Abstract
Pharmaceuticals are now considered to be established contaminants, and their presence in water poses a real risk not only to the marine ecosystem, as they may adversely affect non-target organisms that are exposed to them, but also indirectly to humans. This is particularly true for the model organism considered in this work, Mytilus galloprovincialis (Lamarck, 1819), a suspensivore and bioaccumulating organism that enters the human food chain. Among the most commonly used over-the-counter medicines, anti-inflammatory drugs certainly feature prominently, with acetylsalicylic acid (ASA) at the top. In this work, M. galloprovincialis specimens were exposed to two concentrations of ASA (10 and 100 μg/L) for 10 and 20 days to evaluate possible alterations in the decrease in regulatory volume (RVD) in digestive gland cells and cell viability of both these cells and hemocytes. In addition, the histopathological condition index of the gills and digestive gland was evaluated. The data obtained showed that chronic exposure to ASA did not alter the cell viability of hemocytes and digestive gland cells but alters the physiological mechanisms of volume regulation in the digestive gland and, in addition, a time-dose reaction to ASA in the gills and digestive gland showing numerous alterations such as lipofuscin deposits and hemocyte infiltration was found. These results confirm the potential toxicity to the marine biota, highlighting the necessity to deepen the knowledge regarding the link between over-the-counter pharmaceuticals and non-target organisms.
Keywords: mediterranean mussel, drugs, histology, regulation volume decrease, viability analyses
1 Introduction
The contamination of wastewater and drinking water is a major environmental and public health problem. The main cause of contamination is anthropogenic. The various contaminants present in urban wastewater are called contaminants of emerging concern (CECs) and originate from industrial, hospital, and domestic wastewater, which end up in the marine and terrestrial environment (Rock et al., 2009). However, most of the chemicals released into the environment are not monitored, and their diffusion, interaction, and effects on ecosystems are poorly explored (Carere et al., 2019).
CECs include different classes of chemicals such as drugs, nanomaterials, microplastics, pesticides, flame retardants, perfluorinated compounds, cosmetic products, and many others (Faggio et al., 2016; Pagano et al., 2016, Pagano et al., 2020; Stara et al., 2020; Savoca et al., 2021). Generally, the concentrations of these substances are not reduced in wastewater treatment plants (Capillo et al., 2014, 2018; Albano et al., 2021a; Spanò et al., 2021).
Among drugs, the most commonly found in the aquatic environment are anti-inflammatories (e.g., diclofenac or ibuprofen), antibiotics (e.g., erythromycin, azithromycin), beta-blockers (metoprolol), lipid regulators (gemfibrozil), antidepressants (fluoxetine), antiepileptics (carbamazepine), diuretics, antidiabetics, synthetic hormones (e.g., alpha estradiol), and others (Aliko et al., 2021). Some of these active pharmaceutical ingredients may also have bioaccumulative properties and therefore potentially have the ability to enter the aquatic or terrestrial food chains (Klimaszyk and Rzymski, 2017; Carere et al., 2019) and through biomagnification phenomena may become dangerous to animal and human health (Zenker et al., 2014; XueLi and Hong, 2016; Aliko et al., 2021).
Mussels have also been reported as suitable test organisms for use in ecotoxicological assays due to their wide distribution, resistance to many contaminants, ease in capturing and maintenance under laboratory conditions, and being useful for characterizing the full ecotoxicological potential of drugs.
Acetylsalicylic acid (ASA) is a non-steroidal anti-inflammatory drug among the most widely produced and consumed drugs, in the range of several kilotons per year (Cleuvers, 2004).
ASA is an anti-inflammatory drug that permanently inactivates COX-2 through acetylation of a serine located near the catalytic site of the enzyme. Therefore, the duration of the inhibitory effect of aspirin depends on the rate of de novo synthesis of the enzyme by the target cells after the drug’s rapid disappearance from circulation (FitzGerald and Patrono, 2001; Patrignani and Patrono, 2015).
ASA, like other active pharmaceutical ingredients, has also been found in surface and groundwater, including sources of drinking water (Klimaszyk and Rzymski, 2017). The main source of ASA pollution is from industrial, urban, and agricultural spills, but it has also been found in municipal, livestock, and pharmaceutical and hospital wastewater treatment plants, as reported by the free database of the German Environment Agency, available from: https://www.umweltbundesamt.de/dokument/database-pharmaceuticals-in-the-environment-excel. In European waters, the estimated ASA concentration is 80.4 μg/L (Stuer-Lauridsen et al., 2000).
M. galloprovincialis has been poorly studied in the possible interaction with ASA. Piedade et al. (2020) show that acute exposures do not alter the animal’s oxidative metabolism. In contrast, exposures of M. galloprovincialis to salicylic acid not only reduce respiration capacity but also the normal antioxidant balance (Freitas et al., 2020b, 2019).
Since CECs can persist in the aquatic environment for long periods, the health concern about ASA and ASA-like contaminants is due to their implications for non-target aquatic organisms, that is, organisms that are not intended to be affected by these xenobiotics. Due to their feeding mode, filter-feeder organisms could be particularly exposed and sensitive to this class of pollutants (Deeds et al., 2008; Albano, et al., 2021b; Sauvey et al., 2021). For this reason, the Mediterranean mussel (Mytilus galloprovincialis, Lamarck, 1819) has been chosen as a model organism in this study. Mytilus galloprovincialis is characterized by physiological and cellular mechanisms that can be used as markers to evaluate the possible effects of pollutants (Freitas et al., 2021, 2020b, 2020a, 2019; Pagano et al., 2020, 2017).
The present study aims to assess the effect of two different concentrations of ASA, one less than estimated ASA1: 10 μg/L and the other greater than estimated ASA2: 100 μg/L after chronic exposure (10 and 20 days) on the fitness of M. galloprovincialis to provide the basic knowledge about non-target organisms and ecosystem responses to this contaminant.
2 Materials and Methods
2.1 Experimental Design
Mytilus galloprovincialis specimens, 5.60 ± 0.40 cm shell length, were obtained from the meromitic marine coastal lagoon named ‘‘Faro Lake” from a local bivalve mollusc farm (company Farau Srl, Frutti di Mare, Messina, Italy).
The Faro lagoon is an area exploited for bivalve rearing and cultivation (D’Iglio et al., 2022; Sanfilippo et al., 2022; Savoca et al., 2020).
One hundred-twenty specimens of mussels were maintained in 30 L aquaria filled with continuously aerated brackish water (salinity 32.96 ± 0.31 PSU) in the laboratory with daylight exposure 12 h light:12 h dark and temperature 18 ± 1°C for 7 days acclimation before the start of any experimental procedure.
After acclimation, 30 mussels were randomly selected and placed into each of the six aquaria (three experimental groups in duplicated) containing 20 L continuously aerated brackish water. The mussels were exposed to concentrations of ASA (minimum 99.5%) (Sigma-Aldrich, Darmstadt, Germany): control: 0 μg/L; ASA1: 10 μg/L; ASA2: 100 μg/L for 20 days. Thirteen mussel samples were sampled for laboratory analysis immediately before the transfer for the experimental exposure on 20 L aquaria (T0) after 10 (T1) and 20 (T2) days of exposure to ASA.
2.2 Hemolymph Collection
Hemolymph samples were collected from five mussels from each experimental group. Two pools for each experimental group were used for analyses. The hemolymph was collected from the anterior adductor muscle with a 23-gage needle to a 1-ml plastic syringe. Once collected, it was placed in tubes and immediately centrifuged at 1,000 rpm for 10 min. The pellet was resuspended in 1.5 ml of physiologic saline solution (NaCl 550 mM; KCl 12.5 mM; MgSO4 8 mM; CaCl2 4 mM; glucose 10 mM; HEPES 20 mM; and π= 1,100 mOsm).
