Backgrounds and aims
Single, short stair climbing and descending (SCD) bouts of low to moderate intensity effectively lower postprandial blood glucose but previous reports have found conflicting results on interactions by sex during exercise. We hypothesize that SCD at a self-selected intensity will be equally effective at lowering postprandial blood glucose in males and females. Methods and Results: Thirty subjects (age: 23.8 (3.0) years) performed 0, 1, 3, and 10 min of SCD following consumption of a mixed meal. SCD was performed at a self-selected comfortable pace and all bouts ended at minute 28. Postprandial blood glucose was measured every 15 min for 1 h and analyzed as glucose over time, area under the curve (AUC), and incremental AUC (iAUC) using mixed-design ANOVAs with repeated measures. Although there was no interaction between sex and condition or time (p = .129 to .541) for glucose over time, AUC, or iAUC, there was a main effect for sex for glucose over time (p = .004) and AUC (p = .006), but not iAUC (p = .125). Females had higher blood glucose throughout each trial (22% (13 to 31%), p = .004) but both males' and females’ postprandial blood glucose was lowered following 10 min of SCD relative to the seated control condition. Conclusions: Males and females benefited equally from single, short SCD bouts of low to moderate intensity despite females having higher blood glucose at all time points. Previous findings of sex differences in the attenuating effect of exercise on postprandial blood glucose are likely due to the use of absolute workloads leading to varying relative intensities.
Keywords: Carbohydrate tolerance, Sex differences, Exercise, Glucose, Postprandial
Highlights
-
•
SCD at a self-selected, comfortable pace for 3 min reduced postmeal blood glucose.
-
•
Men and women benefit equally from SCD postprandial glucose attenuations.
-
•
Previous sex differences were likely due to unequal relative intensity of exercise.
Abbreviations list:
- SCD
stair climbing and descending
- PBG
postprandial blood glucose
- AUC
area under the curve
- iAUC
incremental area under the curve
- HbA1c
glycosylated hemoglobin
- OGTT
oral glucose tolerance test
- RPE
ratings of perceived exertion
1. Introduction
Cardiometabolic diseases remain among the top causes of death in developed countries with an associated global economic burden of trillions of dollars. While physical activity ameliorates the risk of cardiometabolic disease, adherence to such interventions is often poor [[1], [2], [3]]. Postprandial blood glucose (PBG) is a compelling target for disease risk reduction due to its linear, independent association with disease and mortality risk in both diabetics and non-diabetics alike, unlike fasting glucose and glycosylated hemoglobin (HbA1c) [[4], [5], [6], [7], [8]]. Single, short, low to moderate intensity stair climbing and descending bouts (SCD) are an effective means of improving postprandial blood glucose, insulin levels, and insulin sensitivity following an oral glucose tolerance test (OGTT) and mixed meal [[9], [10], [11]] with fewer barriers, including time, perceived exertion, cost, and self-efficacy, than other modes of exercise [12]. Previous research has shown SCD interventions after an OGTT are equally effective for males and females, and regardless of cardiorespiratory fitness levels [13,14]. Daily SCD has also been shown to be associated with a lower risk of metabolic syndrome [15]. Health organizations in developed countries have begun promoting SCD interventions to the general population [[16], [17], [18]]. However, it is important to ensure such recommendations are beneficial for the population as a whole, including those often underrepresented in research.
Potential differences in physiological responses by sex are often neglected for a variety of reasons. Whether there are sex differences in the postprandial glucose attenuation following exercise interventions is important and would allow for more targeted public health strategies if necessary. Evidence of sex differences in postprandial glucose lowering exercise interventions is mixed [14,[19], [20], [21], [22], [23]]. A previous study found no sex differences in the postprandial blood glucose lowering effects of SCD following an oral glucose tolerance test (OGTT) [14] but potential sex differences with this intervention following a mixed meal have yet to be determined. This previous intervention used an OGTT and thus lacked external validity as few individuals consume 75 g of dextrose without other food items or macronutrients. A mixed meal reflecting the average macronutrient intake of Americans was used in this study to increase external validity and increase the applicability of the results to real-world scenarios. The main purpose of this paper is to determine whether there are sex differences in the effects of SCD on PBG following a mixed meal. We hypothesize SCD at a self-selected intensity will be equally effective at lowering PBG in males and females.
