Abstract
The most common surgical risk after total thyroidectomy remains the recurrent laryngeal nerve (RLN) injury. Nowadays, the use of intraoperative nerve monitoring systems (IONM) such as the endotracheal tube‐based is recommended to prevent RLN palsy. The use of the nerve monitoring is standardized by dedicated guidelines on the basis of a normal laryngeal anatomy, but previous head and neck surgical procedures may complicate its application. The authors herewith present a case of a non‐conventional use of endotracheal tube‐based IONM in a 72‐year‐old patient who underwent to a second‐stage total thyroidectomy for metastatic papillary cancer incidentally detected after an open partial horizontal laryngectomy (OPHL) extended to one arytenoid (Type IIa + ary left) for squamous cell carcinoma. The use of the endotracheal tube‐based IONM in such particular case where the function of the only remaining arytenoid had to be absolutely preserved was effective in avoiding the RLN accidental injury. The authors reviewed the non‐traditional use of IONM and described the procedure in case of thyroidectomy in patients previously treated by OPHL.
Keywords: intraoperative nerve monitoring, metastatic papillary cancer, OPHL, partial laryngectomy, thyroid cancer
Intraoperative Neuro‐monitoring can offer a paramount surgical benefit in case of unconventional surgical scenarios such as OPHL. It can represent a safe and reliable indicator for the recurrent laryngeal nerve identification when facing with major anatomical changes hazarding laryngeal function.

1. INTRODUCTION
Estimated incidence of thyroid cancer according to GLOBOCAN 2020 is 586,202 new cases in overall population. 1
In Italy, over 34,000 thyroid operations were performed during 2016, 2 , 3 and in the United States, 150,000 thyroidectomies per year are estimated. 4
A critical point of thyroid surgery is the potential recurrent laryngeal nerve (RLN) injury, which can result in either unilateral or bilateral, temporary or permanent vocal cord paresis or paralysis. The British Association of Endocrine and Thyroid Surgeons reported the post‐surgical injury rate at 1.8%, and current rates in tertiary care centers have been reported as low as 0%–1.1%, 5 but these data are definitively underestimated.
RLN injury is an invalidating complication that affects postoperative quality of life: The RLN provides the whole innervation of the crico‐arytenoid unit, which is key for breathing, phonation, and swallowing. 6 , 7 In case of unilateral RLN injuries, the contralateral crico‐arytenoid unit can initially compensate the dysfunction, especially if the adductors tone is preserved, while bilateral lesions have major impact on quality of life frequently requiring tracheostomy. Even though less bothersome, lesions of the superior laryngeal nerve may cause alterations in mucosal sensitivity (in case of lesion of the external branch alone) or disfunction of the cricothyroid muscle with consequent alteration of high pitches and vocal cord de‐tension, which can affect more professional vocal users. 8 Different strategies have been described as to avoid RLN injury.
Indirect approaches to overcome RLN injuries are represented by the visualization of the intraoperative vocal cord movement or palpation of the crico‐arytenoid muscle during RLN stimulation.
The first objective evaluation with electrical stimulation was made on animal models by Shedd and Durham in 1965 who studied the differential pressures on the tube's cuff positioned on the glottis. 9 The need for more reliable tools led to the development of proper forms of neuromonitoring based on electromyography (EMG): direct placement of needles in vocalis muscles either endoscopically or intraoperatively or via the use of endotracheal tube‐based electrodes. The parameters to consider EMG monitoring of the RLN during surgery are the shape of the waveform, the amplitude, the latency, and the threshold. The basic evoked waveform for RLN or Vagus nerve during EMG monitoring is typically biphasic or triphasic. 10
The benefit of electromyographic endotracheal tube monitoring for RLN preservation in standard thyroid surgery has not a statistical evidence, 11 probably because of the very low frequency of permanent RLN paralysis following primary thyroid resections despite the methods of intraoperative RLN identification and monitoring. 12 However, the recommendations of the surgical societies for the standardized application of IONM have been established and incorporated into guidelines.
The main benefit of IONM remains the possible change of resection strategy in case of a “loss of signal” (LOS) after resection of one thyroid lobe in patients with planned bilateral resection, 12 , 13 reducing the risk of the highly invalidating bilateral vocal cord palsy.
