Summary
Background
Historically, the incidence of cardiovascular disease and mortality in persons with Type 1 diabetes (T1D) has been increased compared to the general population. Contemporary studies on time trends of mortality and cardiovascular disease are sparse.
Methods
In this observational study, T1D persons were identified in the Swedish National Diabetes Registry (n=45,575) and compared with matched controls from the general population (n=220,141). Incidence rates from 2002 to 2019 were estimated with respect to mortality and cardiovascular disease in persons with T1D overall and when stratified for prevalent cardiovascular and renal disease relative to controls.
Findings
Mean age in persons with T1D was 32.4 years and 44.9% (20,446/45,575) were women. Age- and sex- adjusted mortality rates declined over time in both groups but remained significantly higher in those with T1D compared to controls during 2017–2019, 7.62 (95% CI 7.16; 8·08) vs. 2.23 (95% CI 2.13; 2.33) deaths per 1,000 person years. Myocardial infarction, heart failure and stroke decreased over time in both groups, with persistent excess risks in the range of 3.4–5.0 times from 2017 to 2019 in those with T1D. T1D persons ≥45 years without previous renal or cardiovascular complications had standardized mortality rates similar or even lower than controls 5.55 (4.51; 6.60) vs.7.08 (6.75; 7.40) respectively in the last time period.
Interpretation
Excess mortality persisted over time in persons with T1D, largely in patients with cardiorenal complications. Improved secondary prevention with a focus on individualized treatment is needed to close the gap in mortality for individuals with T1D.
Funding
This study was financed by grants from the ALF-agreement, NovoNordisk Foundation and the Swedish Heart and Lung Foundation.
Keywords: Type 1 diabetes, Ischemic heart disease, Acute myocardial infarction, Heart failure, Stroke
Research in context.
Evidence before this study
We searched PubMed and Google Scholar for articles published until December 20, 2021 with the search terms “Type 1 diabetes” and “mortality” in the title or the abstract. We found no recent studies evaluating mortality rates in persons with Type 1 diabetes. We found a few earlier studies stratifying persons with Type 1 diabetes on diabetes complications evaluating prognosis over time.
Added value of this study
-
•
Excess risk of mortality, myocardial infarction, heart failure and stroke remain for persons with Type 1 diabetes and rates are 3.4–5.8 times higher when evaluated over 20 years until December 31, 2019.
-
•
Type 1 diabetes is a cardiovascular equivalent with respect to future risk of myocardial infarction.
-
•
Contemporary mortality rates in persons ≥45 years are lower in people with Type 1 diabetes free from cardiorenal complications than for controls without diabetes at the same age and sex.
-
•
Type 1 diabetes persons free from cardiorenal complications but with hyperglycemia have excess risk of myocardial infarction, but the risk attenuates for patients with mean Hba1c≤58 mmol/mol (7.5%) over time and converges to incidence rates in controls.
Implications of all the available evidence
Excess mortality remains in people with Type 1 diabetes, but prognosis needs to be individualized and diversified since large groups without cardiorenal complications show low mortality rates and cardiovascular incidences. To reduce the overall gap in mortality for persons with Type 1 diabetes, improved secondary prevention in patients with cardiorenal complications is urgently needed.
Alt-text: Unlabelled box
Introduction
Cardiovascular disease (CVD) is the major reason for shortened life expectancy in persons with Type 1 diabetes (T1D).1,2 Hyperglycemia, hypertension, smoking, renal dysfunction, and hyperlipidemia accelerate the atherosclerotic process in persons with T1D and increase the risk of cardiovascular events and mortality.3 Accordingly, goals in the care of persons with diabetes include maintaining a low risk factor burden and reaching life expectancy similar to persons without diabetes.4 Despite more aggressive treatment guidelines, mortality trends for persons with T1D have not converged to those found in the general population.
Although multiple studies have described temporal changes in risk factor burden and prognosis in patients with T1D, contemporary analyses are sparse.2,5, 6, 7, 8, 9, 10 A recent evaluation in Sweden lasted only until 2013.2 With more aggressive cardiovascular preventive strategies in place over longer time periods and the recent introduction of more efficient tools for glycemic control (e.g., continuous glucose-monitoring systems and modern insulin delivery systems), glycemic control has improved.11, 12, 13, 14 Updated information on complications and mortality is needed, particularly in light of findings of a marked reduction in amputations in recent years.14
Cohort studies have shown that persons with T1D without renal complications or not having overt hyperglycaemia have mortality rates similar to the general population.15,16 Less recent analyses of patients without renal complications found similar or only slightly elevated mortality rates for persons with T1D.17,18 Despite the impact of these risk factors as well as cardiovascular disease most investigations of whether prognosis improve over time generally treat T1D patients as a homogenous group.
The aim of this study was to estimate mortality and the incidence of cardiovascular disease over time in the past two decades in persons with T1D and controls overall and stratified by prevalent cardiovascular and renal disease.
Methods
Setting and participants
This was a population-based study, of persons with T1D in the Swedish National Diabetes Registry (NDR) and controls identified in the Swedish population registry matched by age, gender, and county. Data were linked using the unique personal identification number of each citizen to the Swedish National Patient Registry (NPR), the Swedish Cause of Death Registry, and the Longitudinal Integration Database for Health Insurance and Labor Market Studies as described previously.1 All patients in the NDR provided informed consent. In the data set used for this study personal identifiers were removed and replaced by codes. All procedures were approved by the Swedish Ethical Review Authority.
