Abstract
The process of bone remodeling is connected with the regulated balance between bone cell populations (including bone-forming osteoblasts, bone-resorbing osteoclasts, and the osteocyte). And the mechanism of bone remodeling activity is related to the major pathway, receptor activator of nuclear factor kappaB (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) signaling axis. Recently, researchers have found a novel cytokine secreted by activated T cells, which is related to osteoclastogenesis in the absence of osteoblasts or RANKL, leading to bone destruction. They name it the secreted osteoclastogenic factor of activated T cells (SOFAT). SOFAT has been proven to play an essential role in bone remodeling, like mediating the bone resorption in rheumatoid arthritis (RA) and periodontitis. In this review, we outline the latest research concerning SOFAT and discuss the characteristics, location, and regulation of SOFAT. We also summarize the clinical progress of SOFAT and assume the future therapeutic target in some diseases related to bone remodeling.
Keywords: Secreted osteoclastogenic factor of activated T cells, Osteoclast, Bone remodeling, Bone resorption, Periodontal disease
1. Introduction
Bone is a dynamically changing tissue that is continuously degraded and built via bone remodeling, the process in which bone cell populations achieve a balance between resorption and deposition episodes [1]. This process consists of three consecutive phases: the initiation of bone resorption by osteoclasts, the transition from catabolism to anabolism, and the termination of bone formation by osteoblasts [2]. Each phase is finely controlled by humoral factors or molecules, which mediate the communication among bone cells to maintain skeletal integrity [3]. While most bone resorption diseases are due to the excessive activity of osteoclasts, leading to the imbalance of bone remodeling [4], such as osteoporosis, periodontal disease, and rheumatoid arthritis (RA) [5]. Thus, a deeper understanding of the regulation in the molecular mechanisms of bone remodeling is crucial to develop better approaches for the prevention and treatment of bone resorption diseases.
The osteoclast is a tissue-specific macrophage polykaryon created by the differentiation of monocyte or macrophage precursors cells at or near the bone surface [5]. There are many ways to affect osteoclast formation, differentiation, or apoptosis, such as receptor activator of nuclear factor kappaB (RANK)/RANK ligand (RANKL)/osteoprotegerin (OPG) pathway. Besides, several humoral factors like tumor necrosis factor (TNF)-α can substitute for RANKL to induce osteoclast formation [6], [7], [8], [9]. However, bone mass loss primally depends on the RANK/RANKL/OPG system, a major regulatory system of osteoclast differentiation induction, activation, and survival [10].
The axis of RANK/RANKL/OPG is considered the major way of osteoclastogenesis in which osteoclast differentiation and activation are triggered by the interaction between RANKL and RANK [11]. This axis is involved in the formation of osteoclasts and related to many bone loss diseases, such as RA, periodontal disease, and postmenopausal osteoporosis [6], in which alterations in the levels of hormones or pro-inflammatory cytokines stimulate bone resorption [12]. Hormones and factors that stimulate bone resorption in vivo induce the expression of RANKL on osteogenic stromal cells [13], [14]. The RANKL polypeptide is a type II transmembrane protein found on the surface of osteoblast or stromal cells. It interacts directly with its cognate receptor, RANK, on the surface of cells in the osteoclast lineage [5], [14], [15]. When RANKL binds to its receptor RANK, tumor necrosis factor receptor-associated factor 6 (TRAF6) is recruited to activate downstream nuclear factor kappa-B (NF-κB)-associated signaling cascades, causing nuclear translocation of NF-κB and the initiation of osteoclast-specific gene transcription [16], [17]. Activation of NF-κB induces activation of c-Fos. Those two factors interact with the nuclear factor of activated T cells (NF-AT) c1 promoter to trigger auto-amplification of NF-ATc1 and the transcription of genes, which mediate the completion of the osteoclast differentiation process [18]. For OPG, a soluble RANKL decoy receptor that is predominantly produced by osteoblasts [19], [20]. While RANKL binding to RANK drives further osteoclast differentiation, fusion, activation, and survival [21], [22], OPG is able to inhibit osteoclastic bone resorption by preventing RANKL from binding with RANK [19], [23], [24]. Thus, the RANKL: OPG ratio is vital in the regulation of bone resorption [25]. And inhibiting the axis of RANKL/RANK can increase bone mass by preventing osteoclastic bone resorption.
