Summary
Background
Antenatal multiple micronutrient supplementation (MMS) with iron, folic acid, and other micronutrients might improve birth outcomes, but it is not currently universally recommended by WHO.
Methods
In this observational cohort study, we surveyed pregnancies for adverse birth outcomes at eight hospitals from July, 2014, to July, 2018, and 18 hospitals from August, 2018, to December, 2020, in Botswana to assess four routine supplementation strategies in women presenting before 24 weeks’ gestation: folic acid only, iron only, iron and folic acid supplementation (IFAS), and MMS. Women with singleton pregnancies; a known HIV status, age, and delivery site; haemoglobin measured within 7 days of presenting to antenatal care; and weight measured within 31 days of presenting to care were included in our analysis. Data were abstracted from the maternity obstetric record (a record of antenatal care) at the time of birth from all women giving birth at selected hospitals throughout the country. We estimated risk differences overall and in key subgroups, adjusting for demographic and clinical factors.
Findings
Between July 6, 2014, and Dec 8, 2020, 96 341 eligible women (21 659 [22·5%] of whom had HIV) were included in the study. 36 334 (37·7%) women initiated iron only supplementation, 1133 (11·8%) initiated folic acid only supplementation, 23 101 (24·0%) initiated IFAS, and 31 588 (32·8%) women initiated MMS. Women who initiated iron only and folic acid only supplementation had higher risks of stillbirth, preterm birth, very preterm birth, low and very low birthweight, and neonatal death compared with women who received IFAS (adjusted risk differences for iron only supplementation vs IFAS ranged from 0·22% [95% CI 0·04 to 0·40] for neonatal death to 2·39% [1·78 to 3·00] for preterm birth; and adjusted risk differences for folic acid only supplementation vs IFAS ranged from 0·77% [−0·80 to 2·34] for neonatal death to 5·75% [1·38 to 10·13] for preterm birth), with greater difference in women with HIV and those aged 35 years and older. Compared with IFAS, women who initiated MMS had lower risks of preterm and very preterm births, and low and very low birthweight (adjusted risk differences ranged from −0·50% [−0·77 to 0·23] for very preterm birth to −1·06% [−1·69 to −0·42] for preterm birth).
Interpretation
Nationwide data from Botswana support improved birth outcomes with MMS compared with IFAS.
Funding
National Institutes of Health, National Institute of Child Health and Human Development, and National Institute of Allergy and Infectious Diseases.
Introduction
Reducing adverse birth outcomes is a global priority established by the Every Newborn Action plan from WHO and UN.1 Identifying and implementing low-cost interventions to reduce adverse birth outcomes is of crucial public health importance. WHO currently recommends daily iron and folic acid supplementation (IFAS) during pregnancy to prevent maternal anaemia, preterm birth, and small for gestational age, but the strength of the evidence varies and uptake of supplementation interventions has been suboptimal.2–4 Multiple micronutrient supplementation (MMS), including iron and folic acid, has also been shown to reduce the risk of adverse birth outcomes,5,6 but it is not universally recommended by WHO, due in part to a scarcity of high quality implementation research on the effectiveness of MMS programmes.7,8 Despite numerous clinical trials of IFAS and MMS, key knowledge gaps remain, including identifying effects in key populations (eg, women with HIV), estimates of effect on rare outcomes (eg, stillbirth), and the effectiveness of antenatal supplementation with real-world data from nationally representative programmes.7
Current WHO guidelines recommending daily IFAS for pregnant women are based primarily on a meta-analysis of 44 randomised clinical trials—which assessed a total of 43 274 women—that compared daily supplements containing iron versus no iron or placebo.9 This meta-analysis found that iron-containing supplements decreased the risk of maternal anaemia, low birthweight, and very preterm birth (<34 weeks’ gestation), but the quality of the evidence was graded as low to moderate. A second meta-analysis of 21 randomised clinical trials—which assessed 142 496 women—found that MMS decreased the risk of low birthweight, preterm birth, and small for gestational age compared with iron (with or without folic acid).5 A secondary analysis—which used individual patient data—of the same studies found MMS was particularly protective against adverse birth outcomes in anaemic and underweight (body-mass index [BMI] <18·5 kg/m2) women.6 Although these trials provide important evidence supporting the use of IFAS and MMS, less is known about the effectiveness of antenatal supplementation strategies in the context of real-world programmes, which could differ due to differences in adherence and access or due to heterogeneous study populations.
The risk of adverse birth outcomes is especially high in sub-Saharan Africa, particularly in women with HIV.10–19 In Botswana, the risk of any adverse birth outcome in women with HIV ranges from 30% to 50%.10,20 We aimed to compare the effectiveness of IFAS, MMS, iron only, and folic acid only supplementation during pregnancy on adverse birth outcomes in Botswana.
Methods
Study design and participants
Tsepamo is a birth outcomes surveillance study in Botswana. In this observational cohort study, data were abstracted from the maternity obstetric record (a record of antenatal care) at the time of delivery from all women giving birth at eight hospitals (in which about 45% of all babies in Botswana were born) from July 6, 2014, to July 31, 2018, and 18 hospitals (in which about 72% of all babies in Botswana were born) from Aug 1, 2018, to Dec 8, 2020. The Tsepamo study captured data on more than 99% of births at the included sites.20,21 In Botswana, approximately 95% of women give birth at a hospital.22 Women with singleton pregnancies who presented for care before 24 weeks’ gestation were eligible for inclusion in our analysis. We additionally required women to have a known HIV status, age, and delivery site, haemoglobin concentration measured within 7 days of presenting to antenatal care (measured in venous blood with auto-analyzer [Sysmex XT-2000i, Kobe, Japan]), and weight measured by a nurse or midwife at the antenatal clinic within 31 days of presenting to care. We excluded women with a haemoglobin concentration of 7·7 g/dL or less or a concentration of 14·6 g/dL or more (<2%), and women for whom supplementation during pregnancy was unknown (>1%).
Procedures
We compared the following antenatal supplementation strategies: no supplementation, folic acid alone, iron alone, IFAS, and MMS. We categorised women according to the health district of the clinic where they received their first antenatal visit. Health districts were categorised into the rural northwest, rural south, urban Gaborone, urban Francistown, and rural east regions. Data extraction was done with single-data entry using automated data checks to limit response options and reduce errors. The study coordinator (MD) regularly audited the source documents to ensure accuracy. Nurses and midwives prescribe supplements to pregnant women at the antenatal visit, and supplements are then filled by clinic pharmacists free of charge. IFAS is standard of care in Botswana, but stock-outs can occur. Accordingly, decisions about what supplementation strategy to prescribe are typically based on a combination of haemoglobin concentration—measured at the first antenatal visit—and supplement availability: women with low haemoglobin concentration are preferentially prescribed IFAS over other supplementation strategies. Women typically initiate supplementation at the second antenatal visit, although nurses and midwives contact women with low haemoglobin concentration to initiate supplementation sooner. Tsepamo research assistants identified the first time a supplement was filled in pregnancy from the maternity card. Therefore, we defined supplementation strategies based on the first filled prescription of a supplement during pregnancy. Dates are included when folic acid was prescribed but not when iron was prescribed. The UN International Multiple Micronutrient Antenatal formulation of MMS was provided and included standard doses of vitamins A, C, D, E, B1, B2, B3, B6, B9 (folic acid), and B12; iron (30 mg); iodine; zinc; selenium; and copper (Kirk Humanitarian, Salt Lake City, UT, USA).23 IFAS contained 60 mg iron and either 0·25 mg or 5 mg folic acid. Iron alone contained 60 mg iron. Folic acid alone contained 5 mg folic acid. Daily supplements were prescribed in quantities of 30 tablets.
