Highlights
-
•
Long term seizure outcome in children following epilepsy surgery is favorable.
-
•
Histopathology is an independent determinant of long-term seizure outcome.
-
•
Long-term seizure outcome remains stable in children with tumours and FCD type 2b, whereas seizure freedom declines with time in children with other types of FCD and cortical malformations.
-
•
Children with moderate to severe developmental disability and younger age of seizure onset have higher seizure recurrence reflecting broader epileptic networks.
Keywords: Focal cortical dysplasia, Epilepsy, Epilepsy surgery, Tumours
Abbreviations: FCD, Focal cortical dysplasia; MRI, Magnetic Resonance Imaging; ASM, Anti seizure medication; DEE, Developmental epileptic encephalopathy; FDG-PET, Fluorodeoxyglucose positron emission tomography; EEG, Electro encephalogram; SPECT, Single-photon emission computed tomography; ECoG, Electrocorticography
Abstract
There is a paucity of data on longitudinal seizure outcome of children undergoing epilepsy surgery. All children (n = 132) who underwent resective epilepsy surgery from January 1998 to December 2015 were identified. Relevant clinical, neurophysiological, imaging, surgical and seizure outcome data were extracted. Multivariable logistic regression analysis and Kaplan-Meier survival with Cox proportional hazard modelling were performed. The mean age at surgery was 7.8 years (range 0.2–17.9). 71% were seizure-free at a mean follow up of 5.3 ± 2.7 years. Of those who were seizure-free, 65 patients were able to completely wean off anti- seizure medications successfully. Using survival analysis, the probability of Engel Class I outcome at one year after surgery was 81% (95% confidence interval [CI] 87%–75%). This dropped to 73% at two years (95% CI 81%–65%), 58% at five years (95% CI 67.8%–48%), and 47% at ten years. Proportional hazard modelling showed that the presence of moderate to severe developmental disability (HR 6.5; p = 0.02) and lack of complete resection (HR 0.4; p = 0.02) maintain association as negative predictors of seizure-free outcome. Our study demonstrates favorable long-term seizure control following pediatric epilepsy surgery and highlights important predictors of seizure outcome guiding case selection and counseling of expectations prior to surgery.
Introduction:
Up to one third of children with epilepsy will have seizures that are drug-resisatnt, defined as failing proper trials of at least two appropriate anti-seizure medications (ASMs) [1]. A 2019 Cochrane review on epilepsy surgery examined 182 studies and found that among 16,756 adults and children who underwent epilepsy surgery, 64% achieved seizure freedom [2]. An abnormal pre-operative MRI, complete surgical resection, absence of focal cortical dysplasia (FCD), presence of tumor or mesial temporal sclerosis and right-sided resection were factors associated with better postsurgical seizure outcomes [2]. Most studies on epilepsy surgery have been performed in adults or mixed adult/pediatric populations or focused on individual types of surgeries or histopathology. This is further compounded by a wide range of follow up with limited information on longitudinal outcomes following epilepsy surgery.. This has hindered the analysis of the presurgical factors that influence the surgical outcome in childhood epilepsies.
In 7–35% of children, seizures recur with a longer duration of follow-up after epilepsy surgery (Suppl Table) [3], [4], [5], [6]. Hence, it is essential to understand long-term seizure outcomes in order to counsel parents to guide preoperative expectations, follow-up, and ASM withdrawal. Unfortunately, there is a paucity of data on longitudinal seizure outcome beyond 2 years and its predictors in children [7]. Supplementary Table 1 summarizes studies which reported seizure outcomes with a mean or median follow up of more than three years. It is important to note that most of these studies were cross-sectional and applied limited statistical methods, such as survival analysis and hazard modeling, to account for variable follow-up durations. Only nine studies performed logistic regression analysis (that would allow an analysis of the relationship between variables), and only three performed Cox proportional hazard modeling using a selected groups of patients for surgeries such as temporal/extratemporal resection and hemispherectomy [3], [8], [9]. There is also significant heterogeneity in the predictive factors depending on the patient population selected in the individual study. For example, the proportion of patients in Engel class I at 5 year follow up varied widely from 33% to 87.5%, with seizure freedom being highest in studies reporting a larger proportion of patients with tumors and mesial temporal sclerosis [5], [10], [11] and the lowest in FCD [3], [4], [12], [13].
In this study, we evaluated the longitudinal seizure outcomes of children and adolescents who had a resective epilepsy surgery over a 17-year period and analyzed presurgical or surgical factors predictive of seizure outcome.We used the statistical methods involving survival analysis and proportional hazard modeling to evaluate the rate, stability, and predictors of seizure freedom while accounting for variation in the duration of follow-up among patients.
Patients and methods
This study was approved by the HREC of Sydney Children’s Hospital Network (LNRSSA/14/SCHN/283). All children and adolescents (under 18yrs) who underwent resective epilepsy surgery at the Children’s Hospital at Westmead between January 1998 to December 2015 were identified using an institutional database. The patients who underwent palliative procedures such as corpus callosotomy were excluded. All patients underwent detailed presurgical evaluation, including video-EEG monitoring and MRI using an epilepsy protocol (high resolution with 3D, T1, and 3D FLAIR sequences). In addition, functional imaging, including Fluorodeoxyglucose positron emission tomography (PET) and subtraction ictal single photon emission computed tomography (SPECT), was performed when EEG and MRI results were non-localized or non-lesional. The epilepsy multimodal data, including clinical characteristics of epilepsy, electrophysiology, MRI, functional imaging and MRI co-registration data such as subtraction ictal SPECT coregistered on MRI and MRI-PET. These, were discussed in the comprehensive multidisciplinary epilepsy surgery meeting, and a consensus was reached regarding concordance and surgical candidacy. In patients where the electroclinical syndrome was consistent with a focal etiology and MRI was unrevaling, invasive monitoring (stereo EEG and subdural grid/depth electrodes) was used to determine the epileptogenic zone. Electrocorticography (ECoG) was used in selected cases where the lesion had ill-defined margins or was close to eloquent cortex (combined with evoked potentials) or where the lesion was known to be associated with FCD. Only those who had at least 12 months of follow-up after surgery were included. One hundred and fifty-eight patients fulfilled the inclusion criteria. Four patients were excluded because there was no presurgical information available. Three were excluded as they had aggressive tumors for which surgery was performed as a palliative procedure. A further 19 patients did not have follow-up information available beyond 12 months. The final cohort consisted of 132 children and adolescents comprising 69 males and 63 females.
