Highlights
-
•
The progression-free survival (PFS) ratio represents a meaningful outcome measure.
-
•
The median PFS-ratio differs significantly between histological subtypes of ovarian carcinoma.
-
•
Thresholds for clinical benefit should be adjusted for clinicopathological factors.
-
•
Treatment response during PFS1 may result in a distorted view of the PFS-ratio.
Keywords: Progression-free survival ratio, Growth-modulation index, Time to progression ratio, Ovarian carcinoma, Ovarian cancer
Abstract
Objective
Clinical efficacy of cytostatic anticancer agents can be determined with the progression-free survival (PFS) ratio. This outcome measure compares PFS achieved by a new treatment (PFS2) to the PFS of the most recent treatment on which the patient has experienced progression (PFS1). Clinical benefit has been defined as a PFS-ratio (PFS2/PFS1) > 1.3. However, in order to demonstrate significant benefit, trial designs require an assumption on the proportion of patients who reach this ratio during palliative options. For ovarian carcinoma, data is lacking to support this assumption. Therefore in this study, we assess the PFS-ratio in recurrent ovarian carcinoma patients treated with current palliative options.
Methods
We included 67 patients with recurrent high-grade serous (HGSC, 73.1%) or low-grade (LGOC, 26.9%) ovarian carcinoma. We determined the median PFS-ratio and investigated the association with clinicopathological characteristics.
Results
Overall, we observed a median PFS-ratio of 0.69. The proportion of patients with a PFS-ratio > 1.3 was 22.4%. For HGSC patients, the median PFS-ratio was significantly lower than for LGOC patients (respectively, 0.58 and 1.26, p = 0.007). Multivariate logistic regression analysis revealed that the LGOC subtype and CA125 tumor marker concentration were independent factors related to a PFS-ratio > 1.3.
Conclusions
Although the PFS-ratio represents a meaningful outcome measure in studies investigating cytostatic anticancer agents, we conclude that it is influenced by tumor histology and biological behavior. In future research, these factors should be taken into account when determining thresholds for clinical benefit in trial designs.
1. Introduction
Advances in medicine and the rapidly expanding number of new targets for anticancer treatment have led to the search for a new outcome measure in clinical trials to optimally determine treatment efficacy. Targeted agents often cause cytostatic instead of cytotoxic effects (Kummar et al., 2006). Therefore, the response rate (i.e., complete (CR) or partial response (PR)) does not fully cover the possible beneficial effects of a certain treatment and therefore might not represent a meaningful primary outcome measure. Since growth inhibition is the expected outcome for many cytostatic agents, time to disease progression gives a better reflection of treatment efficacy (Mick et al., 2000).
To assess the clinical efficacy of cytostatic agents, Von Hoff suggested the growth modulation index, also known as the progression-free survival (PFS) ratio, as outcome measure (von Hoff, 1998). The PFS-ratio compares the PFS established by a new treatment (PFS2) to the PFS of the antecedent treatment after which the patient has experienced progression (PFS1). Clinical benefit was defined as a PFS-ratio (PFS2/PFS1) > 1.3. This paired analysis within individual patients is suggested to compensate for the heterogeneity caused by the type of tumor and patient characteristics as it uses patients as their own control (Mock et al., 2019). In 2010, Von Hoff et al. designed a prospective multicenter study in which molecular profiling was used to select targeted therapy for patients with different types of cancer (Von Hoff et al., 2010). The threshold of 1.3 was used to define clinical benefit under the assumption (null hypothesis) that 15.0% of the patients or less would reach this ratio. With 27.0% of the patients reaching a PFS-ratio > 1.3, the null hypothesis was rejected (p = 0.007) (Von Hoff et al., 2010).
The threshold and assumption that 15.0% of the population or less would have a PFS-ratio > 1.3 by Von Hoff et al. have been adopted by several other clinical trials using the PFS-ratio as outcome measure (Massard et al., 2017, Seeber et al., 2016, Seeber et al., 2019, Belin et al., 2017, Rodon et al., 2019). However, the PFS-ratio threshold of 1.3 and the proportion of 15% have never been substantiated. Previously, Watson et al. investigated the PFS-ratio in a cohort of patients with refractory solid tumors or lymphomas included in several phase 1/1b trials (Watson et al., 2018). They found that 24.2% of the patients reached a PFS-ratio > 1.3. Based on these results, the authors recommend that a more stringent null hypothesis should be set to conclude treatment efficacy in early clinical trials. In addition, they suggested that the thresholds should be adjusted for several other factors, for example patient selection based on actionable alterations (as a higher probability of treatment efficacy could be expected) and tumor biological behavior, such as high differentiation grade (by affecting the time per treatment due to poor prognosis) (Watson et al., 2018).