Adverse birth outcomes measured included stillbirth (fetal death ≥24 weeks’ gestation, summed Apgar score of 0), preterm birth (livebirth or stillbirth <37 weeks’ gestation), very preterm birth (<32 weeks’ gestation), small for gestational age (<10th percentile according to the Intergrowth-21st norms using completed weeks24,25), very small for gestational age (<3rd percentile), neonatal death (before leaving the hospital within 28 days of delivery in liveborn infants), stillbirth or neonatal death (combined endpoint), low birthweight (<2500 g), and very low birthweight (<2000 g). Additional outcomes assessed included third trimester anaemia (haemoglobin <11·0 g/dL at or after 24 weeks’ gestation), caesarean delivery, and short length for gestational age (<10th percentile using Intergrowth-21 norms24,25). Estimated date of delivery was calculated at the first antenatal visit using the reported last menstrual period and confirmed by ultrasound when available. If the last menstrual period date was unknown or suspected to be incorrect, midwives used fundal height measurements. Gestational age at delivery was recorded (in completed weeks) by the midwife using the estimated date of delivery. Infant weight and length were measured immediately after birth.
Statistical analysis
In descriptive analyses, we examined patterns of micronutrient supplementation strategies over time according to the health district of the first antenatal visit.
We used inverse probability weighting to adjust for the following variables measured in the mother at the first antenatal visit: HIV status, haemoglobin concentration measured within 7 days, weight measured within 31 days, clinic health district, age, year, trimester, employment, education, parity, season of presentation, smoking status, and alcohol use. We fit multinomial logistic regression models to estimate stabilised weights. For each outcome, we then used weighted regression models26 to estimate risk differences and risk ratios comparing MMS, iron alone, and folic acid alone with IFAS. The outcome models were conditional on supplementation strategy and baseline haemoglobin concentration as additional protection against confounding by indication. 95% CIs were obtained using a robust variance estimator.27 We also estimated absolute risks by supplementation strategy by standardising the inverse probability weighted risk estimates to the distribution of the first haemoglobin measured in pregnancy. We excluded the no supplementation group in our adjusted analyses because this comparison was likely susceptible to immortal time bias.
We estimated risk differences separately in several key subgroups: trimester of presentation to care, maternal HIV status, weight at presentation to antenatal care, haemoglobin concentration at presentation to antenatal care, age, delivery site (urban [Gaborone or Francistown], rural [all other sites]), parity, education, and employment. We also evaluated a subgroup of HIV-negative women presenting to care before 20 weeks’ gestation to more closely match characteristics of the women included in the largest randomised clinical trial.28
To investigate the potential effect of residual confounding by indication, we did two sensitivity analyses: in one we excluded haemoglobin concentration from the outcome model and in the other we included early pregnancy weight, age, and gestational age at presentation to care in the outcome model. To investigate the potential effect of residual confounding by geographical or programmatic differences, we (1) estimated risk differences separately by health district region, (2) restricted the analysis to 30 antenatal clinics serving more than 500 pregnant women over the study period, (3) included health district region and calendar year in the outcome model, (4) excluded women who gave birth after the COVID-19 lockdown was implemented on April 3, 2020,29 and (5) adjusted for birth site (restricting to the eight sites originally included in Tsepamo; caesarean outcome only). We estimated inverse probability of censoring weights27 to adjust for potential selection bias induced by not having haemoglobin concentration measured in the third trimester for the outcome of maternal anaemia. Finally, we excluded 5400 women whose IFAS regimen contained less than the WHO recommended dose of folic acid (0·4 mg).
Role of the funding source
The funders had no role in study design, data collection, analysis, and interpretation, or manuscript preparation.
Results
96 341 women who gave birth between July 6, 2014, and Dec 8, 2020, were eligible for inclusion, all of whom were included. 4185 (4·3%) did not initiate any micronutrient supplementation during pregnancy, 1133 (1·2%) initiated folic acid supplementation only, 36 334 (37·7%) initiated iron supplementation only, 23 101 (24·0%) initiated IFAS, and 31 588 (32·8%) initiated MMS (appendix p 8). The number of women initiating MMS in pregnancy increased from 1705 (16·6%) of 10 304 women in 2014 to 4501 (52·7%) of 8549 women in 2020. The proportion of women with no supplementation or folic acid supplementation only decreased from 2014 to 2019, but then increased in 2020 (figure 1), reaching nearly 30% in the rural northwest and urban Gaborone regions. The median gestational age at presentation to care was 15·3 weeks (IQR 11·9–18·9). The median time from presentation to care to supplementation initiation (known for 55 798 [60·5%] of 92 156 women) was 28 days (IQR 0–75) overall, 32 days (0–88) for folic acid only, 28 days (0–85) for IFAS, and 25 days (0–58) for MMS. Compared with the other supplementation strategies, women initiating MMS were more likely to present to care in the first trimester, weigh less than 50 kg at presentation, and use alcohol during pregnancy. Women initiating IFAS were more likely to have a haemoglobin concentration of less than 11·0 g/dL at the first antenatal visit compared with the other supplementation strategies (table 1). The included variables were well balanced across the supplementation strategies in the weighted population (appendix p 1).
Figure 1: Supplementation strategy by calendar year and region of first antenatal care visit.
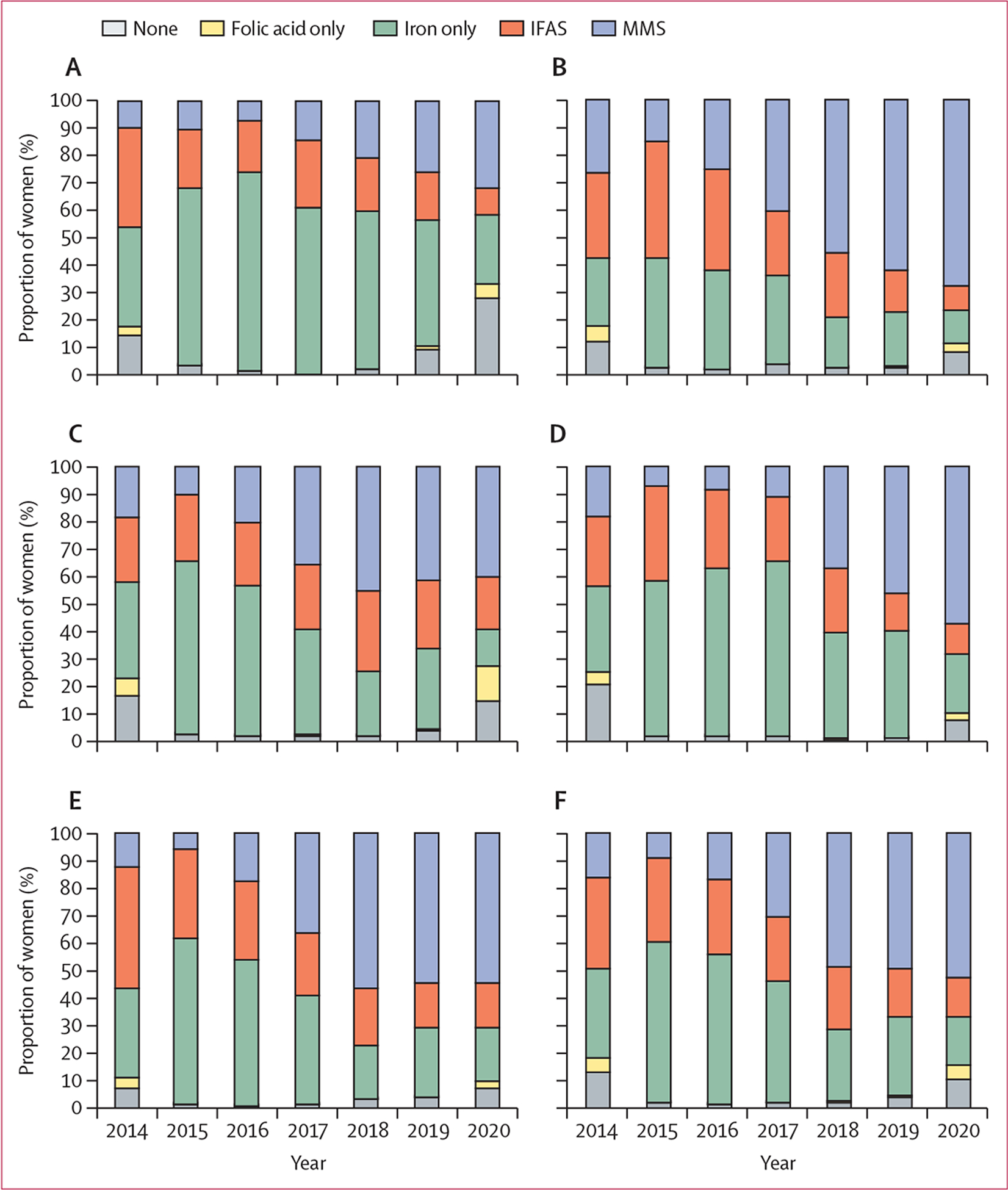
(A) Rural northwest. (B) Rural south. (C) Urban Gaborone. (D) Urban Francistown. (E) Rural east. (F) All regions. Health districts were categorised into five groups: rural northwest (Maun, Okavango, and Chobe health districts), rural south (Ghanzi, Kgalagadi north and south, Kweneng west and east, southern, and Goodhope), urban Gaborone region, urban Francistown region, and rural east (all other health districts). IFAS=iron and folic acid supplementation. MMS=multiple micronutrient supplementation.