2.3 Cell Viability Assays
The experiments used hemolymph and digestive gland cells of mussels. The viability of hemolymph and isolated digestive cells was evaluated by 1) the trypan blue (TB) exclusion method by microscopic observation and 2) the stability of the lysosomal membrane by neutral red (NR) retention assay by microscopic observation, according to Faggio et al. (2016).
2.4 Isolation of Digestive Cells and Regulation of Volume Decrease (RVD) Experiments
Digestive glands of four animals from each group were isolated according to the method of Torre et al. (2013), with slight modifications by Pagano et al. (2017). The cells were observed by using a light microscope (Carl Zeiss Axioskop 20, Wetzlar, Germany) connected to a Canon 550D camera that digitized the image to a PC. Individual cells were selected, and the images were taken at 0 and 3 s in isotonic solution; afterward, the solution was rapidly changed with a hypotonic solution (800 mOsm), and the image was taken every 1 min for the first 10 min after the change of the solution and after every 5 min for 20 min. The profiles of the cells were drawn with the aid of ImageJ (NIH, Bethesda, MD, United States). The data are reported as the relative area Aexp/Ai; indeed, the cell areas for each experimental condition (Aexp) were compared to the areas measured in isotonic solution (Ai) at the beginning of the experiment.
2.5 Histology
Immediately after hemolymph sampling, the gills and digestive glands were quickly removed from ice and stored and fixed in immunofix (paraformaldehyde 4% in phosphate-buffered saline, Bio-Optica, Milan, Italy) for 12 h at room temperature for histopathological condition evaluation. An investigation under histological conditions of digestive glands and gills was performed. Sampled fractions of both tissues from each treatment group were collected in triplicate from three specimens. Tissues were embedded in paraffin and successively sectioned to 5-μm sections by using a rotative microtome (Leica, RM2235). The obtained sections were stained using hematoxylin and eosin for a qualitative histopathological examination using a light microscope (Leitz Diaplan, Germany). For detailed procedures, see Pagano et al. (2016), Lauriano et al. (2019), Zaccone et al. (2015).
2.5.1 Histopathological Condition Indices
For the evaluation of each individual histopathological index (Ih), a semi-quantitative weighted indices approach, initially described by Bernet et al. (1999) for fish and later modified by Costa et al. (2013), was applied. The Ih was calculated for both organs separately (gills and digestive gland) and related to “reaction patterns”: morphological epithelial modifications (gills) and tubule and intertubular tissue alterations (digestive gland). Through microscopic observation of the previously obtained sections, a weight (based on its biological importance) was assigned to each detected alteration, with a value ranging between 1 (minimum severity) and 3 (maximum severity) and a score (degree of dissemination) with values between 0 (alteration not detected) and 6 (alteration diffuse). The weights used have been based on observations collected in this experiment and partially on the literature about both invertebrate Costa et al. (2013) and vertebrate histopathology and are shown in Table1. Further details of the formula for the assessment of histopathological condition indices were reported by Stara et al. (2019).
TABLE 1.
Reaction patterns. Weights assigned to each digestive gland and gill alteration.
| Digestive gland | — | Gill | |
|---|---|---|---|
| Alteration | Weight | Alteration | Weight |
| Tubule alterations | Cellular and morphological changes | ||
| Epithelial cell hyperplasia | 2 | Epithelial cell hyperplasia | 2 |
| Epithelial cell hypertrophy | 2 | Vacuolation | 1 |
| Brown cells | 1 | Hemocyte infiltration | 1 |
| Hemocyte infiltration | 1 | Granulocytoma | 2 |
| Lipofuscin aggregates | 1 | Lipofuscin aggregates | 1 |
| Necrosis | 3 | Fibrosis | 2 |
| Tubule regression | 2 | Necrosis | 3 |
| Intertubular tissue changes | Loss of epithelia | 3 | |
| Hemocyte infiltration | 1 | Lamellar fusion | 1 |
| Lipofuscin aggregates | 1 | Lamellar deformation | 1 |
| Brown cells | 1 | Epithelial detachment | 1 |
| Fibrosis | 2 | — | |
| Necrosis | 3 | — | |
| Granulocytoma | 2 | — | |
2.6 Statistical Analyses
The statistical analyses of results were performed using two-way ANOVA followed by the Bonferroni test for pairwise comparisons among experimental conditions in RVD assay and an unpaired t-test for comparisons in viability assays. Package Prism, Version 8.2.1 (GraphPad Software Ldt., La Jolla, CA 92037, United States) was used for statistical analysis. The data of histopathological indices (Ih) were analyzed using two-way ANOVA followed by Tukey’s post hoc test for multiple comparisons. p-value was set at p < 0.05. Statistical analysis was performed using the software package Prism, Version 8.2.1 (GraphPad Software Ldt., La Jolla, CA 92037, United States).
3 Results
3.1 Cell Viability Assays
As shown in Table 2, hemocytes maintain high viability values throughout the experiment at both drug concentrations. The same trend is evident in Table 3 for the cells of the digestive gland.
TABLE 2.
Percentage of viability hemocytes in Mytilus galloprovincialis exposed to acetylsalicylic acid (control (0 mg/L); ASA1 (10 μg/L); and ASA2 (100 μg/L) by trypan blue (TB) and neutral red (NR) after 10 days (T1) and 20 days (T2) of exposure. One-way ANOVA was used to test the differences between control and treatment and the Tukey test. The values are presented as the mean ± SD (n = 5); significant differences compared with the control group value (p < 0.05).
| Viability assays | Time of exposure | Test group | ||
|---|---|---|---|---|
| Control (0 mg/L) | ASA1 (10 μg/L) | ASA2 (100 μg/L) | ||
| TB | T1 | 100 ± 0 | 92.96 ± 1.00 | 99.00 ± 0.69 |
| T2 | 95.96 ± 0.49 | 95.92 ± 0.23 | 93.91 ± 0.13 | |
| NR | T1 | 100 ± 0 | 91.57 ± 0.75 | 99.00 ± 0.69 |
| T2 | 95.17 ± 0.11 | 97.79 ± 0.41 | 97.42 ± 0.11 | |
TABLE 3.
Percentage of viability of digestive cells in Mytilus galloprovincialis exposed to acetylsalicylic acid [Control (0 mg/L); ASA1 (10 μg/L); and ASA2 (100 μg/L)] by trypan blue (TB) and neutral red (NR) after 10 days (T1) and 20 days (T2) of exposure. The values are presented as the mean ± SE; significant differences compared with the control group value (p < 0.05).One-way ANOVA has been used to test the differences between control and treatment and the Tukey test.
| Viability assays | Time of exposure | Test group | ||
|---|---|---|---|---|
| Control (0 mg/L) | ASA1 (10 μg/L) | ASA2 (100 μg/L) | ||
| TB | T1 | 100 ± 0 | 96.67 ± 0.50 | 97.89 ± 0.26 |
| T2 | 97.33 ± 0.21 | 98.86 ± 0.28 | 98.06 ± 0.18 | |
| NR | T1 | 100 ± 0 | 99.01 ± 0.62 | 98.33 ± 0.92 |
| T2 | 95.03 ± 0.13 | 98.28 ± 0.33 | 98.70 ± 0.03 | |
3.2 RVD Experiment
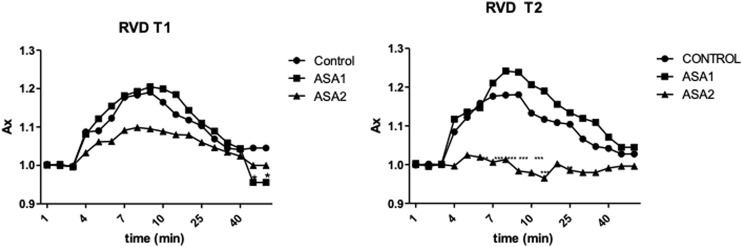
Digestive gland cells of Control and ASA1 organisms after exposure to hypotonic solution increased their volume by approximately 12% and then returned to their initial volume. This response was observable for both T1 and T2. On the other hand, the cells of the ASA2 group behaved differently at the two exposure times: at T1, the cells exposed to hypotonic solution swelled slowly to 10% of their volume and then returned to their initial conditions; at T2, the cells after washing with hypotonic solution were unable to swell (Figure 1).