A retrospective review of each patient’s medical record was performed to obtain demographic information, including gender, neurodevelopmental status and other relevant medical history, including perinatal adverse events, brain infections, febrile convulsions and family history of epilepsy. Presurgical neurodevelopmental status was based on the results of formal neuropsychological assessment when these were available and from medical records. Patients were classified based on their developmental quotient (developmental age/chronological age) as either: normal (>85), mild (75–85), moderate (55–75) or severe disability (<55). Age at seizure onset, seizure type, frequency and duration, presence of developmental and epileptic encephalopathy (DEE) [14], and the number of ASMs trialled was recorded. Reports from presurgical scalp EEG, MRI, FDG-PET and ictal SPECT were reviewed, and findings were classified based on the focality of abnormalities using a standardised template (focal, multifocal, hemispheric or normal).
Surgical details collected included the type of surgery performed, completeness of resection, and use of invasive monitoring. We defined completeness of resection as the complete removal of the epileptogenic zone defined by intraoperative EEG and removal of the lesion based on the postoperative MRI. Acute postoperative seizures were defined as seizures occurring in the first week postoperative period. Information about histopathology was gained from formal histopathology reports. Follow-up information was obtained from outpatient clinic visits. The usual follow-up schedule consisted of outpatient clinic visits at three postoperative months, six months, one year, and then yearly or as needed. The seizure outcome at the time of the last follow up was assessed based on the Engel Classification System [4]. For the purpose of the study, we considered the primary outcome as seizure freedom since surgery at the longest follow-up appointment available for each patient.
Statistical analysis
The data were summarized with descriptive statistics for each variable, including frequency for categorical variables, and mean, median and standard deviations for continuous variables. The seizure outcome at the time of the last follow up was classified as either seizure-free (Engel 1a) or not seizure-free. Each of the presurgical and surgical variables was tested for association with the seizure-free and not seizure-free groups. Univariable analysis was performed using the chi-square test for categorical predictors and logistic regression analysis for continuous predictors of binary outcome of seizure freedom. All presurgical variables with p-value of <0.1 in univariable analysis were included in a multivariable logistic regression model using backward elimination. No adjustment was made for multiple group statistical comparisons, as this study was largely exploratory with an intention to study the predictors of seizure outcome.
In addition, we have performed a time to event analysis to account for the variable follow-up duration after epilepsy surgery. Kaplan- Meier survival analysis was used first to describe the probability of Engel class 1 outcome in the overall group and later by considering each of the risk factors. Predictors of seizure outcomes were explored using the log-rank test, which allowed the identification of potential prognostic indicators. Variables with a p-value of <0.1 on the log rank test were then tested in a multivariable Cox proportional hazards regression model. SAS statistical software version 9.4 was used for analysis.
Results
Medical history
Table 1 summarizes the clinical characteristics of the overall group, as well as the seizure outcome for each presurgical and surgical variable studied. The mean age at seizure onset was 3.5 years (range 1 day-15.7yrs), and the average number of ASM trials preoperatively was 4 (range 1–14). Most patients (78%) had daily seizures, some of which lasted >60 s (39%) prior to surgery. Fifty-one children (39%) had one or more of the etiological factors studied: perinatal adverse event in 19 (14%), history of febrile seizures in 11 (8%), history of CNS infection in 3 (2%) and family history of epilepsy in 18 (14%). At the time of surgery, 97 patients (73%) had normal neurodevelopment or mild disability, while 29 (22%) had a moderate developmental disability and 6 (5%) had a severe developmental disability. Of 43 patients, 39 had DEE, whereas four patients did not have preexisting cognitive difficulties prior to the onset of epilepsy.
Table 1.
Demographic and clinical characteristics of the children according to seizure freedom after their epilepsy surgery.
| a) Presurgical Characteristics | ||||
|---|---|---|---|---|
| OUTCOME |
||||
| Overall group n = 132 (except where specified) | Seizure-free | Not seizure-free | p-value | |
| (n = 94) | (n = 38) | |||
| Gender (males) | 69 (52) | 47 (50) | 22 (58) | 0.41 |
| Mean age at seizure onset (years) | 3.5 (range 0.003–15.7) | 3.9 (0–15.7) | 2.4 (0–14) | 0.04* |
| Mean number ASM’s trialled | 4.0 (range 1–14) | 4 (1–14) | 5 (1–9) | 0.03* |
| Seizure frequency and duration | ||||
| Seizure frequency >1/day | 103 (78) | 73 (78) | 30 (79) | 0.87 |
| Seizure duration >1 min | 51 (39) | 33 (35) | 18 (47) | 0.19 |
| Seizure characteristics (categories not exclusive) | ||||
| Focal | 58 (44) | 46 (49) | 12 (32) | 0.07 |
| Focal impaired awareness | 79 (60) | 63 (67) | 16 (42) | 0.008* |
| Generalized seizures | 46 (35) | 28 (30) | 18 (47) | 0.055 |
| Focal to bilateral tonic clonic | 50 (38) | 34 (36) | 16 (42) | 0.52 |
| History of status epilepticus | 33 (25) | 21 (22) | 12 (32) | 0.27 |
| Presence of DEE | ||||
| Yes | 39 (29.5) | 20 (51) | 19 (48.7) | 0.001* |
| No | 93 (70.5) | 74 (79.5) | 19 (20.5) | |
| MRI findings | ||||