In literature, the use of IONM is also recommended in patients with anatomical laryngeal alterations because of its reliability in high‐risk cases during revision surgery with the loss of the anatomical landmarks 10 , 11 , 14 , 15 , 16 but it has never been described the use of IONM in case of thyroid surgery in patients with a clinical history of partial laryngeal resection. In such scenarios, the preservation of the remaining RLN after partial laryngectomy is mandatory because any injury would lead to the loss of the laryngeal functions with consequent need of total laryngectomy or permanent tracheostomy and/or gastrostomy.
The authors reviewed the non‐traditional use of IONM and described the procedure of thyroidectomy in patients previously treated by open partial horizontal laryngectomy (OPHL) to help clinicians who face this complex and uncommon scenario.
2. CASE REPORT
A 72‐year‐old patient underwent total thyroidectomy for metastatic papillary cancer incidentally detected after a Type IIa + ary left OPHL according to the classification of Succo et al. 17 associated with left selective neck dissection (levels IIa‐III‐IV) and temporary tracheostomy performed at the Unit of Otorhinolaryngology, Department of Surgery, Azienda Ospedaliero‐Universitaria di Cagliari, University of Cagliari, Italy.
After the OPHL surgery, the patient had a good functional recovery with a compensatory movement of the residual crico‐arytenoid unit and he was therefore successfully decannulated. The histological report was diagnostic for squamous cell carcinoma with no evidence of locoregional metastases of squamous cell carcinoma (pT2N0cM0): Thus, there was no indication for adjuvant therapy and the unimodality treatment was achieved. Unexpectedly, one 1.8 mm papillary thyroid cancer nodal metastasis (Thyroglobulin +, TTF1 +) was detected in the level III (pN1b) after neck dissection.
According to the American Thyroid Association (ATA) guidelines and Italian consensus, the high‐risk features of the case mandate for total thyroidectomy with central node dissection. 18 , 19 , 20
The surgical planning was influenced by the risk of losing the only remaining crico‐arytenoid unit secondary to RLN damage in an already operated area. During counseling, the patient was made aware of the high risk of tracheostomy, consequent to right RLN damage, due to anatomical changes and scarring of the surgical bed.
A baseline computed tomography (CT) scan was performed: No focal thyroid lesions were detected nor lymphadenopathies.
In our Department, the endotracheal‐based neuromonitoring device for thyroid surgery is routinely used according with International Neural Monitoring Study Group guidelines. 10
The use of the nerve integrity monitor (NIM 3.0, Medtronics, Inc.) was considered essential to overcome the anatomical difficulties in the identification and preservation of the RLN.
The cooperation and the proactive communication during the preoperative briefing with the anesthetic team were crucial to achieve the best outcome: No Lidocaine gel‐based lubricant nor muscle relaxants after the induction were employed.
The endotracheal tube was placed in the neo‐larynx under endoscopic view, allowing the precise alignment of the right electrode upon the remaining crico‐arytenoid unit (Figure 1).
FIGURE 1.
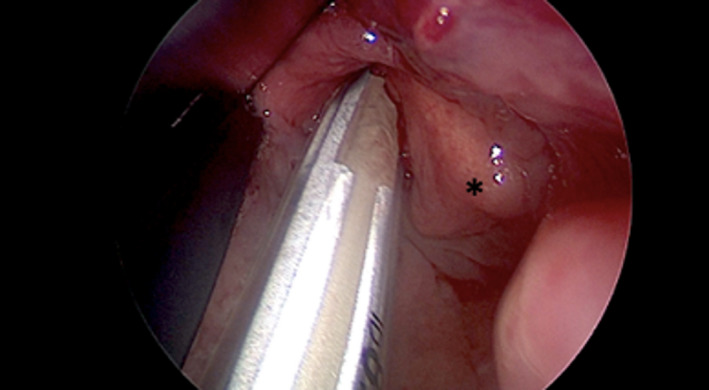
Endoscopic view of endotracheal tube‐based neuromonitoring position: the right electrode (white band) leans on the remnant crico‐arytenoid unit (*)
The standard recommended algorithm 10 for monitoring tube placement was executed:
After intubation, the patient was positioned in head extension; no shoulder roll was placed in order to avoid any tube mispositioning;
The taping of the tube for stabilizing purpose was positioned adherently to the lips and not further up;
It has been waited for the respiratory variations waves to appear in the Medtronic 3.0 NIM monitor;
The tube positioning was once more checked via direct visualization;
A “tap test” was performed, even though there are not available data about its accuracy;
Monitor settings such as electrode's impedance and the event threshold were assessed;
A stimulation on the strap muscles after the raising of the flap was performed in order to evaluate a gross twitching and to confirm the complete myorelaxants' wash‐out.
