Abstract
Bacterial spores are being consumed as probiotics, although little is known about their efficacy or mode of action. As a first step in characterizing spore probiotics, we have studied the persistence and dissemination of Bacillus subtilis spores given orally to mice. Our results have shown that spores do not appear to disseminate across the mucosal surfaces. However, we found that the number of spores excreted in the feces of mice was, in some experiments, larger than the original inoculum. This was an intriguing result and might be explained by germination of a proportion of the spore inoculum in the intestinal tract, followed by limited rounds of cell growth and then sporulation again. This result raises the interesting question of whether it is the spore or the germinated spore that contributes to the probiotic effect of bacterial spores.
The gram-positive soil microorganism Bacillus subtilis has been studied extensively, primarily as a model with which to study cell differentiation and for exploitation in the biotechnology industry. While some Bacillus species are pathogenic (e.g., B. anthracis and some B. cereus strains), B. subtilis has, at most, been associated with opportunistic infections of immunocompromised patients (6, 11, 17). For these reasons, it has received relatively little clinical interest. Bacillus spores, though, are currently available as probiotics and as competitive exclusion agents (CE agents). Probiotics are live bacterial supplements which can enhance the normal intestinal flora, while CE agents are bacteria which can suppress infection and may contain undefined mixtures of more than one bacterial species (7, 8, 19). Ingestion of significant quantities of spores is thought to restore the normal microbial flora following extensive antibiotic usage or illness (13). How this occurs is unclear but could include competitive exclusion of pathogens, whether by immunostimulation or competition for adhesion sites. Spore probiotics are primarily used by humans as an over-the-counter supplement for oral bacteriotherapy and bacterioprophylaxis of mild gastrointestinal disorders, many of which lead to diarrhea (13). In the livestock and poultry industries, probiotics containing Bacillus spores are used extensively; an example is Biogrow (Provita Eurotech Ltd., County Tyrone, Northern Ireland), which contains a mixture of B. subtilis and B. licheniformis spores. With the recent ban on the use of antibiotics as growth promoters in Denmark, the use of probiotics or CE agents as antibiotic alternatives seems likely to increase.
The validity of spores as probiotics or CE agents was recently demonstrated by showing that oral inoculation of 1-day-old chicks with 2.5 × 108 B. subtilis spores suppressed all aspects of infection when chicks were challenged with Escherichia coli 078:K80 (12). One dogma regarding the use of spores as probiotics or CE agents is their mode of action, which presumably must be substantially different from that of the other, better known bacterial supplements, such as Lactobacillus spp., which exist only in the vegetative state. As part of a study to investigate the mode of action of spore probiotics, we have characterized a number of commercially available products for human use (9, 10). Surprisingly, almost all of these were found to carry mislabeled species, raising serious questions about the regulatory procedures in force to control the use of probiotics or CE agents. In this work we have addressed the question of what happens to spores taken orally. Using a murine model, we show that spores do not disseminate in significant numbers beyond the gastrointestinal tract. However, we show that spores can persist in the gastrointestinal tract and may germinate despite the anaerobic environment.
MATERIALS AND METHODS
Preparation of spores.
Spores were prepared from large (200-ml) cultures as described previously (15) using strain SC1712, which carries cat and erm insertions, encoding resistance to chloramphenicol and erythromycin, respectively (note that neither insertion interfered with cell growth or spore formation). Strain SC1712 was derived from prototrophic, wild-type (Spo+) strain PY79 of B. subtilis (20). The spore suspensions were then heated at 65°C for 1 h to kill any residual, nonsporulated cells, and aliquots (0.5 ml) were frozen at −80°C. The number of CFU per milliliter of frozen aliquots was determined and, if necessary, spore suspensions were concentrated further on the day of use (by centrifugation) to produce 108 to 109 CFU/ml.
Fecal counts.
For experiment 1, outbred mice (female, 6 to 8 weeks old) were obtained from the Pasteur Institute, Ho Chi Minh City, Vietnam. For the remaining experiments, pathogen-free female BALB/c mice (6 to 8 weeks old) were purchased from Harlan UK (Oxon, United Kingdom).