| Focal lesion | 82 (62) | 61 (74) | 21 (26) | 0.66 |
| Multifocal lesion | 29 (22) | 18 (62) | 11 (38) | |
| Hemispheric lesion | 11 (8) | 8 (73) | 3 (27) | |
| Normal | 10 (8) | 7 (70) | 3 (30) | |
| Interictal EEG findings (n = 130) | ||||
| Regional discharges | 63 (48) | 50 (79) | 13 (21) | 0.1 |
| Multiregional discharges | 51 (39) | 31 (60) | 20 (39) | |
| Hemispheric discharges | 8 (6) | 5 (63) | 3 (38) | |
| Normal | 8 (6) | 7 (88) | 1 (13) | |
| Ictal EEG findings (n = 120) | ||||
| Regional discharges | 73 (61) | 54 (74) | 19 (26) | 0.46 |
| Multiregional discharges | 34 (27) | 21 (62) | 13 (34) | |
| Hemispheric discharges | 10 (8) | 6 (60) | 4 (40) | |
| Normal | 3 (3) | 2 (67) | 1 (33) | |
| FDG-PET findings (n = 86) | ||||
| Focal hypometabolism | 58 (67) | 43 (74) | 15 (25) | 0.03* |
| Multifocal hypometabolism | 15 (17) | 6 (40) | 9 (60) | 0.016* |
| Hemispheric hypometabolism | 3 (3) | 3 (100) | 0 (0) | 0.98 |
| No localising metabolic focus | 10 (12) | 8 (80) | 2 (20) | 0.69 |
| SPECT findings (n = 67) | ||||
| Focal hyperperfusion | 41 (62) | 31 (76) | 10 (24) | 0.1 |
| Multifocal hyperperfusion | 12 (18) | 5 (42) | 7 (58) | |
| Hemispheric hyperperfusion | 2 (3) | 1 (50) | 1 (50) | |
| No perfusion abnormality | 12 (18) | 6 (50) | 6 (50) | |
| b) Surgery and histopathology characterstics | ||||
|---|---|---|---|---|
| OUTCOME |
||||
| Overall group n=132 (except where specified) | Seizure-free | Not seizure-free | p-value | |
| (n=94) | (n=38) | |||
| Mean age at surgery (years) | 7.8 (0.2–17.9) | 8.3 (0.5–17.9) | 6.6 (0.2–17.9 | 0.66 |
| Mean duration epilepsy to surgery | 4.2 (0.1–15) | 4.3 (0.1–14.6) | 4.2 (0.1–13.4) | 0.83 |
| Surgery side (Left) | 70 (53) | 52 (55) | 18 (47) | 0.79 |
| Surgery location | ||||
| Frontal | 45 (34) | 33 (73) | 12 (27) | 0.89 |
| Temporal | 38 (29) | 27 (71) | 11 (29) | |
| Parietal | 9 (7) | 5 (56) | 4 (44) | |
| Occipital | 5 (4) | 3 (60) | 2 (40) | |
| Insular | 2 (2) | 2 (100) | 0 (0) | |
| Hypothalamic | 1 (0.8) | 1 (100) | 0 (0) | |
| Hemispheric | 13 (10) | 10 (77) | 3 (23) | |
| Multi-lobar | 19 (14) | 13 (68) | 6 (32) | |
| Extent of surgical resection | ||||
| Focal (lesionectomy) | 81 (61) | 63 (78) | 18 (22) | 0.06 |
| Unilobar Lobectomy | 33 (25) | 19 (58) | 14 (42) | |
| Multilobar Lobectomy | 5 (4) | 2 (40) | 3 (60) | |
| Hemispherectomy | 13 (10) | 10 (77) | 3 (23) | |
| Use of invasive monitoring | 23 (17) | 18 (19) | 5 (22) | 0.41 |
| Use of ECoG | 74 (56) | 52 (55) | 22 (58) | 0.79 |
| Complete resection | 116 (88) | 86 (91) | 30 (79) | 0.046* |
| Acute postoperative seizures | 25 (20) | 14 (15) | 11 (29) | 0.06 |
| Histopathology | ||||
| FCD1 | 19 | 9 (47%) | 10 (53) | 0.045* |
| FCD2 | 34 | 26 (77%) | 8 (23%) | |
| FCD3 | 15 | 11 (73%) | 4 (27) | |
| Other cortical malformations | ||||
| -Hemimegalencephaly | 2 | 1 (50%) | 1 (50%) | |
| - Tuberous sclerosis | 7 | 3 (43%) | 4 (57%) | |
| - Heterotopia | 10 | 6 (60%) | 4 (40%) | |
| - Polymicrogyria | 2 | 2 (100%) | 0 (0%) | |
| Tumours | 25 | 23 (92%) | 2 (8%) | |
| Vascular | ||||
| - Previous stroke | 3 | 3 (100%) | (0%) | |
| Vascular malformation | 7 | 5 (71%) | 2 (29%) | |
| Postencephalitis | ||||
| - Rasmussens | 3 | 3 (100%) | 0 (0%) | |
| - Other encephalitis | 1 | 0 (0%) | 1 (100%) | |
| Others | 4 | 2 (50%) | 2 (50%) | |
Abbreviations: OR, Odd Ratio; ASM, Anti seizure medication; GTCS, Generalized tonic clonic seizures; DEE, developmental and epileptic encephalopathy; MRI, Magnetic resonance imaging; EEG, Electroencephalogram; FDG-PET, Fluorodeoxyglucose positron emission tomography; SPECT, Single-photon emission computed tomography; FCD, Focal cortical dysplasia;
*See Results section for more details.
Presurgical EEG and imaging findings
Interictal and ictal scalp EEG results are presented in Table 1a. Invasive monitoring, either using subdural or stereo-EEG electrodes, was used to define the lesion in 23 patients (17%). ECoG at the time of surgery was used in 74 (56%) of patients. All patients had presurgical MRI, and lesions were classified according to their extent (Table 1). Sixty-one cases (46%) were radiologically classified as FCD, 10 (8%) as malformations of the cortex (hemimegalencephaly, polymicrogyria, Tuberous sclerosis) and 26 as tumors (20%). Other radiological diagnoses included vascular lesions (including stroke, encephalomalacia, Sturge Weber syndrome in 13 (10%), post-encephalitic changes (including Rasmussen's syndrome) in 4 (3%) and 8 patients (6%) with other findings (mesial temporal sclerosis, hypothalamic hamartoma, uncertain but definite signal abnormality). Ten patients (8%) had no lesion identified on MRI. Of 86 patients who had FDG-PET, a focal area of hypometabolism was demonstrated in 58 patients (67%). SPECT scan was performed in 67 patients and showed concordant focal hyperperfusion in 41 patients (61%).
Surgical procedures and histopathology
The mean age at surgery was 7.8 years (range 0.2–17.9). The details of resective surgical procedures are presented in Table 1. The types of surgery included extra temporal (n = 61, 46%)), temporal (n = 38, 28.7%), multilobar (n = 19, 14.3%), hemispheric (n = 13, 10%) and hypothalamic (n = 1). Acute postoperative seizures occurred in 25 patients (20%). Eighteen patients (14%) required repeat surgery, with 4 of these patients requiring 3 surgeries in total. Nine patients had an extension of the original resection area, three had lobectomy after failed focal resection and 4 patients proceeded to hemispherectomy. Two patients with Tuberous Sclerosis required resection of additional tubers. One patient had a hypothalamic tumor which required debulking on 3 occasions before she was rendered seizure-free. Two patients went on to have corpus callosotomy due to ongoing drug-resistant seizures. 10 out of 18 patients (55%) became seizure-free after repeat surgery.