The surgical field was fibrotic and the identification of the anatomical landmarks was challenging.
There was no indication for left RLN identification, given the previous surgery. The right RLN was more easily identified at the inferior aspect of the thyroid in the untouched tracheoesophageal groove, although before the entrance into the larynx at the level of the crico‐arytenoid unit, the RLN was identified with the aid of the IONM system. In addition, the bilateral superior laryngeal nerves were not identified during this procedure due to the already discussed fibrotic changes, although both superior laryngeal nerves were anatomically identified during the former procedure (OPHL).
After the removal of the thyroid, the nerve appeared to be intact and responsive at the stimulation (Figure 2): the stimulation was related to an acceptable biphasic wave in the monitor with a stimulation of 0.5–1.0 mA (Figure 3).
FIGURE 2.

RLN isolated
FIGURE 3.

Recurrent laryngeal nerve electric response
No LOS from right RLN has been detected during the whole operation.
Once awake, the patient was eupneic and he underwent in‐theater laryngoscopy confirming the right hemi‐larynx movement and, consequently, the integrity of the right RLN. Therefore, no tracheostomy was required. Postoperative swallowing function and speech were evaluated, and no differences from the preoperative features were noted (Figures 4 and 5).
FIGURE 4.

Post‐op endoscopic features of new larynx during abduction
FIGURE 5.

Post‐op endoscopic features of new larynx during adduction
The patient was discharged 7 days after surgery.
Histology was diagnostic for infiltrative papillary microcarcinoma, classic variant, multicentric (biggest nodule 4 mm), BRAF‐V600E positive in the left lobe. No other lymph nodal metastases were detected. Definitive staging according to AJCC 2018 was pT1a (m) N1b. Adjuvant therapy with Radioiodine was performed.
No relapses were detected during the follow‐up, neither clinically nor radiologically (9 months post‐op).
3. DISCUSSION
Incidental finding of differentiated thyroid cancer (DTC) is not seldom, occurring in 1%–10% of general population. 21 Nodal metastases from differentiated thyroid cancer are rarely detected after neck dissection in patients treated for head and neck malignancies, such as squamous cell carcinoma, occurring approximatively in 0.7%. 22 , 23
Even though the detection of DTC does not affect head and neck patients' prognosis, total thyroidectomy with central compartment neck dissection is mandatory in order to assess a proper staging and a correct therapeutic work‐up. 18 , 21
In 2015, Farrag and co‐workers 24 reported a case series of incidental thyroid findings in patients who underwent laryngectomy. Eight patients out of 67 were reported to have incidental thyroid cancer: four of them were diagnosed with differentiated thyroid cancer after open partial laryngectomy. Key take‐home‐message of the above study is the importance of preoperative assessment of synchronous thyroid tumors in order to avoid a second surgery given the challenge and difficulty of re‐operating the central neck compartment. The authors did not mention any neuromonitoring and reported one case of metastatic papillary cancer (case 2) undertreated with hemi‐thyroidectomy alone due to the significant scarring and noted difficulty in identifying and preserving the RLN.
The present study shows the efficacy of the tube‐based IONM in case of anatomical changes of the larynx with preservation of a single crico‐arytenoid unit. This scenario is challenging because of the technical difficulties due to the postoperative anatomical aberrations.
