Abstract
Emiliania huxleyi is a unicellular marine alga that is considered to be the world's major producer of calcite. The life cycle of this alga is complex and is distinguished by its ability to synthesize exquisitely sculptured calcium carbonate cell coverings known as coccoliths. These structures have been targeted by materials scientists for applications relating to the chemistry of biomedical materials, robust membranes for high-temperature separation technology, lightweight ceramics, and semiconductor design. To date, however, the molecular and biochemical events controlling coccolith production have not been determined. In addition, little is known about the life cycle of E. huxleyi and the environmental and physiological signals triggering phase switching between the diploid and haploid life cycle stages. We have developed laboratory methods for inducing phase variation between the haploid (S-cell) and diploid (C-cell) life cycle stages of E. huxleyi. Plating E. huxleyi C cells on solid media was shown to induce phase switching from the C-cell to the S-cell life cycle stage, the latter of which has been maintained for over 2 years under these conditions. Pure cultures of S cells were obtained for the first time. Laboratory conditions for inducing phase switching from the haploid stage to the diploid stage were also established. Regeneration of the C-cell stage from pure cultures of S cells followed a predictable pattern involving formation of large aggregations of S cells and the subsequent production of cultures consisting predominantly of diploid C cells. These results demonstrate the ability to manipulate the life cycle of E. huxleyi under controlled laboratory conditions, providing us with powerful tools for the development of genetic techniques for analysis of coccolithogenesis and for investigating the complex life cycle of this important marine alga.
Emiliania huxleyi is a unicellular alga that is distinguished by its exquisitely sculptured calcium carbonate cell coverings known as coccoliths (Fig. 1). E. huxleyi is found throughout the world's oceans and forms extensive blooms sometimes greater than 100,000 km2, with cell densities up to 10,000 cells ml−1 (5, 18). It is also considered to be the world's major producer of calcite (35). Because of its relative abundance, widespread distribution, and ability to fix carbon into both organic and biomineralized products, E. huxleyi has attracted the attention of research scientists interested in global carbon cycling (10, 18, 31). E. huxleyi has also been targeted by materials scientists who are interested in the biomineralized skeletons for applications relating to materials chemistry. Such porous shells of calcium carbonate have been targeted by materials scientists as having potential significance as lightweight ceramics, catalyst supports, and robust membranes for high-temperature separation technology (34). Biomedical applications include the use of these biomineralized materials for construction of artificial bone in humans (8, 30, 32), scaffolding supports in tissue engineering (2, 7), complement activation enhancement (29), artificial dental root construction (6, 26), and biomedical implants (34). While a general understanding of some of the ecophysiological aspects of calcification and coccolithogenesis in E. huxleyi has been obtained, little information on the life cycle of this organism is available.
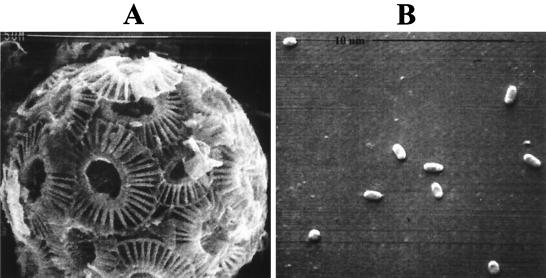
FIG. 1.
Scanning electron micrographs of E. huxleyi strain 1516 life cycle types. (A) Nonmotile C cell showing overlapping coccolith plates. (B) Motile S cells representing a possible gametic stage of the life cycle. Note the relative difference in the sizes of these two cell types involved in phase variation mechanisms described in the text. Magnifications, ca. ×8,000 (A) and ×10,500 (B).
The life cycle of E. huxleyi is complex and involves several different cell types including coccolith-bearing cells (coccolithophores; C cells), nonmotile naked N cells, and motile, scale-bearing swarm cells (S cells), each of which can exist independently and reproduce vegetatively (15, 19) (Fig. 1). In liquid culture, N and S cells frequently arise from C cells. The interrelationships between these different cell types and the mechanisms that govern these phase variation events, however, have not been defined. Flow cytometry data indicate that the DNA complement of the motile S cells is half that of the C cells (15, 24). This suggests that the S- and C-cell types are haploid and diploid with respect to one another and that the S cells may represent a gametic stage. Billiard (4) and Green et al. (15) contend that the C cell and the S cell act as alternate phases of a life cycle involving meiosis and syngamy. Whether E. huxleyi is a monoecious or dioecious alga, however, is not known. While it seems likely that the life cycle of E. huxleyi involves a sexual stage, meiotic division leading to the formation of S cells acting as gametes has never been documented, nor has the actual fusion of the S cells in sexual reproduction. Prior to this study C cells and S cells have only been observed in liquid culture, and the physiological and molecular signals controlling interconversion of these cell types remain unknown (15). To understand the life cycle of E. huxleyi, the environmental and physiological conditions that trigger E. huxleyi C cells to exit the mitotic cycle and initiate sexual reproduction must be defined.
In this report we describe conditions for induction of phase switching from the diploid coccolithophore cell (C-cell) to the haploid swarm cell (S-cell) life cycle stages of marine alga E. huxleyi. We also describe a mechanism for induction of the reverse change, from S cell back to C cell. To our knowledge, this is the first report demonstrating plating of E. huxlyei on solid media, a laboratory condition which we have found induces differentiation of the diploid life cycle stage to the haploid gametic stage. The ability to manipulate the life cycle of E. huxleyi in the controlled laboratory environment, as demonstrated herein, represents a powerful tool for identifying the mechanisms underlying the phase transition from vegetative growth to sexual reproduction in E. huxleyi. In addition, these data will aid in the development of genetic tools for investigating the molecular mechanisms governing the processes of calcium carbonate biomineralization and coccolithogenesis in this organism.
MATERIALS AND METHODS
Media and growth conditions.
