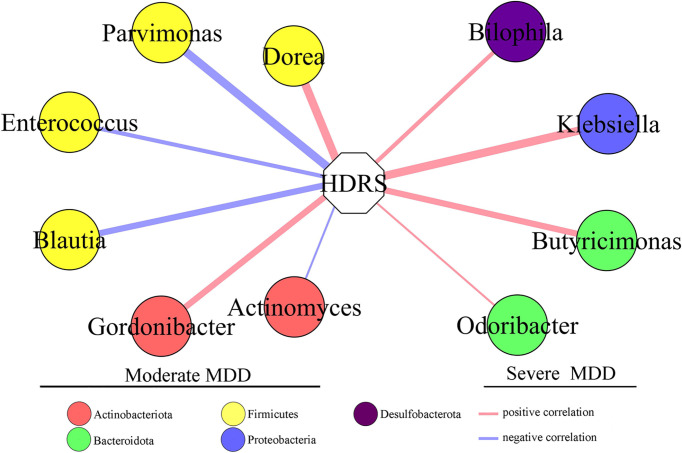
Correlation Between HDRS and Differential Genera
To find out the potential correlations between the severity of depression and gut microbiota, Pearson correlation analysis was used to analyze the correlations between HDRS score and differential genera. The genera significantly correlated with the HDRS score were used to build the correlation network (Figure 4). Six differential genera (Parvimonas, Dorea, Gordonibacter, Blautia, Actinomyces, and Enterococcus) in moderate MDD patients presented significantly positive or negative correlations with the HDRS score. Four differential genera (Klebsiella, Butyricimonas, Bilophila, and Odoribacter) in severe MDD patients presented significantly positive correlations with HDRS.
Figure 4.
Differential genera in moderate and severe MDD patients significantly correlated with HDRS. Six genera (four of them belonged to phylum Firmicutes) in moderate MDD patients were significantly positively or negatively correlated with HDRS. Four genera in severe MDD patients were significantly positively correlated with HDRS. MDD, major depressive disorder; HDRS, Hamilton Depression Rating Scale.
Moderate MDD vs. Severe MDD
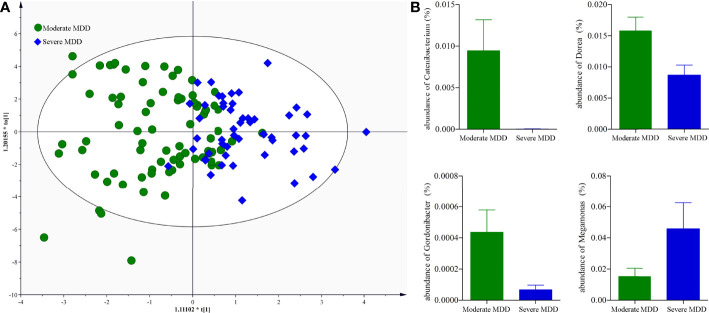
It might be interesting to see the differential genera between moderate and severe MDD patients; thus, we directly used the data from moderate and severe MDD patients to build the OPLS-DA model. Results showed no significant difference on both α-diversity (Shannon, p = 0.51; Simpson, p = 0.47) and β-diversity (p = 0.22) between moderate and severe MDD patients. Meanwhile, after adjusting for age, sex, and BMI, the built OPLS-DA model displayed that moderate and severe MDD patients could not be clearly separated (40.38% severe MDD patients were wrongly assigned into moderate MDD patients) (Figure 5A). However, we still identified four differential genera (Catenibacterium, VIP = 1.73; Dorea, VIP = 2.16; Gordonibacter, VIP = 1.45; Megamonas, VIP = 1.58) between moderate and severe MDD patients. Compared with severe MDD patients, Catenibacterium, Dorea, and Gordonibacter were significantly higher, while Megamonas was significantly lower in the moderate MDD patients (Figure 5B).
Figure 5.
Genus-level analysis of gut microbiota between moderate and severe MDD patients. (A) OPLS-DA model showed that the moderate and severe MDD patients could not be significantly separated; (B) there were four differential genera between the two groups.