2. Methods
Thirty subjects (18 females, age 22.8 (2.8) years; 12 males, age 24.9 (3.4) years) (Table 1) participated. Participants were screened for cardiovascular risk using the Physical Activity Readiness Questionnaire [24] and deemed as low risk for exercise participation [25]. All participants provided written informed consent and the study was approved by the Institutional Review Board at San Diego State University.
Table 1.
Participant demographics.
| Age (y) | Weight (kg) | BMI (kg/m2) | Fasting Blood Glucose (mg/dL) | |
|---|---|---|---|---|
| All (N = 30) | 23.8 (3.0) | 69.8 (12.8) | 23.7 (3.0) | 113 (10) |
| Male (n = 12) | 24.9 (3.4) | 80.0 (10.0) | 24.9 (2.8) | 118 (11) |
| Female (n = 18) | 22.8 (2.8) | 63.7 (9.3) | 23.6 (3.0) | 110 (7) |
Fasting blood glucose was averaged from the participants' four visits. Data presented as mean (SD).
This study was a randomized controlled crossover trial with 4 conditions: a seated control, 1 min SCD, 3 min SCD, or 10 min SCD after a mixed meal. Visits occurred in the morning between the hours of 7 am and 11 am. To minimize potential circadian-related variations subjects were required to schedule all visits at the same time of day (within 1 h). Subjects were also asked to keep the length of their overnight fast consistent (within 1 h), consume the same last meal the night before each visit, avoid caffeine the morning of, and maintain their usual diet and lifestyle habits throughout their participation. Visits were separated by a minimum of 48 h and a maximum of 1 week. The experiment was conducted in the Clinical Nutrition and Physiological Sciences Laboratory at San Diego State University. The lab was well-lit, quiet, and maintained at a temperature of 22 °C. Randomization was performed using a free online random number generator program.
Baseline measurements were obtained before participants were provided with a standardized meal as described below. Participants were asked to finish the entire meal within 15 min. Time to completion of the meal was noted and the subjects were encouraged to consume the meal in the same amount of time (within 1 min) at each subsequent visit. Completion of the meal was denoted as minute 0 and measurements were then taken every 15 min thereafter for 1 h. Participants remained seated for the entire visit except for the prescribed exercise condition.
All exercise bouts ended at minute 28 to allow enough time for measurements at minute 30. Therefore, the 1, 3, and 10 min SCD bouts started at minute 27, 25, and 18 respectively. SCD bouts were performed continuously using an indoor 21-step stairwell. Participants self-selected a comfortable SCD pace (90–110 steps per minute) that was held constant intra-individually for every visit. Adherence to this pace was ensured using a metronome and direct supervision of the participants by investigators.
The standardized meal was created to reflect the average macronutrient intake of adults in the United States. It was comprised of 1 cup of fortified breakfast cereal (Cheerios, General Mills), 1 cup of 2% cow's milk (Trader Joe's), 1 slice of 100% whole wheat bread (Western Hearth), 2 tablespoons of peanut butter (Jif), 1 tablespoon of blackberry jam (Smucker's), and 100 g of a fresh ripe banana (Chiquita) for a total of 650 kilocalories (53% carbohydrate, 33% fat, and 14% protein). The order of consumption of these food items was kept consistent within subjects to minimize the meal order effect [26,27].
Blood glucose measurements were obtained with finger sticks and a glucometer (Nova Max). Measurements were repeated at each time point until two measurements within 15 mg/dL were obtained and then averaged [28].
SPSS version 27 was used for all statistical analyses. Data for measures relating to blood glucose or lactate were normalized by body weight to account for size differences [14]. Blood glucose measurements were analyzed using a 4 (trial) by 5 (time) by 2 (sex) mixed design ANOVA with repeated measures. AUC and iAUC for blood glucose measurements were analyzed using a 4 by 2 mixed design ANOVA with repeated measures. Area under the curve was calculated using the trapezoid rule. Incremental area under the curve was calculated using the trapezoid rule and subtraction of baseline blood glucose levels. Post hoc pairwise comparisons were performed with LSD adjustments. Violations of the assumption of sphericity were corrected with the Greenhouse–Geisser correction if the estimated epsilon (ε) was <0.75 or the Huynh-Feldt correction if > 0.75. The alpha level was set a priori at ꭤ = 0.05 for significance in these secondary analyses of this pre-existing data set. Unless stated otherwise, data are presented as mean (95% CI). Power analysis conducted with G*Power 3.1 [29] based on visually extracted group mean and SEM data from previously published work [30] for independent group comparison yields an estimated effect size of d = 1.12, which for an alpha error probability of 0.05 and two-tailed analysis results in a calculated power of 1-beta error probability >0.8 for a total Sample of N = 28. The current analysis is part of a larger study [9].