Furthermore, recent studies have shown that other cytokines can substitute for RANKL to promote osteoclast differentiation and function, especially in diseases with pathological bone resorption [6]. Those findings proved the possibility of alternative pathways that could induce osteoclast differentiation independent of the RANK/RANKL/OPG axis. Firstly, Kim and his co-workers successfully induced osteoclastogenesis in vitro by preparing osteoclast precursors with macrophage colony-stimulating factor (M-CSF) and transforming growth factor (TGF)-β, and the cells were treated with TNF-α and IL-1 later [26]. Subsequently, Rifas et al. [27] found a novel cytokine secreted by activated T cells, which could induce osteoclastogenesis independent of that major pathway. They named it the secreted osteoclastogenic factor of activated T cells (SOFAT).
SOFAT, a molecular mass of ~ 27 kd, which is identified through biochemical fractionation by Rifas et al. The four peptides of this ~ 27 kd product are identified, and they share homology with a threonine synthase-like protein through the amino acid sequence test. The SOFAT cDNA is also cloned, and this 1002-bp sequence translates a protein sequence with 247 amino acids, identical to the natural product isolated biochemically. Besides, the researchers analyze the genomic structure of SOFAT and find out it is derived from an unusual messenger RNA splice variant coded by the threonine synthase-like 2 (THNSL2) gene homolog, which is a conserved gene remnant coding for threonine synthase, an enzyme that functions only in microorganisms and plants [27]. Although SOFAT could be induced by the genomic structure of THNSL2 and RNA splice variants, SOFAT is independent of pyridoxal-5’-phosphate, unlike threonine synthase.
2. The source, regulation and clinical significance of SOFAT
2.1. The source of SOFAT
It was first believed that only T cells (CD4+ and CD8+) expressed SOFAT [28], and later other studies successively demonstrated that B-lineage cells (including plasma cells) and multinucleated giant cells (MGCs) are the critical sources of SOFAT in inflammatory states [29], [30]. Immunohistochemical analysis shows the expression of SOFAT is companied with the infiltration of lymphocytes in diseased periodontal tissues. Besides, indirect immunofluorescence is used to verify that cell types other than T cells express SOFAT. Although the majority of B-lineage cells are positive for SOFAT staining, not all T cells express SOFAT [29]. For MGCs, SOFAT is positive in these giant cells under specific conditions through immunohistochemistry, indicating that SOFAT could be expressed in cells other than lymphocytes [30].
Another study has demonstrated that the mRNA and protein levels of SOFAT are higher in gingival tissues of the chronic periodontitis (CP) group than that of the non-periodontitis group, using qPCR analysis and ELISA, respectively [31]. Later, the immunohistochemical expression of SOFAT is evaluated in osseous lesions. Results show that SOFAT is positive in the intraosseous lesion group (cherubism, central giant cell lesions, osteoblastomas, cementoblastomas) and peripheral giant cell lesions, except in the periapical foreign body as well as extraosseous lesions (paracoccidioidomycosis and foreign body reaction) [30]. Based on the results above, SOFAT is only positive in osteoclast of osteolytic bone lesions. Thus, the paper concludes that SOFAT is a putative marker of osteoclasts to differentiate them from multinucleated macrophages. Likewise, in the collagen-induced arthritis (CIA) model, the immunohistochemistry of SOFAT exhibits high positive stains in the knee joint of mice. In agreement with this, the mRNA and protein expression of SOFAT are significantly higher in the joints of mice induced by the CIA protocol [32]. Moreover, SOFAT is highly expressed in inflammatory milieus, such as RA, not in non-inflammatory osteoarthritis (OA), suggesting that SOFAT might be a novel biological marker in the inflammatory diseases accompanied by lymphocytic infiltration [32]. In a word, SOFAT could be expressed in gingival tissues (periodontitis), osteolytic bone lesions, and the knee joint’s synovial liquid (RA).
