Abstract
Background:
Personal payments from the pharmaceutical industry to US physicians are common and are associated with changes in physicians’ clinical practice and interpretation of clinical trial results. We assessed temporal trends in industry payments to oncologists, with particular emphasis on payments to authors of oncology clinical practice guideline and on payments related to immunotherapy drugs.
Methods:
We included US physicians with active National Plan and Provider Enumeration System records and demographic data available in the Centers for Medicare & Medicaid Services Physician Compare system who had a specialty type of medical oncology or general internal medicine. Medical oncologists serving on NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Panels were identified manually. Industry payments, and the subset associated with PD-1/PD-L1 drugs, were identified in Open Payments, the federal repository of all transactions of financial value from industry to physicians and teaching hospitals, from 2014 to 2017.
Results:
There were 13,087 medical oncologists and 85,640 internists who received payments. The mean, annual, per-physician value of payments to oncologists increased from $3,811 in 2014 to $5,854 in 2017, and from $444 to $450 for internists; the median payment increased from $152 to $199 for oncologists and remained at $0 for internists. Oncologists who served on NCCN Guidelines Panels received a greater value in payments and experienced a greater relative increase: mean payments increased from $10,820 in 2014 to $18,977 in 2017, and median payments increased from $500 to $1,366. Among companies marketing PD-1/PD-L1 drugs, mean annual per-oncologist payments associated with PD-1/PD-L1 drugs increased from $28 to $773. Total per-oncologist payments from companies marketing PD-1/PD-L1 drugs experienced a 165% increase from 2014 to 2017, compared with a 31% increase among similar companies not marketing PD-1/PD-L1 drugs.
Conclusions:
Pharmaceutical industry payments increased for US oncologists from 2014 to 2017 more than for general internists. The increase was greater among oncologists contributing to clinical practice guidelines and among pharmaceutical companies marketing PD-1/PD-L1 drugs. The increasing flow of money from industry to US oncologists supports ongoing concern regarding commercial interests in guideline development and clinical decision-making.
Background
Financial relationships between US physicians and the pharmaceutical industry are common, particularly among oncologists. In 2014, the first full year of available data from the Open Payments transparency law, which required the public reporting of all transactions of financial value from industry to physicians and teaching hospitals, 40% of US physicians received at least one payment or in-kind value transfer from a drug or devicemanufacturer,1 and this number exceeded 60% for medical oncologists.2 The total dollar value of personal payments from industry to US medical oncologists is >$75 million annually.2
The high prevalence of such relationships has been a source of concern,3,4 because the receipt of industry payments has consistently been associated with changes in physician behavior in ways that are more favorable to industry interests.5 Among physician authors of editorials commenting on clinical trial results, those who have received payments from the relevant companies express views more favorable to the tested product.6,7 Research evaluating the cost-effectiveness of targeted cancer drugs may reach more favorable conclusions when authors have funding from or financial relationships with the drug manufacturer.8 Physicians who have received payments from a drug company are more likely to use that company’s drug over other therapeutic options; this association has been found across many drug classes,9–14 including anticancer drugs.15,16
Because the magnitude of industry payments received by physicians in many specialties—including medical oncology—has increased during the period that Open Payments data have been available,17–21 these findings regarding physician behavior warrant ongoing attention and concern. We decided to investigate several aspects of industry payments that have not been characterized previously for oncologists andmay further our understanding of how these payments function within the current clinical oncology landscape. For example, changes in payments over physicians’ career trajectories may be informative regarding which physicians are believed to be the highest-priority targets for promotional activities by manufacturers, whereas trends in the specific drugs being promoted may indicate a shifting focus of manufacturer promotional strategies. Trends in payments across the calendar year have not been evaluated previously and may provide insight into whether payments are occurring with greater frequency at large academic events, such as the ASCO Annual Meeting, which are attended by both physicians and industry representatives.
Therefore, the goals of this study were to characterize industry payments to physicians with respect to (1) clinical specialties and participation in clinical practice guideline creation, (2) variation across the career trajectory, (3) variation across the calendar year, and (4) temporal trends in payments for newly approved drugs, using PD-1 and PD-L1 agents as a case study. We focused on PD-1 and PD-L1 agents because their recent approval uniquely allows for the evaluation of initial payment patterns for entire classes of drugs and of how these drugs may have shaped the overall payment landscape within medical oncology.