Ovarian carcinoma patients are frequently enrolled in clinical trials due to an advanced stage of disease at diagnosis and high recurrence rate despite successful first-line treatment (Corr et al., 2020). However, appropriate historical data on the PFS-ratio during current palliative options is missing and the lack of validation of the suggested threshold to determine clinical efficacy complicates conceptualization of clinical trials and adoption of possible beneficial new drugs. Therefore, in this study we assess the PFS-ratio in recurrent high-grade serous (HGSC) and low-grade (LGOC) ovarian carcinoma patients treated with current palliative options. Additionally, we investigate clinicopathological characteristics associated with a PFS-ratio > 1.3 in ovarian carcinoma patients and provide recommendations for the implementation of the PFS-ratio and thresholds for clinical benefit in future research.
2. Materials and methods
Between 2003 and 2019, a retrospective cohort study was conducted at the Catharina hospital in Eindhoven, Elisabeth-TweeSteden hospital in Tilburg, Antoni van Leeuwenhoek hospital in Amsterdam and Radboud University Medical Center in Nijmegen, The Netherlands. The study was approved by the Dutch Medical research Ethics committees United (MEC-U, study number W16.108 and W19.175). Patients were included if they were diagnosed with FIGO (Fédération Internationale de Gynécologie et d’Obstétrique) stage IIIC-IV recurrent HGSC or if they were diagnosed with recurrent LGOC, including low-grade serous, mucinous and endometrioid ovarian carcinoma. Patients were excluded if treatment for the primary tumor was not completed, if systemic treatment for recurrent disease was combined with local interventions, e.g., surgery or radiotherapy, if they had been enrolled in a clinical trial during PFS1 or PFS2 or because their medical files did not contain sufficient data for inclusion. Under the conditions that the results of this study did not affect further treatment of the patients and that the majority had succumbed to progressive disease by the time of inclusion, the need for informed consent was waved by the Dutch MEC-U.
The following data were retrieved from medical records of the included patients: age at the time of recurrent disease, body mass index (BMI), histological subtype, FIGO stage at primary diagnosis, BRCA mutation status, primary tumor treatment, CA125 concentration after treatment for primary disease, date and type of first and second systemic treatment for recurrent disease, response to first and second treatment for recurrent disease, platinum-sensitivity status and date of progression after first and second treatment for recurrent disease. PFS1 and PFS2 were defined as the start of respectively first and second treatment for recurrent disease until the moment disease progression occurred. Disease progression was defined as the date progression was described in the medical record by the treating physician based on elevated CA125 serum levels and / or tumor progression seen on CT scan, and was defined as ‘progressive disease’, ‘stable disease’ (SD), ‘partial response’ (PR) or ‘complete response’ (CR) during PFS1 and PFS2. In addition, for patients treated with platinum-containing chemotherapy, we reviewed the platinum-sensitivity status and defined this as ‘refractory’, ‘resistant’ or ‘sensitive’ depending on the treatment response according to the above-mentioned criteria.
Patient characteristics were described using the following tests. Differences in skewed continuous variables were assessed using Mann-Whitney U tests and for normally distributed continuous variables independent T-tests were performed. The correlation between the PFS duration was analyzed by means of a Spearman’s Rank Correlation Coefficient and the differences in PFS-ratio between histological subtypes were analyzed with a Mann-Whitney U test. Univariate and multivariate logistic regression analyses were used to assess relevant clinicopathological characteristics. Statistical significance was defined as p < 0.05. All analysis were performed using IBM SPSS (version 26) and data visualization was performed using R (version 1.1.463).
3. Results
A total of 67 patients were included in this study, including 18 patients with LGOC (twelve serous, five endometrioid and one mucinous) and 49 patients with HGSC. Baseline characteristics and clinical data are described in Table 1.
Table 1.