2.2. The regulation of SOFAT
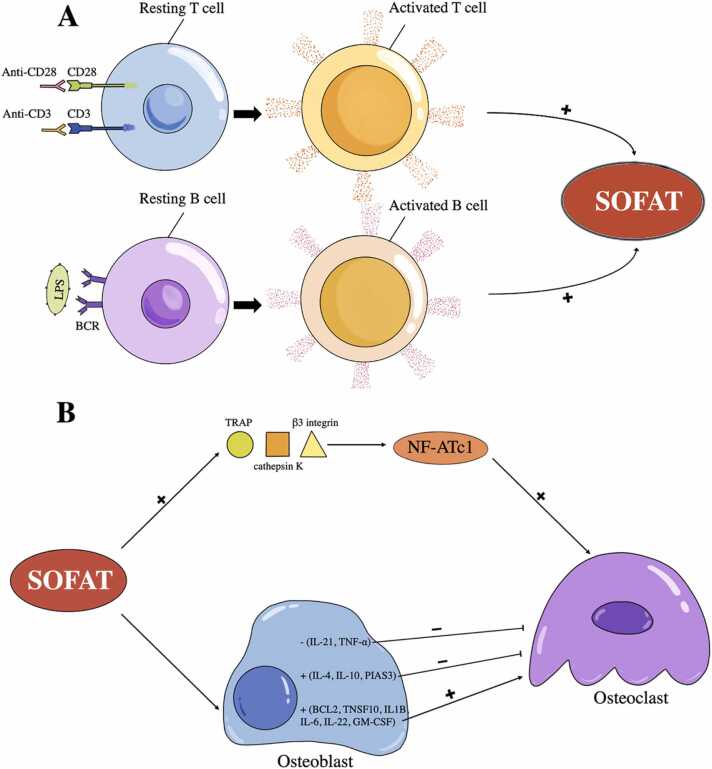
First, SOFAT is secreted by activated T cells in a calcineurin-independent pathway, unlike RANKL, because adding cyclosporin A (CsA) could not prevent activated T cells from secreting SOFAT [33], [34]. Thus, it is suggested that the production of SOFAT by T cells is stimulated by an intracellular pathway different from that of RANKL [27]. Based on the findings that interleukin (IL)-6 modulates the production of T cell-derived cytokines in antigen-induced arthritis (AIA) [35], one editorial suggests the potential existence of a feedback mechanism between activated T cells and SOFAT [28], and the specific pathways involved remain to be elucidated. Besides, the higher expression of SOFAT’s mRNA is detected when using anti-CD3/CD28 (5 μg/ml, respectively) for T cell stimulation and 200 ng/ml lipopolysaccharide for B cell stimulation [29]. In short, specific stimuli promote lymphocytes producing SOFAT in a pathway that differs from RANKL (Fig. 1. A).
Fig. 1.
The regulation of secreted osteoclastogenic factor of activated T cells (SOFAT). (A) Using anti-CD3/CD28 for T cell stimulation and lipopolysaccharide (LPS) for B cell stimulation could induce the production of SOFAT. (B) SOFAT could induce the production of tartrate-resistant acid phosphatase (TRAP), cathepsin K and β3 integrin in the nuclear factor of activated T cells (NF-AT) signal transduction pathway, contributing to the formation of osteoclasts. Besides, the upregulation of cytokines (interleukin (IL)-6 and granulocyte-macrophage-colony stimulating factor (GM-CSF)) and genes (BCL2, TNFSF10, IL-1B, IL-6, and IL-22) in osteoblasts could induce osteoclastogenesis. However, the upregulation of cytokine (IL-10) and genes (IL-4, IL-10, and PIAS3), as well as the downregulation of cytokines (IL-21 and tumor necrosis factor (TNF)-α) in osteoblasts, may seem as the anti-osteoclastogenic effect of SOFAT. BCR, B Cell Receptor; +, increase; −, inhibit.
Second, SOFAT could induce the production of tartrate-resistant acid phosphatase (TRAP), cathepsin K and β3 integrin in the NF-AT signal transduction pathway [27], as well as the expression of cytokines (IL-6, IL-10, and granulocyte-macrophage-colony stimulating factor (GM-CSF)) and genes (BCL2, IL-1B, IL-10, IL-22, IL-2RA, IL-4, IL-6, TNFSF10, and PIAS3) in osteoblasts, while other five genes (IL-2, IL-21, CD4, Csf3R and TNF) are downregulated in osteoblasts by contrast [36] (Fig. 1. B). SOFAT-induced osteoclast formation appears to focus on NF-ATc1, which is consistent with TRAP, cathepsin K, and β3 integrin (all have NF-ATc1 consensus sequences in their gene promoters) induced by SOFAT [37]. Besides, cytokines and genes regulated by SOFAT in osteoblasts could be separated into two parts, osteoclastogenic and anti-osteoclastogenic activities. Stimulatory cytokines must prevail over inhibitory ones to cause bone resorption. For the osteoclastogenic activities, the production of IL-6, GM-CSF, IL-1β, IL-22, and TNFSF10 stimulated by SOFAT could induce osteoclastogenesis, resulting in bone destruction [38], [39], [40], [41]. On the contrary, IL-10, IL-4, and PIAS3 are the components of Janus kinase (Jak) -signal transducer and activator of transcription (STAT) 3 signaling pathway with anti-osteoclastogenic activities [42], [43], [44]. Their upregulation may seem like a SOFAT-induced attempt to prevent the persistent pro-inflammatory cytokines produced. In addition, the downregulation of the genes which encode TNF-α and IL-21 is also viewed as the anti-osteoclastogenic effect of SOFAT [45], [46]. Therefore, SOFAT could modulate osteoblast activities, sustaining the inflammatory condition and causing bone loss.