E. huxleyi strain 1516 was obtained from the Provasoli-Guillard National Center for Culture of Marine Phytoplankton. Stock cultures were maintained by inoculating cells into 75 ml of F/50 or F/2 medium (17) in 250-ml flasks. Cultures were incubated photoautotrophically at 17 to 18°C under cool white fluorescent light (660 μmol · m−2 · s−1) and under either a continuous-light or a discontinuous-light (12-h dark, 12-h light) cycle. Previous reports had shown that coccolith production in E. huxleyi was enhanced in cells grown on F/50 versus F/2 media. Consequently, most experiments described in this study utilized F/50 as the growth medium, unless noted otherwise. Growth on plates was obtained in F/50 media supplemented with 1.5% Agar Select (Sigma Co., St. Louis, Mo.). Other agar sources employed in the plating media (e.g., Difco agar and agarose) inhibited or prevented growth of strain 1516.
PCR amplification of the RubisCO large-subunit and 16S rRNA genes.
Amplification the ribulose-1,5-bisphosphate carboxylase/oxygenase (RubisCO) large-subunit gene from E. huxleyi (14) was obtained with the following primers: 5′-ACTGCTACATGGACTGTAGTA-3′ and 5′-TAGATCTAATGCAGTTTGAAG-3′. Single-colony isolates were picked from agar plates and resuspended in 15 μl of Tris-EDTA buffer; 5 μl of the resuspended cells was then used in a standard PCR with a 10-min hot start at 95°C and a 52°C annealing temperature. A similar strategy was employed for amplification of the 16S nuclear ribosomal DNA gene from E. huxleyi (3) using the following E. huxleyi-specific primers: 5′-AGTCATATGCTTGTCTCA-3′ and 5′-GATAAGGTTCGGACAGCTT-3′.
Induction of phase switching from diploid to haploid stages.
Single colonies of strain 1516 consisting exclusively of S cells were obtained by plating dilutions of late-log- to early-stationary-phase cultures (ca. 6 × 105 to 8 × 105 C cells·ml−1) on F/50 medium plates and incubating them for 48 h at 18°C on a 12-h light, 12-h dark cycle. For experiments where a lawn of S cells was desired, C-cell cultures at densities of 105 cells/ml or greater were plated and incubation was extended to 4 or 5 days to ensure complete transition to the S-cell stage. Phase transition to the S-cell stage was confirmed by phase-contrast microscopy of cells resuspended from the plates. Data on the rate of conversion of C to S cell was obtained by resuspending cells from plates at specific time intervals and counting the C cells present and comparing this number to the original number plated. Duplicate plates were counted for each time point, and the average C-cell number was recorded. The rate of decrease in C-cell number per hour was determined from four independent experiments, and the average rate of C-cell conversion to S cells was recorded as the percent decrease in C-cell number per hour.
Induction of phase switching from haploid stage to diploid stage.
To regenerate the diploid C-cell stage from S cells, the latter were collected from a single plate with ca. 2 ml of F/50 medium, pelleted gently (3 min at 1,500 × g), and then resuspended in a final volume of 100 to 250 μl of F/50 medium. This concentration step was essential for successful S cell-to-C cell phase transition, suggesting that there may be an attrition rate when resuspending S cells from an agar surface and/or that only a small subset of E. huxleyi cells may be capable of gametogenesis. The resuspended cells were then inoculated into 24-well microtiter plates containing 2.5 ml of the same medium. Phase transition from the S- to the C-cell stage was monitored by phase-contrast or Nomarski optics microscopy.
To establish the relative efficiency of regenerating the diploid C-cell stage following phase transition to S cells, known concentrations of C cells (104, 105, and 106 cells·ml−1) were plated and incubated for 4 to 5 days to allow complete transition of each original C-cell population to the S-cell stage. Duplicate plates of the original C-cell populations were employed in all experiments. Following harvesting of S cells from their respective plates, various dilutions of each culture (1:10, 1:25, and 1:50 [vol/vol] final) were inoculated into microtiter wells and incubated at 18°C on a 12-h light, 12-h dark cycle for 10 to 11 days. Direct microscopic counts of C cells were then performed, and densities were compared with the respective original densities of plated C cells. Diploid-cell regeneration efficiency was determined from the results of three independent experiments performed at each of the original C-cell culture densities listed above.
Flow cytometry.
E. huxleyi strain 1516 cells from mid-log- and late-stationary-phase liquid-grown cultures (10 ml, representing a minimum of 1 × 106 to 3 × 106 C cells) were pelleted by centrifugation at 1,600 × g for 15 min at 4°C and washed once with sterile phosphate-buffered saline (PBS; 4.3 mM Na2HPO4 · 7H2O, 1.4 mM KH2PO4, 2.7 mM KCl, 137 mM NaCl [pH 7.3]). Cells were fixed by resuspending them in 90% methanol (MeOH) and passed through a 25½-gauge syringe several times to reduce clumping. Cells were stored in MeOH at −20°C for a minimum of 24 h prior to staining. Large-scale preparation of S-cell cultures was performed by spreading 2 ml of mid-log-phase cells (ca. 1 × 106 to 2 × 106 C cells) onto F/50 agar plates (Nunc; Bio-Assay dish; 245 by 245 by 25 mm) and incubating the plates for 4 to 5 days as described above. Cells were resuspended from plates in 20 ml of F/50 medium and prepared for staining as described for liquid-grown cells. To ensure complete dispersal of the resuspended cells from agar surfaces, S cells were passed several times through a 20-gauge syringe, followed by several passages through a 25½-gauge syringe. Following fixation, the cell samples were harvested and washed in 1 ml of PBS by centrifugation for 30 s at 10,000 × g. Cells were stained in 1 ml of PBS buffer containing propidium iodide (10 or 50 μg · ml−1; Sigma Co.) and RNase A (100 μg · ml−1; Sigma Co.) for 18 to 24 h in the dark at 4°C. Relative DNA contents of C cells and S cells were analyzed with a Becton Dickinson FACSCaliber flow cytometer.
RESULTS
Growth of E. huxleyi strain 1516 in continuous light versus a light-dark incubation cycle.