Discussion
This study was conducted to find the divergent microbes of different MDD severity. The results showed that there were 36 and 27 differential genera in moderate and severe MDD patients, respectively. The differential genera in moderate and severe MDD patients mainly belonged to three (Firmicutes, Actinobacteriota, and Bacteroidota) and two phyla (Firmicutes and Bacteroidota), respectively. Meanwhile, one specific covarying network from phylum Actinobacteriota was identified in moderate MDD patients. In addition, the moderate and severe MDD patients shared no differential genera that were significantly correlated with the HDRS score. Therefore, these findings suggested that although moderate and severe MDD patients shared some common differential genera, the two groups had significantly different microbial signatures.
Currently, clinicians still use the structured clinical interview rather than objective laboratory tests to diagnose MDD. However, the interview method often results in a certain percentage of misdiagnosis (Mitchell et al., 2009) due to the highly heterogeneous of clinical presentation of MDD. One promising way to markedly increase the accuracy of diagnosis is to identify disease biomarkers for objectively diagnosing MDD. In recent decades, much work has been done to identify potential biomarkers for MDD (Dmitrzak-Weglarz et al., 2021; Bai et al., 2021; Huang et al., 2022; Travica et al., 2022). However, few studies have taken the severity of MDD into consideration. In our previous studies, the differential urinary and plasma metabolites related to the severity of MDD were observed (Liu et al., 2015; Chen et al., 2017). Here, we provided an interesting method to identify potential biomarkers for MDD. A panel consisting of five consistently changed genera was found to have fair efficacies in diagnosing MDD patients from HCs.
Gut microbiota could be influenced by many factors, such as dietary habit and antibiotic agents (Farag et al., 2020; Khan et al., 2020; Dordević et al., 2021; Liu et al., 2021). Madison et al. reported that dietary habit could affect the gut microbiota compositions independently or in conjunction with stress (Madison and Kiecolt-Glaser, 2019). Lv et al. found that there was a close relationship between BMI and gut microbiota compositions in Chinese male college students (Lv et al., 2019). Duan et al. observed that the gut microbiota compositions were different in cynomolgus macaques with different ages (Duan et al., 2019). Our previous study found the differential gut microbiota compositions between young and middle-aged MDD patients (Chen et al., 2020). In the present study, our findings further suggested that the gut microbiota compositions could also be affected by the severity of MDD. These results might provide a novel clue for understanding the role of gut microbiota in the onset of depression. However, only gut microbiota at the genus level was analyzed here. Therefore, our identified potential microbial biomarkers—although very promising—were preliminary results and need further validation.
Firmicutes and Bacteroidetes are the two phyla of dominating bacteria in human gut microbiota. Zheng et al. found that the relative abundance of Bacteroidetes was significantly changed in MDD patients compared with HCs (Zheng et al., 2016). Jiang et al. reported that both the relative abundances of Firmicutes and Bacteroidetes were significantly disordered in MDD patients compared with HCs (Jiang et al., 2015). In our previous study, we found that compared with HCs, the relative abundance of Bacteroidetes was significantly increased and decreased in young and middle-aged MDD patients, respectively, and the relative abundance of Firmicutes was only found to be significantly changed in young MDD patients (Chen et al., 2020). In this study, we observed that the differential genera in moderate and severe MDD patients mainly belonged to three (Firmicutes, Actinobacteriota, and Bacteroidota) and two (Firmicutes and Bacteroidota) phyla, respectively. These results indicated that the gut microbiota compositions could be affected by many factors, and further studies on the associations between MDD and gut microbiota should minimize the influence of confounding factors.
The shared differential genus Collinsella by two MDD groups is an important intestinal bacterium to produce ursodeoxycholic acid. Ursodeoxycholic acid has antioxidant and anti-apoptotic effects and can suppress pro-inflammatory cytokines like IL-2 and TNF-α (Hirayama et al., 2021). The close relationships between MDD and inflammation have been reported in many previous studies (Leonard, 2018; Colasanto et al., 2020). Another shared differential genus Faecalibacterium is an important intestinal bacterium to produce butyric acid. Butyric acid is a major short-chain fatty acid (SCFA) produced by gut microbiota (Sun et al., 2021). SCFAs are speculated to play an important role in the cross talk between the gut and brain. Our previous study found associations between disordered hypothalamus neurotransmitters and fecal SCFAs in depressed mice (Wu et al., 2020). These results showed that the identified shared differential genus were worthy of further exploring.