In conclusion, T cells and B cells could secrete SOFAT in a calcineurin-independent pathway, which stimulates the expression of RANKL induced by osteoblasts [29], [33], [34]. Subsequently, by combining with RANK from osteoclast precursor cells, the axis of RANK/RANKL could induce the differentiation of osteoclasts [11]. Besides, SOFAT could induce monocytes to differentiate into osteoclasts independent of exogenous RANKL or osteoblasts [27]. And in the process of differentiation, the NF-AT signal pathway plays a critical role in osteoclastogenesis [37].
2.3. The clinical significance of SOFAT
Periodontal disease comprises a wide range of inflammatory conditions that affect the supporting structures of the teeth (the gingiva, bone, and periodontal ligament), which could lead to tooth loss and contribute to systemic inflammation [47]. In terms of alveolar bone resorption, it is mediated by the balance between osteoclasts and osteoblasts. When the osteoclastic activity overwhelms the osteogenic activity, it will cause an imbalance of bone remodeling, resulting in bone resorption [5]. Besides, one of the critical sources of osteoclastogenetic factors in periodontal disease is activated T cells, which are referred to as effective regulators of bone remodeling [48], [49]. Therefore, SOFAT, as an activated factor secreted by T cells, may promote inflammation and bone remodeling in the case of periodontal infection, affecting the occurrence and development of periodontal disease. To better understand the clinical significance of SOFAT in periodontal disease (Table 1), gingival biopsies are collected from CP patients to demonstrate higher expression of SOFAT compared with healthy individuals [29], [31]. In addition, SOFAT is injected into the maxilla of mice, resulting in the formation of osteoclast-like cells in the periodontal ligament [31]. The research findings above show the osteoclastogenic activity of SOFAT in an animal model in vivo for the first time. In another study, the clinicians select smokers and non-smokers with generalized aggressive periodontitis (GAgP) to receive one-stage full-mouth ultrasonic debridement (OSFMUD) after initial supragingival therapy, and the expression of SOFAT in gingival crevicular fluid only decreases significantly in the non-smoker group [50]. Thus, clinicians propose that smokers have an additional risk factor compared with non-smokers, resulting in differences in the level of cytokines like SOFAT between the two groups. And the reduction of SOFAT corresponds with less bone resorption. When studying the effect of hydrogen sulfide (H2S) in rats with periodontal disease, results show H2S could downregulate the pro-inflammatory and pro-resorptive cytokines like SOFAT in gingival tissues via topical applications of NaHS, making an influence on the bone remodeling process [51]. In general, the researches above suggest that SOFAT may play an essential role in the pathology of periodontitis.
Table 1.
The clinical significance of SOFAT.
| Diseases | Group | Result | Ref. |
|---|---|---|---|
| Periodontitis | Sample: Gingival biopsy 1. Chronic periodontitis patients 2. Non-periodontitis patients |
The mRNA and protein levels of SOFAT are significantly higher in group 1. | [29], [31] |
| Sample: Mice maxilla 1. Injected SOFAT 2. Injected saline solution |
The formation of osteoclast-like cells could be detected in the periodontal ligament in group 1. | [31] | |
| Sample: Gingival biopsy 1. Smokers with GAgP received OSFMUD 2. Non-smokers with GAgP received OSFMUD |
SOFAT expression is significantly lower only in group 2. | [50] | |
| Sample: Rats with periodontal disease 1. Exposed to H2S 2. Treated with saline only |
Gene’s expression shows a significant induction of SOFAT in group 1. | [51] | |
| Arthritis | Intra-articular injection of SOFAT in the mice knee joint | SOFAT can induce joint pain. | [32] |
| Orthodontics | Sample: rat teeth 1. Control group 2. Orthodontic group 3. PBM group |
The level of SOFAT is significantly higher in group 2 and group 3 (the highest). | [55] |
| ETO | Sample: gingival tissues of ETO 1. Sham group 2.0.4 mm group 3.0.7 mm group |
SOFAT is significantly higher in the gingival tissues of group 3. | [57] |
Abbreviations: SOFAT, Secreted osteoclastogenic factor of activated T cells; GAgP, Generalized aggressive periodontitis; OSFMUD, One-stage full-mouth ultrasonic debridement; H2S, Hydrogen sulfide; PBM, Photobiomodulation; ETO, Experimental traumatic occlusion.