Spores (0.2 ml of spore suspension) were administered by intragastric gavage to mice anesthetized by inhalation of halothane. Animals were housed individually in cages with gridded floors. Total feces were collected at various time points and processed immediately for experiment 1 (see below); however, for experiments 2 and 3, total fecal collections were frozen at −80°C until the time of processing. To determine the number of spores in feces, samples were suspended in 10 to 30 ml of phosphate-buffered saline (PBS) at 65°C. Sterile glass beads (2 mm; 3 ml) were added, and the suspension was incubated at 65°C for 1 h with frequent vortexing until there was little remaining residual solid matter. Serial dilutions were then made with PBS (65°C), plated on Difco sporulation medium plates containing chloramphenicol (5 μg/ml) and erythromycin (1 μg/ml), and incubated at 37°C for 2 days. B. subtilis colonies of strain SC1712 were identified by their colony morphology. Spore counts were extrapolated for the total weight of feces collected. In experiment 1, to determine the total number of B. subtilis viable units, including spores and vegetative cells, the same procedure was used, except that feces were suspended in PBS at room temperature and no heat treatment was applied.
Dissemination experiments.
Groups of four mice (6-week-old female BALB/c; Harlan UK) were dosed orally (0.2 ml) with purified spore suspensions (1.83 × 108/ml). As controls, we inoculated groups of four mice with PBS alone. Groups of mice were sacrificed at 12, 86, and 158 h and dissected for selected organs and tissues. Organs were weighed by difference, rinsed with 2 ml of PBS, suspended in PBS (1 ml), and homogenized with glass beads (1 mm) using a Beadbeator (Biospec Inc.) with 10-s cycles and intermittent cooling on ice (10 s) until tissues were completely broken. Homogenized suspensions were heat treated (65°C, 45 min), serially diluted in PBS, and plated for determination of CFU per milliliter. Heat treatment was used to reveal the number of spores present in organs or tissues.
RESULTS AND DISCUSSION
Fate of spores in mice inoculated orally.
As an indicator of the fate and longevity of spores administered orally, we gave a fixed dose of spores of a multiply drug-resistant spo+ strain, SC1712 (Cmr Ermr), to mice and measured the number of spores excreted or, in experiment 1, the number of spores and vegetative cells excreted. In experiment 1 we used outbred mice, and in experiments 2 and 3 we used inbred BALB/c mice. In experiment 1 a large inoculum, 1.75 × 1011 spores, was given, while in experiments 2 and 3, lower doses, 5.97 × 108 and 2.0 × 109 spores, respectively, were given.
Our results from dosing of outbred mice (Table 1) showed that B. subtilis viable units were first detectable 3 h after inoculation; the majority of counts occurred within the first 24 h, although B. subtilis was still detectable in the 4-day (96-h) samples.
TABLE 1.
B. subtilis counts in feces from outbred mice orally inoculated with 1.75 × 1011 spores in experiment 1
| Time (h) | Countsa in mouse:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
4
|
5
|
||||||
| VC + SP | SP | VC + SP | SP | VC + SP | SP | VC + SP | SP | VC + SP | SP | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 3.0 × 102 | 0 | 0 | 4.5 × 103 | 4.5 × 103 | 0 | 1.3 × 103 | 6.1 × 102 | 3.1 × 102 |
| 6 | 3.8 × 106 | 5.8 × 108 | 1.1 × 106 | 3.6 × 105 | 7.2 × 105 | 4.2 × 109 | 1.1 × 108 | 4.0 × 109 | 1.2 × 106 | 4.6 × 109 |
| 9 | 3.9 × 107 | 6.0 × 1010 | 3.0 × 107 | 8.3 × 1010 | 1.2 × 107 | 1.3 × 109 | 1.0 × 109 | 1.5 × 1010 | 4.3 × 107 | 4.0 × 1010 |
| 12 | 9.3 × 107 | 6.5 × 1010 | 1.5 × 107 | 4.2 × 109 | 9.0 × 107 | 3.9 × 108 | 1.9 × 107 | 6.0 × 1011 | 3.3 × 107 | 4.2 × 1011 |
| 24 | 1.1 × 109 | 9.4 × 103 | 5.0 × 104 | 1.4 × 108 | 1.0 × 107 | 6.9 × 109 | 1.3 × 105 | 9.3 × 109 | 5.3 × 104 | 7.4 × 109 |