Previous studies reported that the dominant taxa were different in the different phases of the life cycle (Lim et al., 2015; Vemuri et al., 2018). Our previous study found that there were age-specific differential changes on gut microbiota composition in MDD patients (Chen et al., 2020). In this study, we identified three significantly decreased and one significantly increased genus in severe MDD patients compared with moderate MDD patients. Three of them (Catenibacterium, Dorea, Megamonas) belonged to the phylum Firmicutes. These results indicated that the continuing changes of gut microbiota in moderate MDD patients, especially phylum Firmicutes, might contribute to the deterioration of depression. Therefore, developing personalized treatment methods to timely treat moderate MDD patients might be able to alleviate or delay the progress of depression.
Many studies have reported the microbial markers of depression (Jiang et al., 2015; Chen et al., 2018; Zhou et al., 2020; Yang et al., 2020). Zhou et al. found that gut microbiota-based biomarkers, such as Faecalibacterium and Butyricicoccus, might be helpful for the diagnosis and treatment of postpartum depressive disorder patients (Zhou et al., 2020). Here, Faecalibacterium was also identified as a potential biomarker for MDD. Another study reported that a combinatorial marker panel consisting of bacterial species and fecal metabolite markers could effectively discriminate MDD from HC (Yang et al., 2020). Our previous study found that the suitability of Actinobacteria and Bacteroidia as the sex-specific biomarkers for diagnosing MDD was worthy of further exploring (Chen et al., 2018). Jiang et al. observed that Alistipes and Faecalibacterium might be potential biomarkers for MDD patients (Jiang et al., 2015). Here, Collinsella and Eggerthella belonged to phylum Actinobacteriota and Alistipes belonged to phylum Bacteroidota were identified as potential biomarkers for MDD. Although these results showed a potential and novel method for objective diagnosis of depression, further studies were warranted to evaluate the suitability of gut microbiota as a biomarker for depression.
Several limitations should be mentioned here. Firstly, the number of subjects in each group was relatively small, which requires future studies to validate and support the conclusions. Secondly, although the potential effects of main confounding factors (age, BMI, sex ratio) were eliminated, the effects of other potential factors, such as family history of psychiatric diseases, host genetics, smoking, and dietary habit, were not explored here; thus, future studies were needed to assess the effects of these factors. Thirdly, all the included subjects came from the same place, and thus there might be ethno-specific biases, which could limit the applicability of our conclusion. Fourthly, due to technical reasons, the identification of gut microbiota at the species level was unsuccessful. Therefore, it might also be meaningful to further investigate the differential gut microbiota compositions at the species level. Fifthly, we did not analyze the functions of differential gut microbiota related to the severity of MDD, which was worthy of further exploring using whole-genome sequencing (WGS) or phylogenetic investigation of communities by reconstruction of unobserved states (PICRUST). Sixthly, the “healthy human microbiota” is only a theoretical phenomenon. Due to the complexity of assessing the health status of gut microbiota, the “healthy human microbiota” has not yet been defined. Thus, the microbial biomarkers should be cautiously interpreted. Seventhly, although the sensitivity analysis showed that the results obtained by excluding these 21 MDD patients were similar to the original results, we did not know whether 1 month was enough to remove the effects of antidepressive treatments on gut microbiota; thus, future studies should recruited drug-naïve MDD patients to evaluate our results.