Patient characteristics per histology group.
| Patient characteristics | Total (n = 67) | LGOC (n = 18) | HGSC (n = 49) |
|---|---|---|---|
| Age in years (mean ± SD) | 59.9 ± 12.0 | 57.06 ± 13.8 | 61.0 ± 11.3 |
| BMI in kg/m2 (mean ± SD) | 26.2 ± 4.6 | 25.4 ± 4.5 | 26.5 ± 4.7 |
| FIGO stage primary disease I II III IV Unknown |
1 (1.5%) 2 (3.0%) 52 (77.6%) 9 (13.4%) 3 (4.5%) |
1 (5.6%) 2 (11.1%) 14 (77.8%) 1 (5.6%) |
38 (77.6%) 9 (18.4%) 2 (4.1%) |
| BRCA-mutation None BRCA1 BRCA2 Unknown |
26 (38.8%) 4 (6.0%) 4 (6.0%) 33 (49.3%) |
10 (55.6%) 8 (44.4%) |
16 (31.7%) 4 (8.2%) 4 (8.2%) 25 (51.0%) |
| CA125 after primary treatment (kU/L) (mean ± SD) | 16.4 ± 17.8 | 12.6 ± 6.9 | 17.4 ± 19.7 |
| Treatment response during PFS1 Progressive disease Stable disease Partial response Complete response Unknown |
10 (14.9%) 11 (16.4%) 30 (44.8%) 12 (17.9%) 4 (6.0%) |
3 (16.7%) 5 (27.8%) 7 (38.9%) 2 (11.1%) 1 (5.6%) |
7 (14.3%) 6 (12.2%) 23 (46.9%) 10 (20.4%) 3 (6.1%) |
| Treatment response during PFS2 Progressive disease Stable disease Partial response Complete response Unknown |
16 (23.9%) 21 (31.3%) 18 (26.9%) 4 (6.0%) 8 (11.9%) |
4 (22.2%) 8 (44.4%) 3 (16.7%) 3 (16.7%) |
12 (24.5%) 13 (26.5%) 15 (30.6%) 4 (8.2%) 5 (10.2%) |
Abbreviations: LGOC, low-grade ovarian carcinoma; HGSC, high-grade serous ovarian carcinoma; SD, standard deviation; BMI, body mass index; PFS, progression-free survival.
3.1. Progression-free survival ratio analysis
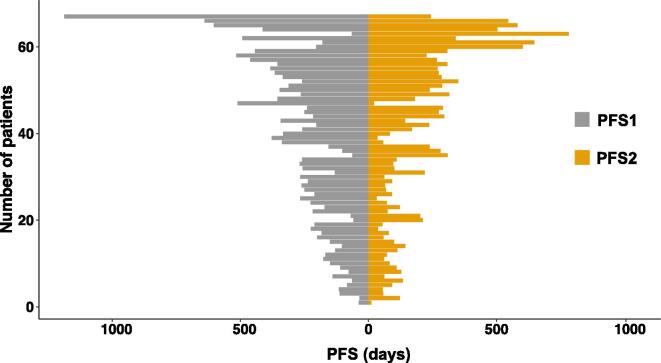
In the overall study population, the median PFS1 was 7.4 months (range 1.1–39.1 months), and the median PFS2 was 4.4 months (range 0.4–25.8 months). There was no correlation between PFS1 and PFS2 (Spearman rho = 0.21, p = 0.087). The PFS-ratio distribution is visualized in Fig. 1. The median PFS-ratio was 0.69 (range 0.04–12.23). The proportion of patients with a PFS-ratio above the 1.3 threshold was 22.4%.
Fig. 1.
Distribution of the progression-free survival (PFS) times in recurrent ovarian carcinoma patients.
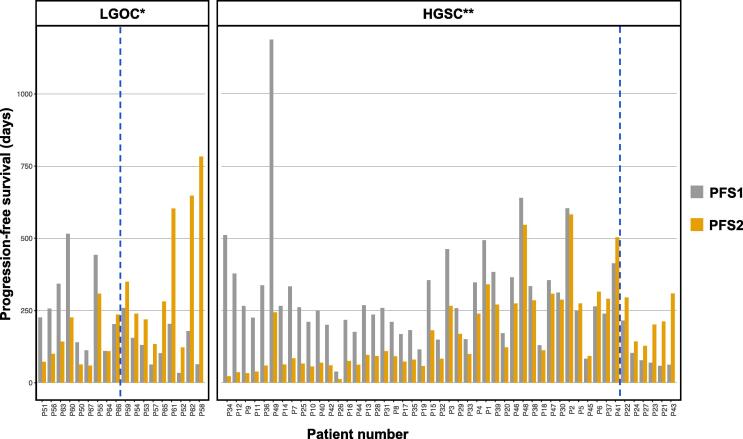
Additionally, we investigated the PFS-ratio per histological subtype. The comparison of PFS1 and PFS2 between histological subtypes is shown in Fig. 2. In the LGOC group, the median PFS1 was 5.5 months, and the median PFS2 was 7.3 months. For HGSC, the median PFS1 and PFS2 were 8.2 months and 3.7 months respectively. The median PFS-ratio in the LGOC group was 1.26 (range 0.32–12.23), with 50.0% of the patients reaching a PFS-ratio > 1.3. In the HGSC group, the median PFS-ratio was significantly lower with 0.58 (range 0.04–4.98), and 12.2% of the patients exceeding the 1.3 threshold (p = 0.007).