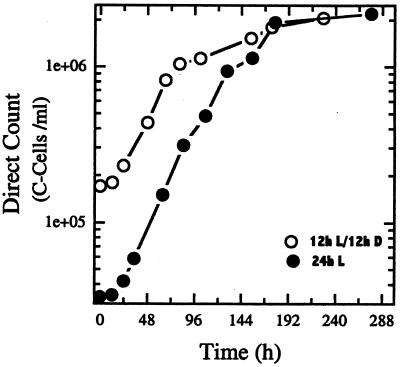
The light-scattering effects of the CaCO3 coccoliths produced by coccolithophores (C cells) prohibit the use of spectrophotometric methods to monitor growth of strain 1516 C cells. Therefore growth was monitored by employing the direct-cell-count method. Figure 2 shows a typical growth curve for E. huxleyi strain 1516 C cells grown in F/50 media under a continuous-light versus a light-dark incubation cycle. Strain 1516 had a generation time of ca. 24 h when incubated under either a 12-h light, 12-h dark cycle or under continuous light. The proportion of swarm cells (S cells) to C cells increased throughout stationary phase (data not shown), supporting previously described microscopic observations suggesting that the production of S cells from C cells was most pronounced during stationary-phase growth (19). The average diameter of the C-cell population increased during stationary phase compared to that during log-phase growth (average cell diameters: log phase, ∼4 to 6 μm; stationary phase, ∼7 to 10 μm). This was presumably due to the continued accumulation of CaCO3 coccolith material in batch cultures of strain 1516 when grown on F/50 media (low levels of phosphate and nitrate) as previously described (21).
FIG. 2.
Growth of E. huxleyi 1516 C cells in medium F/50 under continuous light (●) or a 12-h light, 12-h dark incubation cycle (○). Each culture was incubated at 17 to 18°C, and the minimum generation times were ca. 24 h under both conditions.
Growth of E. huxleyi strain 1516 on solid media.
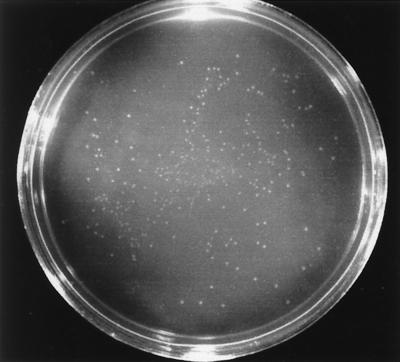
E. huxleyi strain 1516 was grown photosynthetically in liquid batch cultures to late log or early stationary phase. Figure 3 shows the results of a typical plating experiment in which serial dilutions of C-cell batch cultures (ca. 2 × 106 cells·ml−1) were spread onto F/50 plates and incubated under photosynthetic conditions (continuous light, or 12-h light, 12-h dark cycle) for 4 to 5 days. Duplicate plates of each dilution were incubated in the dark as controls; however, no growth was observed on any of the plates under these conditions. Photosynthetically incubated plates showed pinpoint colonies present within 24 to 48 h of incubation, with maximum plate growth obtained after 4 to 5 days (Fig. 3). Calculation of plating efficiency utilizing viable-cell counts was not possible due to a phase transition phenomenon induced upon plating E. huxleyi on solid media. Diploid C cells appeared to differentiate into numerous S cells within 48 h after plating. Two or three distinct colony morphologies were observed on the plates, ranging from very small (<1-mm-diameter) to larger (>5-mm-diameter) colonies (Fig. 3). The color of the colonies ranged from clear to colorless, creamy to opaque, and green to yellow. Pigmented colonies appeared only after extended incubation on agar plates (>2 weeks).
FIG. 3.
Colonies of E. huxleyi cells on agar plates. Late-log-phase cells (ca. 2 × 106 C cells/ml) were diluted to 10−4, and 0.1 ml was plated on an F/50 medium plate and incubated photosynthetically for 72 h. Note the large number of colonies actually present (ca. 1,000) compared to the number expected (ca. 20) based on the original number of C cells plated, indicating production of multiple S cells from a given C cell during phase transition.
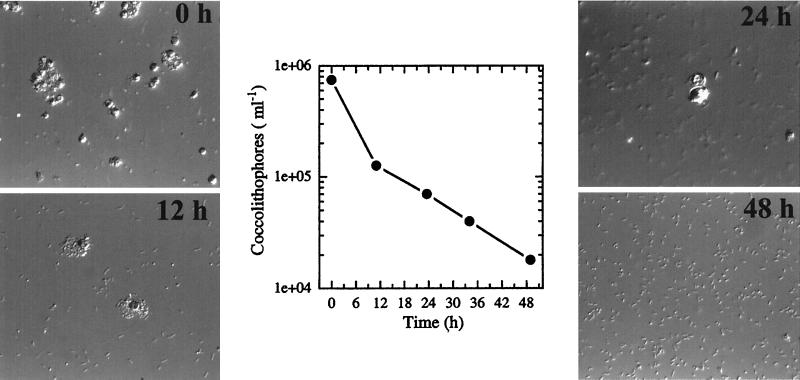
We monitored the fate of plated cells for the presence of coccolith-forming C cells within a given colony microscopically using plane-polarized light birefringence (37, 38). This cell type, however, could be detected only up to approximately 24 to 40 h of incubation on solid media. Microscopic observations of colonies within this time period showed numerous ruptured C cells with tiny S cells present both inside and around the periphery of these C cells (see Fig. 5A, 12 h). In general, coccolith-producing C cells could not be detected from any colony sampled after 48 h of incubation on solid media. At this time all plates contained small (∼0.5- to 1.0-μm-long), often highly motile, S cells exclusively.
FIG. 5.
(A) Nomarski photomicrographs of E. huxleyi strain 1516 C cell-to-S cell phase transition over a 48-h period following plating on F/50 media. Magnifications: 0 h, ×564; 12 h, ×677; 24 h, ×752; 48 h, ×677. (B) Rate of decrease in the numbers of C cells present on agar plates during the experiment shown in panel A. The results from four independent experiments were obtained as described in Materials and Methods and showed an average rate of C cell-to-S cell phase transition of 2 to 3% per h on F/50 plates.