3. Results
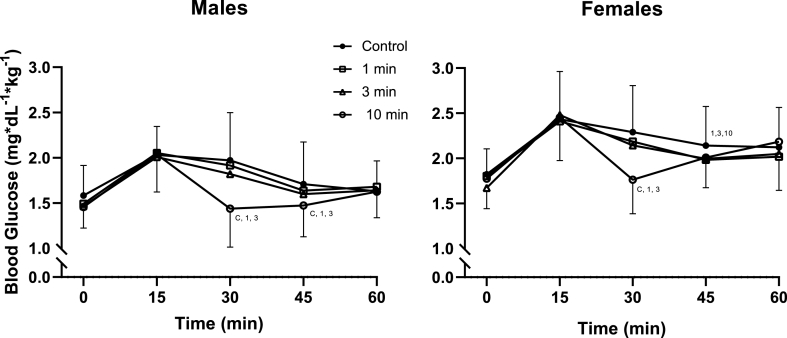
There was a main effect for sex [F (1, 28) = 9.675, p = .004, ηp2 = 0.257] but no interaction between sex and any other tested variable (all p > .129). Females’ blood glucose was higher than males throughout each trial (22% (13% to 31%), p = .004) (Fig. 1).
Fig. 1.
Blood Glucose Over Time in Males and Females
Changes in blood glucose in males (n = 12) and females (n = 18) over the 60-min mixed meal tolerance test for the seated control, 1 min, 3 min, and 10 min stair climbing and descending conditions. The 1 min, 3 min, and 10 min stairclimbing bouts all ended at minute 28. Blood glucose was analyzed using a 4 (trial) by 5 (time) by 2 (sex) mixed design ANOVA with repeated measures. No significant interaction was detected (p > .129). Post hoc pairwise comparisons were performed with LSD adjustments. Symbols denote significant difference (p < .05) from that condition.
Among females, there was an interaction between trial and time [F (6.170, 104.896) = 7.911, p < .001, ηp2 = 0.318]. Compared to the control condition significant differences were seen at minute 30 for the 10 min (−30% (−42% to −19%), p < .001) but not the 1 min (−5% (−13% to 4%), p = .275) or 3 min (−6% (−15% to 2%), p = .128) SCD bouts. The 10 min SCD bout also reduced PBG compared to the 1 min (−24% (−36% to −13%), p < .001) and 3 min (−22% (−32% to -11%, p = .001) SCD bouts. At minute 45, PBG was reduced compared to control following 1 min (−8% (−15% to −1%), p = .023), 3 min (−7% (−12% to −2%),p = .008), and 10 min of SCD (7% (−12% to −1%), p = .019).
Among males, there was an interaction between trial and time [F (4.697, 51.672) = 5.133, p = .001, ηp2 = 0.318]. Compared to the control condition significant differences were seen at minute 30 for the 10 min SCD bout (−37% (−56% to −19%), p = .001) but not the 1 min (−3% (−11% to 5%), p = .449) or 3 min SCD bout (−8% (−16% to 0%), p = .056). At minute 45, PBG was reduced significantly compared to control following the 10 min SCD bout (−16% (−26% to −6%), p = .006) but not the 1 min SCD bout (−4% (−14% to 5%), p = .360) or 3 min SCD bout (−6% (−14% to 1%), p = .090). The 10min SCD bout also differed from the 1 min SCD bout at minutes 30 (−25% (−38% to −11%), p = .002) and 45 (−10% (−17% to −3%, p = .013), and the 3 min SCD bout at minutes 30 (−21% (−34% to −8%), p = .004) and 45 (−8% (−15% to −1%, p = .023).