FIGURE 1.
Relative changes in the area of digestive cells of Mytilus galloprovincialis exposed to a hypotonic solution for both exposure times, T1 and T2. The values are presented as the mean ± SD (n = 4); significant differences compared with the control group value (p < 0.05) (two-way ANOVA test). Control (0 μg L−1) (◆), ASA1. (10 μg L−1) (▪), and ASA2 (100 μg L−1) (▴).
3.3 Histology
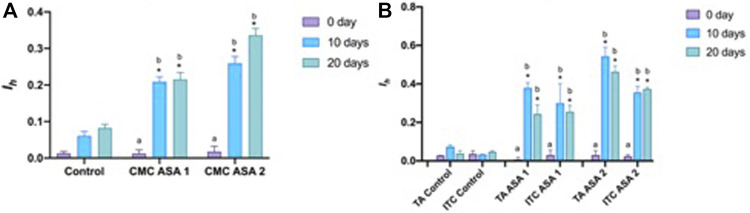
Histopathological alterations detected are shown in Figure 3. Histopathological condition index (Ih) results are shown in Table 4. A time- and concentration-dependent reaction to ASA was detected in both the digestive gland and gills when compared to the control (p < 0.05) (Figure 2). Ih showed a trend dependent on different treatments and exposure times (p < 0.05) for both organs analyzed. In the gills, an increasing trend of Ih was observed, proportionally to increase in the exposure time and ASA concentration, although not statistically significant (Figure 3A). In the digestive gland tissue, no statistically significant differences were obtained comparing digestive tubule changes and intertubular tissue modifications in the group exposed to both concentrations tested, showing a marked decrease in Ih values at 20 days of exposure (Figure 3B).
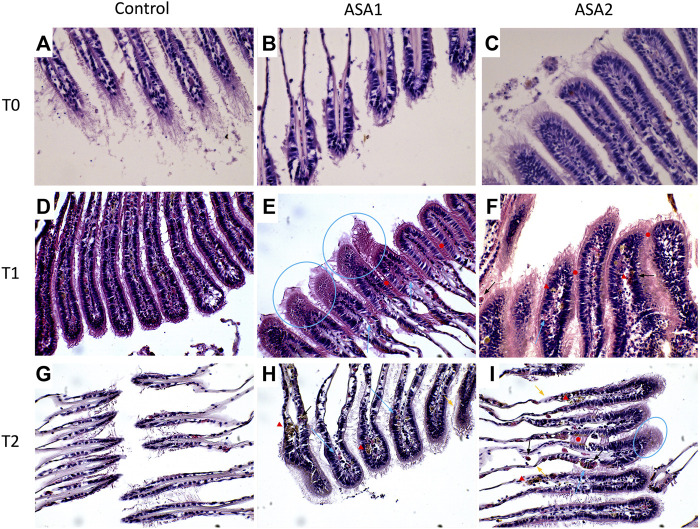
FIGURE 3.
Mytilus galloprovincialis gill sections after ASA exposure. Representative images: (A–C) are, respectively, Control, ASA1, and ASA2, 0-day exposure; (D–F) are, respectively, Control, ASA1, and ASA2, 10-day exposure; (G–I) are, respectively, Control, ASA1, and ASA2, 20-day exposure. ASA1 (ASA 10 μg L−1) and ASA2 (ASA 100 μg L−1). Magnification ×40. Blue arrows highlight hemocyte infiltrations, blue circles highlight vacuolation, red arrowheads highlight lipofuscin aggregates, black arrows highlight granulocyte infiltrations, orange arrows highlight epithelial alterations, and red filled circles highlight lamellar fusion.
TABLE 4.
Histopathological condition index Ih of Mytilus galloprovincialis exposed to experimental concentrations of ASA. The values are presented as the means ± SD (n = 3). Significant differences compared with the control value set as p < 0.05 (*) are shown. ASA1 (ASA 10 μg L−1) and ASA2 (ASA 100 μg L−1).
| Organ | Reaction pattern | Exposure time (days) | Test groups | ||
| Control | ASA1 | ASA2 | |||
| (10 μg L−1) | (100 μg L−1) | ||||
| Digestive gland | Tubule alterations | 0 | 0.028 ± 0.0 | 0.07 ± 0.01* | 0.03 ± 0.02* |
| 10 | 0.07 ± 0.0 | 0.38 ± 0.02* | 0.54 ± 0.04* | ||
| 20 | 0.03 ± 0.01 | 0.24 ± 0.04* | 0.46 ± 0.03* | ||
| Intertubular tissue changes | 0 | 0.03 ± 0.01 | 0.03 ± 0.02* | 0.02 ± 0.0* | |
| 10 | 0.03 ± 0.01 | 0.30 ± 0.10* | 0.355 ± 0.03* | ||
| 20 | 0.04 ± 0.0 | 0.25 ± 0.03* | 0.37 ± 0.0* | ||
| Gills | Cellular and morphological changes | 0 | 0.013 ± 0.05 | 0.012 ± 0.01* | 0.017 ± 0.015* |
| 10 | 0.06 ± 0.013 | 0.20 ± 0.01* | 0.26 ± 0.01* | ||
| 20 | 0.082 ± 0.010 | 0.21 ± 0.01* | 0.33 ± 0.019* | ||
FIGURE 2.
Histopathological condition index (Ih) of tissues from Mytilus galloprovincialis exposed to acetylsalicylic acid (ASA): (A) gills and (B) digestive gland. The values are shown as mean ± SD (n = 3); stars represent significant differences between the treatment and control groups at the same time point. Letters are only present in the case of significant statistical differences. Different small letters indicate significant differences between time points within the same treatment group. Differences were considered significant when p < 0.05 (two-way ANOVA test/Tukey’s multiple comparisons test). ASA1 (ASA 10 μg L−1) and ASA2 (ASA 100 μg L−1). CMC, cellular and morphological changes; TA, tubular alteration; ITC, intertubular tissue changes.
3.3.1 Gills
Various serious alterations due to ASA exposure were recorded in gill tissues during the experiment. The most frequent histological modifications detected were alterations of epithelial structure, lamellar fusion, vacuolation, lipofuscin deposits, and hemocyte infiltration (Figure 3). An increasing trend of alterations was observed in Ih values, proportional to the increase in ASA concentration and exposure time, although no statistically significant differences were highlighted. Contrary to the digestive gland investigation, in the gills, no inversion on the increasing of the Ih trend was detected.