Conclusion
In conclusion, this study found that there were divergent microbial phenotypes between moderate and severe MDD patients. Totally, 36 and 27 differential genera in moderate and severe MDD patients, respectively, were identified. One specific covarying network from phylum Actinobacteriota was identified in moderate MDD patients. In addition, five differential genera (Collinsella, Eggerthella, Alistipes, Faecalibacterium, and Flavonifractor) held promise as the potential biomarkers for diagnosing MDD. Our results may also be helpful for further exploring the role of gut microbiota in the pathogenesis of depression.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA806486.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee of Chongqing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QZ, J-JC, and PX conceived and designed the study; QZ, YW, and W-HS participated in data collection. J-JC and C-JZ analyzed the data. QZ, J-JC, and PX prepared the paper. All authors have read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2017YFA0505700), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2019PT320002), the Natural Science Foundation Project of China (81820108015, 81701360), the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0084), the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant No. KJQN202100420), and the Chongqing Yuzhong District Science & Technology Commission (20190115).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdullaeva Y., Ambika Manirajan B., Honermeier B., Schnell S., Cardinale M. (2021). Domestication Affects the Composition, Diversity, and Co-Occurrence of the Cereal Seed Microbiota. J. Adv. Res. 31, 75–86. doi: 10.1016/j.jare.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Harbi K. S. (2012). Treatment-Resistant Depression: Therapeutic Trends, Challenges, and Future Directions. Patient Prefer. Adherence 6, 369–388. doi: 10.2147/PPA.S29716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S., Xie J., Bai H., Tian T., Zou T., Chen J. J. (2021). Gut Microbiota-Derived Inflammation-Related Serum Metabolites as Potential Biomarkers for Major Depressive Disorder. J. Inflamm. Res. 14, 3755–3766. doi: 10.2147/JIR.S324922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S., Macqueen G. (2004). The Role of the Hippocampus in the Pathophysiology of Major Depression. J. Psychiatry Neurosci. 29 (6), 417–426. [PMC free article] [PubMed] [Google Scholar]
- Chambers E. S., Preston T., Frost G., Morrison D. J. (2018). Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 7 (4), 198–206. doi: 10.1007/s13668-018-0248-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., He S., Fang L., Wang B., Bai S. J., Xie J., et al. (2020). Age-Specific Differential Changes on Gut Microbiota Composition in Patients With Major Depressive Disorder. Aging (Albany NY) 12 (3), 2764–2776. doi: 10.18632/aging.102775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wang R., Duan Z., Yuan X., Ding Y., Feng Z., et al. (2021). Akkermansia Muciniphila Protects Against Psychological Disorder-Induced Gut Microbiota-Mediated Colonic Mucosal Barrier Damage and Aggravation of Colitis. Front. Cell Infect. Microbiol. 11, 723856. doi: 10.3389/fcimb.2021.723856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Zheng P., Liu Y. Y., Zhong X. G., Wang H. Y., Guo Y. J., et al. (2018). Sex Differences in Gut Microbiota in Patients With Major Depressive Disorder. Neuropsychiatr. Dis. Treat. 14, 647–655. doi: 10.2147/NDT.S159322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. J., Zhou C. J., Liu Z., Fu Y. Y., Zheng P., Yang D. Y., et al. (2015). Divergent Urinary Metabolic Phenotypes Between Major Depressive Disorder and Bipolar Disorder Identified by a Combined GC-MS and NMR Spectroscopic Metabonomic Approach. J. Proteome Res. 14 (8), 3382–3389. doi: 10.1021/acs.jproteome.5b00434 [DOI] [PubMed] [Google Scholar]