Liquid cultures and plate cultures of our laboratory strain 1516 were routinely checked for bacterial contamination by streaking onto rich media (e.g., Luria-Bertani media) and incubating them in the dark at 23 and 37°C. Cultures were also checked for contamination by utilizing the BBL Enterotube II Multitest biochemical assay system to detect the presence of bacteria, particularly members of the Enterobacteriaceae and Vibrioaceae. All contamination checks under these conditions were consistently negative. In addition, routine checks of E. huxleyi strain 1516 cultures employing media for growth of marine methylotrophic bacteria (organisms capable of growth on one-carbon compounds) were also consistently negative. No growth on F/50 plates in the dark was observed under these conditions, demonstrating that growth was absolutely dependent on photosynthesis.
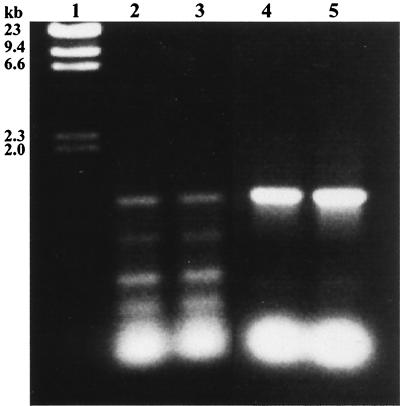
Given the lack of any molecular data regarding life cycle relationships between S cells and C cells observed only from liquid culture, we employed PCR to unequivocally determine the identity of the plated cells. Utilizing the published sequence data of the E. huxleyi RubisCO large-subunit (rbcL) gene, primers hybridizing close to the 5′ and 3′ ends of the gene were constructed. DNA was extracted from liquid-grown cells and amplified using the rbcL gene primers, and the expected 1.2-kb PCR product was generated (Fig. 4, lanes 4 and 5). This product was sequenced and was confirmed to contain the predicted portion of the RubisCO large-subunit gene from E. huxleyi. The PCR product amplified from two single-colony isolates (lanes 2 and 3) was also of the expected 1.2-kb size. Subsequent sequence data for the fragments from these colony isolates also identified the E. huxleyi RubisCO large-subunit gene and thus confirmed the identity of the S cells on plates as E. huxleyi (data not shown). The identity of the plated cells was also confirmed by 16S rRNA sequence analysis of the PCR-amplified rRNA gene product from single colonies (data not shown).
FIG. 4.
PCR amplification of genomic DNA from E. huxleyi strain 1516 with primers specific for the E. huxleyi RubisCO large-subunit gene. DNA extracted from single-colony isolates on F/50 plates (lanes 2 and 3) and from liquid-grown cells (lanes 4 and 5) produced the same 1.2-kb fragment. Subsequent sequencing of these PCR products confirmed the presence of the RubisCO large-subunit gene of E. huxleyi. Lane 1, 23-kb ladder.
Induction of the C cell-to-S cell phase transition on agar plating media.
To further investigate the apparent phase transition of C cells to S cells on agar media, we monitored the fate of plated C cells at various times by Nomarski optics microscopy as well as by direct cell counts. Figure 5 shows the sequence of events from a typical experiment following plating of liquid-grown cultures onto solid media. At time zero, a large population of mid-log-phase C cells was spread onto the surface of an F/50 plate, and a sample taken prior to incubation in the light consisted of predominantly C cells (Fig. 5A). Direct microscopic counts showed a steady decrease in the number of C cells present starting at approximately 6 h after incubation on plates. At 12 h, increasing numbers of S cells, along with ruptured C cells, could be observed (Fig. 5A) and direct counts showed nearly an 80% decrease in the C-cell population (Fig. 5B). By 48 h, 98% of the original C-cell population had differentiated to S cells (Fig. 5A and B). The average rate of C cell-to-S cell phase transition ranged between 2 to 3% per h when late-log- to early-stationary-phase C cells were plated. When C cells at the same concentration were heat treated (10 min at 55°C) prior to plating, phase transition to S cells did not occur, indicating that viable C cells were required for this phase transition to the haploid S-cell stage observed on agar plates (data not shown).
Currently, the number of S cells generated from a single C cell is unknown. However, the results from plating experiments suggest that this number may be quite large. For example, when C cells at a known concentration were plated, the colony number on a given plate was consistently 1 to 3 orders of magnitude higher than the number predicted (calculated from the following equation: original cell concentration = colony number/volume plated × dilution factor; Fig. 3). A further complication with calculating plating efficiency is that liquid cultures of E. huxleyi contain both C cells and S cells, the relative proportion of which varies with the growth phase of the culture. Pure cultures at the C-cell stage have not previously been isolated and maintained in laboratory culture, and cultures consisting exclusively of S cells are described for the first time in this study. We have begun to isolate individual S-cell clones for analysis of life cycle phase transition events and to determine whether E. huxleyi is a monoecious or dioecious alga. Cells from single colonies were repeatedly streaked and restreaked onto F/50 plates to obtain pure cultures of haploid S cells. Several clonal isolates have been maintained on agar plate media for over 2 years. Microscopic observations of all these clonal isolates have indicated the presence of only S cells; regeneration of the diploid C-cell stage on solid media has never been observed. PCR amplification and sequencing of the RubisCO large-subunit and 16S ribosomal DNA genes from these S-cell clones have confirmed these isolates as E. huxleyi (data not shown). To date, preliminary attempts to regenerate the C-cell stage from S-cell clones, either individually or in test cross experiments, have not been successful.
Determination of relative DNA contents of C cells and S cells by flow cytometry.