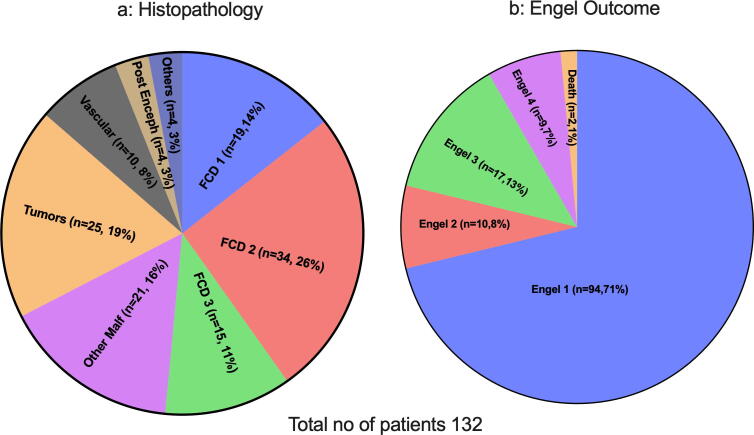
Histopathology details are presented in Fig. 1a and Table 1b. FCD was the most frequent diagnosis occurring in 68 (52%) patients in total. Tumors included dysembryoplastic neuroepithelial tumor in 13 (10%), ganglioglioma in six (5%) and other tumors in a further six patients (5%). One patient had hypothalamic hamartoma, two patients had nonspecific gliotic changes, and a histopathological diagnosis was not available for one patient. Of the patients who had a negative pre-operative MRI, 7 were found to have FCD and 3 had heterotopia.
Fig. 1.
a) Histopathology results and b) surgical outcome using Engel outcome, at last follow up (Mean 5.3 ± 2.7 years).* See results for more details. Abbreviation FCD, Focal cortical dysplasia; Enceph, Encephalitis; Malf, Malformations.
Seizure outcomes and their predictors
The mean duration of postoperative follow-up was 5.3 ± 2.7 years (range 1–16.9 years). Of all patients, 76 had follow up between 1 and 5 years. Forty patients had follow up for >5 yrs, whereas 14 patients were followed up for longer than 14 years. At last follow up, ninety-four patients (71%) were Engel class I (Fig. 1b). This included 10 patients who had required repeat surgery to render them seizure-free. A further 10 patients (8%) were Engel class 2, 17 (13%) were Engel class 3, and 9 (7%) were Engel class 4. Two patients died due to drug-resistant uncontrolled seizures 3 and 7 years after the epilepsy surgery respectively; both had severe DEE. Out of the 94 patients in the Engel class 1 category, 65 (69%) were completely off ASMs at the time of the last follow-up. Of these patients, sixteen patients remained seizure-free for more than five years off ASM and eight patients for more than ten years, latter group fulfilling the ILAE definition of epilepsy cure or resolution. Of 38 patients who had seizure recurrence in follow up, thirty-three patients were still on ASMs.
Comparison of presurgical and surgical variables based on seizure freedom
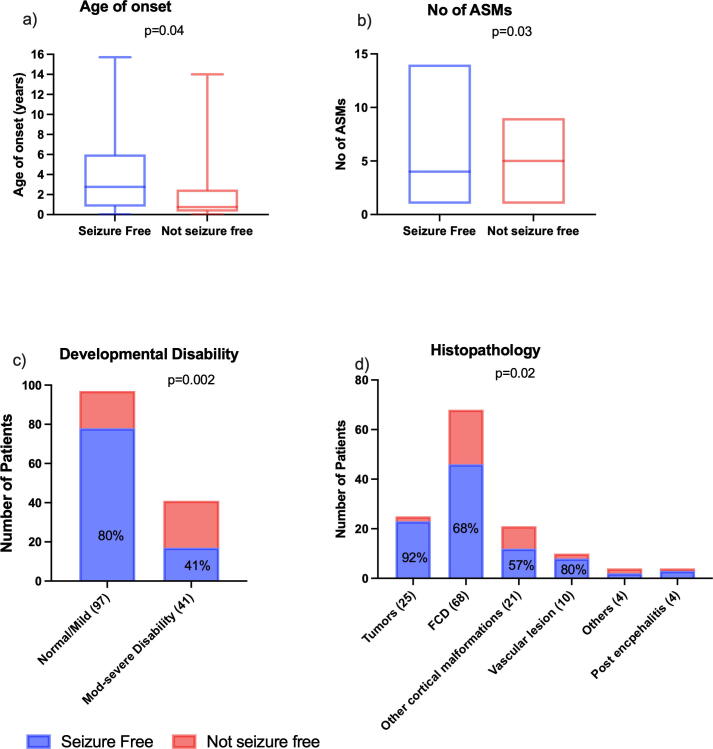
Table 1 and Fig. 2 show the results of univariable analysis based on seizure freedom. Those who had seizure recurrence were more likely to have had earlier onset of seizures, tried more ASMs, and had moderate to severe developmental disability. The presence of focal impaired awareness seizures, unifocal PET, and complete surgical resection were associated with a greater chance of seizure freedom. Children with developmental tumors had lower seizure recurrence compared to those with FCD or other cortical malformations. Further analysis comparing different subtypes of FCD showed that FCD Type 2b had a favorable prognosis compared to those with other subtypes of FCD. Duration of epilepsy, age at surgery, seizure frequency, presence of secondary generalized tonic-clonic seizures, EEG abnormalities, the extent of lesion on MRI, location of surgery, use of ECoG or invasive monitoring and acute postoperative seizures did not influence the surgical outcome.
Fig. 2.
Comparison of seizure outcome by age at seizure onset (A), number of pre-operative ASMs trialled (B), level of pre-operative developmental disability (C) and underlying histopathological diagnosis (D).
On multivariable regression analysis, children with focal impaired awareness seizures were less likely to have seizure recurrence after surgery (Odd's ratio [OR] 0.31, 95% confidence interval [CI] 0.13–0.72, p = 0.007), while children with a moderate or severe developmental disability were more likely to have seizure recurrence compared to those with normal or mild disability (moderate developmental disability: OR 5.37, 95% CI 1.88–15.28, p = 0.002, severe developmental disability: OR 27.9, 95% CI 2.76–28.2, p = 0.005).