Fig. 2.
Comparison of the progression-free survival (PFS) times of the included patients displayed per histological subtype. The patients on the right of the blue line reached a PFS-ratio > 1.3. *LGOC = low-grade ovarian carcinoma; **HGSC = high-grade serous ovarian carcinoma.
Clinicopathological characteristics considered clinically relevant were analyzed by univariate logistic regression to investigate their association with a PFS-ratio > 1.3 (Table 2). A PFS-ratio > 1.3 was correlated with low CA125 concentration after primary treatment (odds ratio (OR) 0.86, 95% CI 0.74–0.99, p = 0.041) and LGOC (OR 7.17, 95% CI 2.04–25.22, p = 0.002). Other factors positively correlated with a PFS-ratio > 1.3 were a platinum-resistant or -refractory response during PFS1 (OR 12.00, 95% CI 1.98–72.74p = 0.007) and immediate progressive disease during PFS1 (OR 0.07, 95% CI 0.01–0.31, p = 0.001). Multivariate logistic regression analysis revealed both LGOC histological subtype (OR 21.03, 95% CI 3.18–139.2, p = 0.002) and CA125 tumor marker concentration (OR 0.82, 95% CI 0.69–0.98, p = 0.028) to be independent factors related to a PFS-ratio > 1.3.
Table 2.
Univariate logistic regression analysis of clinicopathological characteristics related to a progression-free survival (PFS) ratio > 1.3.
| Variable | Number of patients in analysis | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|---|
| Age at time of diagnosis recurrent disease | 67 | 0.99 | 0.94–1.04 | 0.605 |
| Body Mass Index | 50 | 0.99 | 0.85–1.15 | 0.915 |
| BRCA1 or BRCA2 mutation versus no mutation (ref) | 35 | 0.00 | 0.00–0.00 | 0.999 |
| CA125 tumor marker concentration after primary treatment | 56 | 0.86 | 0.74–0.99 | 0.041 |
| Low-grade versus high-grade histological subtype (ref) | 67 | 7.17 | 2.04–25.22 | 0.002 |
| Platinum-resistant/refractory during primary treatment versus platinum-sensitive (ref) | 62 | 1.54 | 0.40–6.00 | 0.535 |
| Platinum-resistant/refractory during PFS1 versus platinum-sensitive (ref) | 43 | 12.00 | 1.98–72.74 | 0.007 |
| Platinum-resistant/refractory during PFS2 versus platinum-sensitive (ref) | 22 | 0.95 | 0.13–7.28 | 0.962 |
| Progressive disease during PFS1 versus response (stable disease, partial or complete response) (ref) | 63 | 0.07 | 0.01–0.31 | 0.001 |
4. Discussion
In this study, we investigated the PFS-ratio in 67 recurrent ovarian carcinoma patients treated with current palliative treatment options in order to determine the thresholds used to measure clinical benefit. Our data showed that a PFS-ratio > 1.3 is achieved in 22.4% of the patients. Moreover, multivariate logistic regression analysis demonstrated that both the LGOC histological subtype and CA125 tumor marker concentrations are associated with a PFS-ratio > 1.3.
