Abstract
Background and Aims
Abnormalities in hematological and biochemical markers are assumed to be associated with the progression of COVID‐19 disease. This meta‐analysis was performed to assess the consequences of abnormalities of biomarkers (D‐dimers, C‐reactive protein [CRP], serum ferritin, lactate dehydrogenase [LDH], random blood sugar [RBS], absolute neutrophil count [ANC], neutrophil to lymphocyte ratio (NLR), serum creatinine, and hemoglobin) in the Bangladeshi COVID‐19 patients.
Methods
The data of biomarker levels in Bangladeshi COVID‐19 patients were gathered from five databases: PubMed, ScienceDirect, Web of Science, Google Scholar and Bangladesh Journals Online between January 2020 to March 2022. Review Manager 5.4 was used for the meta‐analysis, and Egger's test and Begg‐Mazumdar's rank correlation were used to investigate publication bias.
Results
This study included 1542 patients with 567 severe and 975 nonsevere statuses. Based on the accumulated data synthesis, there is a strong correlation between disease severity and different biomarkers, including D‐dimer, CRP, ferritin, LDH, RBS, NLR, and serum creatinine (MD = 1.16, p = 0.0004; MD = 22.97, p = 0.003; MD = 419.26, p < 0.00001; MD = 118.37, p = 0.004; MD = 1.96, p = 0.02; MD = 1.26, p = 0.02; and MD = 0.31, p = 0.008, respectively). A significantly decreased correlation was observed for hemoglobin levels in severe COVID‐19 patients (MD = −0.73, p = 0.10).
Conclusion
The elevated biomarkers level was noticed in severe cases compared to nonsevere patients, revealing that D‐dimer, CRP, ferritin, LDH, RBS, NLR, and serum creatinine are significantly correlated to COVID‐19 severity. Only lower hemoglobin level was found to be associated with COVID‐19 severity.
Keywords: COVID‐19, creatinine, CRP, D‐dimer, meta‐analysis
1. INTRODUCTION
The novel coronavirus (2019‐nCoV) or severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) first appeared in Wuhan, Hubei Province, China, at the end of 2019. Because of its pervasive perspective and virulent impacts, the World Health Organization (WHO) announced a global virus outbreak on March 11, 2020. 1 An estimated 486,974,361 COVID‐19 cases and 6,166,935 deaths have been reported by March 30, 2022, throughout the globe. 2 In Bangladesh, about 1,951,504 cases have been identified, and 29,122 patients have died due to COVID‐19 infection till March 30, 2022. 2 By attaching to the angiotensin‐converting enzyme 2 receptor, this virus is hypothesized to enter the respiratory system via contaminated droplets or mucosal contact. 3 Even though most COVID‐19 sufferers are asymptomatic, some individuals die from severe pneumonia, acute respiratory distress syndrome (ARDS), and multiorgan failure due to COVID‐19 infection. 4 , 5 According to existing research, SARS‐CoV‐2 predominantly infects the upper respiratory system before progressing to the lower airways. 6 The severity of COVID‐19 has been reported to be correlated with older age, being male and comorbidities such as diabetes, abnormally high blood pressure, adiposity, chronic respiratory illnesses, cardiovascular disease, and cancer. 7
Coronavirus is an RNA virus that undergoes rapid mutation and forms new varieties. 8 , 9 Moreover, WHO enlist delta and omicron as currently circulating variants of concern. 10 , 11 However, the vaccination rate has a direct and significant impact on the status of the COVID‐19 epidemic and the ability of health systems to confine it. 12 , 13 , 14 Even though the licensed COVID‐19 vaccine has been demonstrated to be safe and effective, mass vaccination in Bangladesh remains a problem due to the awareness, emotions, and beliefs of the population about the COVID‐19 vaccine and vaccination implementation. 12 , 13 , 14
When evaluating an individual with COVID‐19 infection, biomarkers may aid in the initiation of treatment and continuous surveillance. While biomarkers can improve prediction and outcomes, high diversity in their concentration across individuals may impact the study findings. 15 Several studies have linked higher levels of proinflammatory cytokines in serum with pulmonary inflammation and significant lung damage in SARS and MERS coronavirus infections. 16 , 17 , 18 Numerous research revealed that inflammatory biomarkers such as C‐reactive protein (CRP) and D‐ dimer levels are comparatively predominant in intensive care unit patients than in mild and moderate individuals infected with COVID‐19. 15 , 19 , 20
The flu‐like symptoms of COVID‐19 infection are often followed by changes in the hemogram and inflammatory markers. 21 The level of the inflammatory marker CRP can be utilized to make an early diagnosis of pneumonia. 22 Patients who presented with severe pneumonia had high levels of CRP. 23 D‐dimer levels have been linked to the severity of community‐acquired pneumonia as well as the patient's prognosis. 24 Hemogram‐derived NLR is an emerging inflammatory marker that has been linked to a variety of diseases, including thyroid inflammation, 25 Hashimoto's disease, 26 Irritable Bowel Syndrome, 27 and even COVID‐19 infection. 28 Ferritin as a molecule of signaling and immune system mediator. Several medical disorders, as well as a poorer prognosis for critically ill individuals, are linked to hyperferritinemia. Autopsy results and the clinical progression of some COVID‐19 patients have shown a possible link between COVID‐19 and “Hyperferritinemic Syndrome.” 29 High levels of ferritin have been linked to both an increased risk of developing ARDS and an increased mortality risk. 30 Hemoglobin and red blood cell distribution width were found to be good indicators of recurrent hospitalizations during outbreaks, according to a recent. 31 In the COVID‐19 era, hemogram markers are considered indicative of frail patients. 32 Moreover, CRP is an independent risk factor for comorbid disease like metabolic and autoimmune disorders including autoimmune thyroiditis, 33 stroke, 34 , 35 rheumatoid arthritis, 36 type 2 diabetes, 37 sensory impairments, 38 and cerebrovascular disease. 39 D‐dimer is associated with stroke, 34 , 35 diabetic kidney disease, 40 lung disease, 41 , 42 and neurological deficits. 43 High level of ferritin is also associated with diabetes, 44 , 45 , 46 coronary heart disease, 47 cerebrovascular disease. 48 , 49
The purpose of this meta‐analysis is to highlight the correlation of numerous biomarkers, including D‐dimers, CRP, serum ferritin, lactate dehydrogenase (LDH), random blood sugar (RBS), absolute neutrophil count (ANC), neutrophil to lymphocyte ratio (NLR), serum creatinine, and hemoglobin with the severity of COVID‐19 patients in Bangladesh and how their values change based on the intensity of the illness.