Table 1:
Baseline characteristics measured at the first antenatal visit by supplementation strategy
| No supplementation (n=4185) | Folic acid supplementation only (n=1133) | Iron supplementation only (n=36 334) | Iron and folic acid supplementation (n=23 101) | Multiple micronutrient supplementation (n=31 588) | |
|---|---|---|---|---|---|
| Trimester of first antenatal care visit | |||||
| First (<12 weeks) | 796 (19·0%) | 239 (21·1%) | 8415 (23·2%) | 6130 (26·5%) | 9302 (29·5%) |
| Second (12–24 weeks) | 3389 (81·0%) | 894 (78·9%) | 27 919 (76·8%) | 16 971 (73·5%) | 22 286 (70·6%) |
| HIV status | |||||
| Women with HIV | 858 (20·5%) | 252 (22·2%) | 7905 (21·8%) | 5523 (23·9%) | 7121 (22·5%) |
| Women without HIV | 3327 (79·5%) | 881 (77·8%) | 28 429 (78·2%) | 17 578 (76·1%) | 24 467 (77·5%) |
| Haemoglobin within 7 days of first antenatal care visit (g/dL) | |||||
| Median (IQR) | 12·0 (11·2–12·7) | 11·8 (10·8–12·6) | 12·0 (11·2–12·7) | 11·8 (10·8–12·6) | 12·0 (11·1–12·8) |
| <11 | 838 (20·0%) | 321 (28·3%) | 7029 (19·4%) | 6678 (28·9%) | 6692 (21·2%) |
| ≥11 | 3347 (80·0%) | 812 (71·7%) | 29 305 (80·7%) | 16 423 (71·1%) | 24 896 (78·8%) |
| Weight at first antenatal care visit | |||||
| <50 kg | 475 (11·4%) | 154 (13·6%) | 4793 (13·2%) | 3207 (13·9%) | 5464 (17·3%) |
| 50–80 kg | 2914 (69·6%) | 791 (69·8%) | 25 346 (69·8%) | 16 197 (70·1%) | 21 595 (68·4%) |
| ≥80 kg | 796 (19·0%) | 188 (16·6%) | 6195 (17·1%) | 3697 (16·0%) | 4529 (14·3%) |
| Health district of first antenatal care visit | |||||
| Rural northwest (Maun, Okavango, and Chobe) | 585 (14·0%) | 90 (7·9%) | 4772 (13·1%) | 1811 (7·8%) | 1459 (4·6%) |
| Rural south (Ghanzi, Kgalagadi, Kweneng, southern, and Goodhope) | 536 (12·8%) | 133 (11·7%) | 3712 (10·2%) | 3627 (15·7%) | 6317 (20·0%) |
| Urban Gaborone | 1211 (28·9%) | 448 (39·5%) | 9184 (25·3%) | 5726 (24·8%) | 6991 (22·1%) |
| Urban Francistown | 619 (14·8%) | 122 (10·8%) | 6355 (17·5%) | 3243 (14·0%) | 3307 (10·5%) |
| Rural east | 1234 (29·5%) | 340 (30·0%) | 12 311 (33·9%) | 8694 (37·6%) | 13 514 (42·8%) |
| Age | |||||
| <20 years | 454 (10·9%) | 107 (9·4%) | 4133 (11·4%) | 2379 (10·3%) | 3768 (11·9%) |
| 20–35 years | 2992 (71·5%) | 856 (75·6%) | 26 486 (72·9%) | 16 962 (73·4%) | 22 738 (72·0%) |
| ≥35 years | 739 (17·7%) | 170 (15·0%) | 5712 (15·7%) | 3760 (16·3%) | 5082 (16·1%) |
| Parity | |||||
| One or more children | 2625 (62·7%) | 679 (59·9%) | 21 392 (58·9%) | 13 473 (58·3%) | 18 416 (58·3%) |
| No children | 1554 (37·3%) | 454 (40·1%) | 14 918 (41·1%) | 9613 (41·7%) | 13 150 (41·7%) |
| No data | 6 (0·1%) | 0 | 24 (0·1%) | 15 (0·1%) | 22 (0·1%) |
| Occupation | |||||
| Salaried | 1719 (41·1%) | 492 (43·4%) | 14 075 (38·7%) | 9177 (39·7%) | 11 016 (34·9%) |
| Other or missing | 2466 (58·9%) | 641 (56·6%) | 22 259 (61·3%) | 13 924 (60·3%) | 20 572 (65·1%) |
| Education | |||||
| Secondary or higher | 3839 (91·7%) | 1080 (95·3%) | 33 990 (93·6%) | 21 832 (94·5%) | 29 314 (92·8%) |
| Primary, none, or no data | 346 (8·3%) | 53 (4·7%) | 2344 (6·5%) | 1269 (5·5%) | 2274 (7·2%) |
| Calendar year of first antenatal care visit | |||||
| 2013–16 | 1904 (45·5%) | 559 (49·3%) | 19 282 (53·1%) | 11 571 (50·1%) | 5432 (17·2%) |
| 2017–18 | 655 (15·7%) | 49 (4·3%) | 10 135 (27·9%) | 6969 (30·2%) | 12 318 (39·0%) |
| 2019–20 | 1626 (38·9%) | 525 (46·3%) | 6917 (19·0%) | 4561 (19·7%) | 13 838 (43·8%) |
| Season of first antenatal care visit | |||||
| Rainy (November-March) | 1583 (37·8%) | 443 (39·1%) | 15 136 (41·7%) | 10 020 (43·4%) | 13 633 (43·2%) |
| Dry (April-October) | 2602 (62·2%) | 690 (60·9%) | 21 198 (58·3%) | 13 081 (56·6%) | 17 958 (56·9%) |
| Smoking during pregnancy | |||||
| No | 3871 (92·5%) | 1060 (93·6%) | 34 089 (93·8%) | 21 732 (94·1%) | 29 338 (92·9%) |
| Yes | 72 (1·7%) | 10 (0·9%) | 500 (1·4%) | 241 (1·0%) | 608 (1·9%) |
| No data | 242 (5·8%) | 63 (5·6%) | 1745 (4·8%) | 1128 (4·9%) | 1642 (5·2%) |
| Alcohol during pregnancy | |||||
| No | 3519 (84·1%) | 957 (84·5%) | 31 284 (86·1%) | 19 897 (86·1%) | 26 395 (83·6%) |
| Yes | 426 (10·2%) | 113 (10·0%) | 3309 (9·1%) | 2094 (9·1%) | 3562 (11·3%) |
| No data | 240 (5·7%) | 63 (5·6%) | 1741 (4·8%) | 1110 (4·8%) | 1631 (5·2%) |
The adjusted risk differences and absolute risks for each birth outcome by supplementation strategy are reported in table 2. Unadjusted risks are reported in the appendix (p 2). Compared with IFAS, the risk of stillbirth, preterm birth, very preterm birth, neonatal death, stillbirth or neonatal death, low birthweight, and very low birthweight was greater for women who received folic acid only or iron only supplementation (risk differences for folic acid only vs IFAS ranged from 0·77% [95% CI–0·80 to 2·34] for neonatal death to 5·75% [1·38 to 10·13] for preterm birth; and ranged from 0·22% [0·04 to 0·40] for neonatal death to 2·39% [1·78 to 3·00] for preterm birth for iron only vs IFAS). Women initiating MMS had lower risks of preterm birth, very preterm birth, low birthweight, very low birthweight, and caesarean delivery compared with women initiating IFAS (risk differences ranged from −0·50% [−0·77 to −0·23] for very preterm birth to −1·06% [−1·69 to −0·42] for preterm birth). Compared with IFAS, the risk of maternal third trimester anaemia was higher for those who received folic acid only, and the risk of short fetal length-for-age was larger for those who received iron only. The risks of all other outcomes were similar across supplementation strategies (table 2; figure 2). Risk ratios are shown in the appendix (p 3).