Methods
We linked data across the Open Payments system,22 the National Plan and Provider Enumeration System (NPPES), and the Physician Compare system.23 Open Payments contains data on financial payments from industry to physicians, including dollar amount, the paying company, and the associated drug (if any). Physician Compare includes physician demographic data such as medical school graduation year, physician specialty, and sex.
Starting with Open Payments data for 2014 to 2017, we included individuals who had a physician specialty of either internal medicine and no other subspecialty (“internists”) or medical oncology (defined as medical oncology, hematology/oncology, and gynecologic oncology; “oncologists”). We used internists as our comparison group because they are unlikely to receive industry payments related to cancer drugs, which was our primary area of interest. We linked NPPES records to Physician Compare records by National Provider Identifier (NPI) and then to Open Payments records by physician name and address (Open Payments data do not include NPI). Because physicians who have not received industry payments do not have records in Open Payments, we retained the individuals in the NPPES/Physician Compare data with a relevant specialty type (internal medicine or medical oncology) that did not match to Open Payments. We then excluded those with different specialties between the Open Payments and Physician Compare datasets who were missing data or had a deactivated NPI.
We also identified the subset of medical oncologists who were listed as panelists on ≥1 of the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Panels for the 20 most prevalent cancers in the United States for any amount of time from 2014 to 2017.24 To accomplish this, we manually abstracted lists of NCCN Guidelines Panelists from NCCN Guidelines documents (contributing panelists are listed on the second page of each NCCN Guidelines document) and manually linked them to their Open Payments records.
Our analysis focused on personal payments to physicians (corresponding to the Open Payments category of general payments), including payments and in-kind transfers such as consulting fees, gifts, entertainment, and food and beverages. The Open Payments categories of research payments and ownership interests were not included. We calculated annual, per-physician payments from industry to oncologists, internists, and NCCN Guidelines Panelists. We separately assessed payments to oncologists from pharmaceutical companies marketing a PD-1/PD-L1 drug that was FDA-approved before or during the 2014–2017 study period. In cases where one drug was marketed by 2 companies, such as by parent/subsidiary companies, we included payments from both. For comparison, we also assessed payments from companies that (1) were among the top 20 pharmaceutical companies by market capitalization in 2018, (2) marketed at least one cancer drug, and (3) did not market a PD-1/PD-L1 drug or have one in development. We evaluated both the total payments from each company and the proportion of each drug company’s payments associated with that company’s PD-1/PD-L1 drug (for each industry payment, Open Payments includes the name of the specific drug product, if any, associated with that payment). We used a Mann-Kendall trend test to assess for increasing or decreasing trends in industry payments over the study period. To evaluate the seasonality of payments over the calendar year, we applied time-series decomposition to payment data across the 4 years of the study period. Last, we grouped oncologists by medical school graduation year and assessed trends in payments across the 4-year study period for each graduation year–specific cohort.
This study was submitted to the Memorial Sloan Kettering Cancer Center Institutional Review Board and was approved as exempt research under 45 CFR 46.101(b)(4). All analysis was conducted in R version 3.5.1 (R Foundation for Statistical Computing).
Results
Of 169,603 physicians in the Open Payments system, 87.1% were matched to their NPPES/Physician Compare records. After excluding physicians with missing data, deactivated NPI, and/or specialty mismatch, we found that the analytical cohort had 98,727 physicians, of whom 85,640 were internists and 13,087 were oncologists (supplemental eFigure 1, available with this article at JNCCN.org). Results showed that 65.0% of oncologists and 61.4% of internists were male (Table 1). Among both oncologists and internists, the sample contained relatively similar proportions of physicians who had graduated <11 years, 11 to 20 years, 21 to 30 years, or >30 years before the study period.
Table 1.