The NDR includes near-complete coverage of all persons with T1D in Sweden and information about cardiovascular risk factors, diabetes complications, and treatments1,19 collected during routine hospital visits to outpatient wards or primary care clinics. The epidemiological definition of T1D (diagnosis before age 30 and treatment with insulin) was applied to select persons from the NDR, which has been estimated to be correct in about 97%.19
The study cohort included all patients ≥18 years of age with at least one registration in the NDR between January 1, 2000 and December 31, 2018. Each person with T1D was matched with up to five controls, randomly selected from the general population and matched by age, sex, and county, and censored if diagnosed with T1D. Patients and controls were followed from study start until the first event of each outcome, death, or study end (December 31, 2019). Baseline data were collected at the start of the study period or when the person was first registered in the NDR. Diabetes duration, smoking status, HbA1c, systolic and diastolic blood pressure (SBP and DBP), LDL and HDL cholesterol, Body Mass Index (BMI), albuminuria, and creatinine level were retrieved from the NDR. Microalbuminuria was defined as at least two positive results obtained within 1 year and defined as albumin-to-creatinine ratio of 3–30 mg/mmol (∼30–300 mg/g) or urinary albumin clearance of 20–200 µg/min (∼20–300 mg/L). Macroalbuminuria was defined as an albumin-to-creatinine ratio >30 mg/mmol (∼300 mg/g or greater) or urinary albumin clearance >200 µg/min (>300 mg/L). Creatinine-based eGFR values were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.20
Education and country of birth were retrieved from the Longitudinal Integration Database for Health Insurance and Labor Market Studies and stratified into three educational groups: 1) low (up to 9 years), 2) intermediate (10–12 years), and 3) high (university or college).
Outcomes
Mortality and incidence rates of acute myocardial infarction (AMI), heart failure (HF), and stroke were analyzed for persons with T1D and controls for the following time periods: 2002–2004, 2005–2007, 2008–2010, 2011–2013, 2014–2016, 2017–2019. ICD-9 and ICD-10 codes in the cause of death and Swedish national inpatient registries were used to determine these outcomes (Supplementary Methods).
Stratification by cardiovascular disease, renal complications and risk factors
We first analyzed mortality and incidence rates over time in all persons with T1D compared with all controls. Thereafter, since CVD is relatively rare among younger adults, we further stratified persons ≥45 years with T1D versus controls according to cardiovascular and/or renal disease and/or risk factors (stratification was updated at the start of each time period).
In the second analysis, persons with T1D and controls were stratified according to the presence or absence of CVD (defined as in-hospital diagnosis of AMI, HF, stroke, or atrial fibrillation during the previous 10 years).
In a third analysis, patients with T1D were stratified according to the presence or absence of CVD and/or current renal complications. Two different definitions of renal complications were explored: 1) eGFR<60 ml/min/1.73 m2 and/or micro- or macroalbuminuria; 2) eGFR<60 ml/min/1.73 m2 and/or macroalbuminuria.
In a fourth analysis, we used risk factors (mean levels of available measurements before each time period) to stratify patients with diabetes into “high risk” (presence of cardiovascular and/or renal complications and/or presence of a risk factor) and “low risk”, respectively. The following risk factors were analysed one at a time: HbA1c, SBP, LDL, BMI and current smoking status. Different cut-offs for risk factor levels were explored.
A post-hoc analysis was performed to evaluate the cumulative outcome in the following age-groups: 18 ≤ 35 years, 35 ≤ 50 years, 50 ≤ 65 years and ≥65 years. Incidence rates were age adjusted to the first time period for each age group.
Statistical analysis
Baseline characteristics are described as mean with standard deviation or median with interquartile range for continuous variables and number with percentage for categorical variables. Crude mortality rates and incidences of CVD were estimated as events per 1000 person-years and with Poisson 95% confidence intervals (CI). Mortality and incidence rates for AMI, HF, and stroke in persons with T1D and controls were then age and sex-standardized to the first time period and estimated per 1000 person-years with 95% Poisson CI for each time period.
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Study population
Baseline characteristics for 45,575 persons with T1D and 220,141 matched controls are shown in Table 1. Mean follow up for persons with T1D was 11.8 (SD 6.1) years and for controls 12.2 (SD 6.0) years. For persons with T1D, mean diabetes duration was 18.2 (SD 14.8) years and mean HbA1c was 65.8 (SD 17.0) mmol/mol (8.18 (SD 1.65)%) (Table 1). The proportion of persons with AMI, coronary heart disease, HF, stroke, and cancer at baseline was greater in persons with diabetes compared to controls. During follow-up 5339 (11.7%) persons with T1D and 10,369 (4.7%) controls died (Supplementary Table 1). 73.3% of all deaths among persons with T1D occurred in patients ≥45 years. The number of T1D patients ≥65 years increased from 947 to 3958 persons from the first to the last time period.
Table 1.
Baseline characteristics for 45,575 persons with Type 1 diabetes and 220,141 controls matched on age, sex and county.