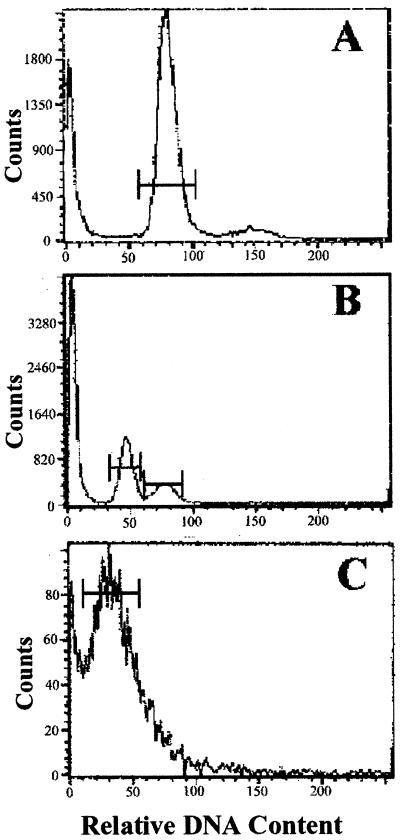
To determine the relative DNA contents of strain 1516 C-cell and S-cell types employed in this study, cells were analyzed by fluorescence flow cytometry. Flow cytometry of log-phase cultures, consisting of C cells, showed a major peak at approximately channel 80 (coefficient of variation [CV], ∼9%) and a smaller peak at ca. channel 160, corresponding to diploid C cells in the G0-plus-G1 and M-plus-G2 phases of the cell cycle, respectively (Fig. 6A). A late-stationary-phase culture containing a large proportion of S cells relative to C cells yielded a large peak at approximately channels 40 to 45 (CV, ∼9.5%), representing the haploid S-cell population, and a smaller diploid cell peak at channel 80 (CV, ∼10%) (Fig. 6B). Histogram analyses of flow cytometry samples of single-cell eukaryotes routinely exhibit statistical CVs of 9 to 12%, as reported here. These data confirmed that S cells of E. huxleyi strain 1516 contain one-half the DNA content of C cells, supporting the data by Green et al. (15) obtained from several geographical isolates of E. huxleyi strains grown in liquid culture.
FIG. 6.
Flow-cytometric analysis of cultures from E. huxleyi strain 1516. Samples were analyzed after fixation in 90% methanol and staining with 50 μg of propidium iodide ml−1. Histograms represent data from the following cultures: log phase, consisting almost exclusively of diploid C cells (A), late stationary phase, containing a large haploid S-cell population and smaller numbers of C cells (B), and pure culture of S cells from agar plates (C). See Results for details.
Analysis of pure cultures of the S cells generated from growth on agar plates confirmed that the peak observed at channel 40 in stationary-phase liquid cultures represented the S-cell stage of E. huxleyi strain 1516. These pure S-cell cultures showed a single (although somewhat noisy) major peak with a statistical mean at approximately channel 40 (Fig. 6C). Flow cytometry measurements of strain 1516 S cells resuspended from agar plates consistently yielded high CVs compared to those observed in liquid culture (30 to 40% versus 8 to 10%, respectively). Although cells were passed several times through a 25½-gauge needle and sonicated prior to staining in an attempt to reduce aggregation, the results were unchanged. The noise most probably reflects aggregation of S cells with agar debris during sample preparation, which is likely to prohibit proportional staining of individual cells and/or lead to inaccurate cell counts during flow cytometry. Several other variables relating to sample preparation were evaluated in an effort to reduce the CVs of plated-cell samples. These included varying the type of fixative, the time of fixation and staining, the concentration of propidium iodide, and the amount of cell material per sample reaction. We also tried the more-sensitive SYBR Green I (Molecular Probes, Inc.), previously described as an effective stain for liquid-grown populations of marine picoplankton (23) under a variety of conditions. However, none of these methods was found to be effective for reducing the CVs of plated S-cell samples. The fact that the haploid S-cell peak can be observed in liquid cultures (Fig. 6B) supports our hypothesis with respect to aggregation during sample preparation.
Regeneration of coccolithophore cell stage from the swarm cell stage.
Figure 7 shows the results of a typical experiment depicting the sequence of events occurring during phase transition from the haploid S-cell stage to the diploid C-cell stage. Following initial inoculation of S cells into liquid media from agar plate cultures, single motile S cells were observed over the first 24 h (Fig. 7, D1). By days 2 and 3, S cells began to aggregate into “packs,” and these packs continued to increase in size through days 5 to 8. Highly motile S cells were observed at the periphery of the packs and appeared to lose motility as the packs increased in size. While these cell aggregates continued to increase in both size and number through days 7 to 10, several large C cells were visible within, or associated with, most S-cell aggregations by days 5 to 8 (Fig. 7, D8). By days 8 to 11, C cells could be observed in almost every S-cell pack. After 14 days the cultures consisted of predominantly C cells and most of the remaining S cells had disappeared from the media by that time (Fig. 7, D14). The fate of these S cells, which apparently do not contribute to C-cell formation, is currently unknown.
FIG. 7.
Nomarski photomicrographs of E. huxleyi strain 1516 during phase transition from the S-cell to the C-cell stage. Note the “packing behavior” of S-cells beginning by about days 2 to 3, resulting in the formation of extremely large aggregations, and the eventual appearance of C cells within and around the S-cell packs by about day 9. Magnifications: D1, ×800; D3, ×320; D8, ×600; D14, ×600.
To establish laboratory conditions for developing genetic techniques for E. huxleyi, we decided to evaluate the relative efficiency of regenerating the diploid C-cell stage from a population of haploid S cells. Regeneration efficiency was determined by plating a known concentration of C cells and incubating them for 4 to 5 days to ensure a complete phase transition to the S-cell stage. Next, these S cells were resuspended from plates and inoculated into microtiter wells (see Materials and Methods). Direct counts of C cells were performed following a 10- to 11-day incubation period on a 12-h light, 12-h dark cycle. Three replicates, each at original C-cell densities of 106, 105, and 104 cells per ml, had C-cell recovery levels of ca. 75, 97, and 98%, respectively, of their original populations. Interestingly, preliminary results from a single experiment under continuous light failed to regenerate the C-cell stage, suggesting the possibility that a dark induction might be involved in this phase transition.