3.3.2 Digestive Gland
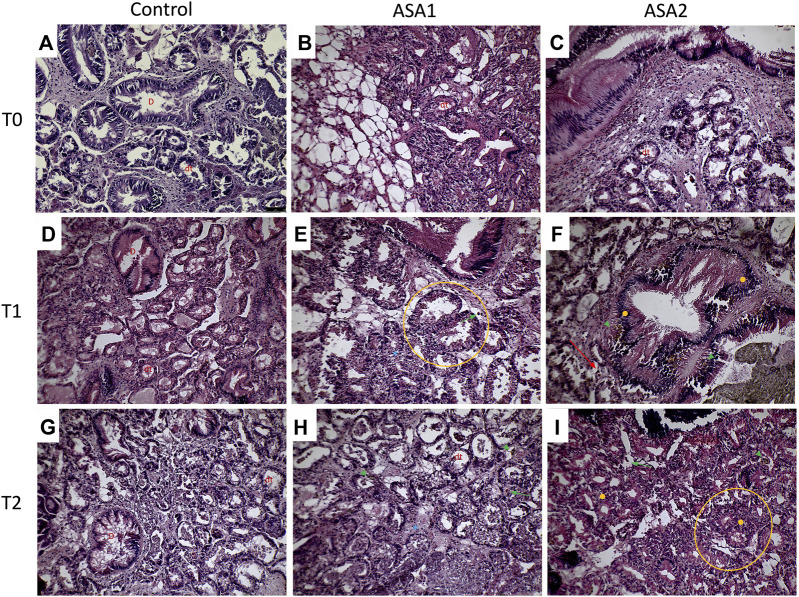
Mainly present digestive gland alterations comprehended lipofuscin aggregates, hemocyte infiltration, and hyperplasia both in digestive tubule and intertubular tissues in exposed specimens. In the most severe cases, tubule regression, hypertrophy, and focal points of necrosis were observed in mussels exposed also to ASA1 (Figure 4). Ih in ASA1-exposed specimens was higher with respect to the ASA2 experiment, except for the sample ASA2 at 20 days of exposure. No significant discrepancies were obtained when comparing digestive tubule changes and intertubular tissue modifications.
FIGURE 4.
Mytilus galloprovincialis digestive gland sections from ASA exposure: representative figures. (A–C) are, respectively, Control, ASA1, and ASA2, 0-day exposure; (D–F) are, respectively, Control, ASA1, and ASA2, 10-day exposure; (G–I) are, respectively, Control, ASA 1, and ASA2, 20-day exposure. ASA 1 (ASA 10 μg L−1) and ASA2 (ASA 100 μg L−1). Magnification ×20. Orange circles highlight digestive tubule alteration, orange filled circles indicate hyperplasia, red arrows show brown cells, green arrowheads show lipofuscin aggregates, green arrows highlight hemocyte infiltrations, and blue asterisks highlight hypertrophy. D identifies duct; dt identifies digestive tubule.
4 Discussion
Acetylsalicylic acid is widely used by humans as an analgesic and is found in wastewater and urban effluents. Despite the larger amount of data on the occurrence of CECs in the aquatic environment, studies assessing their possible adverse effects on aquatic organisms are still poor and relatively limited. Few studies are available on the ASA effects on non-target organisms (Dittrich, 2018; Almeida et al., 2020; Piedade et al., 2020; Siddeswaran et al., 2020); instead, the effects of other anti-inflammatory agents have been studied (Freitas et al., 2019,2020b; Gonzalez-Rey and Bebianno, 2014; Mezzelani et al., 2018). Freitas et al. (2019), (2020a) showed that chronic exposure to salicylic acid (SA) reduces the respiration capacity of mussels and alters normal antioxidant balances and causes neurotoxic damage, and this will be amplified if temperatures are increased.
In our results, the cell viability assays highlighted the lack of interaction between the cells and the ASA. In fact, no significant alterations in cell viability were present at either concentration for any exposure time for both assays tested, and in contrast to other molluscs exposed to non-steroidal anti-inflammatory drugs, the lysosomal membranes were found to be intact (Parolini and Binelli, 2011; Parolini, 2020). Instead, there was a massive presence of hemocytes in the gills and in the digestive gland, demonstrated by histological examinations, which is the first indication of tissue inflammation (de Vico and Carella, 2012).
Bivalve gills are also involved in the alimentation process, filtering water and trapping particulate matter. In the bivalve mollusc’s anatomy, gills represent the first contact with mucosal surfaces by which the organism faces the surrounding water (Azevedo et al., 2015; Stara et al., 2020, 2019). This barrier can be crossed both by substances important for the organism and contaminants present in water (Canesi et al., 2012; Phuong et al., 2017; Azizi et al., 2018). Despite that gills are the primary site of contact with the pollutants, as reported by many authors, the main target of their accumulation and detoxification in bivalve molluscs is represented by the digestive gland (Faggio et al., 2016; Blanco et al., 2021; Stollberg et al., 2021). In addition, the digestive gland is also involved in the metabolism of heavy metals (Viarengo et al., 1981; Caricato et al., 2018). Histopathological modifications on these target tissues have been examined using the Ih as suggested by Costa et al. (2013). The Ih values determined for the histological alteration and reactions evaluated (Table 1) are shown in Table 4 and graphed in Figure 2. Regarding gills, Ih resulted higher, as expected, in ASA2 at 20 days exposure. In the digestive gland, Ih resulted higher in ASA2-treated specimens than ASA1, following a dose-dependent inflammation pattern. It is interesting to note how the Ih values detected for both examined structures of the digestive gland showed an unexpected trend. Indeed, the higher Ih values were, in both ASA1 and ASA2, the higher the exposure will be in the 10-day than in the 20- day experiment. It is also conceivable that for the tissue of the digestive gland, the 10 days exposed specimens suffer an acute reaction that was reduced in the 20-day experiment. It can be assumed that M. galloprovincialis, after an acute inflammatory response can tolerate exposure to ASA, as also reported in a previous study (Pagano et al., 2016; Bayne et al., 1979; Kumar Yadav, 2013.). The histological modifications reported in this study have been confirmed in M. galloprovincialis by our recent studies on the evaluation of the chronic exposure effect of some toxicants, both at acute and sub-lethal concentrations (Stara et al., 2020, 2021). Some other histological alterations were detected and related in this case to an inflammatory response to ASA.
Various stage inflammations have been detected in both organs examined (gills and digestive gland), related in our case to ASA exposure. These alterations could represent a first response to various pollutants and drugs in these organs, as already reported by other authors (Yasmeen, 2019; Abdel-Latif et al., 2020; Couch and Fournie, 2021). Regarding the gill tissue, these inflammations were mainly focal at low concentrations of toxicant characterized by vacuolation and sometimes widespread with infiltration of hemocytes and granulocytes in ASA2. The digestive glands showed a more diffused inflammation characterized by both hemocyte infiltration and hyperplasia, resulting in the loss of physiological anatomy. Nodular inflammations, such as granulocytomas, appear rarely and are not widespread compared to the results of other authors (Kumeiko et al., 2018; Yee-Duarte et al., 2018; Sendra et al., 2021). Considering that nodular inflammations occur from phagocytosis activity of hemocytes after a pathogen’s invasion (Rowley, 1996), that during their activity creates different sizes of aggregates in hemolymph and interstices (Galloway & Depledge, 2001), from the results, as expected, ASA exposure seems to not cause this aggregate formation. Despite this, some other authors have highlighted the aggregative properties of hemocytes under stimulation by acute or chronic exposure to xenobiotics (Auffret and Oubella, 1997; Carella et al., 2015). The brown cells were evident in digestive gland tissue, with higher frequency in intertubule spaces; these cells are highly present in the digestive gland of stressed organisms and are involved in recognition, accumulation, and detoxification of toxicants (Usheva and Frolova, 2006; de Vico and Carella, 2012). In addition, digestive gland cells exposed to hypotonic solutions can normally regulate their volume (Torre et al., 2013), but in the cells exposed to ASA2, there was an interaction response to the pollutant and the cellular mechanisms at T1, with the cells unable to regulate their volume. At T1, after hypostatic exposure, the cells swell less than in the other two conditions, and at T2, they cannot swell at all. M. galloprovincialis, being an osmoconforming organism, alterations in these capacities can be used as a parameter for assessing physiological changes (Pagano et al., 2016, 2017). It is as if long exposure to higher concentration of ASA has blocked the normal ionic efflux, also preventing the swelling of cells exposed to hypotonic concentration, behaving as an ion channel inhibitor as demonstrated by Torre et al. (2013).