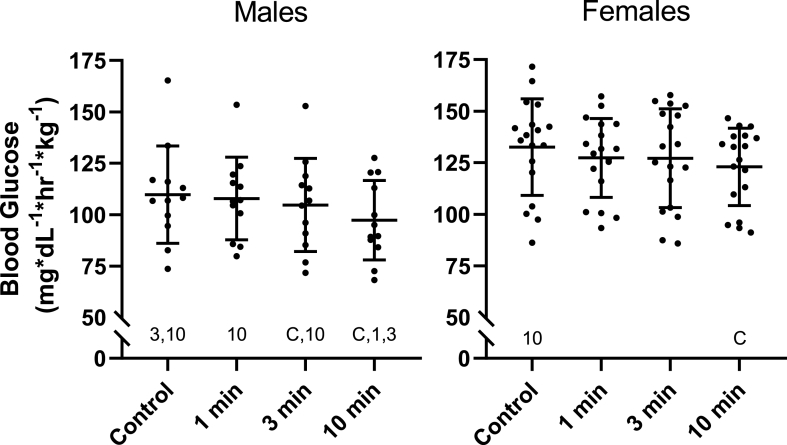
There was no interaction between condition and sex [F (3, 84) = 0.723, p = .541, ηp2 = 0.025] but there was a main effect for sex [F (1, 28) = 8.895, p = .006, ηp2 = 0.241] (Fig. 2).
Fig. 2.
Area Under the Curve for Blood Glucose in Males and Females
Blood glucose excursion in males (n = 12) and females (n = 18) represented by the area under the curve (AUC) over the 60-min mixed meal tolerance test for the seated control, 1 min, 3 min, and 10 min stair climbing and descending conditions. AUC for blood glucose was analyzed using a 4 by 2 mixed design ANOVA with repeated measures. Area under the curve was calculated using the trapezoid rule. Post hoc pairwise comparisons were performed with LSD adjustments. Symbols denote significant difference (p < .05) from that condition.
Females' AUC was higher than males’ during the control (21% (4% to 37%), p = .014), 1 min SCD (18% (4% to 32%), p = .012), 3 min SCD (21% (4% to 39%), p = .016), and 10 min SCD (26% (11% to 41%), p = .001). Among females, there was a main effect for condition [F (3, 51) = 3.992, p = .013, ηp2 = 0.190]. Compared to the control condition the 10 min SCD bout significantly reduced PBG (−7% (−11% to −3%), p < .001) but the 1 min SCD (−4% (−9% to 1%), p = .131) and the 3 min SCD (−4% (-9% to 1%), p = .092) bouts did not. There was also a nonsignificant reduction following the 10 min compared to the 1 min (−3% (-7% to 0%), p = .070) condition.
Among males, there was a main effect for condition [F (3, 33) = 6.826, p = .001, ηp2 = 0.383]. Compared to the control condition there was a significant reduction following the 3 min (−5% (-9% to 0%), p = .041) and 10 min (−11% (−19% to −4%), p = .005) but not 1 min SCD (−2% (−7% to 4%), p = .498) bouts. The 10 min SCD bout also reduced AUC compared to the 1 min (−10% (−17% to −2%), p = .015) and 3 min SCD (−7% (−13% to −1%), p = .031) conditions.
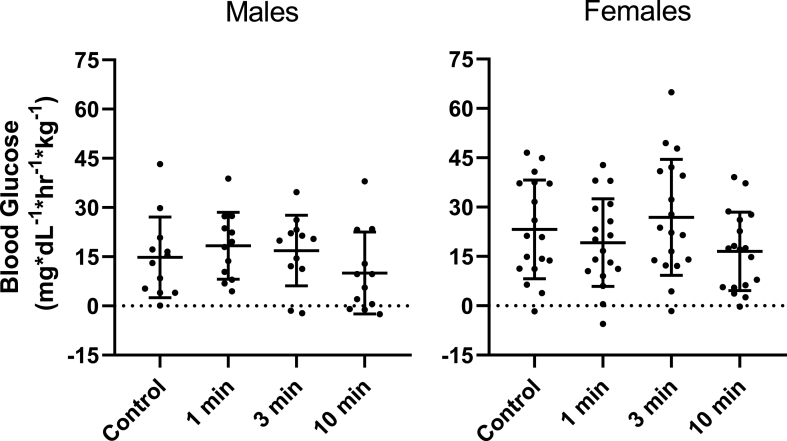
There was no interaction between condition and sex [F (3, 84) = 1.398, p = .249, ηp2 = 0.048] nor a main effect for sex [F (1, 28) = 2.503, p = .125, ηp2 = 0.082] for iAUC (Fig. 3).