DISCUSSION
The lack of detailed information on the life cycle of E. huxleyi has been due primarily to the inability to identify the conditions causing phase variation between the diploid C-cell and haploid S-cell stages. Previous attempts to identify specific mating types of E. huxleyi S cells had focused on gross morphological characteristics, such as scale morphology (4) or flagellum length (15). However, the relative ploidy levels of S cells versus C cells as determined by flow cytometry (15, 16, 24) did not support these morphological criteria. Consequently, we have undertaken a microbiological and molecular approach to address questions relating to phase variation events in the life cycle of E. huxleyi. The goal of our research is to elucidate details of the life cycle of this organism for the development of genetic approaches directed to understanding the processes of biomineralization and coccolithogenesis in E. huxleyi.
Our preliminary studies of the C cell-to-S cell phase variation in E. huxleyi suggested that C cells could be induced to differentiate into S cells upon plating on agar media. However, before undertaking further studies on the life cycle of E. huxleyi, it was necessary to unequivocally determine that the colonies appearing on the F/50 plates consisted of E. huxleyi cells. The evidence presented herein arguing against bacterial contamination as the source of this growth, along with PCR amplification of the E. huxleyi RubisCO (rbcL) and 16S rRNA genes from single colonies, indicated that phase switching from the C-cell stage to the S-cell stage is induced by plating E. huxleyi cells on solid media.
We have determined that an exponentially growing population of C cells will differentiate into S cells very quickly following plating on solid media (rate, ca. 2 to 3% decrease in C cells per h). Analogous types of phase variation responses have been observed in studies of chemotaxis and phototaxis signal transduction in a variety of bacterial species. For example, the photosynthetic nonsulfur purple bacterium Rhodospirillum centenum produces motile cells with a single polar flagellum in liquid media (12, 27, 28), whereas on solid media it produces peritrichously flagellated swarm cells. This differentiation event is dependent on the viscosity of the medium: increasing medium viscosity triggers the production of cells possessing numerous flagella. Swarm cell differentiation in Serratia liquefaciens also appears to be induced by exposure of cells to surfaces of a particular viscosity (20). This environmental signal is thought to be received by an as yet unidentified sensor, initiating a signal transduction cascade which has been demonstrated to proceed via motility master operon flhDC (20, 36). The production of S cells in E. huxleyi from C cells on solid media is particularly intriguing in that it also involves a phase switch from the diploid to the haploid life cycle stage. Flow cytometry data from this study have confirmed that the relative ploidy level of E. huxleyi strain 1516 S cells is indeed half that of C cells. Previous studies of life cycle dynamics of E. huxleyi have inferred relationships between C cells and S cells exclusively from microscopy of “mixed” cultures. Our flow cytometry data of pure cultures of S cells (plated cells), taken together with PCR amplification of the E. huxleyi rbcL and 16S rRNA genes from single colonies and time course Nomarski optics microscopy, have clearly demonstrated diploid-to-haploid phase transition in a marine coccolithophorid for the first time.
The global distribution of E. huxleyi suggests that it must possess the ability to sense environmental changes, including changes in local fluid motion and medium viscosity. Recently studies with transgenic diatom cells have uncovered sensing systems for detecting and responding to osmotic stress, fluid motion, and iron (11). Perhaps there are similarities in the environmental signals and cognate sensor systems affecting cell motility in bacteria and those involved in inducing the E. huxleyi diploid-to-haploid phase transition, resulting in the generation of a motile life cycle stage. The ability to induce this life cycle phase switch in E. huxleyi allows us to investigate the signal transduction mechanisms involved in this process, and we are currently conducting studies to test these hypotheses.
We have not been able to determine, to this point, how many S cells arise from a single C cell. In diatoms and many other unicellular algae, gametes are produced from meiotic nuclear division, followed by multiple mitotic divisions to produce from 8 to over 128 microgametes from a single diploid cell (1, 9). Our preliminary microscopic observations suggest that the number of haploid S cells formed per diploid C cell in E. huxleyi may also be large. This hypothesis was also supported by plating results showing that the “actual” number of colonies observed on agar plates was consistently orders of magnitude higher than the “expected” number based on the original number of C cells plated. However, due to the extremely small size of S cells and their tendency to aggregate when resuspended from an agar surface, we have been unable to obtain accurate counts under the experimental conditions employed, including attempts to quantitate cell number using Coulter counter technology. We are currently evaluating other methodologies (e.g., fluorescence flow cytometry) to establish a quantitative relationship between C-cell and S-cell numbers.
To initiate efforts at unraveling the molecular events, or signal transduction mechanism(s), involved in phase variation in the life cycle of E. huxleyi, we set up experiments to regenerate the diploid C-cell stage from haploid S cells. When S cells were inoculated from plates into liquid media, the sequence of events leading to the formation of C cells followed a predictable pattern, proceeding from single motile S cells, through the formation of large aggregations of S cells, and finally resulting in cultures consisting almost exclusively of C cells (11 to 14 days of incubation). Of particular note regarding this phase transition is the fate of the large numbers of S cells that apparently do not contribute to zygote formation. Although large packs of these S cells can be observed up through ca. days 5 to 9, these cells are barely detectable after about day 11 or 12. A similar situation has been described in studies of gametogenesis in the unicellular marine diatom Thalassiosira weissflogii Grun (1). In studies of this alga, cessation of a dark incubation period required for gamete formation resulted in the disappearance of the large numbers of remaining gametes in the culture over about a 50-h time period. The authors hypothesized that the cells may have died and “disintegrated” over time following removal of the induction signal, and thus the fate of these cells in this well-characterized marine diatom remains unresolved. Nonetheless, there is precedent for this phenomenon, which we observed in this study with E. huxleyi. Considering the lack of any published data on gametogenesis and sexual fusion in E. huxleyi, the fate of these S cells must await future investigation.