Focusing on gill tissues, our analysis revealed an inflammatory status connected to ASA exposure. This reaction was characterized mainly by extended vacuolation, moderate deposits of lipofuscin, hemocyte infiltration, extended lamellar fusion, and modifications of epithelial morphology. Infiltrative inflammations characterized by various stages of hemocyte infiltrations are widely reported in gill tissues of molluscs exposed to environmental toxicants (Carnegie and Meyer, 2021; Khan et al., 2019; Kumeiko et al., 2018; Paviotti-Fischer et al., 2018). As for the digestive gland, the function of infiltrative hemocytes to phagocyte pathogens and/or foreign bodies is to initialize the organism’s response to xenobiotics, starting the multixenobiotic defense mechanism (MXDM) (Pain and Parant, 2003; Parant, 2022). The MXDM system represents a shield for cells and tissues from the adverse effect of toxicants through the reduction of their access and to favor their efflux (Pagano et al., 2016).
Destructive reactions at the expense of gill tissue morphology and functions, such as vacuolation, lamellar fusion, and loss of epithelial morphology, were already reported by several authors in bivalve molluscs, as common reactions to pollutant exposure (Khudhur et al., 2019; Joshy et al., 2022; Khan et al., 2018). The influence of ASA concentration on these modifications has followed a constantly increasing trend during our study. More interesting is their succession in relation to the exposure time. The massive presence of vacuolation found in our study after 10 days of exposure in higher presence suggests that this mechanism may be among the first inflammatory processes. On the contrary, lamellar fusion and modifications of the epithelial normal structure were found at the longest exposure time (20 days). This suggests that the highest functionally more severe modifications occur in the gill tissues of molluscs in a later stage of the inflammatory response process, as reported by other authors (Balamurugan and Subramanian, 2021; Pires et al., 2022).
In bivalve molluscs, lipofuscin formation is related mainly to cellular oxygen consumption (Katz et al., 1984), but several authors have studied how lipofuscin in situ also can represent a signal of primary reaction to the exposure, particularly to heavy metals or other pollutants (Mathew and Damodaran, 1997; Lomovasky et al., 2002; Husmann et al., 2012; Abdel-Latif et al., 2020). In this study, we found a steady increasing trend of the presence of lipofuscin aggregates in gills related both to ASA concentration and exposure time, which highlight its involvement in the generalized inflammatory response. In this case, the lipofuscin accumulation indicates a reaction to the oxidative damage caused by ASA exposure. Considering the lipofuscin more widely also as an age-related pigment linked to oxidation of by-products, further studies with prolonged exposure could also reveal its role in this process in molluscs.
5 Conclusion
The current study examined the chronic effect of acetylsalicylic acid on M. galloprovincialis. Our results show both physiological changes in the organism, such as altered regulation of cell volume and inflammation on a histological level, especially in the digestive gland. These results occur even at concentrations much lower than those estimated in the aquatic environment, reinforcing current assumptions about the need to investigate the effects of water contamination by drugs and/or their derived compounds. Therefore, the aim of this research was to increase knowledge of the ecotoxicological potential of one of the active pharmaceutical ingredients present in the water, acetylsalicylic acid, by studying physiology and possible histological alterations of Mytilus galloprovincialis.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by MP, MA, SS, and FI. The work was supervised by CF and GC. The first draft of the manuscript was written by MP and MA, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abdel-Latif H. M. R., Dawood M. A. O., Menanteau-Ledouble S., El-Matbouli M. (2020). Environmental Transformation of N-TiO2 in the Aquatic Systems and Their Ecotoxicity in Bivalve Mollusks: A Systematic Review. Ecotoxicol. Environ. Saf. 200, 110776. 10.1016/J.ECOENV.2020.110776 [DOI] [PubMed] [Google Scholar]
- Albano M., Panarello G., di Paola D., Capparucci F., Crupi R., Gugliandolo E., et al. (2021a). The Influence of Polystyrene Microspheres Abundance on Development and Feeding Behavior of Artemia salina (Linnaeus, 1758). Appl. Sci. 11, 3352. 10.3390/APP11083352 [DOI] [Google Scholar]
- Albano M., Panarello G., di Paola D., D’Angelo G., Granata A., Savoca S., et al. 2021b. The Mauve Stinger Pelagia noctiluca (Cnidaria, Scyphozoa) Plastics Contamination, the Strait of Messina Case. International Journal of Environmental Studies, 78 (6), 977–982. 10.1080/00207233.2021.1893489 [DOI] [Google Scholar]
- Aliko V., Korriku R. S., Pagano M., Faggio C. (2021). Double-Edged Sword: Fluoxetine and Ibuprofen as Development Jeopardizers and Apoptosis' Inducers in Common Toad, Bufo bufo, Tadpoles. Sci. Total Environ. 776, 145945. 10.1016/j.scitotenv.2021.145945 [DOI] [PubMed] [Google Scholar]
- Almeida Â., Solé M., Soares A. M. V. M., Freitas R. (2020). Anti-Inflammatory Drugs in the Marine Environment: Bioconcentration, Metabolism and Sub-Lethal Effects in Marine Bivalves. Environ. Pollut. 263, 114442. 10.1016/J.ENVPOL.2020.114442 [DOI] [PubMed] [Google Scholar]
- Auffret M., Oubella R. (1997). Hemocyte Aggregation in the Oyster Crassostrea gigas: In Vitro Measurement and Experimental Modulation by Xenobiotics. Comp. Biochem. Physiology Part A Physiology 118, 705–712. 10.1016/S0300-9629(97)00017-0 [DOI] [Google Scholar]
- Azevedo C. C., Guzmán-Guillén R., Martins J. C., Osório H., Vasconcelos V., da Fonseca R. R., et al. (2015). Proteomic Profiling of Gill GSTs in Mytilus galloprovincialis from the North of Portugal and Galicia Evidences Variations at Protein Isoform Level with a Possible Relation with Water Quality. Mar. Environ. Res. 110, 152–161. 10.1016/J.MARENVRES.2015.08.008 [DOI] [PubMed] [Google Scholar]
- Azizi G., Akodad M., Baghour M., Layachi M., Moumen A. (2018). The Use of Mytilus spp. Mussels as Bioindicators of Heavy Metal Pollution in the Coastal Environment. A Review. J. Mater Environ. Sci. 9, 1170–1181. [Google Scholar]