- Chen J. J., Zhou C. J., Zheng P., Cheng K., Wang H. Y., Li J., et al. (2017). Differential Urinary Metabolites Related With the Severity of Major Depressive Disorder. Behav. Brain Res. 332, 280–287. doi: 10.1016/j.bbr.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Coello K., Hansen T. H., Sørensen N., Munkholm K., Kessing L. V., Pedersen O., et al. (2019). Gut Microbiota Composition in Patients With Newly Diagnosed Bipolar Disorder and Their Unaffected First-Degree Relatives. Brain Behav. Immun. 75, 112–118. doi: 10.1016/j.bbi.2018.09.026 [DOI] [PubMed] [Google Scholar]
- Coello K., Hansen T. H., Sørensen N., Ottesen N. M., Miskowiak K. W., Pedersen O., et al. (2021). Affective Disorders Impact Prevalence of Flavonifractor and Abundance of Christensenellaceae in Gut Microbiota. Prog. Neuropsychopharmacol. Biol. Psychiatry 110, 110300. doi: 10.1016/j.pnpbp.2021.110300 [DOI] [PubMed] [Google Scholar]
- Colasanto M., Madigan S., Korczak D. J. (2020). Depression and Inflammation Among Children and Adolescents: A Meta-Analysis. J. Affect. Disord. 277, 940–948. doi: 10.1016/j.jad.2020.09.025 [DOI] [PubMed] [Google Scholar]
- Ding H., Yi X., Zhang X., Wang H., Liu H., Mou W. W. (2021). Imbalance in the Gut Microbiota of Children With Autism Spectrum Disorders. Front. Cell Infect. Microbiol. 11, 572752. doi: 10.3389/fcimb.2021.572752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrzak-Weglarz M., Szczepankiewicz A., Rybakowski J., Kapelski P., Bilska K., Skibinska M., et al. (2021). Expression Biomarkers of Pharmacological Treatment Outcomes in Women With Unipolar and Bipolar Depression. Pharmacopsychiatry 54 (6), 261–268. doi: 10.1055/a-1546-9483 [DOI] [PubMed] [Google Scholar]
- Dong R., Lin H., Chen X., Shi R., Yuan S., Li J., et al. (2021). Gut Microbiota and Fecal Metabolites Associated With Neurocognitive Impairment in HIV-Infected Population. Front. Cell Infect. Microbiol. 11, 723840. doi: 10.3389/fcimb.2021.723840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordević D., Jančíková S., Vítězová M., Kushkevych I. (2021). Hydrogen Sulfide Toxicity in the Gut Environment: Meta-Analysis of Sulfate-Reducing and Lactic Acid Bacteria in Inflammatory Processes. J. Adv. Res. 27, 55–69. doi: 10.1016/j.jare.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Yin B., Li W., Chai T., Liang W., Huang Y., et al. (2019). Age-Related Changes in Microbial Composition and Function in Cynomolgus Macaques. Aging (Albany NY) 11 (24), 12080–12096. doi: 10.18632/aging.102541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Li Z., Yu J., Long Y., Zhao Q., Ding X., et al. (2021). MicroRNAs Secreted by Human Embryos Could be Potential Biomarkers for Clinical Outcomes of Assisted Reproductive Technology. J. Adv. Res. 31, 25–34. doi: 10.1016/j.jare.2021.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M. A., Abdelwareth A., Sallam I. E., El Shorbagi M., Jehmlich N., Fritz-Wallace K., et al. (2020). Metabolomics Reveals Impact of Seven Functional Foods on Metabolic Pathways in a Gut Microbiota Model. J. Adv. Res. 23, 47–59. doi: 10.1016/j.jare.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlizza E., Solmi R., Miglio R., Nardi E., Mattei G., Sgarzi M., et al. (2020). Colorectal Cancer Screening: Assessment of CEACAM6, LGALS4, TSPAN8 and COL1A2 as Blood Markers in Faecal Immunochemical Test Negative Subjects. J. Adv. Res. 24, 99–107. doi: 10.1016/j.jare.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Leitner I., Yazdi K., Gerstgrasser N. W., Tholen M. G., Graffius S. T., Schorb A., et al. (2021). Risk of PTSD Due to the COVID-19 Pandemic Among Patients in Opioid Substitution Treatment. Front. Psychiatry 12, 729460. doi: 10.3389/fpsyt.2021.729460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D., Gao P., Li R., Tan P., Xie J., Zhang R., et al. (2020). Multicenter Assessment of Microbial Community Profiling Using 16S rRNA Gene Sequencing and Shotgun Metagenomic Sequencing. J. Adv. Res. 26, 111–121. doi: 10.1016/j.jare.2020.