2. METHODS
2.1. Literature search strategy and screening
The current meta‐analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses principles. 50 To find relevant published research, we carefully searched four worldwide databases (PubMed, Google Scholar, ScienceDirect, and Web of Science) and one national database (Bangladesh Journals Online) up to March 30, 2022. For excluding identical research, EndNote X 7.0 software was used. During the search, the following keywords were used alone or in combination: “COVID‐19,” “Bangladesh,” “Bangladeshi,” “biological marker,” “CRP,” “D‐Dimer,” “Ferritin,” “LDS,” “RBH,” “ANS,” “NLR,” “serum creatinine,” “hemoglobin level,” “biomarker,” “hematological abnormalities,” “hematological biomarker,” “biochemical markers,” and “Hematological findings.” This meta‐analysis included only Bangladeshi studies. To identify missing research, we evaluated the reference lists of the included publications.
2.2. Eligibility criteria
To be eligible for inclusion, publications must comply with the following requirements:
(1) Research articles published in English peer‐reviewed journal; (2) Studies containing only confirmed COVID‐19 infection in Bangladesh; (3) Cohort and case‐control investigations; (4) Using human research subjects; (5) Data sufficient to compute the MD and 95% confidence interval (CI).
The following are the exclusion criteria:
(1) Research published in languages other than English; (2) Studies published in other populations than Bangladesh; (3) Commentary from experts, editorials, conference abstracts, reviews, and letters; (4) Irrelevant to extracting data; (5) Investigations based on animal data; (6) Overlapping or same publications.
2.3. Data extraction
Three researchers (KKB, MAB, and MAA) independently used eligibility requirements to retrieve data. Independently, they searched for publications, appraised them, and extracted data into an excel file. Another researcher (MSI) resolved any study disagreements that emerged throughout the process. The studies used Rayyan QCRI, a systematic review online software. 51 The authors' names, year of production, number of participants, gender, age, biomarker level, and hematological results of severe and nonsevere cases were retrieved from the related articles.
2.4. Methodological quality assessment
The Newcastle‐Ottawa Scale (NOS) was used to evaluate the qualitative characteristics of prospective observational studies. 52 Any dissension among the investigators was resolved through conversation.
2.5. Statistical analysis
The analysis was conducted using Review Manager 5.4 (The Cochrane Collaboration, Oxford, United Kingdom). Review Manager 5.3 was also used to examine the heterogeneity (χ 2 and I 2). Statistically, significant heterogeneity was defined as p < 0.1 or I 2 > 50%. High heterogeneity was characterized by an I 2 value of 75% and low heterogeneity by 25%. In addition, Egger's regression test and Begg‐Mazumdar's rank correlation were utilized to examine publication biases. The significance level was set at p ≤ 0.05, and while the findings were bigger than predicted, there was no evidence of publication bias.
3. RESULTS
3.1. Study selection, characteristics, and quality assessment
During the first retrieval, 583 publications were detected from five databases (PubMed, Google Scholar, ScienceDirect, Web of Science and Bangladesh Journals Online); however, 213 entries were discarded due to the repetition. After scanning the title and abstract, 257 articles were deleted, and 85 were rejected from the remainder of 113 articles on a different basis. The rigorous assessment and inclusion criteria included eight full‐text papers 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 encompassing 1542 COVID‐19 cases (567 severe and 975 nonsevere) in this meta‐analysis. The smallest sample size was 99, while the highest was 350. Figure 1 depicts the procedure of conducting the literature review, screening, and determining the acceptability of papers for study. All of the listed studies were performed on Bangladeshi COVID‐19 patients between 2020 and 2022, and details are described in Table 1. Based on the NOS, the majority of the included literature was of high quality (scores between 6 and 8), as shown in Table S1.
Figure 1.
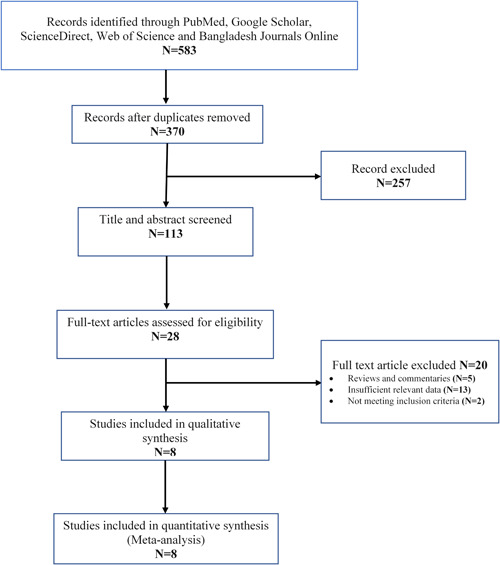
Flow diagram of the study selection process based on the PRISMA guideline. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
Table 1.