- Balamurugan S., Subramanian P. (2021). Histopathology of the Foot, Gill and Digestive Gland Tissues of Freshwater Mussel, Lamellidens marginalis Exposed to Oil Effluent. Austin J. Environ. Toxicol. 7. 1. [Google Scholar]
- Bayne B. L., Moore M. N., Widdows J., Livingstone D. R., Salkeld P. (1979). Measurement of the Responses of Individuals to Environmental Stress and Pollution: Studies with Bivalve Molluscs. Phil. Trans. R. Soc. Lond. B 286, 563–581. 10.1098/RSTB.1979.0046 [DOI] [PubMed] [Google Scholar]
- Bernet D., Schmidt H., Meier W., Burkhardt-Holm P., Wahli T. (1999). Histopathology in Fish: Proposal for a Protocol to Assess Aquatic Pollution. J. Fish. Dis. 22, 25–34. [Google Scholar]
- Blanco J., Mariño C., Martín H., Álvarez G., Rossignoli A. E. (2021). Characterization of the Domoic Acid Uptake Mechanism of the Mussel (Mytilus galloprovincialis) Digestive Gland. Toxins 202113, 458. 10.3390/TOXINS13070458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesi L., Ciacci C., Fabbri R., Marcomini A., Pojana G., Gallo G. (2012). Bivalve Molluscs as a Unique Target Group for Nanoparticle Toxicity. Mar. Environ. Res. 76, 16–21. 10.1016/J.MARENVRES.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Capillo G., Genovese G., Monteleone A., Morabito M., Sanfilippo M., Manganaro A. (2014). From Culture to Application. Agar from Gracilaria gracilis of Ganzirri Lagoon (Sicily, Italy). J. Biol. Res. 87, 5–6. [Google Scholar]
- Capillo G., Savoca S., Costa R., Sanfilippo M., Rizzo C., Lo Giudice A., et al. (2018). New Insights into the Culture Method and Antibacterial Potential of Gracilaria gracilis . Mar. Drugs 16, 492. 10.3390/MD16120492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carella F., Feist S. W., Bignell J. P., de Vico G. (2015). Comparative Pathology in Bivalves: Aetiological Agents and Disease Processes. J. Invertebr. Pathology 131, 107–120. 10.1016/J.JIP.2015.07.012 [DOI] [PubMed] [Google Scholar]
- Carere M., Corti M., di Domenico K., Cristiano W. (2019). “Contaminanti Emergenti Negli Ecosistemi Acquatici,” in Cambiamenti Ambientali Globali e Salute, 19, 15. [Google Scholar]
- Caricato R., Giordano M. E., Schettino T., Lionetto M. G. (2018). Functional Involvement of Carbonic Anhydrase in the Lysosomal Response to Cadmium Exposure in Mytilus galloprovincialis Digestive Gland. Front. Physiol. 9, 319. 10.3389/FPHYS.2018.00319/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie R. B., Meyer G. R. (2021). “Mikrocytosis of Bivalve Molluscs,” in ICES Identification Leaflets for Diseases and Parasites in Fish and Shellfish No. 73. Denmark: ICES, 9. 10.17895/ices.pub.5439 [DOI] [Google Scholar]
- Cleuvers M. (2004). Mixture Toxicity of the Anti-Inflammatory Drugs Diclofenac, Ibuprofen, Naproxen, and Acetylsalicylic Acid. Ecotoxicol. Environ. Saf. 59, 309–315. 10.1016/S0147-6513(03)00141-6 [DOI] [PubMed] [Google Scholar]
- Costa P. M., Carreira S., Costa M. H., Caeiro S. (2013). Development of Histopathological Indices in a Commercial Marine Bivalve (Ruditapes decussatus) to Determine Environmental Quality. Aquat. Toxicol. 126, 442–454. 10.1016/J.AQUATOX.2012.08.013 [DOI] [PubMed] [Google Scholar]
- Couch J. A., Fournie J. W. (2021). Pathobiology of Marine and Estuarine Organisms. Boca Raton, Fl: CRC Press. [Google Scholar]
- D’Iglio C., Natale S., Albano M., Savoca S., Famulari S., Gervasi C., et al. (2022). Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 14, 398. [Google Scholar]
- de Vico G., Carella F. (2012). Morphological Features of the Inflammatory Response in Molluscs. Res. Veterinary Sci. 93, 1109–1115. 10.1016/J.RVSC.2012.03.014 [DOI] [PubMed] [Google Scholar]
- Deeds J., Landsberg J., Etheridge S., Pitcher G., Longan S. (2008). Non-Traditional Vectors for Paralytic Shellfish Poisoning. Mar. Drugs 6, 308–348. 10.3390/MD6020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A. (2018). Effects of Anthropogenic Acetylsalicylic Acid Contamination on Ecological Interactions. iScientist 3, 66–74. [Google Scholar]
- Faggio C., Pagano M., Alampi R., Vazzana I., Felice M. R. (2016). Cytotoxicity, Haemolymphatic Parameters, and Oxidative Stress Following Exposure to Sub-Lethal Concentrations of Quaternium-15 in Mytilus galloprovincialis . Aquat. Toxicol. 180, 258–265. 10.1016/j.aquatox.2016.10.010 [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A., Patrono C. (2001). The Coxibs, Selective Inhibitors of Cyclooxygenase-2. N. Engl. J. Med. 345, 433–442. 10.1056/nejm200108093450607 [DOI] [PubMed] [Google Scholar]
- Freitas R., Silvestro S., Coppola F., Meucci V., Battaglia F., Intorre L., et al. (2019). Biochemical and Physiological Responses Induced in Mytilus galloprovincialis After a Chronic Exposure to Salicylic Acid. Aquat. Toxicol. 214, 105258. 10.1016/j.aquatox.2019.105258 [DOI] [PubMed] [Google Scholar]
- Freitas R., Silvestro S., Coppola F., Costa S., Meucci V., Battaglia F., et al. (2020a). Toxic Impacts Induced by Sodium Lauryl Sulfate in Mytilus galloprovincialis . Comp. Biochem. Physiology Part A Mol. Integr. Physiology 242, 110656. 10.1016/j.cbpa.2020.110656 [DOI] [PubMed] [Google Scholar]
- Freitas R., Silvestro S., Pagano M., Coppola F., Meucci V., Battaglia F., et al. (2020b). Impacts of Salicylic Acid in Mytilus galloprovincialis Exposed to Warming Conditions. Environ. Toxicol. Pharmacol. 80, 103448. 10.1016/j.etap.2020.103448 [DOI] [PubMed] [Google Scholar]
- Freitas R., Coppola F., Meucci V., Battaglia F., Soares A. M. V. M., Pretti C., et al. (2021). The Influence of Salinity on Sodium Lauryl Sulfate Toxicity in Mytilus galloprovincialis . Environ. Toxicol. Pharmacol. 87, 103715. 10.1016/j.etap.2021.103715 [DOI] [PubMed] [Google Scholar]
- Galloway T. S., Depledge M. H. (2001). Immunotoxicity in Invertebrates: Measurement and Ecotoxicological Relevance. Ecotoxicology 10 (1), 5–23. 10.1023/A:1008939520263 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey M., Bebianno M. J. (2014). Effects of Non-Steroidal Anti-Inflammatory Drug (NSAID) Diclofenac Exposure in Mussel Mytilus galloprovincialis . Aquat. Toxicol. 148, 221–230. 10.1016/j.aquatox.2014.01.011 [DOI] [PubMed] [Google Scholar]
- Husmann G., Abele D., Monien D., Monien P., Kriews M., Philipp E. E. R. (2012). The Influence of Sedimentation on Metal Accumulation and Cellular Oxidative Stress Markers in the Antarctic Bivalve Laternula elliptica . Estuar. Coast. Shelf Sci. 111, 48–59. 10.1016/J.ECSS.2012.06.003 [DOI] [Google Scholar]