Earlier studies demonstrated that the thresholds set by Von Hoff et al. were arbitrary and difficult to justify (Wu et al., 2019). Watson et al. suggested that several factors, such as biological behavior, should be taken into account when determining a threshold for meaningful clinical benefit in clinical trials indicating that the PFS-ratio could differ between histological subtypes (Watson et al., 2018). Our results support this theory, as we found a higher median PFS-ratio in patients diagnosed with LGOC as compared to HGSC (1.26 and 0.58, respectively). Similarly, a difference in median PFS-ratio between histological subtypes of ovarian carcinoma has been observed by Gallego et al. (Gallego et al., 2021). In their retrospective study including patients with recurrent ovarian carcinoma treated with bevacizumab-containing regimens they reported a median PFS-ratio of 0.95 for HGSC, 0.97 for LGOC and 2.36 for clear cell ovarian carcinoma patients (Gallego et al., 2021). Furthermore, Von Hoff et al. showed that the number of patients exceeding the 1.3 threshold also varies between types of carcinomas (Von Hoff et al., 2010). They found that 44.0% of the breast cancer patients, 36.0% of the colorectal cancer patients and 20.0% of the ovarian cancer patients reached a PFS-ratio > 1.3. For some types of carcinomas, no patients reached a PFS-ratio > 1.3. In our study population, 12.2% of the HGSC patients and 50.0% of the LGOC patients exceeded this threshold, indicating that the null hypothesis of 15.0% set by Von Hoff et al. might be suitable for recurrent HGSC patients but is less fitting in a study population of recurrent LGOC patients (Von Hoff et al., 2010). Taken together, these results indicate that the threshold for the proportion of patients with a PFS-ratio > 1.3 (i.e., null hypothesis) should be adjusted for the intrinsic differences in tumor behavior between histological subtypes.
In addition to the LGOC subtype, platinum-resistance and progressive disease during PFS1 were also correlated to a PFS-ratio > 1.3 in univariate analysis. This relation can be explained by the fact that immediate progressive disease or a resistant or refractory response to platinum-containing chemotherapy results in a relatively short PFS1-duration. If the PFS2 duration is even slightly longer than PFS1, the PFS-ratio is automatically higher. Vice versa, PR or CR during PFS1 causes this period to last longer, which lowers the chances for the treatment during PFS2 to be considered successful. However, it should not automatically be assumed that the second treatment did not cause response or clinical benefit. A slightly longer PFS2 than PFS1 may result in a PFS-ratio < 1.3, but can still be beneficial for the patient. To overcome the risk of a false positive or false negative classification, others tried to modify the PFS-ratio to better reflect the clinical benefit of a certain treatment (Mock et al., 2019). However, more research in this area is necessary before these modified PFS-ratios can be implemented in future research.
4.1. Strengths and limitations
This is the first study to investigate the PFS-ratio in an ovarian cancer population treated with current palliative options, and thereby critically look at the use of the PFS-ratio and thresholds currently used to determine clinical benefit. However, our study has some limitations. First, the LGOC group is small, which can be explained by the rarity of the disease and our strict in- and exclusion criteria. Secondly, the retrospective study design makes it challenging to collect sufficient medical data and resulted in some exclusions due to missing data or loss to follow-up as patients returned to their referring hospitals2. Thirdly, because there are few studies that have used the PFS-ratio as outcome measure and no studies that investigated the PFS-ratio and its thresholds in an ovarian cancer population treated with currently available treatment options, it is impossible to compare our results to other study populations. Fourthly, because of the heterogeneity in treatment options, it is difficult to compare the PFS-ratio per treatment subgroup due to small sample numbers.
5. Conclusion and future perspectives
The PFS-ratio can represent a meaningful outcome measure when investigating cytostatic anticancer agents. When determining the thresholds to define clinical benefit in trial designs, researchers should take into account that factors related to the prognosis of a disease, such as tumor differentiation grade, could result in a higher or lower PFS, and thus affect the PFS-ratio. Therefore, it can be useful to calculate the PFS-ratio in a study population treated according to current guidelines in order to substantiate the thresholds of clinical benefit when investigating new cytostatic agents.