| Variable | Persons with T1D (n = 45,575) | Controls (n = 220,141) | |
|---|---|---|---|
| Sex | |||
| Women | n (%) | 20,466 (44.9) | 99,776 (45.3) |
| Age (years) | Mean (SD) | 32.4 (14.7) | 31.6 (14.0) |
| Median (IQR) | 28 (20; 42) | 27 (19; 41) | |
| n | 45,575 | 220,141 | |
| Follow-up time (years) | Mean (SD) | 11.8 (6.1) | 12.2 (6.0) |
| Median (IQR) | 12.6 (6.7; 17.6) | 13.4 (7.3; 17.9) | |
| n | 45,477 | 219,854 | |
| Age categories | |||
| 18-<35 years | n (%) | 28,922 (63.5) | 143,614 (65.2) |
| 35-<50 years | n (%) | 9363 (20.5) | 45,660 (20.7) |
| 50-<65 years | n (%) | 5736 (12.6) | 25,642 (11.6) |
| >=65 years | n (%) | 1554 (3.4) | 5225 (2.4) |
| Born in Sweden | n (%) | 40,225 (88.3) | 187,379 (85.1) |
| Education category | |||
| Low | n (%) | 12,882 (36.7) | 62,549 (36.1) |
| Mid | n (%) | 14,712 (42.0) | 71,368 (41.2) |
| High | n (%) | 7463 (21.3) | 39,505 (22.8) |
| Diabetes duration (years) | Mean (SD) | 18.2 (14.8) | |
| Median (IQR) | 15 (7; 28) | ||
| n | 45,575 | ||
| Smoker | n (%) | 5789 (14.3) | |
| BMI (kg/m2) | Mean (SD) | 25.2 (6.4) | |
| Median (IQR) | 24.5 (22.3; 27.2) | ||
| n | 37,053 | ||
| HbA1c (mmol/mol) | Mean (SD) | 65.8 (17.0) | |
| Median (IQR) | 64 (54; 75) | ||
| n | 42,938 | ||
| HbA1c (%) | Mean (SD) | 8.18 (1.56) | |
| Median (IQR) | 8.0 (7.1; 9.0) | ||
| n | 42,938 | ||
| eGFR (CKD-EPI) | Mean (SD) | 107.8 (26.0) | |
| Median (IQR) | 113.1 (93.8; 127.1) | ||
| n | 24,379 | ||
| Albuminuria categories | |||
| No albuminuria | n (%) | 27,483 (82.8%) | |
| Microalbuminuria | n (%) | 3315 (10.0%) | |
| Macroalbuminuria | n (%) | 2389 (7.2%) | |
| LDL (mmol/L) | Mean (SD) | 2.62 (0.85) | |
| Median (IQR) | 2.52 (2.03; 3.1) | ||
| n | 20,269 | ||
| HDL (mmol/L) | Mean (SD) | 1.51 (0.46) | |
| Median (Q1, Q3) | 1.4 (1.2; 1.8) | ||
| n | 20,488 | ||
| Systolic BP (mmHg) | Mean (SD) | 125.4 (16.4) | |
| Median (Q1, Q3) | 120 (115; 135) | ||
| n | 40,689 | ||
| Diastolic BP (mmHg) | Mean (SD) | 73.1 (9.1) | |
| Median (Q1, Q3) | 72 (69; 80) | ||
| n | 40,565 | ||
| Amputation | n (%) | 378 (0.8) | 91 (0.0) |
| CHD | n (%) | 2065 (4.5) | 2200 (1.0) |
| Valve disease | n (%) | 140 (0.3) | 358 (0.2) |
| Heart failure | n (%) | 670 (1.5) | 642 (0.3) |
| Atrial fibrillation | n (%) | 324 (0.7) | 1062 (0.5) |
| AMI | n (%) | 1053 (2.3) | 1117 (0.5) |
| Stroke | n (%) | 725 (1.6) | 958 (0.4) |
| Cancer | n (%) | 937 (2.1) | 3314 (1.5) |
Categorical variables are presented as n (%). Continuous variables are presented as mean (SD) and median (IQR).
Mortality and cardiovascular disease in persons with T1D and controls
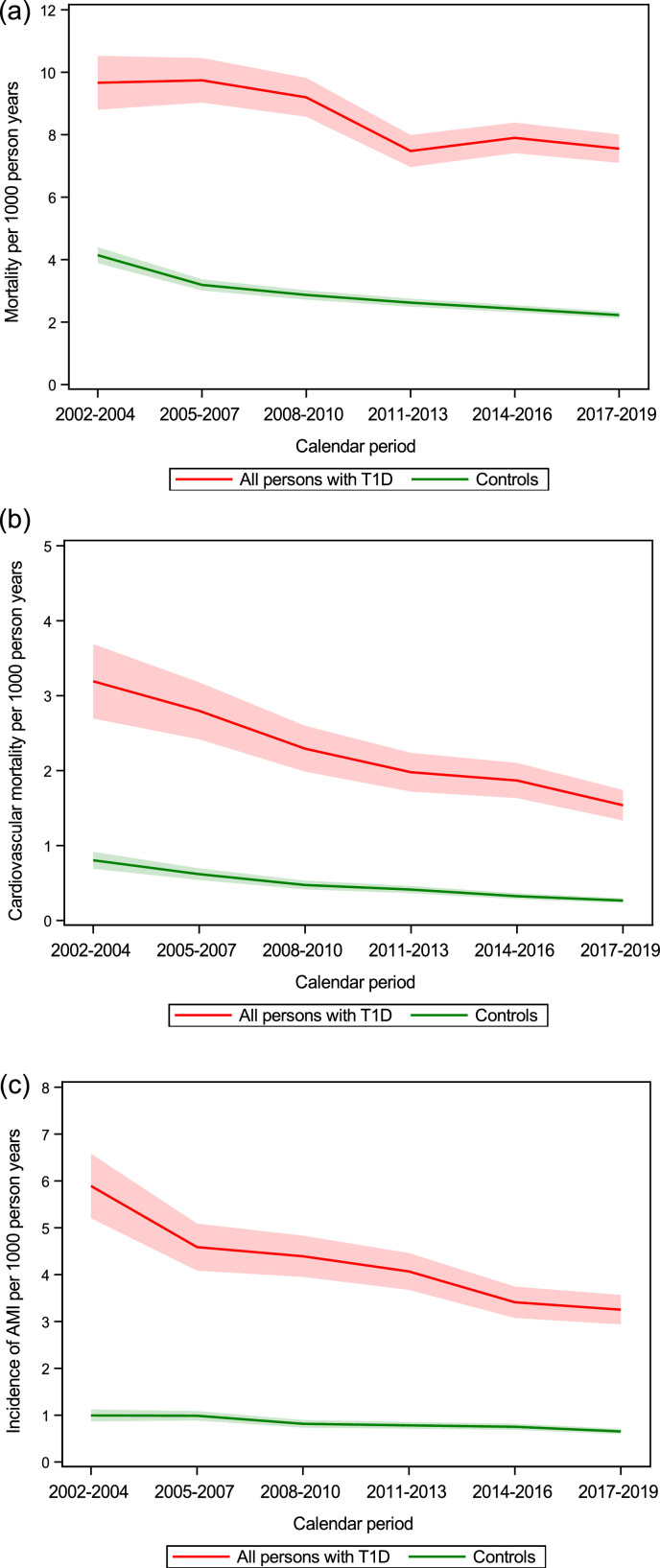
Standardized all-cause mortality rates decreased from the first to the last time period in both persons with T1D and controls, with a persistent gap between groups (Figure 1a). Mortality rates for T1D patients at the beginning and end of follow up were 9.59 (95% CI 8.73–10.44) and 7.62 (95% CI 7.16–8.08) deaths per 1000 person years, respectively. The corresponding mortality rates among controls were 4.11 (95% CI 3.85–4.36) and 2.23 (95% CI 2.13–2.33) deaths per 1000 person years (Supplementary Table 2).