Table 2:
Adjusted risk differences and adjusted absolute risks for each adverse birth outcome by supplementation strategy
| Folic acid supplementation only (n=1133) | Iron supplementation only (n=36 334) | Iron and folic acid supplementation (n=23 101) | Multiple micronutrient supplementation (n=31 588) | |
|---|---|---|---|---|
| Stillbirth | ||||
| Risk difference | 1·71% (−0·83 to 4·26) | 0·56% (0·31 to 0·81) | 0·00 (ref) | −0·06% (−0·32 to 0·19) |
| Risk | 3·51% | 2·36% | 1·80% | 1·74% |
| Preterm birth (<37 weeks) | ||||
| Risk difference | 5·75% (1·38 to 10·13) | 2·39% (1·78 to 3·00) | 0·00 (ref) | −1·06% (−1·69 to −0·42) |
| Risk | 18·43% | 15·07% | 12·68% | 11·63% |
| Very preterm birth (<32 weeks) | ||||
| Risk difference | 0·93% (−0·37 to 2·24) | 0·92% (0·63 to 1·21) | 0·00 (ref) | −0·50% (−0·77 to −0·23) |
| Risk | 3·32% | 3·31% | 2·39% | 1·89% |
| Small for gestational age (<10th percentile) | ||||
| Risk difference | −0·66% (−4·69 to 3·36) | −0·05% (−0·69 to 0·59) | 0·00 (ref) | 0·53% (−0·17 to 1·24) |
| Risk | 14·78% | 15·39% | 15·44% | 15·97% |
| Very small for gestational age (<3rd percentile) | ||||
| Risk difference | 0·16% (−3·01 to 3·34) | 0·20% (−0·21 to 0·61) | 0·00 (ref) | 0·39% (−0·06 to 0·84) |
| Risk | 5·75% | 5·79% | 5·59% | 5·98% |
| Neonatal death (in hospital ≤28 days) * | ||||
| Risk difference | 0·77% (−0·80 to 2·34) | 0·22% (0·04 to 0·40) | 0·00 (ref) | −0·09% (−0·27 to 0·09) |
| Risk | 1·71% | 1·16% | 0·94% | 0·86% |
| Stillbirth or neonatal death * | ||||
| Risk difference | 2·43% (−0·48 to 5·34) | 0·77% (0·47 to 1·08) | 0·00 (ref) | −0·15% (−0·46 to 0·16) |
| Risk | 5·16% | 3·51% | 2·73% | 2·58% |
| Low birthweight (<2500 g) | ||||
| Risk difference | 5·46% (1·09 to 9·83) | 1·24% (0·66 to 1·82) | 0·00 (ref) | −0·99% (−1·59 to −0·38) |
| Risk | 16·92% | 12·70% | 11·46% | 10·48% |
| Very low birthweight (<2000 g) | ||||
| Risk difference | 3·08% (−0·04 to 6·20) | 1·00% (0·63 to 1·38) | 0·00 (ref) | −0·56% (−0·94 to −0·19) |
| Risk | 7·36% | 5·29% | 4·28% | 3·72% |
| 3rd trimester anaemia (haemoglobin <11g/dL) | ||||
| Risk difference | 7·23% (1·27 to 13·18) | −0·95% (−3·51 to 1·62) | 0·00 (ref) | −0·71% (−2·43 to 1·00) |
| Risk | 38·67% | 30·50% | 31·44% | 30·73% |
| Caesarean | ||||
| Risk difference | 3·25% (−1·89 to 8·39) | −0·69% (−1·43 to 0·05) | 0·00 (ref) | −2·67% (−3·47 to −1·87) |
| Risk | 26·94% | 23·00% | 23·69% | 21·02% |
| Short length-for-age (<10th percentile) | ||||
| Risk difference | −0·24% (−3·30 to 2·81) | 0·60% (0·06 to 1·13) | 0·00 (ref) | 0·46% (−0·12 to 1·04) |
| Risk | 9·30% | 10·14% | 9·55% | 10·00% |
Data are risk (%) or risk difference (95% CI). Risk differences are adjusted for HIV status (positive or negative), first haemoglobin concentration in pregnancy (restricted cubic splines with five knots at 9·5 g/dL, 10·2 g/dL, 11·9 g/dL, 13·4 g/dL, and 13·8 g/dL), first weight in pregnancy (restricted cubic splines with five knots at 47·9 kg, 53·5 kg, 62·0 kg, 73·5 kg, and 86·0 kg), region of first antenatal care visit, age (restricted cubic splines with three knots at 19, 27, and 36 years), year of booking (2014–16, 2017–18, and 2019–20), trimester of booking (first [<12 weeks’ gestation] or second [12–24 weeks’ gestation]), employment (salaried, other, or unknown), education (secondary or higher, primary or lower, or missing), parity (first or missing, and second and more), season (dry [April–October], rainy [November–March]), smoking (yes, no, or missing), and alcohol (yes, no, or missing) via inverse probability weighting. The models for each outcome are additionally adjusted for first haemoglobin concentration in pregnancy (modelled linearly). We used log-binomial models to estimate risk ratios and linear probability models (fit by specifying an identity link and binomial distribution) to estimate risk differences. Models did not converge for third trimester anaemia so in this case Poisson models with robust variance were used. Absolute risks are adjusted for the same set of variables and are calculated by standardising the inverse probability weighted risk estimates to the distribution of the first haemoglobin in pregnancy. The mean stabilised inverse probability weight was 1·01 (99th percentile 3·19).
Restricted to liveborn infants (98% of births).
Figure 2: Adjusted risk differences for each adverse birth outcome by supplementation strategy.