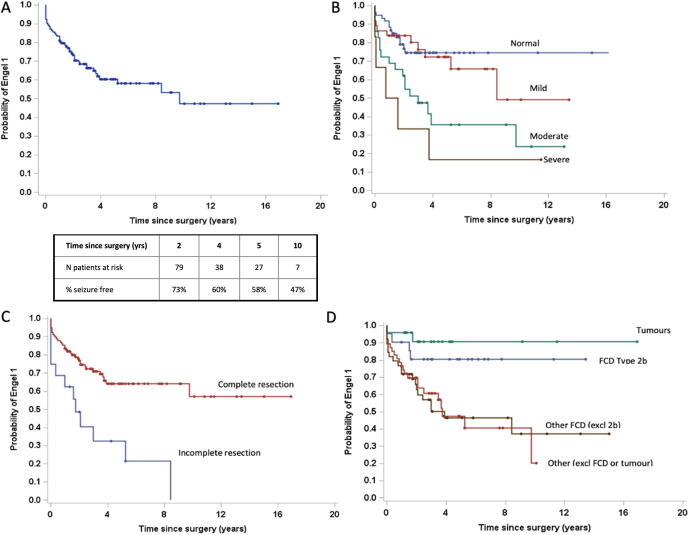
Longitudinal seizure outcome
Fig. 3a shows the longitudinal Engel 1 outcomes for all patients using Kaplan-Meier survival curves. At 1 year, the estimated seizure freedom based on Engel I was 81% (95%CI (87%–75%). This dropped to 73.4% at 2 years (95% CI 81%–65%) and further declined with a longer follow-up: 58% at 5 years (95% CI 67.8%–48%) and 47.3% at 10 years. Fifty percent of seizure recurrences occurred within 12 months of surgery. Seizure freedom remained relatively stable after 4 years postoperatively. Upon applying proportional hazard modelling, the presence of moderate to severe developmental disability (p = 0.01), complete resection (p = 0.01), and histopathology of FCD (p = 0.05) were predictors of a seizure-free outcome. (Fig. 3b-d) (Table 2). On subgroup analysis, the presence of moderate to severe developmental disability (HR 6.5 relative to no disability; CI 1.8–23.7; p = 0.018) and absence of complete resection (HR 0.4; CI 0.2–0.8; p = 0.017) maintain association as negative predictors of seizure-free outcome.
Fig. 3.
Kaplan-Meier survival curves illustrating the cumulative probability of Engel Class 1 outcome at last follow-up for all patients (A) and depending on presurgical level developmental disability (B)(p = 0.01), completeness of resection (C)(p = 0.01), and histopathological diagnosis (D)) (p = 0.05). B-D variables were identified as independent predictors of seizure freedom on Cox proportional hazard modelling*. Abbreviation: excl: Excluding.
Table 2.
Cox proportional hazard models* for postsurgical seizure freedom at last available follow-up. Covariate p-value, adjusted hazard ratio, and 95% CI for hazard ratio are reported.
| Characteristic | p-value | hazard ratio | 95% CI of hazard ratio |
|---|---|---|---|
| Complete Resection | 0.017 | 0.428 | 0.214–0.858 |
| Age of seizure onset | 0.634 | 1.002 | 0.993–1.011 |
| Developmental disability | 0.018 | ||
| Mild | 0.931 | 1.041 | 0.418–2.59 |
| Mod | 0.211 | 1.794 | 0.718–4.48 |
| Severe | 0.005 | 6.471 | 1.765–23.728 |
| PET abnormalities | 0.305 | ||
| Multiregional | 0.333 | 1.509 | 0.657–3.467 |
| Hemispheric | 0.986 | 0.000 | 0.000 |
| Normal | 0.187 | 0.344 | 0.071–1.676 |
| Histopathology | 0.049 | ||
| FCD other than Type2b | 0.121 | 2.451 | 0.789–7.614 |
| Tumour | 0.318 | 0.408 | 0.070–2.368 |
| Other etiology | 0.092 | 2.680 | 0.852–8.427 |
*Normal development, focal PET abnormalities, and FCD type 2b (focal cortical dysplasia) have been used as reference categories for development, PET and histopathology categories. CI, confidence interval.
Discussion
In this study, we describe the long-term seizure outcome in 132 children and adolescents who underwent resective epilepsy surgery at a large tertiary children’s hospital. Our study shows favorable long term seizure outcomes for the majority of patients: 71% of patients were seizure-free with a mean follow up of 5.3 years. Of those patients who were seizure-free, 65 patients were able to completely withdraw ASM treatment successfully. These results are comparable to other studies on children undergoing any type of resective epilepsy surgery [11], [15], [16], [17], [18], [19]. Similarly, long term seizure-free outcome in our study is comparable to adults at 5 yrs (36–79%) and 10 yrs (41%–56%) [18], [20], [21] despite higher numbers of cortical malformations and extratemporal surgeries and lower numbers of mesial temporal sclerosis. However, 49% of the children in our study were seizure- and medication-free compared to 10–40% of adults suggesting children are more likely to achieve seizure freedom with the ability to successfully wean ASMs postoperatively. [18], [22].
In our study, we have reported seizure freedom after adjusting for length of follow-up using Kaplan-Meier survival curves in all types of surgeries. It is apparent from our study that, as time passes, the seizure freedom rate declines: 73.4% at 2 years, 58% at 5 years and 47.3% at 10 years. This contradicts the notion that has previously been suggested that outcome at 2 years is predictive of long-term outcome [23], [24], [25]. In our study, Engel 1 status remained relatively stable after 4 years, after which only 3 patients had seizure recurrence. Previous studies showed that early recurrence is more likely to be due to incomplete resection as evident by seizure freedom in up to half of cases after re-operation in our study [26]. The reason for recurrence in patients with complete resection remains unclear, although it may involve genetic factors, specific patient characteristics, or secondary epileptogenesis [27], [28].