Hematological and biochemical parameters of COVID‐19 patients
| Parameters | Author name (year) | |||||||
|---|---|---|---|---|---|---|---|---|
| Akter et al. (2022) 55 | Bhuiyan et al. (2021) | Islam et al. (2020) | Layla et al. (2021) 54 | Nasir et al. (2021) 58 | Rahman et al. (2021) 59 | Saha et al. (2021) | Sultana et al. (2021) 60 | |
| Total cases | 100 | 100 | 259 | 100 | 99 | 306 | 350 | 228 |
| Severe | 25 | 22 | 81 | 37 | 39 | 108 | 133 | 122 |
| Nonsevere | 75 | 78 | 178 | 63 | 60 | 198 | 217 | 106 |
| Male | ||||||||
| Severe | 17 | 18 | – | 23 | 23 | 30 | 92 | 99 |
| Nonsevere | 43 | 53 | – | 44 | 42 | 95 | 156 | 79 |
| Female | ||||||||
| Severe | 8 | 4 | – | 14 | 9 | 16 | 34 | 33 |
| Nonsevere | 32 | 25 | – | 19 | 18 | 103 | 61 | 27 |
| Age (mean± SD) | ||||||||
| Severe | 50.50 ± 7.70 | 46.67 ± 15.85 | – | – | 65.02 | 54.07 ± 9.82 | 54.19 ± 15.23 | 62.35 ± 10.49 |
| Nonsevere | 42.82 ± 8.07 | 32.33 ± 8.31 | – | – | 57.28 | 45.55 ± 6.34 | 43.03 ± 13.68 | 60.63 ± 16.90 |
| D‐dimer (mg/L) | ||||||||
| Severe | 1.04 ± 1.17 | 0.92 ± 0.97 | 0.61 ± 0.83 | 4.78 ± 3.42 | 1.57 ± 2.01 | 0.76 ± 0.60 | 2.77 ± 1.92 | 5.87 ± 2.61 |
| Nonsevere | 0.34 ± 0.25 | 0.51 ± 0.10 | 0.47 ± 0.7 | 1.09 ± 1.82 | 1.36 ± 2.12 | 0.24 ± 0.99 | 2.04 ± 1.33 | 2.27 ± 1.15 |
| CRP (mg/L) | ||||||||
| Severe | 90.49 ± 73.31 | – | 63.46 ± 58.07 | 23.24 ± 20.81 | 32.13 ± 27.4 | 65.5 ± 72.5 | 47.15 ± 22.71 | 52.38 ± 59.89 |
| Nonsevere | 31.79 ± 47.81 | – | 27.15 ± 29.59 | 10.73 ± 7.08 | 36.14 ± 24.62 | 1.90 ± 1.10 | 39.35 ± 23.22 | 50.98 ± 51.76 |
| Ferritin (ng/ml) | ||||||||
| Severe | 838.95 ± 957.95 | – | 823.58 ± 468.60 | 1003.36 ± 555.50 | 954.19 ± 704.17 | 651.86 ± 793.30 | 1118.05 ± 265.81 | – |
| Nonsevere | 358.88 ± 417.51 | – | 562.21 ± 518.81 | 301.92 ± 220.47 | 841.71 ± 612.28 | 86.41 ± 34.78 | 739.86 ± 142.93 | – |
| LDH (U/L) | ||||||||
| Severe | 441.9 ± 157.51 | – | 410.42 ± 128.04 | 655.70 ± 223.25 | – | – | 379.94 ± 101.91 | – |
| Nonsevere | 266.57 ± 118.85 | – | 370.24 ± 188.53 | 390.93 ± 223.67 | – | – | 344.16 ± 97.55 | – |
| RBS (mmol/L) | ||||||||
| Severe | 11.79 ± 5.11 | – | – | 11.42 ± 5.75 | – | – | – | – |
| Nonsevere | 9.13 ± 5.58 | – | – | 10.05 ± 4.66 | – | – | – | – |
| ANC (×109/L) | ||||||||
| Severe | 6.39 ± 2.16 | 11.57 ± 3.57 | – | – | 7.81 ± 3.99 | – | – | – |
| Nonsevere | 4.86 ± 2.87 | 6.81 ± 6.33 | – | – | 9.64 ± 3.49 | – | – | – |
| NLR | ||||||||
| Severe | 8.90 ± 5.23 | 14.98 ± 39.56 | 4.51 ± 1.63 | – | 9.76 ± 6.68 | – | 2.8 ± 0.32 | – |
| Nonsevere | 6.17 ± 7.29 | 8.35 ± 4.60 | 3.39 ± 1.19 | – | 5.99 ± 3.99 | – | 2.7 ± 0.28 | – |
| Serum creatinine (mg/dl) | ||||||||
| Severe | 0.94 ± 0.26 | – | – | 1.45 ± 1.25 | 2.30 ± 2.57 | 1.15 ± 0.93 | 1.9 ± 0.8 | – |
| Nonsevere | 0.93 ± 0.32 | – | – | 1.00 ± 0.27 | 1.39 ± 1.08 | 0.83 ± 0.27 | 1.5 ± 0.9 | – |
| Hemoglobin (g/dL) | ||||||||
| Severe | 12.57 ± 1.98 | 11.95 ± 1.94 | – | 12.61 ± 5.98 | ||||
| Nonsevere | – | 14.23 ± 1.28 | 12.38 ± 1.86 | – | – | 12.68 ± 1.36 | – | – |
Abbreviations: ANC, absolute neutrophil count; CRP, C‐reactive protein; LDH, lactate dehydrogenase; NLR, absolute neutrophil/absolute lymphocyte or %neutrophil/%lymphocyte; RBS, random blood sugar.
3.2. Presence of D‐dimer (mg/L) level on disease severity
Among the 1542 patients, 567 were severe or critical cases and 975 were nonsevere cases. There was a significant heterogeneity when the D‐dimer level was evaluated between the severe and nonsevere COVID‐19 patients (I 2 = 96%, p < 0.00001). In the meta‐analysis, the random‐effect model was employed, and the findings revealed that severe COVID‐19 patients D‐dimer is significantly higher than nonsevere patients (severe and nonsevere = 36.77% and 63.23%, MD = 1.16, 95% CI = 0.52–1.80, p = 0.0004) (Figure 2).
Figure 2.