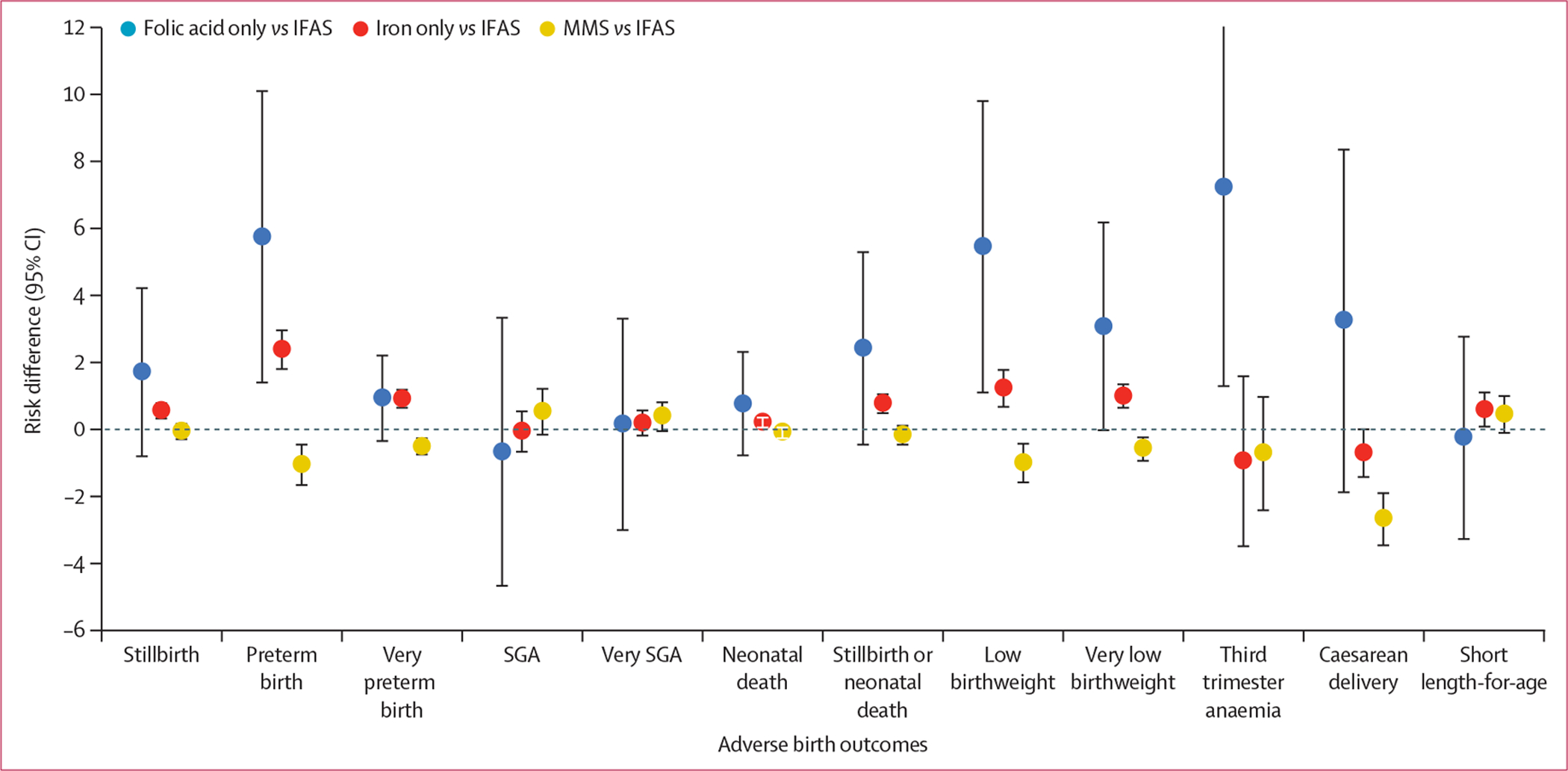
Risk differences are adjusted for HIV status (positive or negative), first haemoglobin concentration in pregnancy (restricted cubic splines with five knots at 9·5 g/dL, 10·2 g/dL, 11·9 g/dL, 13·4 g/dL, and 13·8 g/dL), first weight in pregnancy (restricted cubic splines with five knots at 47·9 kg, 53·5 kg, 62·0 kg, 73·5 kg, and 86·0 kg), region of first antenatal care visit, age (restricted cubic splines with three knots at 19, 27, and 36 years), year of booking (2014–16, 2017–18, and 2019–20), trimester of booking (first [<12 weeks’ gestation] or second [12–24 weeks’ gestation]), employment (salaried, other, or unknown), education (secondary or higher, primary or lower, and missing), parity (first or missing, and second or more), season (dry [April–October], rainy [November–March]), smoking (yes, no, or missing), and alcohol (yes, no, or missing) via inverse probability weighting. The models for each outcome are additionally adjusted for first haemoglobin concentration in pregnancy (modelled linearly). IFAS=iron and folic acid supplementation. MMS=multiple micronutrient supplementation. SGA=small for gestational age.
The adjusted risk differences in each of the key subgroups are summarised in figure 3. For stillbirth and stillbirth or neonatal death, point estimates of risk differences comparing folic acid only and iron only supplementation with IFAS were larger in women presenting to care in the first trimester, women with HIV, and women aged 35 years or older, and risk differences comparing MMS