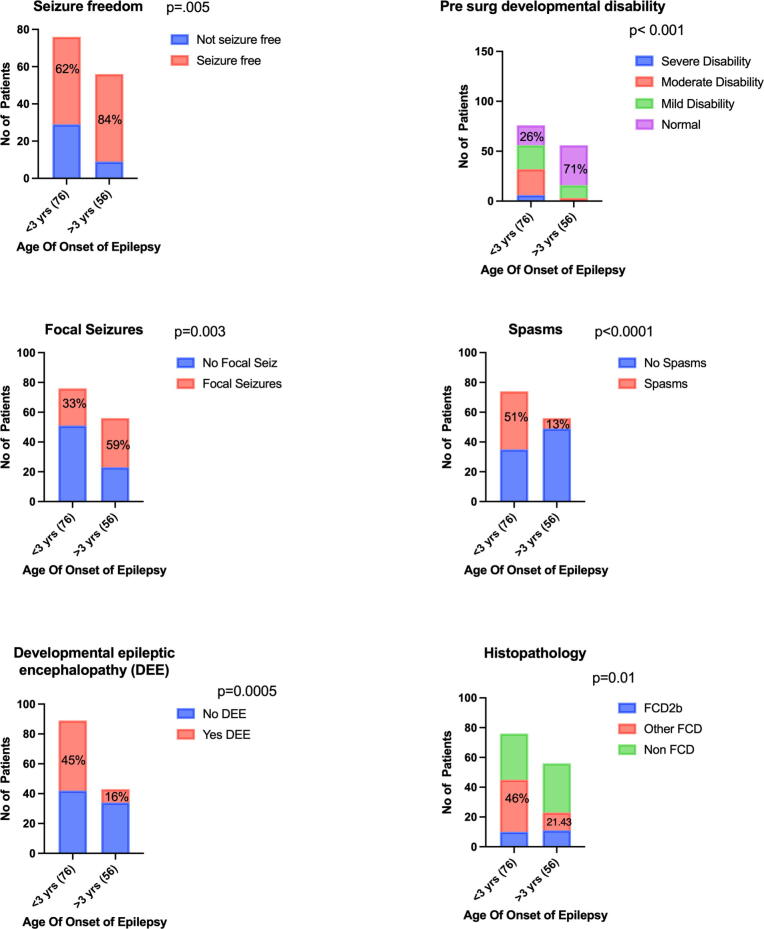
For our cohort of patients, seizure recurrence was associated with younger age at seizure onset, comorbid developmental epileptic encephalopathy, and/or a higher number of failed ASMs. It is likely there is a confounding effect amongst these variables reflecting a subgroup of patients who present in early life with catastrophic epileptic syndromes secondary to widespread epileptogenic networks that are established prenatally or in early postnatal life, and subsequent severe drug resistant epilepsy that is less amenable to surgical intervention [29]. This was further supported by a subgroup analysis comparing children with seizure onset before 3 years of age with those after 3 years of age (supplementary Fig. 1). Those who had seizure onset prior to 3 years of age were more likely to have moderate to severe developmental disability and DEE, whereas older children were more likely to have focal seizures. Children with seizure onset before 3 years of age had a higher prevalence of FCD or other cortical malformations in contrast to older children who had tumors, vascular lesions, postencephalitic lesions or nonlesional etiology.
Moderate to severe developmental disability was strongly predictive of postsurgery seizure recurrence when compared to patients with a normal or mild developmental disability. Furthermore, the rate of seizure freedom decline was greater in children with moderate to severe developmental disabilities. The presence of a developmental disability may explain the severity of underlying brain involvement and epileptic encephalopathy, with extensive epileptogenic networks accounting for decreased seizure freedom. However, it is important not to disregard this group as potential surgical candidates. In fact, 20/39 achieved seizure freedom and a further 12/39 achieved some reduction in seizure frequency (Engel class 2 or 3). This can represent a significant alleviation of the burden of care for these patients, as most had severe drug-resistant epilepsies prior to surgery.
The finding that focal impaired awareness seizure were associated with seizure freedom is not a new finding. This has previously been demonstrated and corresponds to the theory that patients with focal epileptogenic zones, as represented by focal seizure semiologies, are likely to have a clearly defined lesion which can be siccessfully resected, as opposed to those with more diffuse underlying pathology. In our study, age at surgery and duration of epilepsy were not associated with the seizure outcome, similar to other pediatric studies [9], [18].
There was a smaller number of nonlesional cases compared to the published series, possibly due to the advances in MRI technology and use of multimodal imaging [7], [30]. An important negative finding from our study is that the presence of focality of a lesion on MRI did not necessarily correlate with outcome, unlike other studies [31], [2], [32]. We speculate that this may be because of the high use of multimodal investigations and invasive monitoring, which helped to delineate the lesion and lead to a higher chance of complete surgical resection despite nonspecific MRI findings. In our study, 65% of patients underwent PET, and 50% underwent ictal SPECT. This represents a significantly higher use of these investigations compared to that found by Harvey et al. in a survey of 20 pediatric epilepsy centers around the world [33]. Looking specifically at those patients who were MRI negative in our study, 9 out of 10 had invasive monitoring, and the remaining patient had a clear temporal abnormality demonstrated on PET and SPECT and proceeded to a temporal lobectomy. In our study, findings on presurgical FDG-PET scan was found to be associated with seizure recurrence, with those who had multifocal abnormalities having worse long-term seizure outcomes. This finding has not been previously shown in other pediatric studies, but there have been a handful of studies showing the utility of PET in predicting seizure outcome in adult patients where MRI has failed to show a lesion [3], [34], [35]. Taken together, our findings would suggest that all patients presenting with drug-resistant focal seizures should be considered for referral to a pediatric epilepsy surgical centre, even if they initially present with non-focal or nonlesional MRI findings as multimodal investigations, including PET and invasive EEG monitoring, may identify some of these patients as appropriate candidates for successful epilepsy surgery.
As described in our study, completeness of resection is the primary determinant of postoperative seizure freedom as shown by seizure freedom in up to half of the cases after re-operation extending surgical resection [36], [37], In children, temporal resections have consistently been shown to have better outcomes compared to extratemporal resections [2], [15], [31]. Our study did not show any difference in outcomes when comparing the site of surgical resection, either by individual lobe or when temporal resections were compared with extratemporal lesions. Our study demonstrates that the underlying histopathology of the lesion is a more important predictor of long-term seizure outcome, with seizure recurrence higher in FCD and other cortical malformation compared to tumours, similar to previous studies [3], [12], [13], [38], [39]. The rate of seizure freedom remained relatively stable in those with FCD2b and developmental tumors, whereas it declined with time in other FCDs and cortical malformations, highlighting the importance of histopathology in determining the long term seizure outcome [18] (Fig. 3D). It is also interesting to note that even in some children who had extensive lesions, including Rasmussen’s syndrome and hemimegalencephaly, seizure freedom was achieved. This is likely because the extensive nature of these lesions meant that these patients were more likely to undergo hemispherectomy (7 out of 14, 50%), and hence while they attained good seizure outcome, this was also at the cost of permanent postoperative neurological deficit.
Limitations and future directions
The main limitations of this study relate to its retrospective, single center design, making it prone to selection bias, misclassification bias and confounders. While randomized controlled trials would be the gold standard, they are difficult to perform when surgery is the intervention. They would also be limited in ability to define long term outcomes. Realistically, future studies should be of a prospective nature with clearly devised protocols for investigations and follow up. The cohort we have examined is a heterogeneous population with regards to underlying histopathological diagnosis. It is clear that those with simple developmental tumors amenable to surgical resection follow a very different trajectory compared to those with more resistant early onset epileptic encephalopathies. Stratification of patients by different etiologies with separate subgroup analysis may be more useful to prognosticate for an individual patient. However, statistical analysis would be difficult for rare conditions with only a handful of patients (e.g. Rasmussen encephalitis). A further limitation of this study is that we focused on seizure outcomes and did not report on long term quality of life, neuropsychological or educational outcomes. It is known that seizure-freedom is an important determinant of developmental, psychological and quality of life outcomes [40], but it would nevertheless be important to assess these long term outcomes in future studies.