Fig. 3.
Incremental Area Under the Curve for Blood Glucose in Males and Females
Blood glucose excursion in males (n = 12) and females (n = 18) represented by the incremental area under the curve (iAUC) over the 60-min mixed meal tolerance test for the seated control, 1 min, 3 min, and 10 min stair climbing and descending conditions. iAUC for blood glucose was analyzed using a 4 by 2 mixed design ANOVA with repeated measures. Area under the curve was calculated using the trapezoid rule. Incremental area under the curve was calculated using the trapezoid rule and subtraction of baseline blood glucose levels. Post hoc pairwise comparisons were performed with LSD adjustments. Symbols denote significant difference (p < .05) from that condition.
4. Discussion
The lack of an interaction between sex and condition or time suggests that females and males benefit equally from the PBG attenuation provided by single SCD bouts after a mixed meal. This corroborates previous findings in which females and males benefitted equally from a similar intervention following an OGTT [14], as well as others using light and moderate intensity walking [19,20], and light intensity treadmill desk walking [22], but contradicts findings by others who found an interaction by sex after light intensity walking but not simple resistance activities [21] and moderate and vigorous intensity walking [23].
In Dempsey et al. a significant interaction by sex was found after light walking (p = .045) with the reduction in glucose compared to seated control being 2-fold greater in women than men (−58% versus -26%). While the interaction by sex was similar in magnitude following simple resistance activities compared to seated control (−53% versus −31%) it did not reach significance (p = .17). The light walking intervention consisted of 3 min of treadmill walking (zero gradient, 3.2 km · h−1) every 30 min for a total of 12 times [21]. Notably, subjects all performed the treadmill walking at the same absolute speed whereas in our current study subjects were allowed to self-select their SCD pace. This likely led to women performing at a higher relative intensity due to their generally lower VO2 max, muscle mass, and stature. Allowing for self-selection of the exercise intensity increased the external validity of our current study and applicability for public health interventions. The notion that the interaction by sex is an artifact of the differences in relative intensity is further supported by Dempsey et al.‘s findings of no interaction by sex after the simple resistance activities protocol which was comprised of body weight exercises [21].
Bhammer et al. also found a significant interaction between sex and condition with women having a 6%, 13%, and 13% reduction and men having a 3% reduction and 6% and 7% increase in blood glucose following the 3 exercise conditions compared to the seated control. Conditions in their study consisted of a seated control, 30 min of submaximal moderate-intensity walking at 65–75% HR max, 2 min of moderate intensity walking every 20 min (zero gradient, 3.0 miles · h−1), and 2 min of vigorous intensity walking every hour at 3 mph with a gradual increase in grade to 25% or maximum grade reached in participants VO2 max test [23]. While relative intensity may have been equated in the 30 min of submaximal moderate-intensity condition is was likely different in the other two exercise conditions. Without further analyses into interactions by sex within each condition we can only speculate, however its possible an interaction in one or both of the latter two conditions which did not equate relative intensity drove the overall sex by condition interaction.
Dunstan et al. found no interaction between sex and condition (p = .113) comparing light and moderate activity breaks to a seated control. Light activity breaks consisted of 2 min of light (RPE 6 to 9) treadmill walking at 3.2 km/h every 20 min for 5 h. Moderate activity breaks consisted of 2 min of moderate (RPE 12 to 14) treadmill walking at 5.8–7.9 km/h every 20 min for 5 h. A similar relative intensity could have been achieved with their RPE range, however the speed was kept constant for the light activity break condition [20]. Using the same intervention as Dunstan et al. Bailey et al. found no interaction between sex and condition. Their sample size of N = 13 is likely not powered to determine whether sex differences truly exist with this intervention [19]. Champion et al. found no interaction between sex and condition with 20 min of light intensity treadmill walking every hour over 6.5 h for a total of 2 h of walking compared to a seated control [22]. Unlike Bhammer and Demspey, and similar to our intervention, participants were allowed to self-select their intensity with a walking speed between 1.2 and 3.5 km/h.