Figure 1.
(a, b, c) Standardized incidence rates with 95% CI (shadowed area) in persons with T1D and controls over time for (a) mortality, (b) cardiovascular mortality and (c) AMI. See supplementary Table 2 for exact numbers for estimates.
Cardiovascular mortality showed a similar pattern with rates decreasing over time for persons with T1D and controls, with excess cardiovascular mortality in persons with T1D that persisted over time (Figure 1b, Supplementary Table 2). The standardized cardiovascular mortality rates for persons with T1D decreased from 3.16 (95% CI 2.67–3.65) to 1.56 (95% CI 1.36–1.77) deaths per 1000 person years, and the corresponding rates for controls were 0.80 (95% CI 0.69–0.91) and 0.27 (95% CI 0.23–0.30). All-cause and cardiovascular mortality rates were generally higher in men than women for persons with T1D (Supplementary Table 2).
Similar patterns were observed for AMI as well as for HF, and stroke, with improved prognosis for persons with T1D over time but, a gap remained compared with controls (Figure 1 c, Supplementary Figure 1). Standardized incidence rates at the first and last time periods for persons with T1D for AMI were 5.82 (95% CI 5.14–6.50) vs. 3.30 (95% CI 2.98–3.61), HF 4.86 (95% CI 4.24–5.47) vs. 3.75 (95% CI 3.42–4.08), and stroke 4.24 (95% CI 3.66–4.81) vs. 2.46 (95% CI 2.18–2.73) events per 1000 person years (Supplementary Table 2). The corresponding rates for controls were: AMI 0.98 (95% CI 0.86–1.11) vs. 0.66 (95% CI 0.60–0.72), HF 1.04 (95% CI 0.91–1.17) vs. 0.87 (0.81–0.94), and stroke 1.16 (95% CI 1.02–1.30) vs. 0.72 (95% CI 0.66–0.79) events per 1000 person years (Supplementary Table 2).
Mortality and cardiovascular disease in persons with T1D with and without CVD
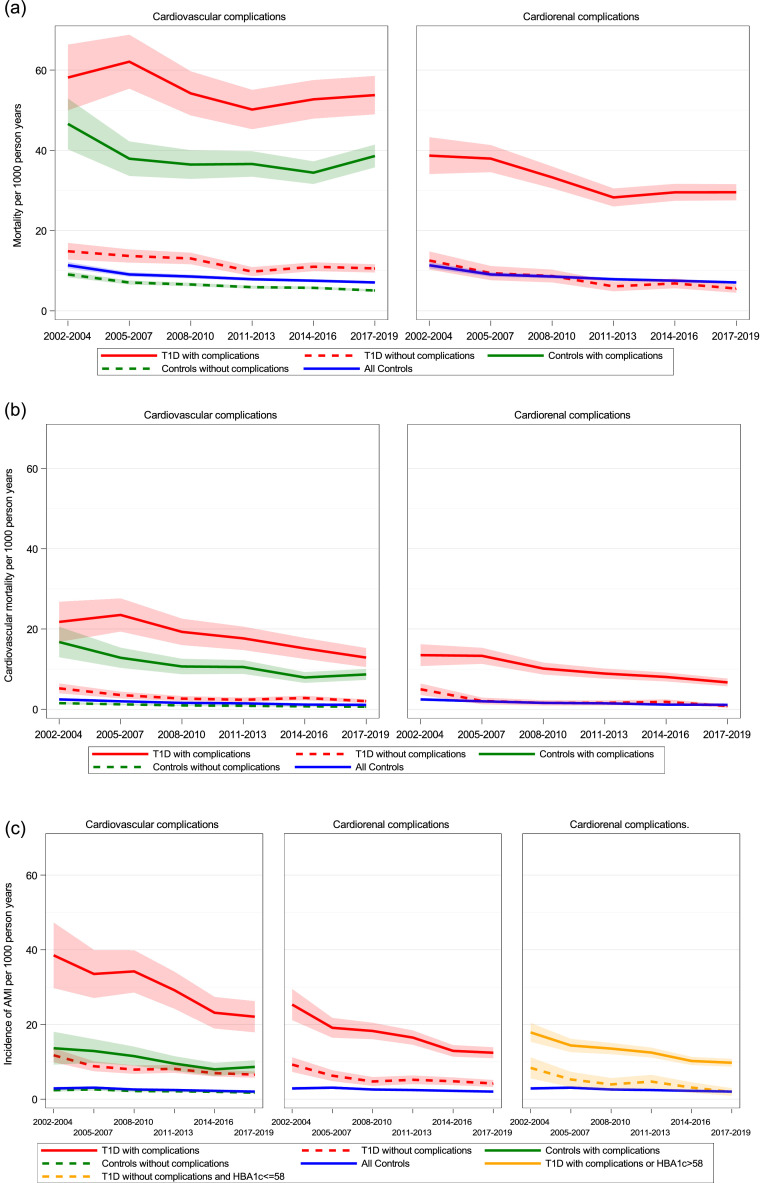
When patients with T1D and controls were stratified according to the presence or absence of CVD, only persons ≥45 years were included. In this population, the proportion of T1D patients with prevalent CVD increased from 20.7% at the beginning of the first time period to 22.2% at the beginning of the last time period (Supplementary Table 3). Over time, standardized mortality rates were highest in T1D patients with established CVD, followed by controls with CVD, T1D patients without CVD and controls free from CVD, and the same trend was observed for cardiovascular mortality (Left panel Figure 2a, 2b). However, for AMI a different pattern was observed such that T1D patients without established CVD had similar risk for AMI over time as controls with established CVD (i.e., T1D was a cardiovascular risk equivalent for AMI (Left panel Figure 2c). The standardized incidence rates of stroke and HF in each time period are presented in Supplementary Table 4 for persons with T1D and controls according to the presence or absence of CVD.