Conclusion
Our study demonstrates favorable long-term seizure control following epilepsy surgery in children with epilepsy and highlights important predictors of outcome. Epilepsy surgery offers a chance to obtain seizure freedom and taper off of chronic ASM in a high proportion of suitable surgical candidates, and our study adds similar results to previous studies on long term surgical outcome in children. Several presurgical and surgical clinical factors serve as predictors of seizure outcome after surgery and can aid optimal patient selection and guide counseling about expectations for long term outcomes. Children with moderate to severe developmental disability, younger age of onset and FCDs (other than FCD type 2b) have higher rates of seizure recurrence, possibly reflecting a more widespread epileptic network. Further prospective studies consisting of national and international, large, homogeneous cohorts are required for accurate data to be collected about long-term seizure outcome and its true relationship to other outcome measures in children after epilepsy surgery.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
Supported by Petre Foundation Scholarship, Epilepsy Research Centre Kids Neuroscience Centre, and Cerebral Palsy Alliance.
Disclosures
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Ethics statement
This study followed research ethics guidelines and was approved by the Human Research Ethics Committees of Sydney Children’s Hospital Network (LNRSSA/14/SCHN/283).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ebr.2022.100561.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Comparison of a) seizure freedom, b) pre-operative developmental disability, c&d) type of seizures, e) developmental epileptic encephalopathy and f) histopathology in children based on the age of onset of epilepsy (under 3 versus over 3 years).
References
- 1.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med. 2011;365(10):919-26. [DOI] [PubMed]
- 2.West S, Nevitt SJ, Cotton J, Gandhi S, Weston J, Sudan A, et al. Surgery for epilepsy. Cochrane Database Syst Rev. 2019;6:CD010541. [DOI] [PMC free article] [PubMed]
- 3.Lopez-Gonzalez M.A., Gonzalez-Martinez J.A., Jehi L., Kotagal P., Warbel A., Bingaman W. Epilepsy surgery of the temporal lobe in pediatric population: a retrospective analysis. Neurosurgery. 2012;70(3):684–692. doi: 10.1227/NEU.0b013e318235183d. [DOI] [PubMed] [Google Scholar]
- 4.Hemb M., Velasco T.R., Parnes M.S., Wu J.Y., Lerner J.T., Matsumoto J.H., et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology. 2010;74(22):1768–1775. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Oijen M., De Waal H., Van Rijen P.C., Jennekens-Schinkel A., van Huffelen A.C., Van Nieuwenhuizen O., et al. Resective epilepsy surgery in childhood: the Dutch experience 1992–2002. Eur J Paediatr Neurol. 2006;10(3):114–123. doi: 10.1016/j.ejpn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 6.Jarrar R.G., Buchhalter J.R., Meyer F.B., Sharbrough F.W., Laws E. Long-term follow-up of temporal lobectomy in children. Neurology. 2002;59(10):1635–1637. doi: 10.1212/01.wnl.0000034759.53430.1c. [DOI] [PubMed] [Google Scholar]
- 7.Widjaja E., Jain P., Demoe L., Guttmann A., Tomlinson G., Sander B. Seizure outcome of pediatric epilepsy surgery: systematic review and meta-analyses. Neurology. 2020;94(7):311–321. doi: 10.1212/WNL.0000000000008966. [DOI] [PubMed] [Google Scholar]
- 8.Moosa A.N., Gupta A. Outcome after epilepsy surgery for cortical dysplasia in children. Childs Nerv Syst. 2014;30(11):1905–1911. doi: 10.1007/s00381-014-2556-7. [DOI] [PubMed] [Google Scholar]
- 9.D'Argenzio L., Colonnelli M.C., Harrison S., Jacques T.S., Harkness W., Scott R.C., et al. Seizure outcome after extratemporal epilepsy surgery in childhood. Dev Med Child Neurol. 2012;54(11):995–1000. doi: 10.1111/j.1469-8749.2012.04381.x. [DOI] [PubMed] [Google Scholar]
- 10.Skirrow C., Cross J.H., Cormack F., Harkness W., Vargha-Khadem F., Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76(15):1330–1337. doi: 10.1212/WNL.0b013e31821527f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benifla M., Otsubo H., Ochi A., Weiss S.K., Donner E.J., Shroff M., et al. Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery. 2006;59(6):1203–1214. doi: 10.1227/01.NEU.0000245615.32226.83. [DOI] [PubMed] [Google Scholar]
- 12.Phi J.H., Cho B.-K., Wang K.-C., Lee J.Y., Hwang Y.S., Kim K.J., et al. Longitudinal analyses of the surgical outcomes of pediatric epilepsy patients with focal cortical dysplasia. J Neurosurg Pediatr. 2010;6(1):49–56. doi: 10.3171/2010.3.PEDS09497. [DOI] [PubMed] [Google Scholar]
- 13.Babini M., Giulioni M., Galassi E., Marucci G., Martinoni M., Rubboli G., et al. Seizure outcome of surgical treatment of focal epilepsy associated with low-grade tumors in children. J Neurosurg Pediatr. 2013;11(2):214–223. doi: 10.3171/2012.11.PEDS12137. [DOI] [PubMed] [Google Scholar]
- 14.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cossu M., Lo Russo G., Francione S., Mai R., Nobili L., Sartori I., et al. Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia. 2008;49(1):65–72. doi: 10.1111/j.1528-1167.2007.01207.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.-K., Wang K.-C., Hwang Y.-S., Kim K.J., Chae J.H., Kim I.-O., et al. Epilepsy surgery in children: outcomes and complications. J Neurosurg Pediatr. 2008;1(4):277–283. doi: 10.3171/PED/2008/1/4/277. [DOI] [PubMed] [Google Scholar]
- 17.Vadera S., Kshettry V.R., Klaas P., Bingaman W. Seizure-free and neuropsychological outcomes after temporal lobectomy with amygdalohippocampectomy in pediatric patients with hippocampal sclerosis. J Neurosurg Pediatr. 2012;10(2):103–107. doi: 10.3171/2012.4.PEDS1233. [DOI] [PubMed] [Google Scholar]