| 48 | 3.9 × 105 | 1.7 × 105 | 6.8 × 105 | 2.3 × 104 | 9.5 × 107 | 3.7 × 1010 | 1.8 × 105 | 2.6 × 105 | 1.3 × 105 | 3.1 × 105 |
| 72 | 6.7 × 105 | 3.3 × 104 | 0 | 0 | 2.4 × 105 | 7.0 × 105 | 3.8 × 105 | 2.3 × 1 | ||
| 05 | 6.0 × 104 | 1.0 × 105 | ||||||||
| 96 | 8.7 × 102 | 1.3 × 103 | 0 | 0 | 1.4 × 103 | 1.7 × 103 | 0 | 0 | 0 | 7.2 × 102 |
| Total | 1.24 × 109 | 1.26 × 1011 | 4.68 × 107 | 8.73 × 1010 | 2.08 × 108 | 4.98 × 1010 | 1.13 × 109 | 6.28 × 1011 | 7.74 × 107 | 4.72 × 1011 |
| Ratio relative to inoculum | 0.0071 | 0.72 | 0.00027 | 0.50 | 0.0012 | 0.28 | 0.0065 | 3.59 | 0.00044 | 2.70 |
Counts of B. subtilis SC1712 obtained from fecal collection experiments. VC + SP, number of vegetative cells plus number of spores in total sample collected; SP, number of spores in sample collected. In the same experiment, feces from control groups of four mice were examined at the same time points, and no counts for B. subtilis SC1712 (VC + SP or SP) were detected (data not shown for control groups).
These results revealed two apparent paradoxes. First, the count for spores and vegetative cells was almost always lower than the count for spores alone. Since heat-treated and untreated feces were always prepared at the same time, this result was intriguing. This phenomenon has been observed previously (10), and it has been postulated that the heat treatment used to process spores actually “activates” the spores and so enhances their germination and outgrowth. This notion is consistent with established studies showing that “heat activation” is a prerequisite for efficient and synchronous germination (4). In the untreated suspensions then, a substantial number of spores (up to 2 log units) are unable to germinate and therefore produce an apparently lower count. Thus, the count for spores more accurately reflects the actual, or real, number of viable units.
We found from preliminary trials that without heat treatment, a substantial number of different bacterial species (up to five in outbred mice) were able to grow on agar plates containing two antibiotics (chloramphenicol and erythromycin). Despite this contamination, we could discern B. subtilis SC1712 simply from its colony morphology (and presence in high numbers at high serial dilutions). Heat treatment, though, reduced the background almost completely and, in fact, virtually no other bacterial species (i.e., spore formers) were detectable in fecal samples.
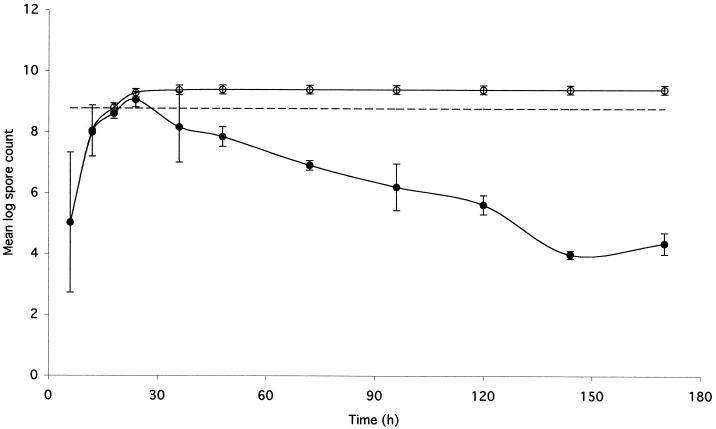
The second intriguing observation was that for two mice (mice 4 and 5), the total accumulated number of spores excreted over 96 h was higher (more than three times) than the original inoculum. One straightforward explanation is, of course, experimental error, although as discussed below this seems unlikely. The other possibility is that the counts were in fact real, in which case the only plausible explanation is that a proportion of the spores had germinated, undergone one or more rounds of growth and replication, and then formed spores. This explanation would at first seem most unlikely, but before coming to any firm conclusions, we repeated this experiment with inbred BALB/c mice. Two of these experiments are shown in Tables 2 and 3. In these experiments, we used smaller inocula of spores (5.97 × 108 and 2.0 × 109) but maintained sampling for 7 days. In experiment 2 (Table 2 and Fig. 1), the results showed that with each mouse the cumulative number of spores excreted was larger (by a factor of 4.40 ± 1.53 [mean and standard error]) than the initial inoculum. Strikingly, considerable numbers of spores were still being detected in the feces on day 7. In experiment 3 (Table 3), we did not observe an increase in the number of spores excreted (0.41 ± 0.18), yet we did observe substantial numbers of spores in the feces on day 7 of sampling.