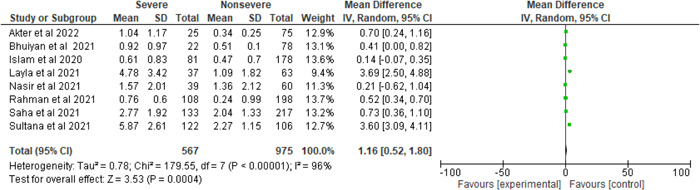
Pooled effects of the linkage of D‐dimer (mg/L) with COVID‐19 patients
3.3. Effect of CRP (mg/L) level on severity
Figure 3 illustrates the ratio of severe and nonsevere patients (0.61:1), and a considerable heterogeneity is found (I 2 = 93%, p < 0.00001). COVID‐19 patients who were in a critical state showed 22.97 mg/L higher CRP level compared to those who were not in critical condition (severe and nonsevere = 37.80% and 62.2%, MD = 22.97, 95% CI = 8.04–37.90, p = 0.003).
Figure 3.
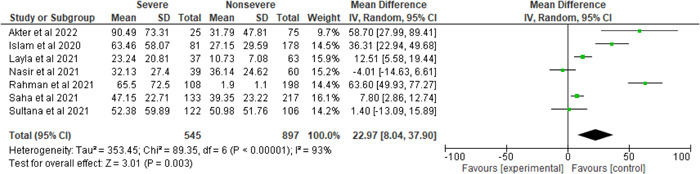
Pooled effects of the linkage of CRP (mg/L) with COVID‐19 patients. CRP, C‐reactive protein.
3.4. The influence of ferritin (ng/ml) on severity
Among the five studies, the percentages of critical and noncritical cases were 34.84% and 65.16%, respectively, and showed remarkable heterogenicity compared with ferritin levels (I 2 = 79%, p = 0.0002). Moreover, 419.26 ng/ml elevated association was observed for ferritin levels in the individuals with the severe condition than in the nonsevere state (severe vs. nonsevere 34.84% vs. 65.16%, MD= 419.26, 95% CI = 283.46–555.05, p < 0.00001) (Figure 4).
Figure 4.
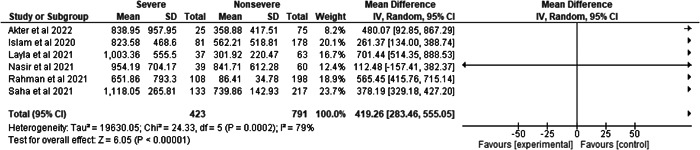
Pooled effects of the linkage of ferritin (ng/ml) with COVID‐19 patients
3.5. Impact of LDH (U/L) and RBS (mmol/L) level on severity
Figures 5 and 6 depict the LDH (U/L) and RBS (mmol/L) levels in severe and nonsevere patients infected with SARS‐CoV‐2. The number of severe and nonsevere patients is 276 and 533 for LDH (U/L) whereas 62 and 138 for RBS (mmol/L), respectively. However, it is translucent that there was a significant heterogeneity compared with LDH (U/L) in the severe and nonsevere group (I 2 = 92%, p < 0.00001), whereas RBS (mmol/L) did not show significant heterogeneity when compared to the severe and nonsevere group (I 2 = 0%, p = 0.43). LDH level was 118.37 U/L higher in severe patients than in nonsevere patients (severe vs. nonsevere 34.12% vs. 65.88%, MD = 118.37, 95% CI = 38.60–198.14, p = 0.004), whereas RBS level was observed higher in severe cases as compared to nonsevere cases and the result was statistically significant (severe vs. nonsevere 31% vs. 69%, MD = 1.96, 95% CI = 0.36–3.57, p = 0.02).
Figure 5.
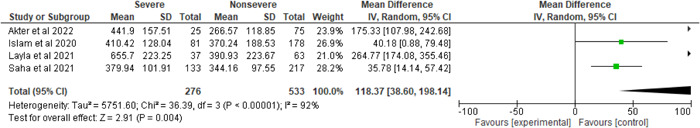
Pooled effects of the linkage of LDH (U/L) with COVID‐19 patients. LDH, lactate dehydrogenase.
Figure 6.

Pooled effects of the linkage of RBS (mmol/L) with COVID‐19 patients. RBS, random blood sugar.
3.6. Effect of ANC (×109/L) level on severity
The percentages of severe and nonsevere patients in the three studies were 27.13% and 72.87%, respectively, as seen in Figure 7 and demonstrated considerable heterogeneity compared to ANC levels (I 2 = 93%, p < 0.00001). Furthermore, SARS‐CoV‐2‐infected individuals with the severe condition had 1.42 × 109/L higher ANC levels than nonsevere individuals (severe vs. nonsevere 27.13% vs. 72.87%, MD = 1.42, 95% CI = −1.77–4.62, p = 0.38).
Figure 7.
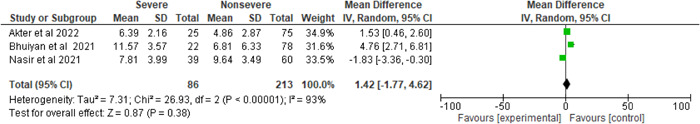
Pooled effects of the linkage of ANC (×109/L) with COVID‐19 patients. ANC, absolute neutrophil count
3.7. Effect of NLR level on severity
There was considerable heterogeneity when the NLR level was compared between the severe and nonsevere COVID‐19 patients (I 2 = 90%, p < 0.00001). Using the random‐effect model, the meta‐analysis found that NLR levels were considerably higher in the severe patients than in the nonsevere patients. A greater NLR level was seen in individuals with severe COVID‐19 disease compared to those without severe illness (severe vs. nonsevere: 33.04% vs. 66.96%, MD = 1.26, 95% CI = 0.24–2.27, p = 0.02) (Figure 8).
Figure 8.