Inflammatory bone disease, such as arthritis, is characterized by massive lymphocyte infiltration because of the initial and uncontrolled inflammatory environment [52]. And a variety of factors exacerbate inflammation, which is responsible for irrecoverable bone loss and pain. Recently, one original research has appointed that intra-articular injection with SOFAT in the knee joint could significantly decrease the mechanical threshold in the hind paw of mice (Table 1). Particularly, this nociceptive response initiates after 3 h of injection, and a second injection assay could sustain this nociceptive stage for up to 8 days [32]. The delay in initiating the mechanical hyperalgesia may attribute to SOFAT does not stimulate neuronal fibers directly. Indeed, SOFAT induces the production of IL-6 in the synovium [27], and this inflammatory molecule could activate the nociceptor sensory neurons, which account for chronic pain [53]. Therefore, the relation between SOFAT and joint pain may explain the critical role of SOFAT in inflammatory bone diseases, such as RA.
The biomechanics of orthodontic tooth movement is based on mechanical forces inducing the periodontal ligament and, subsequently, the biological process of alveolar bone remodeling [54]. In the study of photobiomodulation (PBM)’s effect on tooth movement in mice, the level of SOFAT is significantly higher in the tissues obtained from the maxilla in the orthodontic and PBM groups. In contrast, the control group shows no tooth movement (Table 1). Moreover, for the PBM group, the level of SOFAT is still significantly higher compared with the orthodontic group, accelerating the process of orthodontics [55]. Metabolic activity is highly dynamic during the induced tooth movement, and orthodontic forces over the periodontal ligament increase bone turnover activity through osteoclastogenesis [54]. Thus, it is suggested that laser irradiation could regulate the expression of SOFAT, which plays a critical role in the orthodontic movement by inducing osteoclast formation, resulting in bone remodeling activity, especially in the compression area.
Another study is about the effect of experimental traumatic occlusion (ETO) induced by metal crowns on alveolar bone loss. Previous research has demonstrated that as occlusal trauma increases, a state of inflammatory hyperalgesia is established in the temporomandibular joint, as well as inflammatory mediators on gingival tissue [56]. Likewise, results show that SOFAT is higher in gingival tissues of ETO groups, especially in the 0.7-mm hyperocclusion group, while the 0.4-mm hyperocclusion group could not evoke the secretion of SOFAT (Table 1) [57]. Thus, more significant traumatic injury on the periodontal ligament caused by the higher crown could stimulate the expression of SOFAT, confirming the view that high-intensity trauma may trigger alternative pathways to perpetuate chronic inflammation and bone loss.
3. Discussion
Bone is a living organ that undergoes remodeling throughout life, and bone remodeling results from the action of osteoblasts and osteoclasts [1]. While SOFAT is a novel cytokine, it can induce osteoclastic bone resorption in a RANKL-independent manner [27]. We have summarized the location of SOFAT in different types of cells (T cells, B cells, and MGCs) and diseases (like periodontal disease and RA) and the regulation in osteoclastogenic activity (divided into two parts). Moreover, we also elucidate the clinical significance of SOFAT through various studies (in CP patients, arthritic models, etc.). In terms of the findings above, SOFAT might have an important role in the process of bone remodeling, especially in bone resorption diseases like periodontal disease [40].
However, the current studies have several limitations. Firstly, the studies show that T cells, B cells, and MGCs are the critical sources of SOFAT [29], but whether SOFAT in other types of cells, such as periodontal tissue cells, is unclear. Secondly, we should further detect the impact of SOFAT in other non-inflammatory bone resorption diseases, such as osteoporosis. Therefore, SOFAT, as a novel cytokine, still needs greater effort to elucidate the complex mechanisms and characters behind it.
Although RANKL is a key cytokine for physiological osteoclastogenesis and is important in bone resorption, SOFAT could exacerbate osteoclast formation and bone destruction independent of RANKL [36]. According to this, using RANKL-targeting pharmacological anti-resorptive agents alone may be insufficient to prevent bone loss owing to the action of SOFAT [29]. Thus, inhibiting SOFAT-induced osteoclast formation is an important strategy for preventing and treating bone resorption diseases. Since various monomers derived from traditional Chinese herbs show antiosteoporosis by inhibiting osteoclastogenesis [58], [59], we speculate those herbs may be promising agents for osteoclast-related diseases, probably with fewer side effects and better efficacy.