While there were many differences between these various interventions and ours including total amount of time of the intervention, total amount of time glucose was measured over, time between glucose measurements, and inclusion of a multiple meals, we find the most compelling reason for a presence of interaction by sex to be the non-individualized intensity of the exercise interventions. If the exercise intensity is not self-selected, but rather held constant for all subjects, women, having less muscle mass, lower VO2 max, and shorter statue, would be working at a higher relative intensity potentially explaining the greater reductions in blood glucose. Furthermore, these other experiments did not adjust for bodyweight after giving identical amounts of food to participants resulting in smaller individuals, namely women, being challenged with greater amounts of carbohydrates. In our current paper we normalized blood glucose by bodyweight in order to account for size differences and the standardized meal size as shown previously [14].
These SCD bouts were rated subjectively by participants as very light to light as described in a previous publication [9] though objective measures of this SCD intervention indicated a moderate intensity [10]. Among both females and males 3 min of SCD reduced post-exercise PBG with longer bouts producing greater reductions. While a single minute reduced PBG among females, the reduction was not statistically significant for males likely due to the smaller sample size and subsequent reduction in statistical power. The magnitude of the post-meal peak PBG reduction following 10 min of SCD was similar, if not greater, than that of similar intensity interventions including 30 min of cycling [31] and 40 min of walking [32]. The SCD bouts were planned to be performed immediately before the peak PBG but participants peaked earlier than expected as described in a previous publication [9]. It is possible this led to a partial flooring effect and even greater reductions may be possible had the exercise bouts preempted peak PBG as planned.
According to the reference ranges of the American Diabetes Association, the fasting glucose (113 (10) mg/dL) of our participants would indicate prediabetes. This in contrast to the participants' postprandial glucose response with males and females having a PBG of 126 (16) mg/dL and 127 (25) mg/dL, respectively, at 1-h. These values are below the American Diabetes Association's OGTT 2-h PBG threshold for prediabetes of 140 mg/dL [33]. Participants of the current study performed a mixed meal tolerance test, not an OGTT, but displayed similar responses to the participants in our previous study which did utilize an OGTT. In this previous study, a similar cohort of young adults in apparent good health had a fasting blood glucose of 109 (8) mg/dL and a 1-h OGTT PBG of 158 mg/dL [10]. Unpublished pilot data showed participants returned to baseline by 2 h after their OGTT. Compared to a different set of glucometers (Contour Next), the glucometers used in this experiment (NovaMax) had higher glucose levels and variability (mean (SD): 126 (12) vs 110 (3) mg/dL; mean difference (95% CI): 14% (6%–23%), p = .003) from the same blood sample in a two-tailed student's t-test. Assuming the latter glucometer is correct, this discrepancy could account for the elevated values. While the average BMI was within the normal range, several participants would be categorized as overweight. Many of the participants in our study were students in the Exercise and Nutritional Sciences department and/or physically active. Thus, the BMI values could be skewed higher by higher than average muscle mass. Nevertheless, we can not rule out the possibility of participants having undiagnosed prediabetes. The CDC estimated a prediabetes prevalence of 28% for US adults between the ages of 18 and 44 years from 2017 to 2020 with 50% of these being undiagnosed [34]. However, whether or not people were (pre)diabetic based on fasting blood glucose should not affect the study outcomes or analysis as it represents a repeated measures design, and the effects of exercise are robust in people across the glucose tolerance spectrum.
Strengths of the current study include its randomized crossover design, the external validity of mixed meal matching population intake, and corroboration of previous findings in a separate cohort. There are several limitations to our study. The timing of the exercise intervention missed the peak PBG and thus likely underestimated the potential magnitude of glucose reduction. The homogenous cohort studied herein warrants replication in other populations to confirm our results. The presence of undiagnosed diabetes in our participants could not be confirmed in our study. Body composition measures were not taken and would have given a better insight into the possibility of undiagnosed diabetes and the accuracy of BMI classifications.
Future studies should examine the inter- and intra-individual variability of PBG to determine whether personalized timing of postprandial exercise interventions is feasible for public health strategies.
5. Conclusion
Stair climbing and descending at a self-selected comfortable pace reduces postprandial blood glucose in both men and women. SCD may not be suitable for populations at greater risks of falls or with preexisting joint issues, however, these results further highlight the potential value of SCD bouts as a public health intervention. The need for highly generalizable physical activity interventions is greatly needed as the CDC's 2022 National Diabetes Statistics Report estimates 49% of American adults have some form of diabetes [35].
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval
The study was approved by the Institutional Review Board at San Diego State University.