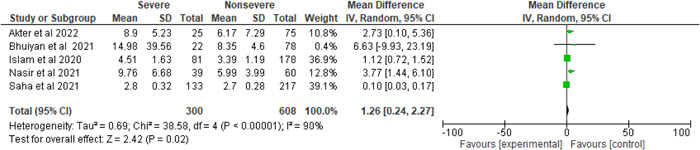
Pooled effects of the linkage of NLR with COVID‐19 patients. NLR, neutrophil to lymphocyte ratio.
3.8. Effect of serum creatinine (mg/dl) level on severity
There was a significant heterogeneity when the serum creatinine level was compared across COVID‐19 individuals (severe and nonsevere) (I 2 = 79%, p = 0.0007). The results showed that serum creatinine levels are elevated in the severe group compared to the nonsevere cohort (severe vs. nonsevere, 34.03% vs. 65.97%, MD = 0.31, 95% CI = 0.08–0.54, p = 0.008) (Figure 9).
Figure 9.
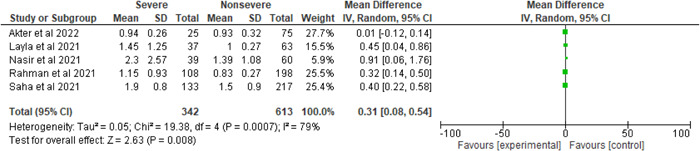
Pooled effects of the linkage of serum creatinine (mg/dl) with COVID‐19 patients.
3.9. Effect of hemoglobin (g/dl) level on severity
Of the eight studies, three studies included hemoglobin in their studies. The percentages of severe and nonsevere cases were 37.73% and 68.27%. Figure 10 elucidates significant heterogeneity between the severe and nonsevere groups in terms of hemoglobin levels (I 2 = 71%, p = 0.03). However, severe patients had a lower haemoglobin level than nonsevere individuals (severe vs. nonsevere, 37.73% vs. 68.27%, MD = −0.73, 95% CI = −1.61–0.14, p = 0.10).
Figure 10.

Pooled effects of the linkage of hemoglobin (g/dl) with COVID‐19 patients
3.10. Publication bias
Our meta‐analysis investigated the publication bias using Begg‐Mazumdar's and Egger's assessment. Both analyses yielded no significant publication bias (Table S2).
4. DISCUSSION
COVID‐19 has brought worldwide public health disaster, and the sickness it produced has a widely varied clinical appearance. Moreover, its consequences are disseminated worldwide and are claiming millions of lives. Bangladesh is analogous to the rest of the world in that age, gender, and comorbidities are related to COVID‐19 infections. Nevertheless, in comparison to other nations, the likelihood of dying from COVID‐19 in Bangladesh is low, presumably because of the demographic features of our population, with just 10–15% of the population aged over 50 years. 7 , 55 , 61 , 62 To our knowledge, this is the first meta‐analysis to look at numerous biochemical and hematological indicators in SARS‐CoV‐2 infected people in Bangladesh. We collected eight independent studies from January 2020 to March 2022 that reported severity, inflammatory biomarkers, hematological markers, and varied outcomes on 1542 COVID‐19 patients from Bangladesh.
In our study, we observed that critical patients have higher D‐dimer (MD = 1.16, p = 0.0004) levels compared to nonsevere patients. An equivalent conclusion was also noted earlier in some other studies. 7 , 63 , 64 , 65 The elevated D‐dimer level in COVID‐19 is due to excessive clotting and hypoxia. As a result of D‐dimer being a consequence of fibrin breakdown, its presence in severe stages may also signify blood clots and deep venous thrombosis (DVT). COVID‐19 patients who have venous thromboembolism episodes (both DVT and pulmonary embolism) had elevated D‐dimer levels in their blood. 66
It is also discernible that CRP, a commonly measured inflammatory marker, is elevated in most COVID‐19 patients and is connected to the degree of severity (MD = 22.97, p = 0.003). CRP levels can rise quickly due to inflammation (reaction to inflammatory cytokines such as IL‐6, IL‐1, or TNF), cell damage, or tissue injury. As a result, significantly high serum CRP levels in severe conditions insinuate an inflated inflammatory response, consistent with elevated serum proinflammatory cytokines in COVID‐19 patients. 67
In our study, the value of serum ferritin was shown to be statistically significant (MD = 419.26, p < 0.00001) compared with critical and noncritical cases. Several other studies concluded that serum ferritin is significantly increased in patients with a severe course of the disease than in those who are not critically ill, documenting its position as the most potential biomarker in foreseeing COVID‐19 severity. 68 , 69
The forest plot described that the LDH, an enzyme that transforms lactate to pyruvate, was considerably higher in severe cases than that in less severe patients (MD = 118.37, p = 0.004), and our result is consistent with other findings. 70 , 71 Khalid et al. 72 also found a link between a high level of LDH and a severe COVID‐19 infection. Besides, RBS was shown to have a substantial relationship with disease severity and was statistically noteworthy (MD = 1.96, p = 0.02). On the contrary, research demonstrates that hyperglycemia is a risk factor that leads to the severity and death of COVID‐19 victims. 73 This research revealed no significant difference in the ANC label between people with moderate and severe COVID‐19 cases (MD = 1.42, p = 0.38). NLR was a helpful biomarker primarily used in tumor‐related disorders, autoimmune disorders, infectious bacterial pneumonia, and tuberculosis. 74 , 75 , 76 , 77 Our study found that the NLR for individuals with severe illnesses was much higher than for those with less severe illness (MD = 1.26, p = 0.02). Besides, our study is analogous to some different studies. 78 , 79
The level of creatinine indicates the health of the kidneys. Our findings demonstrate that higher creatinine levels are significantly related to severe COVID‐19 (MD = 0.31, p = 0.008), suggesting that SARS‐CoV‐2 infection may cause acute inflammation. Previous meta‐analyses have produced conclusions that are congruent with ours. 80 , 81 , 82 Our meta‐analysis found decreased hemoglobin levels in the Bangladeshi population infected with COVID‐19 (MD = −0.73, p = 0.10), and these findings are similar to other meta‐analyses. 82 , 83
There are several strengths of this meta‐analysis. Numerous meta‐analyses have already been published on the correlation of biochemical and hematological irregularities with the intensity of COVID‐19 patients from various countries. However, no meta‐analysis has been published on the relationship between hematological and biochemical malformations with the severity of COVID‐19 infected Bangladeshi patient inhabitants. This is the first comprehensive systematic review and meta‐analysis. The literature covered in this study is of excellent quality, the methodology is meticulous, and the study's outcomes are very plausible. All relevant cohort and case‐control investigations are included in this meta‐analysis and the number of participants is quite good for performing a meta‐analysis. We employed a heterogeneity investigation and publication bias assessment during our meta‐analysis.