- 18.Lamberink H.J., Otte W.M., Blümcke I., Braun K.P.J., Aichholzer M., Amorim I., et al. Seizure outcome and use of antiepileptic drugs after epilepsy surgery according to histopathological diagnosis: a retrospective multicentre cohort study. Lancet Neurol. 2020;19(9):748–757. doi: 10.1016/S1474-4422(20)30220-9. [DOI] [PubMed] [Google Scholar]
- 19.Dwivedi R., Ramanujam B., Chandra P.S., Sapra S., Gulati S., Kalaivani M., et al. Surgery for drug-resistant epilepsy in children. N Engl J Med. 2017;377(17):1639–1647. doi: 10.1056/NEJMoa1615335. [DOI] [PubMed] [Google Scholar]
- 20.Ryvlin P., Cross J.H., Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13(11):1114–1126. doi: 10.1016/S1474-4422(14)70156-5. [DOI] [PubMed] [Google Scholar]
- 21.Tellez-Zenteno J.F., Dhar R., Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128(Pt 5):1188–1198. doi: 10.1093/brain/awh449. [DOI] [PubMed] [Google Scholar]
- 22.Edelvik A., Flink R., Malmgren K. Prospective and longitudinal long-term employment outcomes after resective epilepsy surgery. Neurology. 2015;85(17):1482–1490. doi: 10.1212/WNL.0000000000002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jutila L., Immonen A., Mervaala E., Partanen J., Partanen K., Puranen M., et al. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry. 2002;73(5):486–494. doi: 10.1136/jnnp.73.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foldvary N., Nashold B., Mascha E., Thompson E.A., Lee N., McNamara J.O., et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54(3):630. doi: 10.1212/wnl.54.3.630. [DOI] [PubMed] [Google Scholar]
- 25.Fauser S., Bast T., Altenmuller D.-M., Schulte-Monting J., Strobl K., Steinhoff B.J., et al. Factors influencing surgical outcome in patients with focal cortical dysplasia. J Neurol Neurosurg Psychiatry. 2008;79(1):103–105. doi: 10.1136/jnnp.2007.116038. [DOI] [PubMed] [Google Scholar]
- 26.Siegel A.M., Cascino G.D., Meyer F.B., McClelland R.L., So E.L., Marsh W.R., et al. Resective reoperation for failed epilepsy surgery: seizure outcome in 64 patients. Neurology. 2004;63(12):2298–2302. doi: 10.1212/01.wnl.0000147476.86575.a7. [DOI] [PubMed] [Google Scholar]
- 27.Jehi L., Yehia L., Peterson C., Niazi F., Busch R., Prayson R., et al. Preliminary report: Late seizure recurrence years after epilepsy surgery may be associated with alterations in brain tissue transcriptome. Epilepsia Open. 2018;3(2):299–304. doi: 10.1002/epi4.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Najm I., Jehi L., Palmini A., Gonzalez-Martinez J., Paglioli E., Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54(5):772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- 29.Duchowny M., Jayakar P., Levin B. Aberrant neural circuits in malformations of cortical development and focal epilepsy. Neurology. 2000;55(3):423–428. doi: 10.1212/wnl.55.3.423. [DOI] [PubMed] [Google Scholar]
- 30.Yu T., Zhang G., Kohrman M.H., Wang Y., Cai L., Shu W., et al. A retrospective study comparing preoperative evaluations and postoperative outcomes in paediatric and adult patients undergoing surgical resection for refractory epilepsy. Seizure. 2012;21(6):444–449. doi: 10.1016/j.seizure.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Englot D.J., Rolston J.D., Wang D.D., Sun P.P., Chang E.F., Auguste K.I. Seizure outcomes after temporal lobectomy in pediatric patients. J Neurosurg Pediatr. 2013;12(2):134–141. doi: 10.3171/2013.5.PEDS12526. [DOI] [PubMed] [Google Scholar]
- 32.Miserocchi A., Cascardo B., Piroddi C., Fuschillo D., Cardinale F., Nobili L., et al. Surgery for temporal lobe epilepsy in children: relevance of presurgical evaluation and analysis of outcome. J Neurosurg Pediatr. 2013;11(3):256–267. doi: 10.3171/2012.12.PEDS12334. [DOI] [PubMed] [Google Scholar]
- 33.Harvey A.S., Cross J.H., Shinnar S., Mathern G.W. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia. 2008;49(1):146–155. doi: 10.1111/j.1528-1167.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 34.LoPinto-Khoury C., Sperling M.R., Skidmore C., Nei M., Evans J., Sharan A., et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53(2):342–348. doi: 10.1111/j.1528-1167.2011.03359.x. [DOI] [PubMed] [Google Scholar]
- 35.Uijl S.G., Leijten F.S., Arends J.B., Parra J., van Huffelen A.C., Moons K.G. The added value of [18F]-fluoro-D-deoxyglucose positron emission tomography in screening for temporal lobe epilepsy surgery. Epilepsia. 2007;48(11):2121–2129. doi: 10.1111/j.1528-1167.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 36.Paolicchi J.M., Jayakar P., Dean P., Yaylali I., Morrison G., Prats A., et al. Predictors of outcome in pediatric epilepsy surgery. Neurology. 2000;54(3):642. doi: 10.1212/wnl.54.3.642. [DOI] [PubMed] [Google Scholar]
- 37.Perry M.S., Dunoyer C., Dean P., Bhatia S., Bavariya A., Ragheb J., et al. Predictors of seizure freedom after incomplete resection in children. Neurology. 2010;75(16):1448–1453. doi: 10.1212/WNL.0b013e3181f88114. [DOI] [PubMed] [Google Scholar]
- 38.Edwards J.C., Wyllie E., Ruggeri P.M., Bingaman W., Luders H., Kotagal P., et al. Seizure outcome after surgery for epilepsy due to malformation of cortical development. Neurology. 2000;55(8):1110–1114. doi: 10.1212/wnl.55.8.1110. [DOI] [PubMed] [Google Scholar]
- 39.Lewis E.C., Duchowny M. In: Long-Term Outcomes of Epilepsy Surgery in Adults and Children. Malmgren K., Baxendale S., Cross J.H., editors. Springer International Publishing; Cham: 2015. Long-term seizure and antiepileptic drug outcomes after epilepsy surgery in children; pp. 43–70. [Google Scholar]
- 40.Perry M.S., Duchowny M. Surgical versus medical treatment for refractory epilepsy: outcomes beyond seizure control. Epilepsia. 2013;54(12):2060–2070. doi: 10.1111/epi.12427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.