Figure 2.
Standardized incidence rates for mortality (a), cardiovascular mortality (b), and myocardial infarction (c) with 95% CI (shadowed area) in controls ≥45 years, in controls ≥45 years with and without cardiovascular complications (left panel) and in T1D persons ≥45 years with and without cardiovascular or cardiorenal complications (right panel) and in (c) in T1D persons ≥45 years with and without cardiovascular or cardiorenal complications or elevated HbA1c (right panel). See Supplementary Tables 4 and 6 for exact numbers of estimates.
In persons with T1D age 35 ≤ 50 years and with cardiovascular complications, incidence rates for cardiovascular mortality, AMI, HF and stroke were similar or higher than for T1D persons age ≥65 years without cardiovascular complications (Supplementary Table 5). This suggests that the presence of cardiovascular complications rather than age is the main driver for risk in persons with T1D.
Mortality and cardiovascular disease in persons with T1D with and without cardiorenal disease
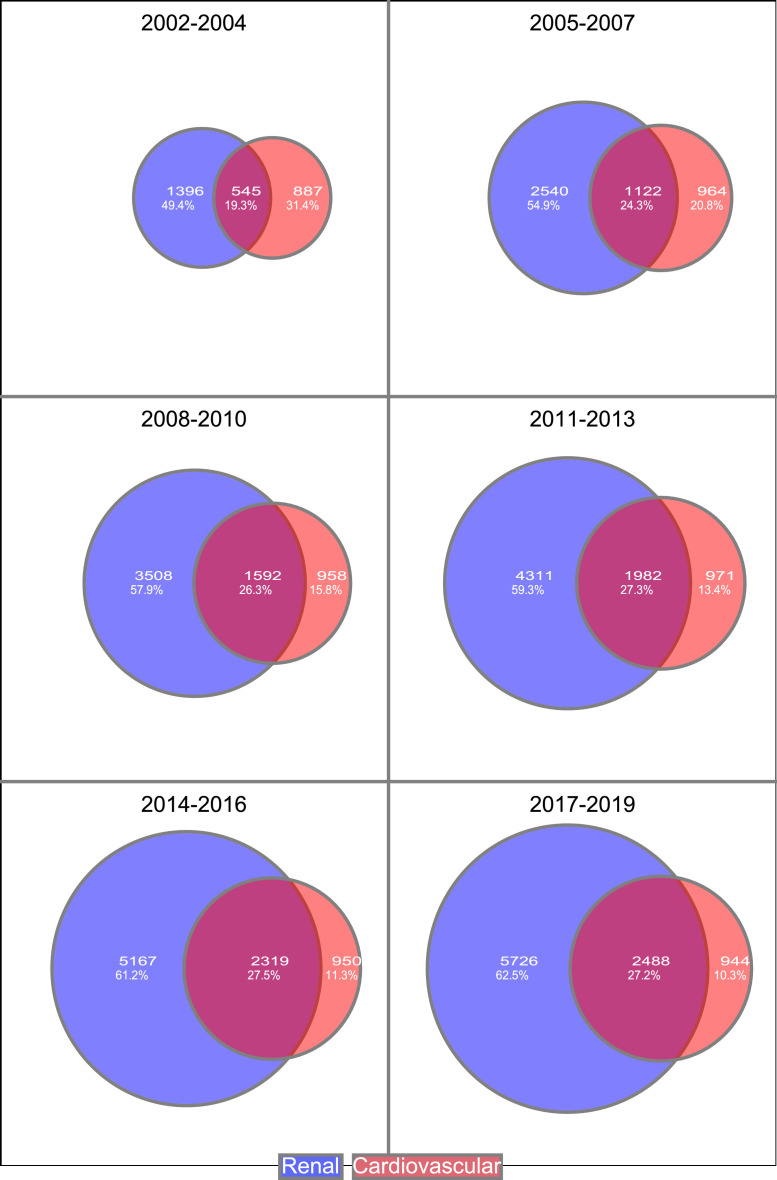
Among persons with T1D ≥45 years, 40.9% had established CVD and/or renal complications (eGFR<60 ml/min, micro- or macroalbuminuria) at the beginning of the first time period compared with 59·3% at the beginning of the last time period (Supplementary Table 3). The proportion of persons in this group with only renal complications increased over time (Figure 3).
Figure 3.
Persons with T1D ≥45 years with cardiorenal complications categorized in; persons with only cardiovascular complications, in persons with only renal complications and in proportion of persons with both complications.
Standardized mortality rates in T1D patients free from cardiorenal disease were similar over time as well as in all controls ≥45 years, with even slightly lower mortality in T1D persons during the last time period (5.55 (95% CI 4.51–6.60) vs. 7.08 (95% CI 6.75–7.40)) deaths per 1000 person years (Right panel Figure 2a, Supplementary Table 6).
HF showed a similar pattern as for mortality, with similar event rates among persons with T1D free from cardiorenal disease and all controls (Right panel, Supplementary Figure 2a). In contrast, incidences of AMI were consistently higher for persons with T1D free from cardiorenal complications compared with all controls ≥45 years (Middle panel Figure 2c).