TABLE 2.
B. subtilis counts in feces from inbred mice orally inoculated with 5.97 × 108 spores of SC1712 in experiment 2
| Time (h) | Countsa in mouse:
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 6 | 1.15 × 105 | 1.85 × 108 | 1.75 × 103 | 3.55 × 103 |
| 12 | 6.5 × 106 | 3.0 × 108 | 1.7 × 108 | 2.7 × 108 |
| 18 | 3.85 × 108 | 2.4 × 108 | 6.04 × 108 | 4.69 × 108 |
| 24 | 2.6 × 109 | 1.18 × 109 | 7.3 × 108 | 8.3 × 108 |
| 36 | 8.0 × 108 | 3.55 × 106 | 1.2 × 109 | 1.34 × 108 |
| 48 | 6.72 × 107 | 1.04 × 108 | 2.58 × 107 | 1.43 × 108 |
| 72 | 8.83 × 106 | 1.12 × 107 | 4.91 × 106 | 9.54 × 106 |
| 96 | 5.93 × 106 | 3.79 × 105 | 3.22 × 105 | 8.99 × 106 |
| 120 | 7.14 × 105 | 8.62 × 105 | 2.12 × 105 | 2.38 × 105 |
| 144 | 1.21 × 104 | 1.27 × 104 | 6.9 × 103 | 8.3 × 103 |
| 170 | 6.6 × 104 | 1.47 × 104 | 2.48 × 104 | 1.01 × 104 |
| Total | 3.87 × 109 | 2.02 × 109 | 2.73 × 109 | 1.86 × 109 |
| Ratio relative to inoculum | 6.49 | 3.39 | 4.58 | 3.12 |
Pathogen-free female BALB/c mice (6 to 8 weeks old) were used. Only the count for spores is shown. In the same experiment, feces from control groups of four mice were examined at the same time points, and no counts for B. subtilis SC1712 were detected (data not shown for control groups).
TABLE 3.
B. subtilis counts in feces from inbred mice orally inoculated with 2.0 × 109 spores of strain SC1712 in experiment 3
| Time (h) | Countsa in mouse:
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 24 | 1.03 × 109 | 8.40 × 108 | 1.09 × 109 | 1.86 × 108 | 3.93 × 108 | 8.40 × 108 |
| 48 | 1.92 × 107 | 1.18 × 107 | 3.54 × 106 | 4.32 × 106 | 3.12 × 107 | 5.31 × 107 |
| 72 | 1.56 × 107 | 8.16 × 104 | 8.20 × 107 | 1.80 × 105 | 2.40 × 105 | 1.33 × 107 |
| 96 | 4.14 × 105 | 1.16 × 105 | 2.93 × 107 | 3.00 × 104 | 2.20 × 107 | 9.00t× 103 |
| 120 | 6.00 × 105 | 4.53 × 105 | 8.94 × 105 | 3.00 × 103 | 1.20 × 104 | 5.70 × 104 |
| 144 | 5.70 × 104 | 9.25 × 104 | 6.00 × 103 | 1.84 × 104 | 1.20 × 104 | 1.20 × 104 |
| 168 | 3.60 × 105 | 2.40 × 104 | 6.30 × 106 | 2.82 × 104 | 2.50 × 104 | 1.40 × 104 |
| Total | 1.06 × 109 | 8.52 × 108 | 1.21 × 109 | 1.91 × 108 | 7.27 × 108 | 9.06 × 108 |
| Ratio relative to inoculum | 0.53 | 0.43 | 0.61 | 0.09 | 0.36 | 0.45 |
Pathogen-free female BALB/c mice (6 to 8 weeks old) were used. Only the count for spores is shown. In the same experiment, feces from control groups of two mice were examined at the same time points, and no counts for B. subtilis SC1712 were detected (data not shown for control groups).