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama M., Nishiwaki H., Hamaguchi T., Ito M., Ueyama J., Maeda T., et al. (2021). Intestinal Collinsella may Mitigate Infection and Exacerbation of COVID-19 by Producing Ursodeoxycholate. PLoS One 16 (11), e0260451. doi: 10.1371/journal.pone.0260451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Yin H., Wan X. X., Fu B., Tang B., Lei J. (2022). Maternal Plasma Serotonin Level Not Suitable as Postpartum Depression Diagnostic Biomarker: Results From a Prospective Cohort Study. J. Affect. Disord. 298 (Pt A), 284–291. doi: 10.1016/j.jad.2021.11.001 [DOI] [PubMed] [Google Scholar]
- Jiang H., Ling Z., Zhang Y., Mao H., Ma Z., Yin Y., et al. (2015). Altered Fecal Microbiota Composition in Patients With Major Depressive Disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Khan I., Pathan S., Li X. A., Leong W. K., Liao W. L., Wong V., et al. (2020). Far Infrared Radiation Induces Changes in Gut Microbiota and Activates GPCRs in Mice. J. Adv. Res. 22, 145–152. doi: 10.1016/j.jare.2019.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshkam Z., Aftabi Y., Stenvinkel P., Paige Lawrence B., Rezaei M. H., Ichihara G., et al. (2021). Recovery Scenario and Immunity in COVID-19 Disease: A New Strategy to Predict the Potential of Reinfection. J. Adv. Res. 31, 49–60. doi: 10.1016/j.jare.2020.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács Z., Glover L., Reidy F., MacSharry J., Saldova R. (2021). Novel Diagnostic Options for Endometriosis-Based on the Glycome and Microbiome. J. Adv. Res. 33, 167–118. doi: 10.1016/j.jare.2021.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston L., von Wolff A. (2011). Not as Golden as Standards Should be: Interpretation of the Hamilton Rating Scale for Depression. J. Affect. Disord. 128 (1-2), 175–177. doi: 10.1016/j.jad.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Kumstel S., Vasudevan P., Palme R., Zhang X., Wendt E. H. U., David R., et al. (2020). Benefits of non-Invasive Methods Compared to Telemetry for Distress Analysis in a Murine Model of Pancreatic Cancer. J. Adv. Res. 21, 35–47. doi: 10.1016/j.jare.2019.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B. E. (2018). Inflammation and Depression: A Causal or Coincidental Link to the Pathophysiology? Acta Neuropsychiatr. 30 (1), 1–16. doi: 10.1017/neu.2016.69 [DOI] [PubMed] [Google Scholar]
- Lim E. S., Zhou Y., Zhao G., Bauer I. K., Droit L., Ndao I. M., et al. (2015). Early Life Dynamics of the Human Gut Virome and Bacterial Microbiome in Infants. Nat. Med. 21 (10), 1228–1234. doi: 10.1038/nm.3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu S., Tang Y., Pu Z., Xiao H., Gao J., et al. (2021). Intragastric Administration of Casein Leads to Nigrostriatal Disease Progressed Accompanied With Persistent Nigrostriatal-Intestinal Inflammation Activited and Intestinal Microbiota-Metabolic Disorders Induced in MPTP Mouse Model of Parkinson's Disease. Neurochem. Res. 46 (6), 1514–1539. doi: 10.1007/s11064-021-03293-2 [DOI] [PubMed] [Google Scholar]
- Liu L., Wang H., Rao X., Yu Y., Li W., Zheng P., et al. (2021). Comprehensive Analysis of the Lysine Acetylome and Succinylome in the Hippocampus of Gut Microbiota-Dysbiosis Mice. J. Adv. Res. 30, 27–38. doi: 10.1016/j.jare.2020.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zheng P., Zhao X., Zhang Y., Hu C., Li J., et al. (2015). Discovery and Validation of Plasma Biomarkers for Major Depressive Disorder Classification Based on Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 14 (5), 2322–2330. doi: 10.1021/acs.jproteome.5b00144 [DOI] [PubMed] [Google Scholar]