- Joshy A., Sharma S. R. K., Mini K. G., Gangadharan S., Pranav P. (2022). Histopathological Evaluation of Bivalves from the Southwest Coast of India as an Indicator of Environmental Quality. Aquat. Toxicol. 243, 106076. 10.1016/J.AQUATOX.2022.106076 [DOI] [PubMed] [Google Scholar]
- Katz M., Robinsonjr W., Herrmann R., Groome A., Bieri J. (1984). Lipofuscin Accumulation Resulting from Senescence and Vitamin E Deficiency: Spectral Properties and Tissue Distribution. Mech. Ageing Dev. 25, 149–159. 10.1016/0047-6374(84)90137-4 [DOI] [PubMed] [Google Scholar]
- Khan M. I., Khisroon M., Khan A., Gulfam N., Siraj M., Zaidi F., et al. (2018). Bioaccumulation of Heavy Metals in Water, Sediments, and Tissues and Their Histopathological Effects on Anodonta cygnea (Linea, 1876) in Kabul River, Khyber Pakhtunkhwa, Pakistan. BioMed Res. Int. 2018, 1–10. 10.1155/2018/1910274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B., Adeleye A. S., Burgess R. M., Smolowitz R., Russo S. M., Ho K. T. (2019). A 72˗h Exposure Study with Eastern Oysters (Crassostrea virginica) and the Nanomaterial Graphene Oxide. Enviro Toxic Chem. 38, 820–830. 10.1002/ETC.4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khudhur S. M., Shekha Y. A., Ahmed Shekha Y. (2019). Histopathological and Biochemical Biomarker Response of Mussel, Unio pictorum, to Carbamate Pesticide Carbaryl: A Laboratory Study. Indian J. Animal Res. 1. 1. 10.18805/ijar.B-1157 [DOI] [Google Scholar]
- Klimaszyk P., Rzymski P. (2017). Water and Aquatic Fauna on Drugs: What Are the Impacts of Pharmaceutical Pollution? International Symposium on Water in Environment. Cham: Springer, 255–278. [Google Scholar]
- Kumar Yadav S. (2013). Dose-Response Models to Understand Toxicodynamics for Pollutants in Ecosystems. Int. J. Environ. Sci. Dev. Monit. 1. 1. [Google Scholar]
- Kumeiko V. V., Sokolnikova Y. N., Grinchenko A. v., Mokrina M. S., Kniazkina M. I. (2018). Immune State Correlates with Histopathological Level and Reveals Molluscan Health in Populations of Modiolus kurilensis by Integral Health Index (IHI). J. Invertebr. Pathology 154, 42–57. 10.1016/J.JIP.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Lamarck (1819). “Non è Una Citazione ma è il Nome Dello Scopritore Della Specie,” in Non si Inserisce la Citazione in Questi Casi. [Google Scholar]
- Lauriano E. R., Pergolizzi S., Aragona M., Montalbano G., Guerrera M. C., Crupi R., et al. (2019). Intestinal Immunity of Dogfish Scyliorhinus canicula Spiral Valve: A Histochemical, Immunohistochemical and Confocal Study. Fish Shellfish Immunol. 87, 490–498. [DOI] [PubMed] [Google Scholar]
- Lomovasky B. J., Morriconi E., Brey T., Calvo J. (2002). Individual Age and Connective Tissue Lipofuscin in the Hard Clam Eurhomalea exalbida . J. Exp. Mar. Biol. Ecol. 276, 83–94. 10.1016/S0022-0981(02)00240-X [DOI] [Google Scholar]
- Mathew S., Damodaran R. (1997). Lipofuscin as Physiological Indicator of Heavy Metal Stress in Sunetta scripta (Yellow Clam) and Perna viridis (Green Mussel). IJMS 26 (1). 1. March 1997. [Google Scholar]
- Mezzelani M., Gorbi S., Regoli F. (2018). Pharmaceuticals in the Aquatic Environments: Evidence of Emerged Threat and Future Challenges for Marine Organisms. Marine Environ. Res. 140, 41–60. 10.1016/j.marenvres.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Pagano M., Capillo G., Sanfilippo M., Palato S., Trischitta F., Manganaro A., et al. (2016). Evaluation of Functionality and Biological Responses of Mytilus galloprovincialis After Exposure to Quaternium-15 (Methenamine 3-Chloroallylochloride). Molecules 21, 144. 10.3390/molecules21020144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano M., Porcino C., Briglia M., Fiorino E., Vazzana M., Silvestro S., et al. (2017). The Influence of Exposure of Cadmium Chloride and Zinc Chloride on Haemolymph and Digestive Gland Cells from Mytilus galloprovincialis . Int. J. Environ. Res. 11, 207–216. 10.1007/s41742-017-0020-8 [DOI] [Google Scholar]
- Pagano M., Stara A., Aliko V., Faggio C. (2020). Impact of Neonicotinoids to Aquatic Invertebrates-In Vitro Studies on Mytilus galloprovincialis: A Review. Jmse 8, 801. 10.3390/jmse8100801 [DOI] [Google Scholar]
- Pain S., Parant M. (2003). Le mécanisme de défense multixénobiotique (MDMX) chez les bivalves. Comptes Rendus Biol. 326, 659–672. 10.1016/S1631-0691(03)00156-2 [DOI] [PubMed] [Google Scholar]
- Parant M. (2022). Ecole Doctorale Sciences ET Ingenierie Des Resources Naturelles SIReNA. [Google Scholar]
- Parolini M., Binelli A. (2011). Sub-Lethal Effects Induced by a Mixture of Three Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) on the Freshwater Bivalve Dreissena polymorpha . Ecotoxicology 21 (2 21), 379–392. 10.1007/S10646-011-0799-6 [DOI] [PubMed] [Google Scholar]
- Parolini M. (2020). Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Acetylsalicylic Acid, Paracetamol, Diclofenac, Ibuprofen and Naproxen Towards Freshwater Invertebrates: A Review. Sci. Total Environ. 740, 140043. 10.1016/J.SCITOTENV.2020.140043 [DOI] [PubMed] [Google Scholar]
- Patrignani P., Patrono C. (2015). Cyclooxygenase Inhibitors: From Pharmacology to Clinical Read-Outs. Biochimica Biophysica Acta (BBA) - Mol. Cell Biol. Lipids 1851, 422–432. 10.1016/j.bbalip.2014.09.016 [DOI] [PubMed] [Google Scholar]
- Paviotti-Fischer E., Lopes-Torres E. J., Santos M. A. J., Brandolini S. V. P. B., Pinheiro J. (2018). Xiphidiocercariae from Naturally Infected Lymnaea columella (Mollusca, Gastropoda) in Urban Area: Morphology and Ultrastructure of the Larvae and Histological Changes in the Mollusc Host. Braz. J. Biol. 79, 446–451. 10.1590/1519-6984.182501 [DOI] [PubMed] [Google Scholar]
- Phuong N. N., Zalouk-Vergnoux A., Kamari A., Mouneyrac C., Amiard F., Poirier L., et al. (2017). Quantification and Characterization of Microplastics in Blue Mussels (Mytilus edulis): Protocol Setup and Preliminary Data on the Contamination of the French Atlantic Coast. Environ. Sci. Pollut. Res. 25 (7 25), 6135–6144. 10.1007/S11356-017-8862-3 [DOI] [PubMed] [Google Scholar]
- Piedade F., Bio S., Nunes B. (2020). Effects of Common Pharmaceutical Drugs (Paracetamol and Acetylsalicylic Acid) Short Term Exposure on Biomarkers of the Mussel Mytilus spp. Environ. Toxicol. Pharmacol. 73, 103276. 10.1016/j.etap.2019.103276 [DOI] [PubMed] [Google Scholar]