FIG. 1.
Excretion of spores in feces of mice immunized orally. Shown is a graphic presentation of the data from experiment 2 (Table 2) to evaluate the fate and longevity of spores following oral inoculation of inbred mice. Shown are the cumulative spore counts (○) and the temporal spore counts (●) obtained from feces collected at the indicated time points after mice were immunized with purified spore suspensions. Spore counts are averages for the four mice shown in Table 2. Standard error bars are shown. The broken line shows the number of spores inoculated (5.97 × 108).
Since both experiments 2 and 3 were done with inbred mice, we performed a simple statistical analysis. Analysis of variance indicated that the results of experiments 2 and 3 were significantly different (P < 0.01). For experiment 1, where we used outbred mice, we found that for some mice we observed an increase in spore counts (mice 4 and 5) and for others we observed a decrease (mice 1, 2, and 3). When mice 1, 2, and 3 from experiment 1 were considered to be one group, the average ratio of spore counts to inoculum was 0.50 ± 0.216. In a comparison with the data of experiment 3 (where spore counts also decreased), a t test revealed a P value of >0.10, demonstrating that there was no significant difference in these data. Similarly, for mice 4 and 5 of experiment 1, a comparison of the average ratio of spore counts to inoculum (3.14 ± 0.63) with the data of experiment 2 (where an increase in spore counts also was observed) revealed a P value of >0.10, again showing no significant difference between spore count data. While the sample sizes are small, our analyses appear to show that the results obtained are not dependent upon the mouse genetic background.
A number of factors could severely limit the accuracy of these experiments. First, between fecal collections, excreted spores might be able to germinate in the feces and undergo successive rounds of growth and division. Our experimental procedure for the detection of spores used a high temperature (65°C; 60 min) to homogenize fecal matter; this temperature is sufficient to kill all vegetative cells. Thus, if spores had germinated, then they would have had to resporulate (an 8-h process) in order to survive the fecal homogenization. We examined 12-h fecal samples containing spores. Spore counts were determined with a portion of this sample, after which the feces were stored at room temperature for 12 and 24 h and spore counts were determined. We found that the spore counts were unchanged (data not shown); therefore, the spores do not appear to germinate in feces in detectable numbers.
A second factor that could introduce error was the fecal extraction procedure itself. Complete homogenization of feces was sometimes difficult to obtain, so it is possible that the actual spore counts were higher than those obtained here but not lower. Another factor which would decrease, but not increase, spore counts is fluid loss during intragastric inoculation.
Our interpretation which explains the increased spore counts in experiments 1 and 2 is that a proportion of spores germinate in the gut. We base this explanation on the following: (i) the total number of spores excreted is, on average, larger than that inoculated; (ii) substantial numbers of spores are still present in the feces 7 days after the initial inoculation, when we might otherwise expect to see complete clearance; (iii) there is a gradual decline in spore counts over time; and (iv) if experimental error were attributed to this procedure at each step, then the spore counts should be dramatically decreased.
We have repeated our experiments using inbred mice in their entirety several times and have found that an increase in spore counts is observed on some occasions but not on others. What is important, though, is that an increase is observed. We were initially skeptical of these results as well as perplexed but have come to the conclusion that on some occasions, spores can germinate and then resporulate. That spores do not always germinate in measurable amounts may in some way reflect the constitution of the mouse, that is, the physiological conditions of the gastrointestinal tract.
We are now attempting, using the molecular technique of reverse transcription-PCR, to show definitively that the spores germinate. However, in the absence of molecular results, is there any work that supports the data we have presented? First, and foremost, there is the assumption that B. subtilis is a strict aerobe. However, given glucose and nitrate as a terminal electron acceptor, B. subtilis has been shown to grow anaerobically (14). It seems unlikely that spores could germinate in the acidic conditions of the stomach; however, the upper intestinal tract, being rich in nutrients, might reasonably be expected to allow spore germination. Ligated loop studies have shown that spores can germinate in the intestine (13). Moreover, studies on the fate of B. natto, the probiotic agent contained in the Japanese product Natto, have shown that the number of B. natto CFU recovered from the feces of animals inoculated with this organism was between 246 and 1,133% larger than the original inoculum (13). These studies with weaned piglets showed that spores could germinate and multiply to a limited degree in the upper intestinal tract. If intestinal colonization occurs, then it must be very limited, since most of the inoculum had transited within 24 h. What is interesting from our studies is that 103 to 104 spores are still detectable 7 days after inoculation. If spores can germinate to some limited degree and then undergo limited rounds of replication, then these processes may account for the continued excretion 7 days later, although these cells must have formed spores.