- Lu L., Tang M., Li J., Xie Y., Li Y., Xie J., et al. (2021). Gut Microbiota and Serum Metabolic Signatures of High-Fat-Induced Bone Loss in Mice. Front. Cell Infect. Microbiol. 11, 788576. doi: 10.3389/fcimb.2021.788576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Qin X., Jia H., Chen S., Sun W., Wang X. (2019). The Association Between Gut Microbiota Composition and BMI in Chinese Male College Students, as Analysed by Next-Generation Sequencing. Br. J. Nutr. 122 (9), 986–995. doi: 10.1017/S0007114519001909 [DOI] [PubMed] [Google Scholar]
- Madison A., Kiecolt-Glaser J. K. (2019). Stress, Depression, Diet, and the Gut Microbiota: Human-Bacteria Interactions at the Core of Psychoneuroimmunology and Nutrition. Curr. Opin. Behav. Sci. 28, 105–110. doi: 10.1016/j.cobeha.2019.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Liu S., Liang H., Wang G., Wang T., Luo S., et al. (2021). Ca2+-Activated Cl- Channel TMEM16A Inhibition by Cholesterol Promotes Angiogenesis in Endothelial Cells. J. Adv. Res. 29, 23–32. doi: 10.1016/j.jare.2020.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-de-Souza D. (2014). Proteomics, Metabolomics, and Protein Interactomics in the Characterization of the Molecular Features of Major Depressive Disorder. Dialogues Clin. Neurosci. 16 (1), 63–73. doi: 10.31887/DCNS.2014.16.1/dmartins [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A. J., Vaze A., Rao S. (2009). Clinical Diagnosis of Depression in Primary Care: A Meta-Analysis. Lancet 374 (9690), 609–619. doi: 10.1016/S0140-6736(09)60879-5 [DOI] [PubMed] [Google Scholar]
- Ongür D., Drevets W. C., Price J. L. (1998). Glial Reduction in the Subgenual Prefrontal Cortex in Mood Disorders. Proc. Natl. Acad. Sci. U. S. A. 95 (22), 13290–13295. doi: 10.1073/pnas.95.22.13290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante C. M., Lightman S. L. (2008). The HPA Axis in Major Depression: Classical Theories and New Developments. Trends Neurosci. 31 (9), 464–468. doi: 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Qiao C. M., Sun M. F., Jia X. B., Li Y., Zhang B. P., Zhao L. P., et al. (2020). Sodium Butyrate Exacerbates Parkinson's Disease by Aggravating Neuroinflammation and Colonic Inflammation in MPTP-Induced Mice Model. Neurochem. Res. 45 (9), 2128–2142. doi: 10.1007/s11064-020-03074-3 [DOI] [PubMed] [Google Scholar]
- Rajput C., Sarkar A., Sachan N., Rawat N., Singh M. P. (2021). Is Gut Dysbiosis an Epicenter of Parkinson's Disease? Neurochem. Res. 46 (3), 425–438. doi: 10.1007/s11064-020-03187-9 [DOI] [PubMed] [Google Scholar]
- Rana T., Behl T., Sehgal A., Srivastava P., Bungau S. (2021). Unfolding the Role of BDNF as a Biomarker for Treatment of Depression. J. Mol. Neurosci. 71 (10), 2008–2021. doi: 10.1007/s12031-020-01754-x [DOI] [PubMed] [Google Scholar]
- Sun X., Wang D., Wei L., Ding L., Guo Y., Wang Z., et al. (2021). Gut Microbiota and SCFAs Play Key Roles in QingFei Yin Recipe Anti-Streptococcal Pneumonia Effects. Front. Cell Infect. Microbiol. 11, 791466. doi: 10.3389/fcimb.2021.791466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian T., Mao Q., Xie J., Wang Y., Shao W.-h., Zhong Q, et al. (2022). Multi-Omics Data Reveals the Disturbance of Glycerophospholipid Metabolism Caused by Disordered Gut Microbiota in Depressed Mice. J. Adv. Res 39, 135–145.. doi: 10.1016/j.jare.2021.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X. Y., Xing J. W., Zheng Q. Q., Gao P. F. (2021). 919 Syrup Alleviates Postpartum Depression by Modulating the Structure and Metabolism of Gut Microbes and Affecting the Function of the Hippocampal GABA/Glutamate System. Front. Cell Infect. Microbiol. 11, 694443. doi: 10.3389/fcimb.2021.694443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S. M., Mohajeri M. H. (2021). The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 13 (3), 732. doi: 10.3390/nu13030732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travica N., Berk M., Marx W. (2022). Neurofilament Light Protein as a Biomarker in Depression and Cognitive Function. Curr. Opin. Psychiatry 35 (1), 30–37. doi: 10.1097/YCO.0000000000000756 [DOI] [PubMed] [Google Scholar]