The comparatively low number of diploid C cells generated from within groups of hundreds of S cells suggests that not all S cells may be capable of acting as gametes. This situation is not uncommon among unicellular eukaryotes, where sexual reproduction involves genes responsible for both determination of mating type and mate recognition to ensure fusion of specific mating types (13, 22). In diatoms, the percentage of cells induced to undergo spermatogenesis was shown to be dependent on three factors: (i) an induction signal (e.g., shift from dim light or darkness) (1), (ii) a decrease in cell size to less than 30 to 40% of its maximum (9), and (iii) the stage of the cell cycle (1). Apparently, once a diatom cell proceeds through an inducible region of G1 in dim light or darkness, completion of spermatogenesis then requires the possession of a particular genetic constitution. The situation in yeasts is also complex. In these organisms (e.g., Saccharomyces cerevisiae), meiosis occurs only in cells that are heterozygous at the mating locus and an environmental signal involving nutrient limitation is also a prerequisite for sex cell formation (25). The life cycle of marine coccolithophorids is complex, and many factors may be involved in the control of phase variation between haploid and diploid life cycle stages and in mating cell development, gamete recognition, and sexual fusion. Some researchers have even hypothesized that a haploid S cell in the G2 phase of the life cycle might give rise to a diploid C cell (15, 33). However, this hypothesis could not be experimentally addressed utilizing DNA flow cytometry and microscopy of liquid-grown E. huxleyi cultures as the research tools. Efforts are under way in our laboratory to employ our clonal S-cell isolates in phase transition experiments to determine whether E. huxleyi is a monoecious or dioecious alga. Our ability to manipulate the life cycle stages of this organism under laboratory-controlled conditions will be particularly useful for designing experimental approaches for addressing these types of questions.
These studies have established, for the first time, laboratory conditions for inducing phase switching between the haploid S-cell and diploid C-cell life cycle stages of marine coccolithophorid E. huxleyi. We have discovered that C cells can be induced to differentiate into S cells upon exposure of cells to a solid surface and that this life cycle stage may be maintained indefinitely under these conditions. We have also shown that the C-cell stage can be regenerated from the S-cell stage. To date, most information on the life cycle of E. huxleyi has only been phenomenologically demonstrated, and little is known about the ecology and environmental signals that induce phase switching in this marine alga. These data provide us with a potentially powerful tool for generating recessive mutations in E. huxlyei and for investigating the environmental factors and signal transduction mechanisms responsible for phase variation events in the life cycle of this ecologically and biomedically important marine alga.
ACKNOWLEDGMENTS
We thank William Porter for providing the scanning electron micrographs of E. huxleyi. We also thank Dennis Young of the Flow Cytometry Core Facility at the Internal Medicine Division, University of California San Diego, for his helpful suggestions.
This work was supported, in part, by a National Institutes of Health MBRS grant.
REFERENCES
- 1.Armbrust E V, Olson R J, Chisholm S W. Role of light and the cell cycle on the induction of spermatogenesis in a centric diatom. J Phycol. 1990;26:470–478. [Google Scholar]
- 2.Arnaud E, Morieux C, Wybier M, de Vernejoul M C. Osteogenesis induced by the combination of growth factor, fibrin, glue and coral; towards a substitute of autologous bone graft. An experimental study on a rabbit. Ann Chir Plast Esthetique. 1994;39:491–498. [PubMed] [Google Scholar]
- 3.Bhattacharya D, Medlin L, Wainright P O, Ariztia E, Bibeau C, Stickel S K, Sogin M L. Algae containing chlorophylls a + c are paraphyletic: molecular evolutionary analysis of the chromophyta. Evolution. 1992;46:1801–1817. doi: 10.1111/j.1558-5646.1992.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 4.Billard C. Life cycles. Syst Assoc Spec Vol. 1994;51:167–186. [Google Scholar]
- 5.Brown C W, Yoder J A. Coccolithophorid blooms in the global ocean. J Geophys Res. 1994;99:7467–7482. [Google Scholar]
- 6.Comprasse G, Comprasse S, Gill G A. Substitution of the dental root by aquatic invertebrate skeletons in animals and man. C R Acad Sci Ser III. 1988;307:485–491. [PubMed] [Google Scholar]
- 7.Damien C J, Christel P S, Benedict J J, Patat J L, Guillemen G. A composite of natural coral, collagen, bone protein, and basic fibroblast growth factor tested in a rat subcutaneous model. Ann Chir Gynaecol Suppl. 1993;207:117–128. [PubMed] [Google Scholar]
- 8.Doherty M J, Schlag G, Schwarz N, Mollan R A, Nolan P C, Wilson D J. Biocompatibility of xenogenic bone, commercially available coral, a bioceramic and tissue sealant for human osteoblasts. Biomaterials. 1994;15:601–608. doi: 10.1016/0142-9612(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 9.Edlund M B, Stoermer E F. Ecological, evolutionary, and systematic significance of diatom life histories. J Phycol. 1997;33:897–918. [Google Scholar]
- 10.Fabry V J, Robins L L. Large scale production of calcium carbonate: coccolithophorids and cyanobacteria. In: Paul J, Pradier C, editors. Carbon dioxide chemistry: environmental issues. Cambridge, United Kingdom: Royal Society of Chemistry; 1994. pp. 301–304. [Google Scholar]
- 11.Falciatore A, d'Alcala M R, Croot P, Bowler C. Perception of environmental signals by a marine diatom. Science. 2000;288:2363–2366. doi: 10.1126/science.288.5475.2363. [DOI] [PubMed] [Google Scholar]