Spore formation in B. subtilis is well studied and occurs primarily as a response to nutrient depletion (5, 16). The decision to sporulate requires other inputs, such as the cell having reached the correct stage in replication and pheromonal information from other B. subtilis cells. As such, many cells will not normally sporulate, although in the laboratory sporulation efficiencies can reach 80 to 90%. In the absence of entering the sporulation life cycle, the vegetative cell will either lyse or transit the gastrointestinal tract. Indeed, recent work has shown B. subtilis to be sensitive to bile salts in the gastrointestinal tracts of mice (18). From our results, we can only infer that spore formation is occurring. When the spore germinates in a nutrient-rich environment, it will be quickly translocated to a more hostile environment, where spore formation may be a reasonable strategy for survival. Interestingly, other spore-forming organisms are found in the intestinal tract (2), and spore formation has been shown to initiate in the ileum as part of the life cycle of the gram-positive bacterium Metabacterium polyspora in guinea pigs (1).
Dissemination of spores.
Although B. subtilis is considered nonpathogenic, it is important to address the ethical issues of potential spore dissemination across the mucosal epithelium, since spores are currently being consumed as probiotics. Accordingly, we administered spores (SC1712; 1.83 × 108 CFU/ml) to groups of 12 mice by intragastric gavage (4 mice were also inoculated with PBS as controls; data not shown). Mice were housed in groups of four in cages with gridded floors to prevent coprophagia. Four mice from each group were sacrificed at 12 h and at 3.5 and 7.5 days. Selected organs and tissues were dissected, and spore counts were determined from homogenized sections as described previously (3). As observed earlier, we found from trial experiments that we could not determine the total number of B. subtilis viable units, so we heat treated homogenized sections and counted spores only. Average spore counts for each group of four mice are shown in Table 4. We found that oral immunization produced extremely low levels of dissemination, other than in the lungs and gut, a result which can be accounted for by the inoculation procedure. It should be noted that the numbers of spores detected in the mesenteric lymph nodes, liver, and spleen must be considered insignificant, since previous work has shown that approximately 50 viable units is the limit of accurate quantification for these organs (3).
TABLE 4.
Dissemination of sporesa
| Organ | Counts at the indicated time (h):
|
||
|---|---|---|---|
| 12 | 84 | 180 | |
| Blood | 1 | 1 | 1 |
| Lungs | 62 | 2 | 5 |
| Liver | 11 | 12 | 7 |
| Spleen | 25 | 1 | 1 |
| Kidneys | 1 | 6 | 6 |
| Mesenteric lymph nodes | 37 | 1 | 1 |
| Stomach | 2.4 × 105 | 41 | 33 |
| Duodenum | 3.2 × 105 | 105 | 92 |
| Jejunum | 2.7 × 105 | 524 | 39 |
| Ileum | 4.2 × 104 | 312 | 18 |
| Cecum | 2.5 × 106 | 5.9 × 103 | 371 |
| Colon | 1.9 × 106 | 6.6 × 103 | 251 |
Groups of four mice (6 weeks old, female BALB/c) were inoculated by intragastric gavage with purified spore suspensions (0.2 ml; 1.83 × 108/ml). Organs were dissected, homogenized, and heat treated, and bacterial spore counts were determined as described in Materials and Methods. As controls, we inoculated groups of four mice with PBS (data not shown).
These results show that spores do not appear to disseminate substantially following oral dosing, consistent with the results of other studies (18). Moreover, the persistence that we observed from analyses of feces suggests that B. subtilis is a transient resident of the gut. Although our procedure measured only viable spores, it is possible that inactive or killed spores were present in selected organs. Similarly, if spores did germinate, then we could not account for the dissemination of vegetative cells.