- Vemuri R., Gundamaraju R., Shastri M. D., Shukla S. D., Kalpurath K., Ball M., et al. (2018). Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. BioMed. Res. Int. 2018, 4178607. doi: 10.1155/2018/4178607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun P., Han F., Wang C., Wang Y., Wang X., et al. (2021). Transcriptome Sequencing Identifies Potential Biomarker for White Matter Lesions Diagnosis in the Hypertension Population. Neurochem. Res. 46 (8), 2079–2088. doi: 10.1007/s11064-021-03346-6 [DOI] [PubMed] [Google Scholar]
- Wu M., Tian T., Mao Q., Zou T., Zhou C. J., Xie J., et al. (2020). Associations Between Disordered Gut Microbiota and Changes of Neurotransmitters and Short-Chain Fatty Acids in Depressed Mice. Transl. Psychiatry 10 (1), 350. doi: 10.1038/s41398-020-01038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Cho H., Lin B. M., Pillai M., Heimisdottir L. H., Bandyopadhyay D., et al. (2021). Improved Metabolite Prediction Using Microbiome Data-Based Elastic Net Models. Front. Cell Infect. Microbiol. 11, 734416. doi: 10.3389/fcimb.2021.734416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Zheng P., Li Y., Wu J., Tan X., Zhou J., et al. (2020). Landscapes of Bacterial and Metabolic Signatures and Their Interaction in Major Depressive Disorders. Sci. Adv. 6 (49), eaba8555. doi: 10.1126/sciadv.aba8555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Gao H. C., Qi Z. G., Jia J. M., Li F. F., Chen J. J., et al. (2013). Peripheral Metabolic Abnormalities of Lipids and Amino Acids Implicated in Increased Risk of Suicidal Behavior in Major Depressive Disorder. Metabolomics 9 (3), 688–696. doi: 10.1007/s11306-012-0474-9 [DOI] [Google Scholar]
- Zheng P., Wang Y., Chen L., Yang D., Meng H., Zhou D., et al. (2013). Identification and Validation of Urinary Metabolite Biomarkers for Major Depressive Disorder. Mol. Cell Proteomics 12 (1), 207–214. doi: 10.1074/mcp.M112.021816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Yang J., Li Y., Wu J., Liang W., Yin B., et al. (2020). Gut Microbial Signatures Can Discriminate Unipolar From Bipolar Depression. Adv. Sci. (Weinh.) 7 (7), 1902862. doi: 10.1002/advs.201902862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y., et al. (2019). The Gut Microbiome From Patients With Schizophrenia Modulates the Glutamate-Glutamine-GABA Cycle and Schizophrenia-Relevant Behaviors in Mice. Sci. Adv. 5 (2), eaau8317. doi: 10.1126/sciadv.aau8317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Zeng B., Zhou C., Liu M., Fang Z., Xu X., et al. (2016). Gut Microbiome Remodeling Induces Depressive-Like Behaviors Through a Pathway Mediated by the Host's Metabolism. Mol. Psychiatry 21 (6), 786–796. doi: 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zhong Z., Chen W., Gao H., Che N., Xu M., Yang L., et al. (2021). Fecal Microbiota Transplantation Exerts a Protective Role in MPTP-Induced Parkinson's Disease via the TLR4/PI3K/AKT/NF-κb Pathway Stimulated by α-Synuclein. Neurochem. Res. 46 (11), 3050–3058. doi: 10.1007/s11064-021-03411-0 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Chen C., Yu H., Yang Z. (2020). Fecal Microbiota Changes in Patients With Postpartum Depressive Disorder. Front. Cell Infect. Microbiol. 10, 567268. doi: 10.3389/fcimb.2020.567268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., He M., Liu Z., Qin Z., Wang Z., Duan L. (2020). Shared Genetic Susceptibilities for Irritable Bowel Syndrome and Depressive Disorder in Chinese Patients Uncovered by Pooled Whole-Exome Sequencing. J. Adv. Res. 23, 113–121. doi: 10.1016/j.jare.2020.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA806486.