Consent to participate
All participants provided written informed consent.
Consent for publication
All authors approve of the submitted manuscript.
CRediT authorship contribution statement
Jeff M. Moore: designed research, conducted research, analyzed data or performed statistical analysis; , wrote paperand, had primary responsibility for final content. Cameron Vinoskey: conducted research. Hannah Salmons: conducted research. Shirin Hooshmand: provided essential reagents and materials, analyzed data, or performed statistical analysis; and. Jochen Kressler: designed research, analyzed data or performed statistical analysis, wrote paper.
Declaration of competing interest
None.
References
- 1.World Health Organization . WhoInt; 2018. Physical activity.https://www.who.int/news-room/fact-sheets/detail/physical-activity [Google Scholar]
- 2.Guthold R., Stevens G.A., Riley L.M., Bull F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Global Health. 2018;6:e1077–e1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 3.CDC . Centers for Disease Control and Prevention; 2021. Physical activity data & Statistics.https://www.cdc.gov/physicalactivity/data/index.html [Google Scholar]
- 4.Jiang J., Zhao L., Lin L., Gui M., Aleteng Q., Wu B., et al. Postprandial blood glucose outweighs fasting blood glucose and HbA1c in screening coronary heart disease. Sci Rep. 2017;7 doi: 10.1038/s41598-017-14152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Temelkova-Kurktschiev T.S., Koehler C., Henkel E., Leonhardt W., Fuecker K., Hanefeld M. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23:1830–1834. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 6.Lind M., Tuomilehto J., Uusitupa M., Nerman O., Eriksson J., Ilanne-Parikka P., et al. The association between HbA1c, fasting glucose, 1-hour glucose and 2-hour glucose during an oral glucose tolerance test and cardiovascular disease in individuals with elevated risk for diabetes. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalot F., Pagliarino A., Valle M., Martino L.D., Bonomo K., Massucco P., et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: lessons from the san luigi gonzaga diabetes study. Diabetes Care. 2011;34:2237–2243. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pareek M., Bhatt D.L., Nielsen M.L., Jagannathan R., Eriksson K.-F., Nilsson P.M., et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: a prospective population-based cohort study. Diabetes Care. 2018;41:171–177. doi: 10.2337/dc17-1351. [DOI] [PubMed] [Google Scholar]
- 9.Moore J., Salmons H., Vinoskey C., Kressler J. A single one-minute, comfortable paced, stair-climbing bout reduces postprandial glucose following a mixed meal. Nutr Metabol Cardiovasc Dis. 2020;30 doi: 10.1016/j.numecd.2020.06.020. 1967–72. [DOI] [PubMed] [Google Scholar]
- 10.Bartholomae E., Johnson Z., Moore J., Ward K., Kressler J. Reducing glycemic indicators with moderate intensity stepping of varied, short durations in people with pre-diabetes. J Sports Sci Med. 2018;17:680–685. [PMC free article] [PubMed] [Google Scholar]
- 11.Moore J., Bartholomae E.M., Ward K., Hooshmand S., Kressler J. Three minutes moderate-intensity stair walking improves glucose and insulin but not insulin sensitivity or total antioxidant capacity. Nutr Metabol Cardiovasc Dis. 2021 doi: 10.1016/j.numecd.2021.10.016. 0. [DOI] [PubMed] [Google Scholar]
- 12.Trost S.G., Owen N., Bauman A.E., Sallis J.F., Brown W. Correlates of adults' participation in physical activity: review and update. Med Sci Sports Exerc. 2002;34:1996–2001. doi: 10.1097/00005768-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Moore J.M., Bartholomae E., Ward K., Kressler J. Postprandial glucose response moderation by cardiorespiratory fitness following short exercise bouts. J Sports Med Phys Fit. 2020 doi: 10.23736/S0022-4707.20.10426-2. [DOI] [PubMed] [Google Scholar]