The information presented in this meta‐analysis must be considered along with several limitations. First, the meta‐analysis cases are retrospective investigations and were not randomized clinical studies. Second, the total number of studies in the analysis is low (eight studies) if we compare with other meta‐analyses with large number of articles. Thirdly, just the combination of nine markers was mentioned to foreshadow the severity of COVID‐19 patients, and coming research may investigate amalgamating more additional biomarkers in the same way. Other concomitant conditions such as diabetes, heart disease, lung disease, kidney disease were not considered in this meta‐analysis. Fourth, all indicators and measures have only been examined once upon admission; thus, variations within these parameters could not be assessed. Despite these drawbacks, the results of our meta‐analysis are more consistent and comprehensive, allowing us to monitor patients' conditions and detect severe patients earlier.
5. CONCLUSION
The most promising indicators in determining the severity of COVID‐19 infections are biochemical and hematological indicators. Our meta‐analysis found that hematological and biochemical biomarkers‐ D‐dimer, CRP, ferritin, LDH, RBS, NLR, and serum creatinine are significantly linked with the severity of COVID‐19 cases. At the same time, the hemoglobin levels of patients with severe sickness are lower than those with less severe COVID‐19 infestations. This analysis aids in identifying individuals at high risk for COVID‐19‐related complications and assists in appropriate medication and care to be given quickly and effectively. This research also pays particular attention to these risk variables and aids in developing individualized therapy regimens for COVID‐19 patients in Bangladesh. Nevertheless, this aspect has to be analyzed further to expedite the commencement and optimization of COVID‐19 therapy.
AUTHOR CONTRIBUTIONS
Khokon Kanti Bhowmik: data curation; methodology; software; writing—original draft. Md. Abdul Barek: data curation; methodology; software; validation; writing—original draft. Md. Abdul Aziz: data curation; methodology; project administration; resources; validation; writing—review and editing. Mohammad Safiqul Islam: conceptualization; formal analysis; methodology; software; supervision; validation; writing—review and editing. Mohammad Safiqul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and any discrepancies from the study as planned (and, if relevant, registered) have been explained.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
TRANSPARENCY STATEMENT
Mohammad Safiqul Islam affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
The authors wish to thank the Laboratory of Pharmacogenomics and Molecular Biology, Department of Pharmacy, Noakhali Science and Technology University, Sonapur 3814, Noakhali, Bangladesh for their generalized support, unwavering motivation, academic supervision, constructive comments, affectionate sensation, and positive recommendations.
Bhowmik KK, Barek MA, Aziz MA, Islam MS. A systematic review and meta‐analysis of abnormalities in hematological and biochemical markers among Bangladeshi COVID‐19 cases. Health Sci. Rep. 2022;5:e728. 10.1002/hsr2.728
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
REFERENCES
- 1. Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID‐19 cases: a meta‐analysis with 55 studies and 10014 cases. Heliyon. 2020;6:e05684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Worldometers.info . COVID‐19 coronavirus pandemic. 2022. https://www.worldometers.info/coronavirus/ [Google Scholar]
- 3. Uddin MS, Millat MS, Baral PK, et al. The protective role of vitamin C in the management of COVID‐19: a review. Egypt Public Health Assoc. 2021;96:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C‐reactive protein, procalcitonin, D‐dimer, and ferritin in severe coronavirus disease‐2019: a meta‐analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80:401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS‐CoV‐2. Nat Rev Microbiol. 2021;19:155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Islam S, Islam T, Islam MR. New coronavirus variants are creating more challenges to global healthcare system: a brief report on the current knowledge. Clin Pathol. 2022;15:2632010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haque A, Pant AB. Mitigating Covid‐19 in the face of emerging virus variants, breakthrough infections and vaccine hesitancy. J Autoimmun. 2022;127:102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Tracking SARS‐CoV‐2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 12. Islam MR, Hasan M, Nasreen W, Tushar MI, Bhuiyan MA. The COVID‐19 vaccination experience in Bangladesh: findings from a cross‐sectional study. Int J Immunopathol Pharmacol. 2021;35:20587384211065628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bari MS, Hossain MJ, Ahmmed F, et al. Knowledge, perception, and willingness towards immunization among Bangladeshi population during COVID‐19 vaccine rolling period. Vaccines. 2021;9:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Islam MR. Urgent call for mass immunization against coronavirus in Bangladesh. Sci Prog. 2021;104:368504211058562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID‐19—a systematic review. Life Sci. 2020;254:117788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS‐CoV infection in humans is associated with a pro‐inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hossen MS, Barek MA, Jahan N, Safiqul Islam M. A review on current repurposing drugs for the treatment of COVID‐19: reality and challenges. SN Compr Clin Med. 2020;2:1777‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID‐19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaudhary R, Garg J, Houghton DE, et al. Thromboinflammatory biomarkers in COVID‐19: systematic review and meta‐analysis of 17,052 patients. MCP:IQ&O. 2021;5:388‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aktas G. A comprehensive review on rational and effective treatment strategies against an invisible enemy; SARS Cov‐2 infection. Exp Biomed Res. 2020;3:293‐311. [Google Scholar]