with IFAS were larger in magnitude in women who weighed 80 kg or more and women aged 35 years or older. For preterm birth and low birthweight, risk differences comparing MMS, iron only, and folic acid only with IFAS were larger in magnitude in women with HIV and women aged 35 years or older. Risk differences for preterm birth and low birthweight were also larger in magnitude comparing MMS with IFAS in those with primary education or lower; risk differences were also larger comparing folic acid only with IFAS in those who had a haemoglobin concentration less than 11 g/dL. Risk differences for small for gestational age comparing folic acid only with IFAS were larger in women with HIV and in women aged 35 years or older. Risk differences for neonatal death comparing folic acid only with IFAS were larger in women who weighed less than 50 kg, those with a haemoglobin concentration less than 11 g/dL, and those younger than 20 years. Of the 58 230 (63·2%) women without HIV presenting to care before 20 weeks’ gestation, risk differences were generally attenuated (figure 3; appendix pp 4–7).
Figure 3: Adjusted risk differences for adverse birth outcomes by supplementation strategy and key subgroups.
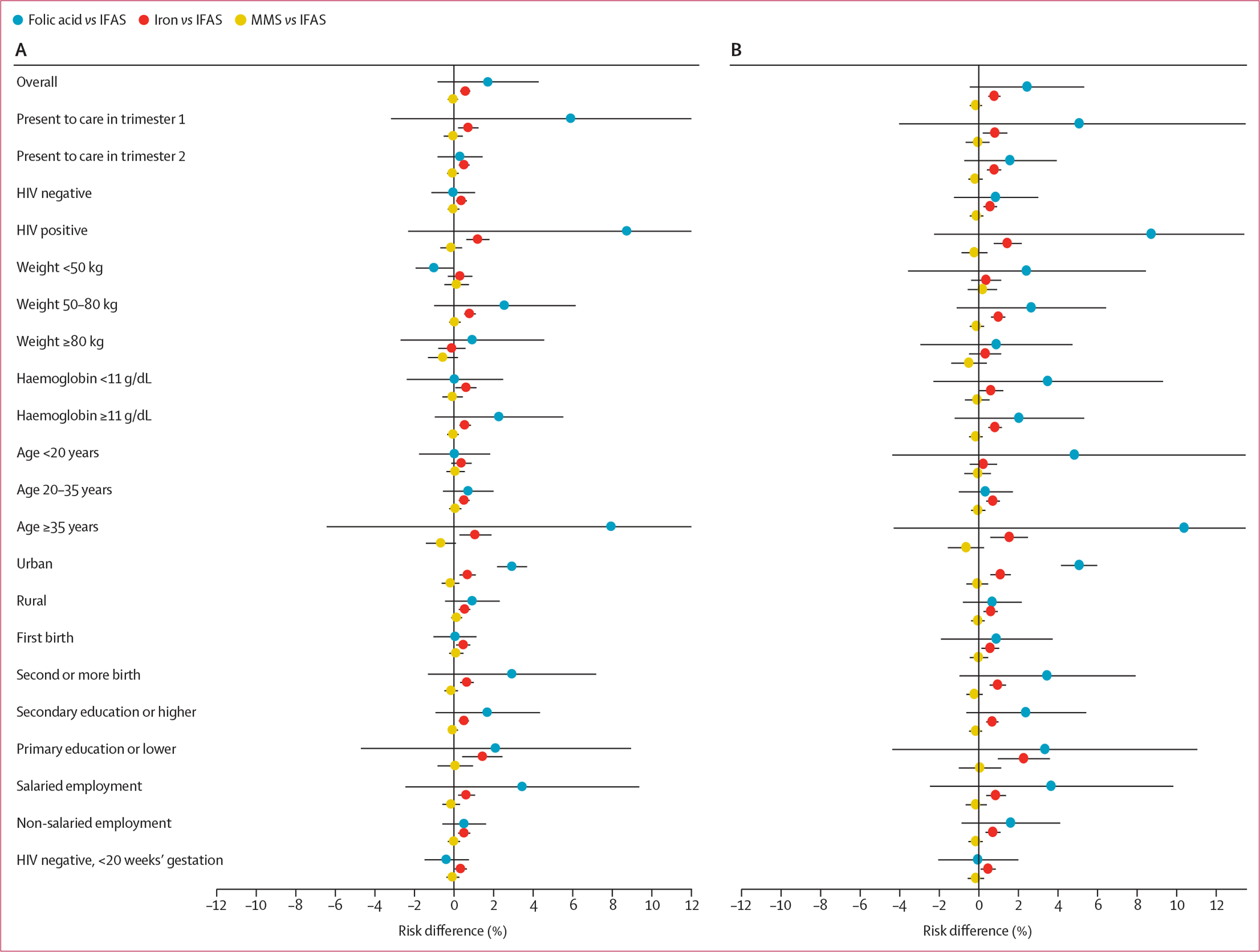
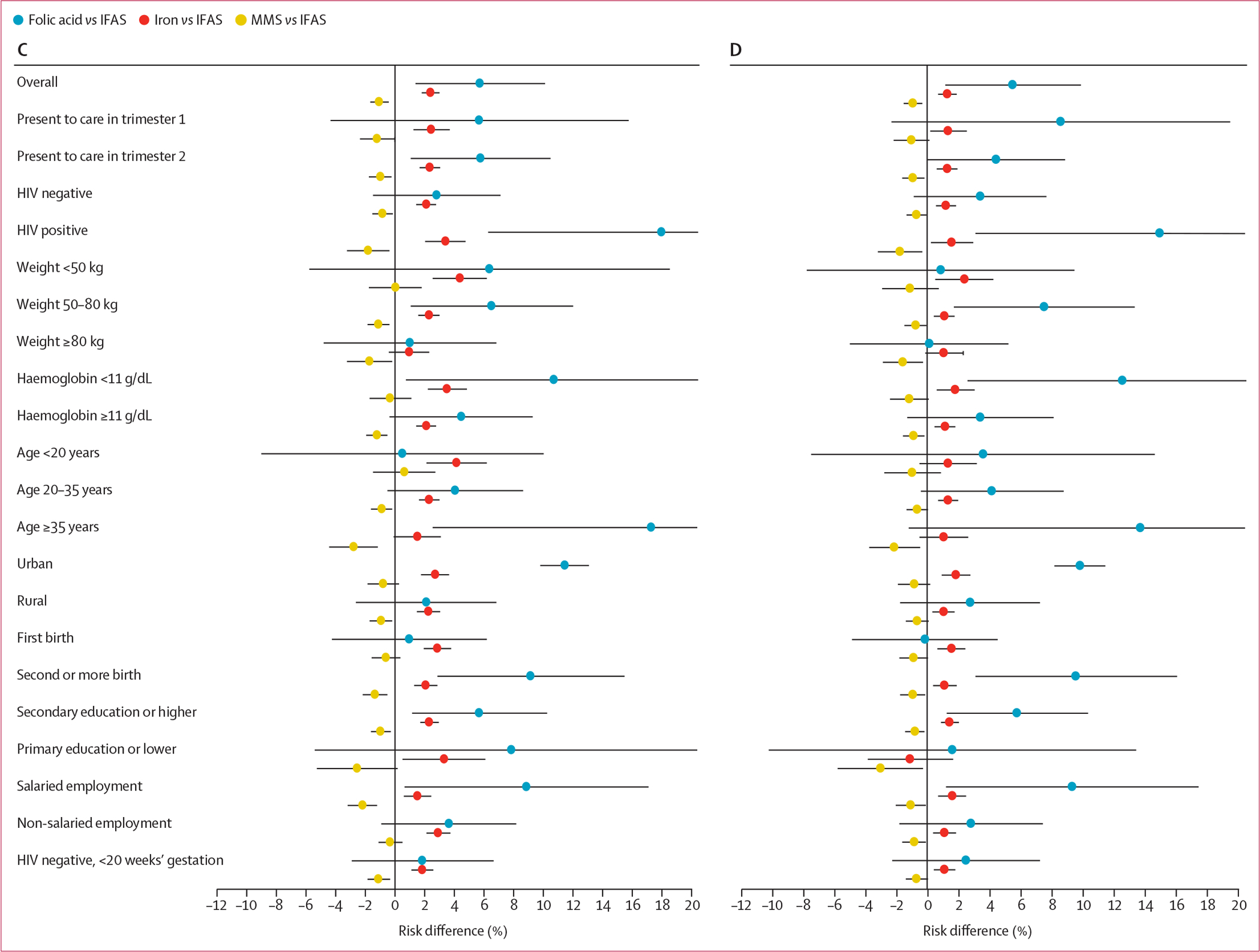
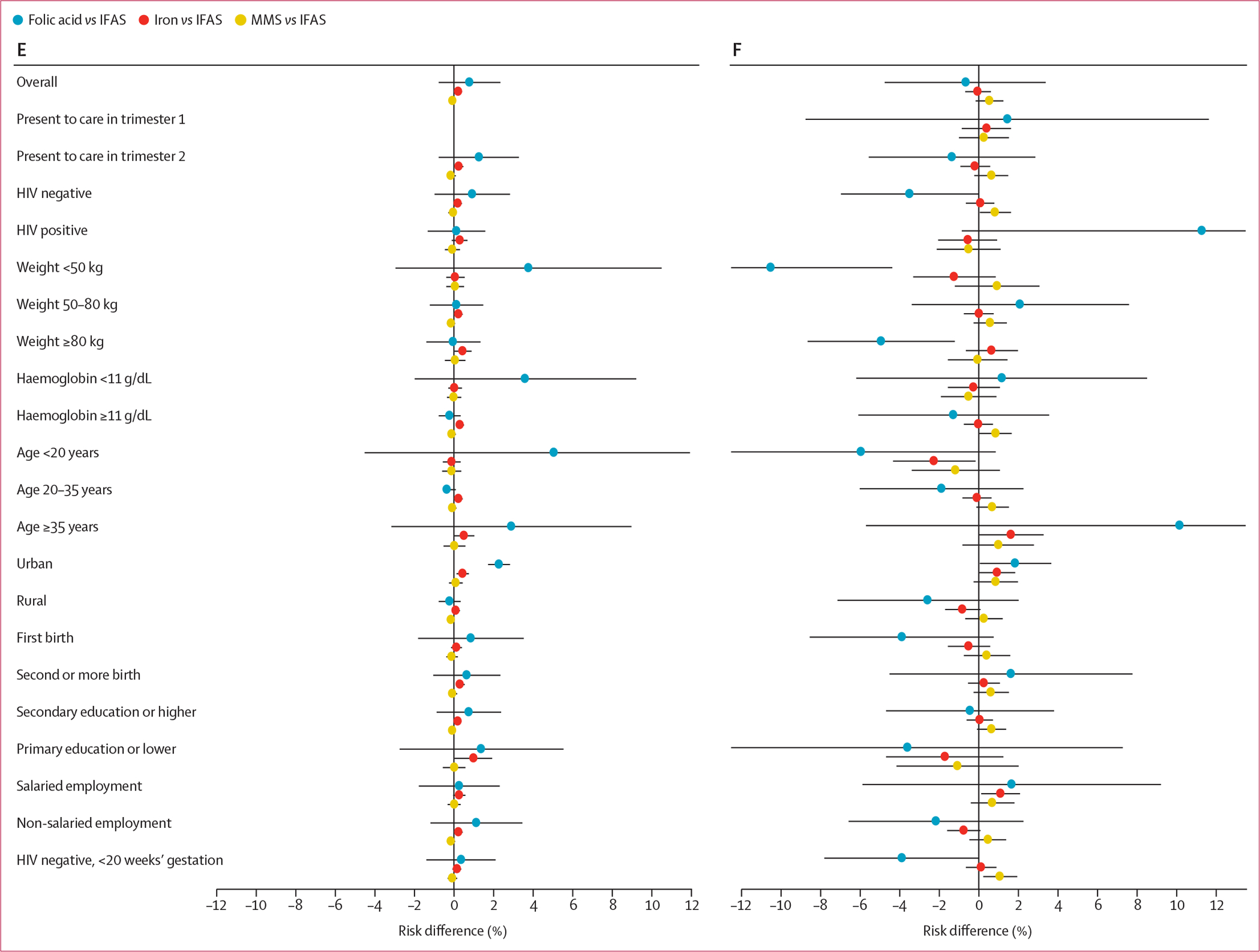
(A) Stillbirth. (B) Stillbirth or neonatal death. (C) Preterm birth. (D) Low birthweight. (E) Neonatal death. (F) Small for gestational age. IFAS=iron and folic acid supplementation. MMS=multiple micronutrient supplementation.