- 14.Bartholomae E.M., Moore J., Ward K., Kressler J. Sex differences in postprandial glucose response to short bouts of exercise: a randomized controlled trial. J Sci Med Sport. 2019;22:181–185. doi: 10.1016/j.jsams.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Whittaker A.C., Eves F.F., Carroll D., Roseboom T.J., Ginty A.T., Painter R.C., et al. Daily stair climbing is associated with decreased risk for the metabolic syndrome. BMC Publ Health. 2021;21:923. doi: 10.1186/s12889-021-10965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC . Centers for Disease Control and Prevention; 2020. Prompts to encourage physical activity.https://www.cdc.gov/physicalactivity/activepeoplehealthynation/strategies-to-increase-physical-activity/prompts-to-encourage-physical-activity.html [Google Scholar]
- 17.Overview | Physical activity in the workplace | Guidance | NICE n.d. https://www.nice.org.uk/guidance/ph13
- 18.Centre for Health Protection Department of health - benefits of stair climbing n.d. https://www.chp.gov.hk/en/static/90006.html
- 19.Bailey D.P., Broom D.R., Chrismas B.C.R., Taylor L., Flynn E., Hough J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl Physiol Nutr Metabol. 2016;41:324–331. doi: 10.1139/apnm-2015-0462. [DOI] [PubMed] [Google Scholar]
- 20.Dunstan D.W., Kingwell B.A., Larsen R., Healy G.N., Cerin E., Hamilton M.T., et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care. 2012;35:976–983. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey P.C., Blankenship J.M., Larsen R.N., Sacre J.W., Sethi P., Straznicky N.E., et al. Interrupting prolonged sitting in type 2 diabetes: nocturnal persistence of improved glycaemic control. Diabetologia. 2017;60:499–507. doi: 10.1007/s00125-016-4169-z. [DOI] [PubMed] [Google Scholar]
- 22.Champion R.B., Smith L.R., Smith J., Hirlav B., Maylor B.D., White S.L., et al. Reducing prolonged sedentary time using a treadmill desk acutely improves cardiometabolic risk markers in male and female adults. J Sports Sci. 2018;36:2484–2491. doi: 10.1080/02640414.2018.1464744. [DOI] [PubMed] [Google Scholar]
- 23.Bhammar D.M., Sawyer B.J., Tucker W.J., Gaesser G.A. Breaks in sitting time: effects on continuously monitored glucose and blood pressure. Med Sci Sports Exerc. 2017;49:2119–2130. doi: 10.1249/MSS.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 24.Warburton D.E.R., Jamnik V.K., Bredin S.S.D., Gledhill N. The physical activity readiness Questionnaire for everyone (PAR-Q+) and electronic physical activity readiness medical examination (ePARmed-X+) Health Fit J Can. 2011;4:3–17. doi: 10.14288/hfjc.v4i2.103. [DOI] [Google Scholar]
- 25.Riebe D., Franklin B.A., Thompson P.D., Garber C.E., Whitfield G.P., Magal M., et al. Updating ACSM's recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473–2479. doi: 10.1249/MSS.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 26.Shukla A.P., Andono J., Touhamy S.H., Casper A., Iliescu R.G., Mauer E., et al. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5 doi: 10.1136/bmjdrc-2017-000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino K., Sakurai M., Takeshita Y., Takamura T. Consuming carbohydrates after meat or vegetables lowers postprandial excursions of glucose and insulin in nondiabetic subjects. J Nutr Sci Vitaminol. 2018;64:316–320. doi: 10.3177/jnsv.64.316. [DOI] [PubMed] [Google Scholar]
- 28.ISO 15197:2013(en) In vitro diagnostic test systems — requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus n.d. https://www.iso.org/obp/ui/#iso:std:iso:15197:en
- 29.Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 30.Dempsey P.C., Larsen R.N., Sethi P., Sacre J.W., Straznicky N.E., Cohen N.D., et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016;39:964–972. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- 31.Aadland E., Høstmark A.T. Very light physical activity after a meal blunts the rise in blood glucose and insulin. Open Nutr J. 2008;2 [Google Scholar]
- 32.Nygaard H., Tomten S.E., Høstmark A.T. Slow postmeal walking reduces postprandial glycemia in middle-aged women. Appl Physiol Nutr Metabol. 2009;34:1087–1092. doi: 10.1139/H09-110. [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Association American. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2020;44 doi: 10.2337/dc21-S002. S15–33. [DOI] [PubMed] [Google Scholar]
- 34.Prevalence of Prediabetes Among Adults | Diabetes | CDC 2022. https://www.cdc.gov/diabetes/data/statistics-report/prevalence-of-prediabetes.html
- 35.National Diabetes Statistics Report | Diabetes | CDC 2022. https://www.cdc.gov/diabetes/data/statistics-report/index.html