Role of the funding source
This work was supported by the Natural Science Foundation of Hubei Province of China [Grant nos.: 2019CFB688, 2021CFB056]; the National Natural Science Foundation of China [Grant no.: 81300883]; Wuhan Innovative Talent Development Funds 2014; and Scientific Research Foundation for Returned Scholars, Tongji Hospital 2016.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the library of Tongji Medical College for providing open access resources. Furthermore, the authors thank Dr. Ming Jiang for her valuable contribution to the language help and writing assistance.
Conflict of Interest
None declared.
Footnotes
Scientific field of dental science: Periodontal Research.
References
- 1.Hadjidakis D.J., Androulakis I.I. Bone remodeling. Ann N Y Acad Sci. 2006;1092:385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo K., Irie N. Osteoclast-osteoblast communication. Arch Biochem Biophys. 2008;473(2):201–209. doi: 10.1016/j.abb.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Negishi-Koga T., Takayanagi H. Bone cell communication factors and semaphorins. Bone Rep. 2012;1:183. doi: 10.1038/bonekey.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodan G.A., Martin T.J. Therapeutic approaches to bone diseases. Science. 2000;289(5484):1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- 5.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 6.Feng W., Guo J., Li M. RANKL-independent modulation of osteoclastogenesis. J Oral Biosci. 2019;61(1):16–21. doi: 10.1016/j.job.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komine M., Kukita A., Kukita T., Ogata Y., Hotokebuchi T., Kohashi O. Tumor necrosis factor-alpha cooperates with receptor activator of nuclear factor kappaB ligand in generation of osteoclasts in stromal cell-depleted rat bone marrow cell culture. Bone. 2001;28(5):474–483. doi: 10.1016/s8756-3282(01)00420-3. [DOI] [PubMed] [Google Scholar]
- 9.Azuma Y., Kaji K., Katogi R., Takeshita S., Kudo A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275(7):4858–4864. doi: 10.1074/jbc.275.7.4858. [DOI] [PubMed] [Google Scholar]
- 10.Udagawa N., Koide M., Nakamura M., Nakamichi Y., Yamashita T., Uehara S., et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J Bone Min Metab. 2021;39(1):19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 11.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 12.Boyce B.F., Xing L., Shakespeare W., Wang Y., Dalgarno D., Iuliucci J., et al. Regulation of bone remodeling and emerging breakthrough drugs for osteoporosis and osteolytic bone metastases. Kidney Int Suppl. 2003;85:S2–S5. doi: 10.1046/j.1523-1755.63.s85.2.x. [DOI] [PubMed] [Google Scholar]
- 13.Hofbauer L.C., Khosla S., Dunstan C.R., Lacey D.L., Boyle W.J., Riggs B.L. The roles of osteoprotegerin and osteoprotegerin ligand in the paracrine regulation of bone resorption. J Bone Min Res. 2000;15(1):2–12. doi: 10.1359/jbmr.2000.15.1.2. [DOI] [PubMed] [Google Scholar]
- 14.Theill L.E., Boyle W.J., Penninger J.M. RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol. 2002;20:795–823. doi: 10.1146/annurev.immunol.20.100301.064753. [DOI] [PubMed] [Google Scholar]
- 15.Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama T., Fukushima H., Nakao K., Shin M., Yasuda H., Weih F., et al. Processing of the NF-kappa B2 precursor p100 to p52 is critical for RANKL-induced osteoclast differentiation. J Bone Min Res. 2010;25(5):1058–1067. doi: 10.1359/jbmr.091032. [DOI] [PubMed] [Google Scholar]
- 17.Novack D.V., Yin L., Hagen-Stapleton A., Schreiber R.D., Goeddel D.V., Ross F.P., et al. The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis. J Exp Med. 2003;198(5):771–781. doi: 10.1084/jem.20030116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takayanagi H., Kim S., Koga T., Nishina H., Isshiki M., Yoshida H., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 19.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Lüthy R., et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda H., Shima N., Nakagawa N., Mochizuki S.I., Yano K., Fujise N., et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139(3):1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 21.Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397(6717):315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda H., Shima N., Nakagawa N., Yamaguchi K., Kinosaki M., Mochizuki S., et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O'Brien C.A. Matrix-embedded cells control osteoclast formation. Nat Med. 2011;17(10):1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakashima T., Hayashi M., Fukunaga T., Kurata K., Oh-Hora M., Feng J.Q., et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17(10):1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 25.Kenkre J.S., Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 26.Kim N., Kadono Y., Takami M., Lee J., Lee S.H., Okada F., et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med. 2005;202(5):589–595. doi: 10.1084/jem.20050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifas L., Weitzmann M.N. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-independent manner. Arthritis Rheum. 2009;60(11):3324–3335. doi: 10.1002/art.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotake S., Nanke Y., Yago T., Kawamoto M., Yamanaka H. Human osteoclastogenic T cells and human osteoclastology. Arthritis Rheum. 2009;60(11):3158–3163. doi: 10.1002/art.24886. [DOI] [PubMed] [Google Scholar]
- 29.Jarry C.R., Martinez E.F., Peruzzo D.C., Carregaro V., Sacramento L.A., Araújo V.C., et al. Expression of SOFAT by T- and B-lineage cells may contribute to bone loss. Mol Med Rep. 2016;13(5):4252–4258. doi: 10.3892/mmr.2016.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cândido-Soares L.E., Martinez E.F., de Araújo V.C., Araújo N.S., Freitas N.S., Napimoga M.H. SOFAT as a putative marker of osteoclasts in bone lesions. Appl Immunohistochem Mol Morphol. 2019;27(6):448–453. doi: 10.1097/PAI.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 31.Jarry C.R., Duarte P.M., Freitas F.F., de Macedo C.G., Clemente-Napimoga J.T., Saba-Chujfi E., et al. Secreted osteoclastogenic factor of activated T cells (SOFAT), a novel osteoclast activator, in chronic periodontitis. Hum Immunol. 2013;74(7):861–866. doi: 10.1016/j.humimm.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Napimoga M.H., Dantas Formiga W.D., Abdalla H.B., Trindade-da-Silva C.A., Venturin C.M., Martinez E.F., et al. Secreted osteoclastogenic factor of activated T cells (SOFAT) is associated with rheumatoid arthritis and joint pain: initial evidences of a new pathway. Front Immunol. 2020;11:1442. doi: 10.3389/fimmu.2020.01442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong B.R., Josien R., Lee S.Y., Sauter B., Li H.L., Steinman R.M., et al. TRANCE (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T cells, is a dendritic cell-specific survival factor. J Exp Med. 1997;186(12):2075–2080. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rifas L., Avioli L.V. A novel T cell cytokine stimulates interleukin-6 in human osteoblastic cells. J Bone Min Res. 1999;14(7):1096–1103. doi: 10.1359/jbmr.1999.14.7.1096. [DOI] [PubMed] [Google Scholar]
- 35.Wong P.K., Quinn J.M., Sims N.A., van Nieuwenhuijze A., Campbell I.K., Wicks I.P. Interleukin-6 modulates production of T lymphocyte-derived cytokines in antigen-induced arthritis and drives inflammation-induced osteoclastogenesis. Arthritis Rheum. 2006;54(1):158–168. doi: 10.1002/art.21537. [DOI] [PubMed] [Google Scholar]
- 36.Napimoga M.H., Demasi A.P., Jarry C.R., Ortega M.C., de Araújo V.C., Martinez E.F. In vitro evaluation of the biological effect of SOFAT on osteoblasts. Int Immunopharmacol. 2015;26(2):378–383. doi: 10.1016/j.intimp.2015.04.033. [DOI] [PubMed] [Google Scholar]
- 37.Takayanagi H. The role of NFAT in osteoclast formation. Ann N Y Acad Sci. 2007;1116:227–237. doi: 10.1196/annals.1402.071. [DOI] [PubMed] [Google Scholar]
- 38.Kung Sutherland M.S., Lipps S.G., Patnaik N., Gayo-Fung L.M., Khammungkune S., Xie W., et al. SP500263, a novel SERM, blocks osteoclastogenesis in a human bone cell model: role of IL-6 and GM-CSF. Cytokine. 2003;23(1–2):1–14. doi: 10.1016/s1043-4666(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 39.Mori T., Miyamoto T., Yoshida H., Asakawa M., Kawasumi M., Kobayashi T., et al. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23(11):701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 40.Yen M.L., Tsai H.F., Wu Y.Y., Hwa H.L., Lee B.H., Hsu P.N. TNF-related apoptosis-inducing ligand (TRAIL) induces osteoclast differentiation from monocyte/macrophage lineage precursor cells. Mol Immunol. 2008;45(8):2205–2213. doi: 10.1016/j.molimm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Sabat R., Ouyang W., Wolk K. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov. 2014;13(1):21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 42.Owens J.M., Gallagher A.C., Chambers T.J. IL-10 modulates formation of osteoclasts in murine hemopoietic cultures. J Immunol. 1996;157(2):936–940. [PubMed] [Google Scholar]