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Nienke van de Kruis: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft. Phyllis van der Ploeg: Conceptualization, Methodology, Formal analysis, Investigation, Visualization, Validation, Writing – review & editing. Jody H.C. Wilting: Investigation, Validation. M. Caroline Vos: Investigation, Validation. Anna M.J. Thijs: Validation. Joanne de Hullu: Validation. Petronella B. Ottevanger: Validation. Christianne Lok: Validation. Jurgen M.J. Piek: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kummar S., Gutierrez M., Doroshow J.H., Murgo A.J. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br. J. Clin. Pharmacol. 2006;62(1):15–26. doi: 10.1111/j.1365-2125.2006.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick R., Crowley J.J., Carroll R.J., et al. Phase II Clinical Trial Design for Noncytotoxic Anticancer Agents for Which Time to Disease Progression Is the Primary Endpoint. Control. Clin. Trials. 2000;21(4):343–359. doi: 10.1016/s0197-2456(00)00058-1. [DOI] [PubMed] [Google Scholar]
- von Hoff D. There are no bad anticancer agents, only bad clinical trial designs–twenty-first Richard and Hinda Rosenthal Foundation Award Lecture. Clin Cancer Res. 1998;4:1079–1086. [PubMed] [Google Scholar]
- Mock A., Heilig C.E., Kreutzfeldt S., et al. Community- driven development of a modified progression- free survival ratio for precision oncology. ESMO Open. 2019;4:e000583. doi: 10.1136/esmoopen-2019-000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D.D., Stephenson J.J., Rosen P., Loesch D.M., Borad M.J., Anthony S., Jameson G., Brown S., Cantafio N., Richards D.A., Fitch T.R., Wasserman E., Fernandez C., Green S., Sutherland W., Bittner M., Alarcon A., Mallery D., Penny R. Pilot Study Using Molecular Profiling of Patients’ Tumors to Find Potential Targets and Select Treatments for Their Refractory Cancers. J. Clin. Oncol. 2010;28(33):4877–4883. doi: 10.1200/JCO.2009.26.5983. [DOI] [PubMed] [Google Scholar]
- Massard C., Michiels S., Ferté C., et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017;7(6):586–595. doi: 10.1158/2159-8290.cd-16-1396. [DOI] [PubMed] [Google Scholar]
- Seeber A., Gastl G., Ensinger C., Spizzo G., Willenbacher W., Kocher F., Leitner C., Willenbacher E., Amann A., Steiner N., Eisterer W., Voss A., Russell K., Zwierzina H. Treatment of patients with refractory metastatic cancer according to molecular profiling on tumor tissue in the clinical routine: an interim-analysis of the ONCO-T-PROFILE project. Genes & Cancer. 2016;7(9-10):301–308. doi: 10.18632/genesandcancer.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeber A., Chahine G., Nasr F., Dean A., Miranova M., Jameson G., Robert N., Gastl G., Zwierzina H. Treatment According to a Comprehensive Molecular Profiling Can Lead to a Better Outcome in Heavily Pretreated Patients With Metastatic Cancer. The Cancer Journal. 2019;25(2):73–79. doi: 10.1097/PPO.0000000000000358. [DOI] [PubMed] [Google Scholar]
- Belin L., Kamal M., Mauborgne C., Plancher C., Mulot F., Delord J.-P., Gonçalves A., Gavoille C., Dubot C., Isambert N., Campone M., Trédan O., Ricci F., Alt M., Loirat D., Sablin M.-P., Paoletti X., Servois V., Le Tourneau C. Randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer: cross-over analysis from the SHIVA trial. Ann. Oncol. 2017;28(3):590–596. doi: 10.1093/annonc/mdw666. [DOI] [PubMed] [Google Scholar]
- Rodon J., Soria J.-C., Berger R., Miller W.H., Rubin E., Kugel A., Tsimberidou A., Saintigny P., Ackerstein A., Braña I., Loriot Y., Afshar M., Miller V., Wunder F., Bresson C., Martini J.-F., Raynaud J., Mendelsohn J., Batist G., Onn A., Tabernero J., Schilsky R.L., Lazar V., Lee J.J., Kurzrock R. Genomic and transcriptomic profiling expands precision cancer medicine: the WINTHER trial. Nat. Med. 2019;25(5):751–758. doi: 10.1038/s41591-019-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S., Menis J., Baldini C., Martin-Romano P., Michot J.-M., Hollebecque A., Armand J.-P., Massard C., Soria J.-C., Postel-Vinay S., Paoletti X. Time to progression ratio in cancer patients enrolled in early phase clinical trials: time for new guidelines? Br. J. Cancer. 2018;119(8):937–939. doi: 10.1038/s41416-018-0245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr B.R., Moroney M., Sheeder J., Eckhardt S.G., Sawyer B., Behbakht K., Diamond J.R. Survival and clinical outcomes of patients with ovarian cancer who were treated on phase 1 clinical trials. Cancer. 2020;126(19):4289–4293. doi: 10.1002/cncr.33073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Chen L.i., Wei J., Weiss H., Miller R.W., Villano J.L. Phase II trial design with growth modulation index as the primary endpoint. Pharm. Stat. 2019;18(2):212–222. doi: 10.1002/pst.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego A., Ramon-Patino J., Brenes J., Mendiola M., Berjon A., Casado G., Castelo B., Espinosa E., Hernandez A., Hardisson D., Feliu J., Redondo A. Bevacizumab in recurrent ovarian cancer: could it be particularly effective in patients with clear cell carcinoma? Clin. Transl. Oncol. 2021;23(3):536–542. doi: 10.1007/s12094-020-02446-z. [DOI] [PubMed] [Google Scholar]