- 22. Warusevitane A, Karunatilake D, Sim J, Smith C, Roffe C. Early diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C‐reactive protein. PLoS One. 2016;11:e0150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L. C‐reactive protein levels in the early stage of COVID‐19. Med Mal Infect. 2020;50:332‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dai RX, Kong QH, Mao B, et al. The mortality risk factor of community acquired pneumonia patients with chronic obstructive pulmonary disease: a retrospective cohort study. BMC Pulm Med. 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Afsin H, Aktas G. Platelet to lymphocyte and neutrophil to lymphocyte ratios are useful in differentiation of thyroid conditions with normal and increased uptake. Ethiop J Health Dev. 2021;35:1‐5. [Google Scholar]
- 26. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil‐to‐lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras. 2017;63:1065‐1068. [DOI] [PubMed] [Google Scholar]
- 27. Aktas G, Duman T, Atak B, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Fam Med Prim Care Rev. 2020;22:107‐110. [Google Scholar]
- 28. Aktas G. Hematological predictors of novel coronavirus infection. Rev Assoc Med Bras. 2021;67:1‐2. [DOI] [PubMed] [Google Scholar]
- 29. Gómez‐Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID‐19 patients–is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin‐6 in COVID‐19 induced pneumonia and macrophage activation syndrome‐like disease. Autoimmun Rev. 2020;19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Atak Tel B, Kahveci G, Bilgin S, et al. Haemoglobin and red cell distribution width levels in internal medicine patients indicate recurrent hospital admission during COVID‐19. Fam Med Prim Care Rev. 2022;24:32‐36. [Google Scholar]
- 32. Atak Tel BM, Bilgin S, Kurtkulagi O, et al. Frailty in diabetic subjects during COVID‐19 and its association with HbA1c, mean platelet volume and monocyte/lymphocyte ratio. Clin Diabetol. 2022;11:119‐126. [Google Scholar]
- 33. Chakrabarti S. Thyroid functions and bipolar affective disorder. J Thyroid Res. 2011;2011:306367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montaner J, Perea‐Gainza M, Delgado P, et al. Etiologic diagnosis of ischemic stroke subtypes with plasma biomarkers. Stroke. 2008;39:2280‐2287. [DOI] [PubMed] [Google Scholar]
- 35. Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. Cerebrovasc Dis. 2009;27:247‐253. [DOI] [PubMed] [Google Scholar]
- 36. Pope JE, Choy EH. C‐reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51:219‐229. [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta‐analysis. Diabetes Care. 2013;36:166‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Micoulaud‐Franchi JA, Faugere M, Boyer L, et al. Elevated C‐reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165:94‐96. [DOI] [PubMed] [Google Scholar]
- 39. Di Napoli M, Elkind MS, Godoy DA, Singh P, Papa F, Popa‐Wagner A. Role of C‐reactive protein in cerebrovascular disease: a critical review. Expert Rev Cardiovasc Ther. 2011;9:1565‐1584. [DOI] [PubMed] [Google Scholar]
- 40. Domingueti CP, Fóscolo RB, Dusse LMS, et al. Association of different biomarkers of renal function with D‐dimer levels in patients with type 1 diabetes mellitus (renal biomarkers and D‐dimer in diabetes). Arch Endocrinol Metab. 2018;62:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fruchter O, Yigla M, Kramer MR. D‐dimer as a prognostic biomarker for mortality in chronic obstructive pulmonary disease exacerbation. Am J Med Sci. 2015;349:29‐35. [DOI] [PubMed] [Google Scholar]
- 42. Ishikawa G, Acquah SO, Salvatore M, Padilla ML. Elevated serum D‐dimer level is associated with an increased risk of acute exacerbation in interstitial lung disease. Respir Med. 2017;128:78‐84. [DOI] [PubMed] [Google Scholar]
- 43. Juli C, Amalia L, Gamayani U, Atik N. D‐dimer level associates with the incidence of focal neurological deficits in cerebral venous thrombosis patients. J Blood Med. 2020;11:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Forouhi NG, Harding AH, Allison M, et al. Elevated serum ferritin levels predict new‐onset type 2 diabetes: results from the EPIC‐Norfolk prospective study. Diabetologia. 2007;50:949‐956. [DOI] [PubMed] [Google Scholar]
- 45. Raj S, Rajan G. Correlation between elevated serum ferritin and HbA1c in type 2 diabetes mellitus. Int J Res Med Sci. 2013;1:12‐15. [Google Scholar]
- 46. Chen X, Scholl TO, Stein TP. Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: The Camden study. Diabetes Care. 2006;29:1077‐1082. [DOI] [PubMed] [Google Scholar]
- 47. Williams MJA, Poulton R, Williams S. Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis. 2002;165:179‐184. [DOI] [PubMed] [Google Scholar]
- 48. Garton A, Gupta VP, Christophe BR, Connolly ES Jr. Biomarkers of functional outcome in intracerebral hemorrhage: interplay between clinical metrics, CD163, and ferritin. J Stroke Cerebrovasc Dis. 2017;26:1712‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kuragano T, Joki N, Hase H, et al. Low transferrin saturation (TSAT) and high ferritin levels are significant predictors for cerebrovascular and cardiovascular disease and death in maintenance hemodialysis patients. PLoS One. 2020;15:e0236277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1‐e34. [DOI] [PubMed] [Google Scholar]
- 51. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wells GA, Shea B, O'Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Oxford; 2000. [Google Scholar]
- 53. Islam MK, Hossain MM, Sharif MM, Hasan P, Molla MMA, Amin MR. Routine blood report and common laboratory parameters in COVID‐19: experience from Bangladesh. J Med. 2021;22:132‐138. [Google Scholar]
- 54. Layla KN, Yeasmin S, Khan SA, et al. Biochemical parameters of mild, moderate & severe COVID‐19 patients. N a J Adv Res. 2021;11:031‐039. [Google Scholar]