- 12.Favinger J, Stadtwald R, Gest H. Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Antonie Leeuwenhoek. 1989;55:291–296. doi: 10.1007/BF00393857. [DOI] [PubMed] [Google Scholar]
- 13.Ferris P J, Pavlovic C, Fabry S, Goodenough U W. Rapid evolution of sex-related genes on Chlamydomonas. Proc Natl Acad Sci USA. 1997;94:8634–8639. doi: 10.1073/pnas.94.16.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujiwara S, Sawada M, Someya J, Minaka N, Kawachi M, Inoue I. Molecular phylogenetic analysis of rbcL in the Prymnesiophyta. J Phycol. 1994;30:863–871. [Google Scholar]
- 15.Green J C, Course P A, Tarran G A. The life cycle of Emiliania huxleyi: a brief review and a study of ploidy levels analysed by flow cytometry. J Mar Syst. 1996;9:33–44. [Google Scholar]
- 16.Green J C, Hori T. Flagella and flagellar roots. Syst Assoc Spec Vol. 1994;51:47–71. [Google Scholar]
- 17.Guillard R R L. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H, editors. Culture of marine invertebrate animals. New York, N.Y: Plenum Press; 1975. pp. 29–60. [Google Scholar]
- 18.Holligan P M, Fernandez E, Aiken J, Balch W M, Boyd P, Burkill P H, Finch M, Groom S B, Malin G, Muller K, Purdie D A, Robinson C, Trees C, Turner S M, van der Wal P. A biogeochemical study of the coccolithophore Emiliania huxleyi in the North Atlantic. Global Biogeochem Cycles. 1993;7:879–900. [Google Scholar]
- 19.Klaveness D. Coccolithus huxleyi (Lohman) Kampter. I. Morphological investigations on the vegetative cell and the presence of coccolith formation. Protistologica. 1972;8:335–346. [Google Scholar]
- 20.Lindum P W, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M. N-Acyl-l-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J Bacteriol. 1998;180:6384–6388. doi: 10.1128/jb.180.23.6384-6388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linschooten C, van Bleijswijk J D L, van Emburg P R, de Vrind J P M, Kempers E S, Westbroek P, de Vrind-de Jong E W. Role of the light-dark cycle and medium composition on the production of coccoliths by Emiliania huxleyi (Haptophyceae) J Phycol. 1991;27:82–86. [Google Scholar]
- 22.Luporini P, Vallesi A, Miceli C, Bradshaw R A. Chemical signaling in ciliates. J Eukaryot Microbiol. 1995;42:208–212. doi: 10.1111/j.1550-7408.1995.tb01567.x. [DOI] [PubMed] [Google Scholar]
- 23.Marie D, Partensky F, Jacquet S, Vaulot D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol. 1997;63:186–193. doi: 10.1128/aem.63.1.186-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medlin L K, Barker G L A, Campbell L, Green J C, Hayes P K, Marie D, Wrieden S, Vaulot D. Genetic characterisation of Emiliania huxleyi (Haptophyta) J Mar Syst. 1996;9:13–31. [Google Scholar]
- 25.Mitchell A P. Two switches govern entry into meiosis in yeasts. In: Haseltine F, First N, editors. Meiotic inhibition: molecular control of meiosis. New York, N.Y: Alan R. Liss, Inc.; 1988. pp. 47–66. [PubMed] [Google Scholar]
- 26.Piatelli A, Podda G, Scarano A. Clinical and histological results in alveolar ridge enlargement using coralline calcium carbonate. Biomaterials. 1997;18:623–627. doi: 10.1016/s0142-9612(96)00158-5. [DOI] [PubMed] [Google Scholar]
- 27.Ragatz L, Jiang Z-Y, Bauer C E, Gest H. Macroscopic phototactic behavior of the purple photosynthetic bacterium Rhodospirillum centenum. Arch Microbiol. 1995;163:1–6. doi: 10.1007/BF00262196. [DOI] [PubMed] [Google Scholar]
- 28.Ragatz L, Jiang Z-Y, Bauer C E, Gest H. Phototactic purple bacteria. Nature. 1994;370:104. [Google Scholar]
- 29.Remes A, Williams D F. Relationship between chemotaxis and complement activation by ceramic biomaterials. Biomaterials. 1991;12:661–667. doi: 10.1016/0142-9612(91)90114-p. [DOI] [PubMed] [Google Scholar]
- 30.Ripamonti U, Ma S S, van den Heever B, Reddi A H. Osteogenin, a bone morphogenetic protein, adsorbed on porous hydroxyapatite substrata, induces rapid bone differentiation in calvarial defects of adult primates. Plast Reconstr Surg. 1992;90:382–393. doi: 10.1097/00006534-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Sikes C S, Fabry V J. Photosynthesis, CaCO3 deposition, coccolithophorids and the global carbon cycle. In: Tolbert N E, Preiss J, editors. Photosynthetic carbon metabolism and regulation of atmospheric CO2 and O2. New York, N.Y: Oxford University Press; 1994. pp. 217–233. [Google Scholar]
- 32.Stupp S I, Braun P V. Molecular manipulation of microstructures: biomaterials, ceramics, and semiconductors. Science. 1997;277:1242–1248. doi: 10.1126/science.277.5330.1242. [DOI] [PubMed] [Google Scholar]
- 33.van Bleijswijk J D L, Velduis M J W. In situ gross growth rates of Emiliania huxleyi in enclosures with different phosphate loadings revealed by diel changes in DNA content. Mar Ecol Prog Ser. 1995;121:271–277. [Google Scholar]
- 34.Walsh D, Mann S. Fabrication of hollow porous shells of calcium carbonate from self-organizing media. Nature. 1995;377:320–323. [Google Scholar]
- 35.Westbroek P, de Jong E W, van der Wal P, Borman A H, de Vrind J P M, Kok D, de Bruijn W C, Parker S B. Mechanism of calcification in the marine alga Emiliania huxleyi. Philos Trans R Soc Lond. 1984;304:435–444. [Google Scholar]
- 36.Young G M, Smith M J, Minnich S A, Miller V L. The Yersinia enterocolitica motility master regulatory operon, flhDC, is required for flagellin production, swimming motility, and swarming motility. J Bacteriol. 1999;181:2823–2833. doi: 10.1128/jb.181.9.2823-2833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young J R. Variation in Emiliania huxleyi coccolith morphology in samples from the Norweigian EHUX mesocosm experiment, 1992. Sarsia. 1994;79:417–425. [Google Scholar]
- 38.Young J R, Kucera M, Chung H-W. Automated coccolith biometrics on captured light microscope images of Emiliania huxleyi. In: Whatley R, Moguilevsky A, editors. Microfossils and oceanic environments. Aberystwyth, United Kingdom: University of Wales, Aberystwyth Press; 1996. pp. 261–280. [Google Scholar]