In our Department, the current practice regarding RLN neuromonitoring is represented by the use of endotracheal tube‐based electrodes according to NIM guidelines. 10 , 13 An important parameter to recognize potential intraoperative neural damage is the response amplitude. In standard conditions, the amplitude response during stimulation (measured from the apex of a positive waveform to the lowest point of the next phase of the waveform) ranges between 100 and 800 μV and may vary within the same patient and among patients and it correlates to the number of muscle fibers recruited. 10 The ranging of the amplitude during surgery, within the same patient, may be influenced by the amount of blood and fluids in the surgical field, the variation of probe‐nerve contacts due to the interposition of tissues and fascia, the variation of temperature in the surgical field, or the misplacement of the surface electrodes mounted to the endotracheal tube. 10 In the present case, the amplitude after stimulation was 203 μV, in range with standard values. Other important parameters to consider in the electrophysiologic RNL monitoring setting, as abovementioned, are the threshold, the latency, and the shape of the waveform. The threshold value is the minimal current that, when applied to the nerve, triggers the EMG response. Human RLN and Vagus nerve have a threshold of 0.3–0.4 mA when dry and dissected from their fascia. 10 The stimulation response starts at threshold and progressively increases reaching maximum amplitude toward 0.8 mA, pass that point, there is no further intensification of response given that all nerve fibers are already recruited. This phenomenon supports the use of a suprathreshold stimulation current of 1 mA during dissection, while a 2 mA stimulation is justified only for initial mapping of the RLN when there are still tissues overlining the nerve. 10 Latency is defined as the distance between the stimulation spike to the first evoked waveform deflection. As a parameter, latency, is still to be well defined in surgical monitoring literature, it is believed to depend on the distance of the stimulated point from the ipsilateral surface electrode (vocal cord). 10 The mean latencies observed in literature are for RLN 3.97 msec, for EBSLN 3.5 msec, for right Vagus 5.4 msec, and for left Vagus 8.1 msec. 10 Our case showed a latency of 1.25 msec. A study enrolling a solid number of patients (111) undergoing thyroid surgery pointed‐out that the mean pre‐thyroidectomy latency was 1.98 ± 0.52 ms (range: 0.9–3.3). These data are consistent with our result confirming the integrity of the nerve. 25
All these parameters are used intraoperatively to recognize a true waveform from false‐positive waveforms due to artifacts. The biphasic shape of the waveform is characteristic enough to differentiate artifacts‐related waveforms such as those related to cable manipulation, respiratory artifacts, thermal effects, non‐neural shunt stimulations, or striking of two metal instruments within the surgical field. 10 Our resulting waveform was, as already mentioned, an acceptable biphasic wave.
Loss of signal is defined as the absence of EMG activity (electrical silence) or the substantial reduction of EMG activity (<100 μV) after stimulation of the Vagus or of the RLN. To correctly attribute the LOS to neural damage and further proceed with decision‐making regarding the treatment of contralateral site, the surgical equipment has to exclude that LOS is due to device malfunction or neuromuscular blockage. Thus, in case of LOS, an intraoperative evaluation algorithm is used and the first step is checking the presence or absence of laryngeal twitch differentiating stimulation‐side problems (which may imply true neural damage) to recording‐side problems which are generally due to endotracheal‐tube malposition, 10 although in our case there was no glottis, the contact with the remaining right arytenoid was adequate for a standard recording.
The absence of LOS during all the surgical steps correctly predicted the anatomical integrity of the right RLN, documented post‐operatively via laryngoscopy and functional assessment tools, preserving the functional outcome of the former OPHL.
Different unconventional uses of IONM are mentioned in literature. Mushiake et al. reported a case of RLN monitoring during an esophageal cancer operation in a patient with double aortic arch Ninomiya et al. 15 mentioned the usefulness of IONM for the treatment of thoracic esophageal cancer in a patient with a right aortic arch. 16 Another paradigmatic adaptation of IONM in case of anatomical abnormality is reported from Kitigawa et al.: They emphasized the effectiveness of intraoperative nerve monitoring in case of thoracic esophageal squamous cell carcinoma with aberrant subclavian artery. 16
The advantages offered by the use of IONM are not clearly stated in literature. The main bias of these data can be represented by the high surgical experience of several centers reporting their own data where there is no statistically significant difference between the intraoperative visualization alone versus the IONM in reducing the rate of RLN injury.