- 55. Akter A, Ahmed T, Tauheed I, et al. Disease characteristics and serological responses in patients with differing severity of COVID‐19 infection: A longitudinal cohort study in Dhaka, Bangladesh. PLoS Negl Trop Dis. 2022;16:e0010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kumar Saha S, Nuruzzaman M, Biswas S, Saha A, Obayedur Rahman M, Asaduzzaman M. Assessment of COVID‐19 cases by haematological and biochemical markers: A tertiary care hospital study in Dhaka, Bangladesh. Eur J Prev Med. 2021;9:133‐139. [Google Scholar]
- 57. Bhuiyan MN, Giti S, Hossen MSM, Rahman MM, Zannat MNE, Chokroborty S. Haematologic profile abnormalities and coagulopathy associated with Covid‐19: a prospective study of 100 patients. J Bangladesh Coll Phys Surg. 2020;38:61‐66. [Google Scholar]
- 58. Nasir M, Perveen RA, Nazneen R, Zahan T, Ahmad SN, Chowdhury AS. Paradox of predictors in critically ill COVID‐19 patients: outcome of a COVID‐dedicated intensive care unit. medRxiv . 2021. doi:10.1101/2021.04.23.21256009
- 59. Rahman MA, Shanjana Y, Tushar MI, et al. Hematological abnormalities and comorbidities are associated with COVID‐19 severity among hospitalized patients: experience from Bangladesh. PLoS One. 2021;16:e0255379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sultana GNN, Srivast A, Akhtaar K, et al. Studying C‐reactive protein and D‐dimer levels in blood may prevent severe complications in Bangladeshi COVID‐19 patients. Res Squ . 2021. doi:10.21203/rs.3.rs‐812399/v1 [DOI] [PMC free article] [PubMed]
- 61. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging. 2020;12:6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Barmania F, Mellet J, Ryder MA, et al. Studying C‐reactive protein and D‐dimer levels in blood may prevent severe complications in Bangladeshi COVID‐19 patients. Eur J Hum Genet . 2022:1‐9. 10.1038/s41431-022-01089-8 [DOI] [PMC free article] [PubMed]
- 63. Simadibrata DM, Lubis AM. D‐dimer levels on admission and all‐cause mortality risk in COVID‐19 patients: a meta‐analysis. Epidemiol Infect. 2020;148:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Paliogiannis P, Mangoni AA, Dettori P, Nasrallah GK, Pintus G, Zinellu A. D‐dimer concentrations and COVID‐19 severity: a systematic review and meta‐analysis. Front Public Health. 2020;8:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhan H, Chen H, Liu C, et al. Diagnostic value of D‐Dimer in COVID‐19: a meta‐analysis and meta‐regression. Clin Appl Thromb Hemost. 2021;27:10760296211010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aloisio E, Chibireva M, Serafini L, et al. A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID‐19 severity. Arch Path Lab. 2020;144:1457‐1464. [DOI] [PubMed] [Google Scholar]
- 67. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Alroomi M, Rajan R, Omar AA, et al. Ferritin level: a predictor of severity and mortality in hospitalized COVID‐19 patients. Immun Inflamm Dis. 2021;2021(9):1648‐1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cheng L, Li H, Li L, et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34:e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li C, Ye J, Chen Q, et al. Elevated lactate dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID‐19. Aging. 2020;12:15670‐15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID‐19 severity marker. Am J Emerg Med. 2020;45:638‐639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Khalid A, Ali Jaffar M, Khan T, et al. Hematological and biochemical parameters as diagnostic and prognostic markers in SARS‐COV‐2 infected patients of Pakistan: a retrospective comparative analysis. Hematology. 2021;26:529‐542. [DOI] [PubMed] [Google Scholar]
- 73. Lazarus G, Audrey J, Wangsaputra VK, Tamara A, Tahapary DL. High admission blood glucose independently predicts poor prognosis in COVID‐19 patients: a systematic review and dose‐response meta‐analysis. Diabetes Res Clin Pract. 2021;171:108561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ying HQ, Deng QW, He BS, et al. The prognostic value of preoperative NLR, d‐NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:1‐8. [DOI] [PubMed] [Google Scholar]
- 75. Saeed AM, Rosati LM, Narang A, et al. Elevated absolute monocyte count, absolute neutrophil count, and neutrophil‐to‐lymphocyte ratio as prognostic factors in locally advanced pancreatic cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93:E157. [Google Scholar]
- 76. Jeon Y, Lee W‐I, Kang SY, Kim MH. Neutrophil‐to‐monocyte‐plus‐lymphocyte ratio as a potential marker for discriminating pulmonary tuberculosis from nontuberculosis infectious lung diseases. Lab Med. 2019;50:286‐291. [DOI] [PubMed] [Google Scholar]
- 77. Uslu AU, Küçük A, Şahin A, et al. Two new inflammatory markers associated with disease activity Score‐28 in patients with rheumatoid arthritis: neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio. Int J Rheum Dis. 2015;18:731‐735. [DOI] [PubMed] [Google Scholar]
- 78. Vafadar Moradi E, Teimouri A, Rezaee R, et al. Increased age, neutrophil‐to‐lymphocyte ratio (NLR) and white blood cells count are associated with higher COVID‐19 mortality. Am J Emerg Med. 2021;40:11‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yang A‐P, Liu J‐P, Tao W‐Q, Li HM. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen Y‐T, Shao S‐C, Hsu C‐K, Wu IW, Hung MJ, Chen YC. Incidence of acute kidney injury in COVID‐19 infection: a systematic review and meta‐analysis. Crit Care. 2020;24:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yang X, Jin Y, Li R, Zhang Z, Sun R, Chen D. Prevalence and impact of acute renal impairment on COVID‐19: a systematic review and meta‐analysis. Crit Care. 2020;24:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mudatsir M, Fajar JK, Wulandari L, et al. Predictors of COVID‐19 severity: a systematic review and meta‐analysis. F1000Res. 2020;9:1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lippi G, Mattiuzzi C. Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. 2020;42:116‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.