When haemoglobin was excluded from the outcome model, the risk difference for maternal third trimester anaemia comparing iron only with IFAS was larger in magnitude, suggesting confounding by indication, but the risk differences were similar to those obtained in the primary analysis for all other outcomes. Risk differences were generally similar by health district region (appendix pp 9–10). When restricting the analyses to antenatal clinics serving more than 500 women (30 234 women), risk differences were attenuated comparing folic acid only with IFAS. None of the other sensitivity analyses yielded appreciably different results.
Discussion
In the largest programmatic study of antenatal micronutrient supplementation strategies and birth outcomes to date, we found that pregnant women who initiated IFAS had a lower risk of stillbirth, preterm birth, very preterm birth, neonatal death, stillbirth or neonatal death, low birthweight, and very low birthweight, compared with women who initiated iron only or folic acid only supplementation. Pregnant women who initiated MMS had a lower risk of preterm birth, very preterm birth, low birthweight, very low birthweight, and caesarean birth compared with women who initiated IFAS. Risk differences were largest in women with HIV and women aged 35 years or older. This observational study provides some of the first real-world evidence comparing four micronutrient supplementation strategies and highlights the importance of improving antenatal micronutrient supplementation coverage globally.
Our finding that women who initiated IFAS had improved birth outcomes compared with women who initiated folic acid only supplementation is consistent with a previous meta-analysis that found a decreased risk of low birthweight, preterm birth, and neonatal death in pregnant women who took iron-containing supplements compared with those who took supplements without iron or placebo.9 However, our comparisons and outcomes were slightly different from those made in the meta-analysis.9 This finding is also supported by an established literature on the link between iron-deficiency anaemia and adverse birth outcomes.30,31 We also found that women who initiated IFAS had improved birth outcomes compared with women who initiated iron only supplementation, which is consistent with a large observational study in China, which found that antenatal folic acid supplementation was associated with reduced risks of low birthweight and small for gestational age, even when initiated later in pregnancy.32 Folic acid supplementation is known to reduce the risk of neural tube defects when initiated preconceptionally or early in pregnancy; it has also been shown to reduce homocysteine concentrations, which could in turn improve birth outcomes.33 However, the previous meta-analysis9 did not directly compare IFAS with iron only supplementation, and caution should be taken when interpreting this finding. Our finding that women who initiated MMS had lower risk of some birth outcomes compared with women who initiated IFAS is somewhat consistent with a previous meta-analysis,5 which found a decreased risk of low birthweight, preterm birth, and small for gestational age in women who received MMS compared with those who received iron (with or without folic acid) supplementation.5 Although we found a decreased risk of low birthweight and preterm birth, we did not find a decreased risk of small for gestational age between women who received MMS and those who received IFAS. The finding is also supported by the established literature linking deficiencies in micronutrients (other than iron and folic acid) with poor pregnancy outcomes and poor fetal growth.5,31,34,35
Our study was the first to explore HIV as a possible effect modifier. We found that risk differences were larger in magnitude in women with HIV than they were in any other subgroup. We identified several additional subgroups defined by maternal age (≥35 years), low (<50 kg) and high (≥80 kg) weight, and anaemia, for which risk differences were larger in magnitude in women who received iron only and folic acid only supplementation than in those who received IFAS. Comparing MMS with IFAS, risk differences were also larger in women aged 35 years and older. In an individual patient data meta-analysis of 17 trials that compared MMS with IFAS, reductions in adverse birth outcomes were larger in women with anaemia or low BMI at the start of supplementation.6 In our study, some estimates were larger in magnitude in women with anaemia (preterm birth and neonatal death) or in women with low weight (neonatal death) when comparing those who received folic acid only supplementation with those who received IFAS, but not when comparing women who received MMS with those who received IFAS.
There are several potential explanations for why our results partly differed from the meta-analyses of MMS versus IFAS. Differences in the distribution of effect modifiers across study populations might lead to different results. Our study included a representative sample of pregnant women in Botswana, a population with high HIV prevalence. The meta-analysis was weighted heavily by two large trials, one from Bangladesh28 and another from Indonesia,36 where HIV prevalence is less than 1%.37 Differences in adherence could lead to different results. In our study, we had information on the first supplement that was prescribed and filled, but not on subsequent prescriptions or on adherence. Therefore, we estimated the effect of initiating supplementation in a real-world setting, whereas previous trials estimated the effect of random assignment to receive supplementation. It is possible that adherence to supplementation was higher in the trials because women were provided with the supplements as part of their participation in the study.38 For example, stock-outs were known to have occurred throughout our study period and could have substantially affected supplementation adherence (through supplementation discontinuation or switching); this was unlikely to be an issue in the randomised trials. Finally, women in our study generally initiated supplementation later in pregnancy than many women enrolled in the trials. It is possible that the duration of supplementation did not reach an adequate threshold to have an effect on certain outcomes, such as small for gestational age.
Confounding in our study might also explain why the results partly differed from previous randomised trials. Although we were able to measure and adjust for the primary clinical indication for supplementation (haemoglobin concentration), calendar year, geographical location, and other potential confounders, these factors might not completely capture why an individual was prescribed one type of supplementation, and confounding by unknown individual-level, provider-level, and clinic-level factors cannot be ruled out. We hypothesise that women were prescribed no supplementation or inadequate supplementation (eg, with folic acid alone) due to stock-outs, but ideally this would be confirmed by reviewing procurement receipts at antenatal clinic pharmacies. Distinguishing spontaneous from indicated preterm birth might have provided insights into the role of confounding by provider-level and clinic-level factors, but accurate data on reasons for preterm birth were not available. Women who presented to antenatal care with low haemoglobin concentrations were more likely to be prescribed IFAS than other regimens. Although our analyses adjusted for haemoglobin concentration and evaluated subgroups defined by haemoglobin concentration, residual confounding by indication might still exist. Our study has additional limitations. The timing of supplementation initiation was not always known. Because the median gestational age at presentation to care was 15·3 weeks and the median time to initiate supplementation was 28 days after presentation, we were not able to evaluate the effect of initiating supplementation very early in pregnancy or before conception. Finally, measurement error for gestational age dating was possible because we typically relied on reported last menstrual period.
Our findings support the importance of current IFAS guidelines for improving birth outcomes and suggest that enhanced supplementation with MMS might add benefit. Although WHO recommends IFAS universally, fewer than 60% of pregnant women in Botswana received supplementation consistent with this guideline during our study. The increased risk of adverse birth outcomes in women who initiated folic acid only might represent a lower bound for the risk of adverse birth outcomes in women who initiated no supplements at all, which is concerning given that stock-outs during the COVID-19 pandemic led to nearly 30% of women in certain regions not receiving any supplementation in 2020. More research is needed to identify strategies to improve micronutrient supplementation supply chains.
In conclusion, our findings support IFAS as an essential part of antenatal care to reduce adverse birth outcomes, with greater benefits in women with HIV and women aged 35 years and older. Our findings suggest that MMS might be better than IFAS in terms of the risk of preterm birth, very preterm birth, low birthweight, and very low birthweight. We also found no evidence that MMS was harmful for any outcome or any subgroup. These findings might be useful for programmes considering implementation of MMS to improve birth outcomes.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for meta-analyses published between Jan 1, 2015, and Dec 31, 2018, synthesising data from randomised clinical trials of iron and folic acid supplementation (IFAS) or multiple micronutrient supplementation (MMS) and adverse birth outcomes with the search terms “oral iron supplementation”, “multiple-micronutrient supplementation”, “pregnancy”, and “cochrane”. WHO recommends that all pregnant women receive daily IFAS during pregnancy to reduce the risk of maternal anaemia and adverse birth outcomes. The IFAS recommendation is based primarily on a meta-analysis of 44 randomised clinical trials (43 274 women) that found a decreased risk of anaemia at term, severe postpartum anaemia, low birthweight, and very preterm birth in women taking supplements containing iron compared with supplements with no iron or placebo. A meta-analysis of 21 randomised clinical trials (142 496 women) found that MMS containing iron, folic acid, and additional micronutrients decreased the risk of low birthweight, preterm birth, and small for gestational age compared with IFAS alone. Given these findings, WHO recommended MMS in the context of rigorous research and noted the need for research to establish the effectiveness of supplementation on adverse birth outcomes in real-world settings. Additional knowledge gaps also include identifying effects of supplementation in key populations, such as women with HIV, and estimates of effects on rare outcomes such as stillbirth.