A meta‐analysis published in 2013 by Pisanu et al. 26 showed that the RLN permanent palsy rate was 0.79% in the IONM group and 0.92% in the VA (Visualization Alone) group. More recently, a meta‐analysis conducted by Wong et al. 27 focused on the high‐risk cases only (multiple surgery cases, thyroidectomies for malignancy, thyrotoxicosis, and retrosternal goiter), like in the present case. Their results seem underline a statistically significant advantage in the use of IONM for reducing overall, transient and permanent palsy in re‐operations and thyroidectomy for malignancy. 27 Even though there is a lack of diriment data about the need of IONM, the present case can be considered as a high‐risk operation, fitting the parameters listed on the aforementioned metanalysis.
Despite the fact that IONM guidelines could not be strictly followed due to the anatomical changes of the larynx where the monitoring tube was positioned, the intraoperative EMG patterns were in agreement with the standard electrophysiological findings: biphasic waveforms after stimulation and absence of LOS.
The tube‐based IONM performance enabled a safer approach. The precise stimulation response of the right RLN gave the reassurance about its integrity avoiding tracheostomy.
4. CONCLUSIONS
In experienced hands, IONM is not mandatory, but its use seems to be helpful even during conventional surgery. IONM could offer also a paramount surgical benefit in case of unconventional scenarios because it represents a safe and reliable indicator for the RLN identification and localization when facing with major anatomical changes.
AUTHOR CONTRIBUTIONS
Carta Filippo drafted the main text and contributed to clinical postoperative management. Marrosu Valeria, Pinto Valeria drafted the main text. Tatti Melania actively contributed to clinical care and follow‐up, and helped in literature collection. Mauro Bontempi helped in literature search. Mariani Cinzia: corrected english grammar. Puxeddu Roberto performed the surgery, contributed to clinical management of the patient, and revised the manuscript.
FUNDING INFORMATION
The authors received no financial support for the present research.
CONFLICT OF INTEREST
The authors declare that this manuscript was conceived and written by the cited authors, they do not have any financial interests to disclose, and they confirm no conflict of interest concerning this manuscript.
CONSENT
Written informed consent was obtained from patient's parents to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENTS
The authors received no financial support for the present research.
Filippo C, Valeria M, Valeria P, et al. Intraoperative recurrent laryngeal nerve monitoring in unconventional thyroid surgery. Clin Case Rep. 2022;10:e06137. doi: 10.1002/ccr3.6137
Carta Filippo, Marrosu Valeria and Pinto Valeria equally contributed to the manuscript.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Data source: Global cancer Observatory. International Research on Cancer . 2021. http://gco.iarc.fr/
- 2. Italian Ministry of Health , Annual Report on hospital admissions . 2016. http://www.salute.gov.it/portale/documentazione/p6_2_2_1.jsp?lingua=italiano&id=2651
- 3. Canu GL, Medas F, Cappellacci F, et al. Can thyroidectomy be considered safe in obese patients? a retrospective cohort study. BMC Surg. 2020;20(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Al‐Qurayshi Z, Robins R, Hauch A, et al. association of surgeon volume with outcomes and cost savings following thyroidectomy: a national forecast. JAMA Otolaryngol Head and Neck Surg. 2016;142(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 5. Chadwick D., Kinsman R., Walton P. The British Association of Endocrine and Thyroid Surgeons Fourth National Audit Report . 2012.