- Pires D., Grade A., Ruano F., Afonso F. (2022). Histopathologic Lesions in Bivalve Mollusks Found in Portugal: Etiology and Risk Factors. Jmse 2022 10, 133. 10.3390/JMSE10020133 [DOI] [Google Scholar]
- Rock M., Buntain B. J., Hatfield J. M., Hallgrímsson B. (2009). Animal-Human Connections, “one Health,” and the Syndemic Approach to Prevention. Soc. Sci. Med. 68, 991–995. 10.1016/J.SOCSCIMED.2008.12.047 [DOI] [PubMed] [Google Scholar]
- Rowley A. F. (1996). The Evolution of Inflammatory Mediators. Mediat. Inflamm. 5, 3–13. 10.1155/S0962935196000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo M., Albano M., Manganaro A., Capillo G., Spanò N., Savoca S. (2022). Spatiotemporal Organic Carbon Distribution in the Capo Peloro Lagoon (Sicily, Italy) in Relation to Environmentally Sustainable Approaches. Water 14, 108. 10.3390/w14010108 [DOI] [Google Scholar]
- Sauvey A., Denis F., Hégaret H., le Roy B., Lelong C., Jolly O., et al. (2021). Interactions Between Filter-Feeding Bivalves and Toxic Diatoms: Influence on the Feeding Behavior of Crassostrea gigas and Pecten maximus and on Toxin Production by Pseudo-Nitzschia. Toxins 2021, 13(8), 577. 10.3390/TOXINS13080577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoca S., Grifó G., Panarello G., Albano M., Giacobbe S., Capillo G., et al. (2020). Modelling Prey-Predator Interactions in Messina Beachrock Pools. Ecol. Model. 434, 109206. 10.1016/J.ECOLMODEL.2020.109206 [DOI] [Google Scholar]
- Savoca S., Matanović K., D'Angelo G., Vetri V., Anselmo S., Bottari T., et al. (2021). Ingestion of Plastic and Non-Plastic Microfibers by Farmed Gilthead Sea Bream (Sparus aurata) and Common Carp (Cyprinus carpio) at Different Life Stages. Sci. Total Environ. 782, 146851. 10.1016/J.SCITOTENV.2021.146851 [DOI] [Google Scholar]
- Sendra M., Sparaventi E., Novoa B., Figueras A. (2021). An Overview of the Internalization and Effects of Microplastics and Nanoplastics as Pollutants of Emerging Concern in Bivalves. Sci. Total Environ. 753, 142024. 10.1016/J.SCITOTENV.2020.142024 [DOI] [PubMed] [Google Scholar]
- Siddeswaran S., Umamaheswari S., Ramesh M. (2020). Toxicity Assessment of Acetylsalicylic Acid to a Freshwater Fish Cyprinus carpio: Haematological, Biochemical, Enzymological and Antioxidant Responses. Handb. Environ. Chem. 96, 191–215. 10.1007/698_2020_549 [DOI] [Google Scholar]
- Spanò N., di Paola D., Albano M., Manganaro A., Sanfilippo M., D’Iglio C., et al. (2021). Growth Performance and Bioremediation Potential of Gracilaria gracilis (Steentoft, L.M. Irvine & Farnham, 1995). Int. J. Environ. Stud. 1. 1–13. 10.1080/00207233.2021.1954775 [DOI] [Google Scholar]
- Stara A., Pagano M., Capillo G., Fabrello J., Sandova M., Vazzana I., et al. (2019). Assessing the Effects of Neonicotinoid Insecticide on the Bivalve Mollusc Mytilus galloprovincialis . Sci. Total Environ. 700, 134914. [DOI] [PubMed] [Google Scholar]
- Stara A., Pagano M., Capillo G., Fabrello J., Sandova M., Vazzana I., et al. (2020). Assessing the Effects of Neonicotinoid Insecticide on the Bivalve Mollusc Mytilus galloprovincialis . Sci. Total Environ. 700, 134914. 10.1016/j.scitotenv.2019.134914 [DOI] [PubMed] [Google Scholar]
- Stara A., Pagano M., Albano M., Savoca S., di Bella G., Albergamo A., et al. (2021). Effects of Long-Term Exposure of Mytilus galloprovincialis to Thiacloprid: A Multibiomarker Approach. Environ. Pollut. 289, 117892. 10.1016/J.ENVPOL.2021.117892 [DOI] [PubMed] [Google Scholar]
- Stollberg N., Kröger S. D., Reininghaus M., Forberger J., Witt G., Brenner M. (2021). Uptake and Absorption of Fluoranthene from Spiked Microplastics into the Digestive Gland Tissues of Blue Mussels, Mytilus edulis L. Chemosphere 279, 130480. 10.1016/J.CHEMOSPHERE.2021.130480 [DOI] [PubMed] [Google Scholar]
- Stuer-Lauridsen F., Birkved M., Hansen L. P., Holten Lützhøft H.-C., Halling-Sørensen B. (2000). Environmental Risk Assessment of Human Pharmaceuticals in Denmark After Normal Therapeutic Use. Chemosphere 40, 783–793. 10.1016/S0045-6535(99)00453-1 [DOI] [PubMed] [Google Scholar]
- Torre A., Trischitta F., Corsaro C., Mallamace D., Faggio C. (2013). Digestive Cells from Mytilus galloprovincialis show a Partial Regulatory Volume Decrease Following Acute Hypotonic Stress Through Mechanisms Involving Inorganic Ions. Cell Biochem. Funct. 31, 489–495. 10.1002/cbf.2925 [DOI] [PubMed] [Google Scholar]
- Usheva L. N., Frolova L. T. (2006). Morphofunctional Changes of the Digestive Gland in the Bivalve Mollusk Crenomytilus grayanus (Dunker, 1853) in Normal Conditions and after Parasitic Invasion by Trematodes. Russ. J. Mar. Biol. 32 (2 32), 96–105. 10.1134/S1063074006020040 [DOI] [Google Scholar]
- Viarengo A., Zanicchi G., Moore M. N., Orunesu M. (1981). Accumulation and Detoxication of Copper by the Mussel Mytilus galloprovincialis Lam: A Study of the Subcellular Distribution in the Digestive Gland Cells. Aquat. Toxicol. 1, 147–157. 10.1016/0166-445X(81)90011-4 [DOI] [Google Scholar]
- XueLi W., Hong G. (2016). A Review of Study on Bioaccumulation and Biomagnification of Persistent Organic Pollutants in Terrestrial Food Chain Using Modeling Method. J. Ecol. Rural Environ. 32, 531–538. [Google Scholar]
- Yasmeen S. (2019). Cadmium Induced Histopathological Alterations in Female Gonad of Freshwater Bivalve Mollusks, Lamellidens marginalis During Summer Season. Ijbi 01, 73–77. 10.46505/ijbi.2019.1207 [DOI] [Google Scholar]
- Yee-Duarte J. A., Ceballos-Vázquez B. P., Arellano-Martínez M., Camacho-Mondragón M. A., Uría-Galicia E. (2018). Histopathological Alterations in the Gonad of Megapitaria squalida (Mollusca: Bivalvia) Inhabiting a Heavy Metals Polluted Environment. J. Aquat. Anim. Health 30, 144–154. 10.1002/AAH.10015 [DOI] [PubMed] [Google Scholar]
- Zaccone G., Fudge D. S., Winegard T. M., Capillo G., Kuciel M., Funakoshi K. (2015). Confocal Imaging and Phylogenetic Considerations of the Subcutaneous Neurons in the Atlantic Hagfish Myxine glutinosa . Acta Zool. (Stockh) 96, 209–217. [Google Scholar]
- Zenker A., Cicero M. R., Prestinaci F., Bottoni P., Carere M. (2014). Bioaccumulation and Biomagnification Potential of Pharmaceuticals with a Focus to the Aquatic Environment. J. Environ. Manag. 133, 378–387. 10.1016/J.JENVMAN.2013.12.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.