In further analysis performed to compare the impact of renal complications in persons with T1D ≥45 years, another definition of renal disease was applied. Persons with T1D ≥45 years were characterized as having renal disease if decreased eGFR or macroalbuminuria was present. With this definition of renal disease, incidence rates of all outcomes were greater in persons with T1D without cardiorenal disease compared to controls (Supplementary Table 7).
HbA1c, blood pressure, BMI, and smoking
Since T1D patients free from cardiorenal complications had an excess risk of AMI over time, we evaluated whether certain cut-offs for traditional risk factors influenced the excess risk (Supplementary Figure 3). Levels of HbA1c, blood pressure, LDL cholesterol, BMI, and the proportion of smoking in T1D patients over time are shown in Table 2. When high risk was defined as cardiorenal disease and/or mean SBP over time >130, >135 and >140 mmHg, no clear converging trend for low risk patients was found for AMI. In separate analyses, when high risk was defined as cardiorenal disease and/or mean LDL cholesterol >2.0, >2.5, and >3.0 mmol/l, BMI >27.5 kg/m2 and >30 kg/m2 and smoking, no or only small effects were found (Supplementary Figure 3). In contrast, a monotonic pattern was found with converging trends when high risk was defined as patients with cardiorenal disease and/or high HbA1c levels (65, 62, and 58 mmol/mol), and for patients with mean HbA1c <7.5% (58 mmol/mol) free from cardiorenal complications, no excess risk of AMI was found (Right panel Figure 2c and Supplementary Figure 3 a).
Table 2.
Risk factors over time in all persons with T1D and in persons with T1D ≥45 years with cardiorenal disease in the first and last time period.
| Risk factor | Persons | 2002–2004 | 2008–2010 | 2017–2019 |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | All T1D | 128.8 (15.7) n = 19,081 |
125.9 (13.5) n = 27,815 |
125.6 (12.6) n = 37,155 |
| T1D ≥45 years with cardiorenal disease | 139.9 (16.2) n = 2492 |
134.9 (13.9) n = 5330 |
133.2 (12.5) n = 7939 |
|
| LDL (mmol/l) | All T1D | 2.7 (0.8) n = 15,451 |
2.7 (0.7) n = 24,266 |
2.5 (0.8) n = 35,195 |
| T1D ≥45 years with cardiorenal disease | 2.7 (0.8) n = 2145 |
2.5 (0.7) n = 4865 |
2.2 (0.8) n = 7622 |
|
| HbA1c (mmol/mol) | All T1D | 65.0 (13.6) n = 19,177 |
64.8 (13.5) n = 28,035 |
61.3 (13.5), n = 37,690 |
| T1D ≥45 years with cardiorenal disease | 66.4 (12.5) n = 2503 |
64.6 (12.0) n = 5382 |
62.3 (11.8) n = 8044 |
|
| Age (years) | All T1D | 39.0 (13.8) n = 20,864 |
39.5 (15.1) n = 30,446 |
39.9 (16.3) n = 41,438 |
| T1D ≥45 years with cardiorenal disease | 56.8 (8.4) n = 2828 |
58.1 (9.0) n = 6058 |
59.9 (9.9) n = 9158 |
|
| BMI (kg/m2) | All T1D | 25.3 (3.8) n = 18,370 |
25.7 (4.3) n = 26,515 |
26.2 (4.7) n = 33,053 |
| T1D ≥45 years with cardiorenal disease | 25.8 (4.1) n = 2400 |
26.2 (4.5) n = 5110 |
26.8 (4.9) n = 7084 |
|
| Smoking (%) | All T1D | 15.8% | 15.5% | 13.9% |
| T1D ≥45 years with cardiorenal disease | 16.3% | 15.0% | 11.9% | |
| Diabetes Duration (years) | All T1D | 24.1 (14.0) n = 20,308 | 24.8 (14.9) n = 29,537 |
25.6 (16.0) n = 39,817 |
| T1D ≥45 years with cardiorenal disease | 40.5 (10.6) n = 2828 |
41.7 (11.0) n = 6058 |
43.7 (11.8) n = 9158 |
Categorical variables are presented as n (%). Continuous variables are presented as mean (SD). For risk factors in all periods see Supplementary Table 8.
Discussion
In this population-based study of persons with T1D over two decades, mortality rates and incidences of AMI, HF, and stroke decreased over time. However, the gap between T1D persons and controls did not converge due to improvements in prognosis in the general population. By contrast, people with T1D ≥45 years of age without previous cardiovascular or renal complications (constituting approximately 50% of this group over time) had mortality rates similar to persons ≥45 years without diabetes, as well as low risks of AMI, HF, and stroke.
Previous studies have evaluated excess risks of mortality and CVD over time for persons with diabetes in different geographic regions showing excess risks on a group level but only a few studies have distinguished patients by cardiorenal complications or other risk factors.15, 16, 17, 18,21, 22, 23 Whether diabetes is a cardiovascular risk equivalent has been debated, i.e., whether persons with diabetes free from coronary artery disease have similar risk of myocardial infarction as persons in the general population with established coronary disease.24 This has primarily been evaluated in persons with type 2 diabetes (T2D) and has been an important basis for whether they should receive more aggressive primary preventive treatment. We found T1D to be a cardiovascular risk equivalent with respect to AMI highlighting the need for aggressive CVD prevention in this patient group. For HF, the impact of renal complications was even stronger than when AMI was evaluated.
Renal complications
Renal complications are a marker of past elevated glucose levels and a key explanatory variable for excess mortality in persons with T1D.1,15, 16, 17, 18 The gap in mortality between persons with T1D and the general population would likely converge if mortality and cardiovascular risk could be reduced in patients with renal complications. In the current study, renal complications had a marked impact on mortality, implying that aggressive prevention is needed at early stages of renal complications.