Added value of this study
In the largest programmatic evaluation of antenatal micronutrient supplementation strategies and birth outcomes to date, we estimated the comparative effectiveness of four antenatal supplementation strategies on adverse birth outcomes in 96 341 women (21 659 [22·5%] of whom had HIV) who presented to antenatal care before 24 weeks gestation in Botswana. We found that women who initiated IFAS had lower risks of most adverse birth outcomes compared with women who initiated iron only or folic acid only supplementation, and differences were greatest in women with HIV and women aged 35 years or older. Women who initiated MMS had lower risks of preterm and very preterm birth and low and very low birthweight compared with women who initiated IFAS.
Implications of all the available evidence
Antenatal supplementation with IFAS and MMS might substantially decrease the risk of adverse and severe adverse birth outcomes, especially in women with HIV and women 35 years of age or older. Our findings support IFAS as an essential component of antenatal care and indicate that MMS might be superior to IFAS in terms of reducing the risk of preterm birth and low birthweight, which is in agreement with evidence from randomised trials. These findings might be useful for programmes considering implementation of MMS to improve birth outcomes.
Acknowledgments
This study was funded by National Institutes of Health (NIH) and National Institute of Child Health and Human Development (NICHD; R01 HD080471, K23 HD088230-01A1, and K01 HD100222-01A1) and NIH and National Institute of Allergy and Infectious Diseases (K24AI131924) grants. We would like to thank the Tsepamo Study team, including our research assistant, the maternity staff and administrators at the 18 participating hospitals, the members of the Botswana Ministry of Health and Wellness, in particular, the department of HIV/AIDS Prevention and Care, and the department of Maternal and Child Health, and the NICHD for their support. This study would not have been possible without the support of the leadership of the Botswana-Harvard AIDS Institute Partnership. We also acknowledge the funding and support from the NICHD and National Institute of Allergy and Infectious Diseases.
Footnotes
See Comment page e780
See Online for appendix
Declaration of interests
We declare no competing interest.
References
- 1.WHO. Every newborn: an action plan to end preventable deaths. 2019. https://www.who.int/initiatives/every-newborn-action-plan (accessed July 19, 2021).
- 2.WHO. WHO Recommendations on antenatal care for a positive pregnancy experience. Geneva; World Health Organization, 2016. [PubMed] [Google Scholar]
- 3.Kamau MW, Mirie W, Kimani S. Compliance with iron and folic acid supplementation (IFAS) and associated factors among pregnant women: results from a cross-sectional study in Kiambu County, Kenya. BMC Public Health 2018; 18: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mbhenyane X, Cherane M. Compliance with the consumption of iron and folate supplements by pregnant women in Mafikeng local municipality, North West province, South Africa. Afr Health Sci 2017; 17: 657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haider BA, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2017; 4: CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ER, Shankar AH, Wu LS, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health 2017; 5: e1090–100. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Nutritional interventions update: multiple micronutrient supplements during pregnancy. 2020. https://www.who.int/publications/i/item/9789240007789 (accessed July 19, 2021). [PubMed]
- 8.WHO. WHO recommendations on antenatal care for a positive pregnancy experience. 2016. https://www.who.int/publications/i/item/9789241549912 (accessed July 19, 2021). [PubMed]
- 9.Peña-Rosas JP, De-Regil LM, Garcia-Casal MN, Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst Rev 2015; 7: CD004736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012; 206: 1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zash R, Souda S, Chen JY, et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother-to-child transmission of HIV in Botswana. J Acquir Immune Defic Syndr 2016; 71: 428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend CL, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and premature delivery in diagnosed HIV-infected women in the United Kingdom and Ireland. AIDS 2007; 21: 1019–26. [DOI] [PubMed] [Google Scholar]
- 13.Suy A, Martínez E, Coll O, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS 2006; 20: 59–66. [DOI] [PubMed] [Google Scholar]
- 14.Ekouevi DK, Coffie PA, Becquet R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d’Ivoire. AIDS 2008; 22: 1815–20. [DOI] [PubMed] [Google Scholar]
- 15.Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretrovial therapy during pregnancy: a single-center cohort study. J Infect Dis 2007; 196: 558–61. [DOI] [PubMed] [Google Scholar]
- 16.Sibiude J, Warszawski J, Tubiana R, et al. Premature delivery in HIV-infected women starting protease inhibitor therapy during pregnancy: role of the ritonavir boost? Clin Infect Dis 2012; 54: 1348–60. [DOI] [PubMed] [Google Scholar]
- 17.de Vincenzi I Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis 2011; 11: 171–80. [DOI] [PubMed] [Google Scholar]
- 18.Kakkar F, Boucoiran I, Lamarre V, et al. Risk factors for pre-term birth in a Canadian cohort of HIV-positive women: role of ritonavir boosting? J Int AIDS Soc 2015; 18: 19933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed S, Kim MH, Abrams EJ. Risks and benefits of lifelong antiretroviral treatment for pregnant and breastfeeding women: a review of the evidence for the Option B+ approach. Curr Opin HIV AIDS 2013; 8: 474–89. [DOI] [PubMed] [Google Scholar]
- 20.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171: e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381: 827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Botswana: WHO statistical profile. 2015. http://www.who.int/gho/countries/bwa.pdf?ua=1&ua=1 (accessed Oct 21, 2019).
- 23.Humanitarian Kirk. UNIMMAP formula, packaging, and labeling guidance. 2021. https://kirkhumanitarian.org/wp-content/uploads/2019/08/UNIMMAP-MMS-Formula-Packaging-and-Labeling-Specifications.pdf (accessed July 21, 2021).
- 24.Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet 2014; 384: 857–68. [DOI] [PubMed] [Google Scholar]
- 25.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH. INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016; 387: 844–45. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 2005; 162: 199–200. [DOI] [PubMed] [Google Scholar]
- 27.Hernán M, Robins JM. Causal inference: what if. Boca Raton: Chapman & Hall and CRC, 2020. [Google Scholar]
- 28.West KP Jr, Shamim AA, Mehra S, et al. Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA 2014; 312: 2649–58. [DOI] [PubMed] [Google Scholar]
- 29.Caniglia EC, Magosi LE, Zash R, et al. Modest reduction in adverse birth outcomes following the COVID-19 lockdown. Am J Obstet Gynecol 2021; 224: 615.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW. Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ 2013; 346: f3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Black RE. Micronutrients in pregnancy. Br J Nutr 2001; 85 (suppl 2): S193–97. [DOI] [PubMed] [Google Scholar]
- 32.Li N, Li Z, Ye R, Liu J, Ren A. Impact of periconceptional folic acid supplementation on low birth weight and small-for-gestational-age infants in China: a large prospective cohort study. J Pediatr 2017; 187: 105–10. [DOI] [PubMed] [Google Scholar]
- 33.McNulty B, McNulty H, Marshall B, et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. Am J Clin Nutr 2013; 98: 92–98. [DOI] [PubMed] [Google Scholar]
- 34.West KP Jr, Katz J, Khatry SK, et al. Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. The NNIPS-2 Study Group. BMJ 1999; 318: 570–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christian P, Labrique AB, Ali H, et al. Maternal vitamin A and β-carotene supplementation and risk of bacterial vaginosis: a randomized controlled trial in rural Bangladesh. Am J Clin Nutr 2011; 94: 1643–49. [DOI] [PubMed] [Google Scholar]
- 36.The Supplementation with Multiple Micronutrients Intervention Trial (SUMMIT) Study Group. Effect of maternal multiple micronutrient supplementation on fetal loss and infant death in Indonesia: a double-blind cluster-randomised trial. Lancet 2008; 371: 215–27. [DOI] [PubMed] [Google Scholar]
- 37.WHO. HIV country profile 2019. 2019. https://cfs.hivci.org/country-factsheet.html (accessed May 20, 2021).
- 38.Swanson SA, Holme Ø, Løberg M, et al. Bounding the per-protocol effect in randomized trials: an application to colorectal cancer screening. Trials 2015; 16: 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