- 43.Joosten L.A., Lubberts E., Helsen M.M., Saxne T., Coenen-de Roo C.J., Heinegård D., et al. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. 1999;1(1):81–91. doi: 10.1186/ar14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hikata T., Takaishi H., Takito J., Hakozaki A., Furukawa M., Uchikawa S., et al. PIAS3 negatively regulates RANKL-mediated osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblasts. Blood. 2009;113(10):2202–2212. doi: 10.1182/blood-2008-06-162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cenci S., Weitzmann M.N., Roggia C., Namba N., Novack D., Woodring J., et al. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–1237. doi: 10.1172/JCI11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwok S.K., Cho M.L., Park M.K., Oh H.J., Park J.S., Her Y.M., et al. Interleukin-21 promotes osteoclastogenesis in humans with rheumatoid arthritis and in mice with collagen-induced arthritis. Arthritis Rheum. 2012;64(3):740–751. doi: 10.1002/art.33390. [DOI] [PubMed] [Google Scholar]
- 47.Krauss J.L., Potempa J., Lambris J.D., Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000. 2010;52(1):141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campbell L., Millhouse E., Malcolm J., Culshaw S. T cells, teeth and tissue destruction – what do T cells do in periodontal disease. Mol Oral Microbiol. 2016;31(6):445–456. doi: 10.1111/omi.12144. [DOI] [PubMed] [Google Scholar]
- 49.Figueredo C.M., Lira-Junior R., Love R.M. T and B cells in periodontal disease: new functions in a complex scenario. Int J Mol Sci. 2019;20(16):3949. doi: 10.3390/ijms20163949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Genaro Modanese D., Tiosso-Tamburi R., Furletti de Goes V.F., de Cássia Bergamaschi C., Martinez E.F., Napimoga M.H., et al. Clinical and immunoinflammatory evaluation of one-stage full-mouth ultrasonic debridement as a therapeutic approach for smokers with generalized aggressive periodontitis: a short-term follow-up study. J Periodontol. 2016;87(9):1012–1021. doi: 10.1902/jop.2016.150632. [DOI] [PubMed] [Google Scholar]
- 51.Niederauer A.J., Guimarães R.A., Oliveira K.L., Pires A.R., Jr., Demasi A.P., Ferreira H.H., et al. H2S in periodontal immuneinflammatory response and bone loss: a study in rats. Acta Odontol Lat. 2019;32(3):164–171. [PubMed] [Google Scholar]
- 52.Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 53.Verri W.A., Jr., Cunha T.M., Parada C.A., Poole S., Cunha F.Q., Ferreira S.H. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharm Ther. 2006;112(1):116–138. doi: 10.1016/j.pharmthera.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Krishnan V., Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofac Orthop. 2006;129(4) doi: 10.1016/j.ajodo.2005.10.007. [469.e1-32] [DOI] [PubMed] [Google Scholar]
- 55.Jettar V., Napimoga M.H., Freitas F., Clemente-Napimoga J.T., Suzuki S.S., Montalli V.A., et al. Effects of photobiomodulation on SOFAT, a T-cell-derived cytokine, may explain accelerated orthodontic tooth movement. Photochem Photobiol. 2018;94(3):604–610. doi: 10.1111/php.12878. [DOI] [PubMed] [Google Scholar]
- 56.Abdalla H.B., Clemente-Napimoga J.T., Bonfante R., Hashizume C.A., Zanelli W.S., de Macedo C.G., et al. Metallic crown-induced occlusal trauma as a protocol to evaluate inflammatory response in temporomandibular joint and periodontal tissues of rats. Clin Oral Invest. 2019;23(4):1905–1912. doi: 10.1007/s00784-018-2639-z. [DOI] [PubMed] [Google Scholar]
- 57.Abdalla H.B., Clemente-Napimoga J.T., Trindade-da-Silva C.A., Alves L.J., Prats R.D.S., Youssef A., et al. Occlusion heightened by metal crown cementation is aggressive for periodontal tissues. J Prosthodont. 2021;30(2):142–149. doi: 10.1111/jopr.13235. [DOI] [PubMed] [Google Scholar]
- 58.He J.B., Chen M.H., Lin D.K. New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch Osteoporos. 2017;12(1):14. doi: 10.1007/s11657-016-0301-4. [DOI] [PubMed] [Google Scholar]
- 59.Feng R., Ding F., Mi X.H., Liu S.F., Jiang A.L., Liu B.H., et al. Protective effects of ligustroflavone, an active compound from Ligustrum lucidum, on diabetes-induced osteoporosis in mice: a potential candidate as calcium-sensing receptor antagonist. Am J Chin Med. 2019;47(2):457–476. doi: 10.1142/S0192415X1950023X. [DOI] [PubMed] [Google Scholar]