Lower blood pressure and treatment with RAAS-inhibitors are recommended for patients with renal complications to reduce further renal progression. Moreover, persons at high risk of AMI, stroke, and HF likely benefit from very strict blood pressure control, although this has been debated among people with T2D.25 Lower blood lipid targets than the overall targets of LDL 2.5 mmol/l (97 mg/dl) may be warranted for individuals with T1D and renal complications who are not yet on dialysis.
SGLT2-inhibitors have shown a preventive effect regarding advanced renal complications, HF, and cardiovascular mortality not only in patients with T2D but also in people without diabetes.26, 27, 28, 29, 30 Similar preventive effects, not acting via the glucose-lowering effect, but among other mechanisms via reduction of intraglomerular pressure are likewise beneficial for patients with T1D.31 Future studies are needed to evaluate the effect of SGLT-2 inhibitors in T1D persons with cardiovascular and cardiorenal disease. As in T2D guidelines, clinicians ought to characterize T1D patients as at high- or low-risk to a greater extent.32 Moreover, other cardiorenal preventive treatments need further investigation, including finerenone which has shown beneficial effects in persons with T2D and renal complications.33,34
Risk factors and cardiorenal complications
Persons with T1D free from cardiovascular and renal complications showed slightly increased risk of AMI compared with persons without diabetes. Hence, some patients seem to convert from low-risk to high-risk via AMI. When exploring traditional risk factors the strongest association existed with glucose control. Patients with historical mean HbA1c ≤7.5% (58 mmol/mol) without cardiorenal complications had no excess risk of AMI. The strong association between AMI and HbA1c may be explained by other risk factors (hypertension and LDL cholesterol levels) that were well-treated overall whereas HbA1c 60–65 mmol/mol were nearly double compared to levels in the general population in Sweden with mean HbA1c 34 mmol/mol.35
The importance of glucose control to prevent a patient from converting to a high-risk profile is supported by the fact that hyperglycemia is a prerequisite for diabetic nephropathy.3 Continuous glucose monitoring (CGM) and advanced insulin pumps connecting CGM with an insulin pump for adjusting insulin delivery may reduce cardiorenal complications by improving glucose control. In a recent study including patients from four countries, renal complications were still common in patients with diabetes onset over the last 20 years, and the majority of patients had glucose levels above target.36 Hence, improved prevention in people with T1D and cardiorenal complications should be the focus of attention in clinical practice and research to reduce the gap in mortality over time.
Strengths and limitations
A strength of the current study is the population-based design comprising nearly all persons with T1D in Sweden over 2 decades including information on diabetes complications and risk factors. This study has several limitations. First, due to the registry-based study design, it is inevitable that some data are missing, for example information on renal complications was less comprehensive during the first 3 years of follow-up. Moreover, coverage of the NDR in the first time periods was lower but improved over time to include almost all persons with T1D during the last decade. It should also be acknowledged that the NDR does not contain data regarding race and ethnicity, so these variables were not accounted for in the analyses. Second, information on CVD in age- and sex-matched controls was available but levels of blood pressure, blood lipids, BMI, and smoking were not. Third, information about renal complications was not available in controls, although renal complications are known to be relatively rare in persons without diabetes. Fourth, information on use of medication to prevent and treat renal complications and hyperlipidemia were not considered in the analysis. This may be of interest in future analyses, especially in high risk patients. Finally, in real-life evaluations as the current, data registration depends on when examinations are performed in clinical practice and not evaluated at specific predefined time points.
Conclusions
Mortality and cardiovascular disease prognosis is improving in persons with T1D but clear excess risks remain overall compared with individuals without diabetes. With respect to mortality, the prognosis of persons with T1D and without cardiorenal complications is similar to persons without diabetes. Increased focus on prevention in patients with renal complications, and improved glucose control in the T1D population overall, are likely key factors to reducing the overall gap in mortality for persons with T1D compared with the general population. Accordingly, a differentiated treatment focus is needed in future care of T1D patients.
Contributors
ML and SH contributed to the conception and design of the study. AR, JL, ML and SH were responsible for acquisition of the data. The manuscript was drafted by SH and ML. AR, HW, JL, MAP, ML, MOW, SH, and PE participated in the analysis and interpretation of data, and also in the critical revision of the paper and gave final approval to submit the manuscript for the publication. SH, the corresponding author, and ML had full access to all study data and takes responsibility for data integrity and the accuracy of the data analysis.
Data sharing statement
The data that support the findings of this study are available from the National Board of Health and Welfare in Sweden and from the Swedish National Diabetes Registry but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available.
Declaration of interests
HW has served on safety or advisory boards for ScanCLAD, SweVAD and Xvivo. MOW has served on advisory boards or lectured for MSD, Lilly, Novo Nordisk, and Sanofi, and has organized a professional regional meeting sponsored by Eli Lilly, Rubin Medical, Sanofi, Novartis, and Novo Nordisk. MAP has Research Grant Support from Novartis and has been a consultant to AstraZeneca, Boehringer Ingelheim, Eli Lilly Alliance, Corvidia, DalCor, GlaxoSmithKline, Lexicon, NHLBI CONNECTs (Master Protocol Committee), Novartis, Novo Nordisk, Peerbridge and Sanofi; and has equity in DalCor. ML has received research grants from Eli Lilly and Novonordisk and been a consultant or received honoraria from Astra Zeneca, Boehringer Ingelheim, Eli Lilly and Novonordisk. No other potential conflicts of interest relevant to this article were reported.
Acknowledgments
We want to thank the staff at the Swedish National Diabetes Registry and all staff who collected data and made linkages possible with other registries. We also want to acknowledge the late Ann-Marie Svensson for her contribution to the manuscript. This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement [ALFGBG-717211] and [ALFGBG-966173], Region Västra Götaland, grants from the Novo Nordisk foundation, Swedish Heart and Lung Foundation [20180589], [20210679], Swedish Research Council [2018-02527] [VRREG 2019-00193], and the Gothenburg Society of Medicine.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100469.