- 6. Leden V, Moore P. The mechanics of the cricoarytenoid joint. Arch Otolaryngol. 1961;73:541‐550. [DOI] [PubMed] [Google Scholar]
- 7. Sato K. Functional Histoanatomy of the Human Larynx. Springer Nature; 2018. [Google Scholar]
- 8. Berger G, Kosztyla‐Hojna B, Chyczewski L. Impact of the anatomy of laryngeal nerves on intraoperative neuromonitoring results in surgery of thyroid gland and functional results after partial laryngectomies. Pol Przegl Chir. 2018;91(2):30‐37. [DOI] [PubMed] [Google Scholar]
- 9. Shedd DP, Durham C. Electrical identification of the recurrent laryngeal nerve. I. Response of the canine larynx to electrical stimulation of the recurrent laryngeal nerve. Ann Surg. 1966;163:47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Randolph GW, Dralle H, International Intraoperative Monitorig Study Group Dralle H., with the International Intraoperative Monitoring Study Group , et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline Statement. Laryngoscope. 2011;121(1):S1‐S16. [DOI] [PubMed] [Google Scholar]
- 11. Godballe C, Madsen AR, Sorensen CH, et al. Risk factors for recurrent nerve palsy after thyroid surgery: a national study of patient treated at Danish departments of ENT Head and Neck Surgery. Eur Arch Otorhinolaryngol. 2014;271(8):2267‐2276. [DOI] [PubMed] [Google Scholar]
- 12. Angeletti F, Musholt PB, Musholt TJ. Continuous intraoperative neuromonitoring in thyroid surgery. Surg Technol Int. 2015;27:79‐85. [PubMed] [Google Scholar]
- 13. Schneider R, Randolph GW, Dionigi G. International neural monitoring study group guideline 2018 Part I: staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope. 2018;128(3):S1‐S17. [DOI] [PubMed] [Google Scholar]
- 14. Mushiake S, Taniguchi K, Lee SW, et al. The usefulness of intraoperative neurological monitoring for esophageal cancer with double aortic arch; a case report. BMC Surg. 2020;20(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ninomiya Y, Oguma J, Ozawa S, et al. Thoracoscopic esophagectomy with left recurrent laryngeal nerve monitoring for thoracic esophageal cancer in a patient with a right aortic arch: a case report. Surg Case Rep. 2020;6(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitahawa H, Iwabu J, Yokota K, et al. Intraoperative neurological monitoring during neck dissection for esophageal cancer with aberrant subclavian artery. Anticancer Res. 2019;39(6):3203‐3205. [DOI] [PubMed] [Google Scholar]
- 17. Succo G, Peretti G, Piazza C, et al. Open partial horizontal laryngectomies: a proposal for classification by the working committee on nomenclature of the European Laryngological Society. Eur Arch Otorhinolaryngol. 2014;271(9):2489‐2496. [DOI] [PubMed] [Google Scholar]
- 18. Pacini F, Basolo F, Bellantone R, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statement of six Italian societies. J Endocrinol Invest. 2018;41:849‐876. [DOI] [PubMed] [Google Scholar]
- 19. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McLeod D.S.A, Zhang L, Durante C, Cooper D.S. Contemporary debates in adult papillary thyroid cancer management. Oxford Academic; 2019. https://academic.oup.com/edrv [DOI] [PubMed] [Google Scholar]
- 21. Lenzi R, Marchetti M, Muscatello L. Incidental nodal metastasis of differentiated thyroid carcinoma in neck dissection specimens from head and neck cancer patients. J Laryngol Otol. 2017;131:368‐371. [DOI] [PubMed] [Google Scholar]
- 22. Ansari‐Lari MA, Westra WH. The prevalence and significance of clinically unsuspected neoplasms in cervicale lymph nodes. Head Neck. 2003;25(10):841‐847. [DOI] [PubMed] [Google Scholar]
- 23. Clark RL, Hickey RC, Ibanez ML, Ballantyne AJ. Thyroid cancer discovered incidentally during treatment of unrelated head and neck cancer: review of 16 cases. Ann Surg. 1966;163(5):665‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farrag TY, Lin FR, Cummings CW, et al. Importance of routine evaluation of the thyroid gland prior to open partial laryngectomy. Arch Otolaryncol Head Neck Surg. 2006;132(10):1047‐1051. [DOI] [PubMed] [Google Scholar]
- 25. Ozemir IA, Ozyalvac F, Yildiz G, Eren T, Aydin‐Ozemir Z, Alimoglu O. Importance of latency and amplitude values of recurrent laryngeal nerve during thyroidectomy in diabetic patients. Int J Surg. 2016;35:172‐178. [DOI] [PubMed] [Google Scholar]
- 26. Pisanu A, Porceddu G, Podda M, Cois A, Uccheddu A. Systematic review with meta‐analysis of studies comparing intraoperative neuromonitoring of recurrent laryngeal nerves versus visualization alone during thyroidectomy. J Surg Res. 2014;188:152‐161. [DOI] [PubMed] [Google Scholar]
- 27. Wong KP, Mak KL, Wong CKH, Lang BHH. Systematic review and meta‐ analysis on intra‐operative neuro‐monitoring in high‐risk thyroidectomy. Int J Surg. 2017;38:21‐30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