In conclusion, our results are important because they address the dogma of how spore probiotics function. Although the mode of action remains unclear, we have presented evidence that spores may germinate in the gut. As such, they may function in the same way as other probiotic bacteria, such as the lactobacilli, and possibly exert their probiotic action by a metabolic effect following spore germination. Alternatively, germination of spores may simply be an incidental feature of the spore entering a nutrient-rich environment, and the mode of action may be unique to the spore state.
ACKNOWLEDGMENTS
This work was supported by grants from the Wellcome Trust (to S.M.C.) and the European Union (to S.M.C. and E.R.).
We thank Pham Thi Nguyen Thuy, Gabriella Casula, and Dana Cohen for assistance in this project.
REFERENCES
- 1.Angert E R, Losick R M. Propagation by sporulation in the guinea pig symbiont Metabacterium polyspora. Proc Natl Acad Sci USA. 1998;95:10218–10223. doi: 10.1073/pnas.95.17.10218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benno Y, Endo K, Mizutani T, Namba Y, Komori T, Mitsuoka T. Comparison of fecal microflora of elderly persons in rural and urban areas of Japan. Appl Environ Microbiol. 1989;55:1100–1105. doi: 10.1128/aem.55.5.1100-1105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter P H, Collins F M. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dring G J, Gould G W. Sequence of events during rapid germination of spores of Bacillus subtilis. J Gen Microbiol. 1971;65:101–104. doi: 10.1099/00221287-65-1-101. [DOI] [PubMed] [Google Scholar]
- 5.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farrar W E. Serious infections due to “non-pathogenic”organisms of the genus Bacillus. Am J Med. 1963;34:134–141. doi: 10.1016/0002-9343(63)90047-0. [DOI] [PubMed] [Google Scholar]
- 7.Fuller R. Probiotics in human medicine. Gut. 1991;32:439–442. doi: 10.1136/gut.32.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 9.Green D H, Wakeley P R, Page A, Barnes A, Baccigalupi L, Ricca E, Cutting S M. Characterization of two Bacillus probiotics. Appl Environ Microbiol. 1999;65:4288–4291. doi: 10.1128/aem.65.9.4288-4291.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoa N T, Baccigalupi L, Huxham A, Smertenko A, Van P H, Ammendola S, Ricca E, Cutting S M. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol. 2000;66:5241–5247. doi: 10.1128/aem.66.12.5241-5247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihde D C, Armstrong D. Clinical spectrum of infection due to Bacillus species. Am J Med. 1973;55:839–845. doi: 10.1016/0002-9343(73)90266-0. [DOI] [PubMed] [Google Scholar]
- 12.La Ragione R M, Casula G, Cutting S M, Woodward M J. Bacillus subtilis spores competitively exclude Escherichia coli 078:K80 in poultry. Vet Microbiol. 2001;79:133–142. doi: 10.1016/s0378-1135(00)00350-3. [DOI] [PubMed] [Google Scholar]
- 13.Mazza P. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll Chim Farm. 1994;133:3–18. [PubMed] [Google Scholar]
- 14.Nakano M M, Zuber P. Anaerobic growth of a “strict aerobe” (Bacillus subtilis) Annu Rev Microbiol. 1998;52:165–190. doi: 10.1146/annurev.micro.52.1.165. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1990. pp. 391–450. [Google Scholar]
- 16.Piggot P J, Coote J G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976;40:908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reller L B. Endocarditis caused by Bacillus subtilis. Am J Clin Pathol. 1973;60:714–718. doi: 10.1093/ajcp/60.5.714. [DOI] [PubMed] [Google Scholar]
- 18.Spinosa M R, Braccini T, Ricca E, De Felice M, Morelli L, Pozzi G, Oggioni M R. On the fate of ingested Bacillus spores. Res Microbiol. 2000;151:361–368. doi: 10.1016/s0923-2508(00)00159-5. [DOI] [PubMed] [Google Scholar]
- 19.Tannock G W, editor. Probiotics: a critical review. Norfolk, United Kingdom: Horizon Scientific Press; 1999. [Google Scholar]
- 20.Youngman P, Perkins J, Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984;12:1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]