Appendix. Supplementary materials
References
- 1.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 2.Rawshani A, Rawshani A, Franzén S., et al. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376(15):1407–1418. doi: 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 3.DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi: 10.1016/S0140-6736(18)31320-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S111–S134. doi: 10.2337/dc20-S010. [DOI] [PubMed] [Google Scholar]
- 5.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with Type 1 diabetes, 2008–2010. JAMA. 2015;313(1):37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind M, Garcia-Rodriguez LA, Booth GL, et al. Mortality trends in patients with and without diabetes in Ontario, Canada and the UKfrom 1996 to 2009: a population-based study. Diabetologia. 2013;56(12):2601–2608. doi: 10.1007/s00125-013-3063-1. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group for the DCCT/EDIC Research Group. Orchard TJ, Nathan DM, et al. Association between 7 years of intensive treatment of Type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45–53. doi: 10.1001/jama.2014.16107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with Type 1 diabetes. N Engl J Med. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groop PH, Thomas M, Feodoroff M, Forsblom C, Harjutsalo V, FinnDiane Study Group Excess mortality in patients with Type 1 diabetes without albuminuria-separating the contribution of early and late risks. Diabetes Care. 2018;41(4):748–754. doi: 10.2337/dc17-1618. [DOI] [PubMed] [Google Scholar]
- 10.Gagnum V, Stene LC, Leivestad T, Joner G, Skrivarhaug T. Long-term mortality and end-stage renal disease in a Type 1 diabetes population diagnosed at age 15–29 years in Norway. Diabetes Care. 2017;40(1):38–45. doi: 10.2337/dc16-1213. [DOI] [PubMed] [Google Scholar]
- 11.Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in Type 1 diabetes. N Engl J Med. 2019;381(18):1707–1717. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lind M, Polonsky W, Hirsch IB, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with Type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017;317(4):379–387. doi: 10.1001/jama.2016.19976. [DOI] [PubMed] [Google Scholar]
- 13.https://www.ndr.nu
- 14.Hallström S, Svensson AM, Pivodic A, et al. Risk factors and incidence over time for lower extremity amputations in people with Type 1 diabetes: an observational cohort study of 46,088 patients from the Swedish national diabetes registry. Diabetologia. 2021;64(12):2751–2761. doi: 10.1007/s00125-021-05550-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahlén E, Pivodic A, Wedel H, Dahlqvist S, Kosiborod M, Lind M. Glycemic control, renal complications, and current smoking in relation to excess risk of mortality in persons with Type 1 diabetes. J Diabetes Sci Technol. 2016;10(5):1006–1014. doi: 10.1177/1932296816652901. Published 22 August 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in Type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh epidemiology of diabetes complications study. Diabetologia. 2010;53(11):2312–2319. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groop P.H., Thomas M.C., Moran J.L., et al. The presence and severity of chronic kidney disease predicts all-cause mortality in Type 1 diabetes. Diabetes. 2009;58(7):1651–1658. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jørgensen M.E., Almdal T.P., Carstensen B. Time trends in mortality rates in Type 1 diabetes from 2002 to 2011. Diabetologia. 2013;56(11):2401–2404. doi: 10.1007/s00125-013-3025-7. [DOI] [PubMed] [Google Scholar]
- 19.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Glycemic control and cardiovascular disease in 7,454 patients with Type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR) Diabetes Care. 2010;33(7):1640–1646. doi: 10.2337/dc10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harjutsalo V, Forsblom C, Groop PH. Time trends in mortality in patients with Type 1 diabetes: nationwide population based cohort study. BMJ. 2011;343:d5364. doi: 10.1136/bmj.d5364. Published 8 September 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset Type 1 diabetes: the Allegheny County Type 1 diabetes registry. Diabetes Care. 2010;33(12):2573–2579. doi: 10.2337/dc10-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carstensen B, Kristensen JK, Ottosen P, Borch-Johnsen K. Steering group of the national diabetes register. The Danish national diabetes register: trends in incidence, prevalence and mortality. Diabetologia. 2008;51(12):2187–2196. doi: 10.1007/s00125-008-1156-z. [DOI] [PubMed] [Google Scholar]
- 24.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with Type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 25.Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. BMJ. 2016;352:i717. doi: 10.1136/bmj.i717. Published 24 February 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillip M, Mathieu C, Lind M, et al. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled Type 1 diabetes: pooled 52-week outcomes from the DEPICT-1 and -2 studies. Diabetes Obes Metab. 2021;23(2):549–560. doi: 10.1111/dom.14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 28.Groop PH, Dandona P, Phillip M, et al. Effect of dapagliflozin as an adjunct to insulin over 52 weeks in individuals with Type 1 diabetes: post-hoc renal analysis of the DEPICT randomised controlled trials. Lancet Diabetes Endocrinol. 2020;8(10):845–854. doi: 10.1016/S2213-8587(20)30280-1. [DOI] [PubMed] [Google Scholar]
- 29.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 30.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 31.Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. doi: 10.2337/dcS15-3006. [DOI] [PubMed] [Google Scholar]
- 32.Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in Type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney disease outcomes in Type 2 diabetes. N Engl J Med. 2020;383(23):2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 34.Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with finerenone in kidney disease and Type 2 diabetes. N Engl J Med. 2021;385(24):2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 35.Sofizadeh S, Pehrsson A, Ólafsdóttir AF, Lind M. Evaluation of reference metrics for continuous glucose monitoring in persons without diabetes and prediabetes. J Diabetes Sci Technol. 2020 doi: 10.1177/1932296820965599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dena M, Svensson AM, Olofsson KE, et al. Renal complications and duration of diabetes: an international comparison in persons with Type 1 diabetes. Diabetes Ther. 2021;12(12):3093–3105. doi: 10.1007/s13300-021-01169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.