Physician Characteristics
| Characteristic | Oncologists | Internists |
|---|---|---|
| Overall, n | 13,087 (13.03%) | 85,640 (86.7%) |
| Open Payments specialty | ||
| Internal medicine | 0 | 85,640 (100.0%) |
| Medical oncology | 2,953 (22.6%) | 0 |
| Hematology/Oncology | 7,828 (59.8%) | 0 |
| Gynecologic oncology | 908 (6.9%) | 0 |
| Multiple oncology specialties | 1,398 (10.7%) | 0 |
| Unknown | 0 | 0 |
| Sex | ||
| Male | 8,508 (65.0%) | 52,563 (61.4%) |
| Female | 4,579 (35.0%) | 33,077 (38.6%) |
| Unknown | 0 | 0 |
| Years since medical school graduation as of 2014 | ||
| <11 | 3,394 (25.7%) | 26,788 (28.7%) |
| 11–20 | 4,099 (31.3%) | 22,823 (26.6%) |
| 21–30 | 2,976 (22.7%) | 20,144 (23.5%) |
| >30 | 2,618 (20.0%) | 15,885 (18.5%) |
| Unknown | 0 | 0 |
The proportion of oncologists who received any payments within the year increased from 65.1% in 2014 to 68.7% in 2017, and the proportion who received >$10,000 within the year increased from 7.0% to 10.2% (Table 2). In comparison, in 2014, 44.1% and 0.4% of internists received any payment and >$10,000 payments, respectively, and no increases were observed across the study period. The mean value of industry payments received by oncologists increased from $3,811 in 2014 to $5,854 in 2017, whereas for internists the increase was from $444 to $450. “Food and beverage” was the most common type of payment across both specialties in each of the 4 years, although for oncologists the categories “compensation for services” and “consulting fee” reflected substantially higher payment amounts than “food and beverage.”
Table 2.
Characteristics of Payments to Physicians
| Oncologists (n=13,087) |
Internists (n=85,640) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2014 | 2015 | 2016 | 2017 | |
|
| ||||||||
| Number of physicians receiving payments | ||||||||
|
| ||||||||
| Any payments, n (%) | 8,517 (65.1) | 8,816 (67.4) | 8,880 (67.9) | 8,979 (68.7) | 37,789 (44.1) | 38,293 (44.7) | 37,404 (43.7) | 37,271 (43.5) |
|
| ||||||||
| >10,000, n (%) | 916 (7.0) | 1124 (8.6) | 1273 (9.7) | 1337 (10.2) | 376 (0.4) | 448 (0.5) | 428 (0.5) | 432 (0.5) |
|
| ||||||||
| >100,000, n (%) | 88 (0.7) | 110 (0.8) | 134 (1.0) | 149 (1.1) | 32 (0.03) | 45 (0.05) | 31 (0.04) | 37 (0.04) |
|
| ||||||||
| Number of payments | ||||||||
|
| ||||||||
| Mean | 21.4 | 24.5 | 27.0 | 28.8 | 15.3 | 14.5 | 13.9 | 14.0 |
|
| ||||||||
| Median | 3 | 4 | 4 | 4 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Median, any paymentsa | 13 | 13 | 15 | 15 | 10 | 9 | 9 | 9 |
|
| ||||||||
| Maximum | 702 | 829 | 686 | 921 | 615 | 652 | 570 | 629 |
|
| ||||||||
| Food and beverage | 17.8 | 20.2 | 22.6 | 24.2 | 14.6 | 13.8 | 13.5 | 13.5 |
|
| ||||||||
| Compensationb | 0.7 | 0.7 | 0.8 | 0.9 | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||||||
| Consulting fee | 0.6 | 0.6 | 0.7 | 0.7 | 0.1 | 0.1 | 0 | 0 |
|
| ||||||||
| Honoraria | 0.1 | 0 | 0.1 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Travel and lodging | 1.5 | 1.8 | 2.1 | 2 | 0.1 | 0.1 | 0.1 | 0.1 |
|
| ||||||||
| All other | 0.8 | 1 | 0.8 | 1 | 0.5 | 0.4 | 0.2 | 0.3 |
|
| ||||||||
| Value of payments (USD) | ||||||||
|
| ||||||||
| Mean | $3,811 | $4,698 | $5,553 | $5,854 | $444 | $491 | $443 | $450 |
|
| ||||||||
| Median | $152 | $173 | $191 | $199 | $0 | $0 | $0 | $0 |
|
| ||||||||
| Median, any paymentsa | $648 | $647 | $735 | $795 | $247 | $231 | $225 | $219 |
|
| ||||||||
| Maximum | $650,100 | $661,038 | $1,962,297 | $923,595 | $264,866 | $351,395 | $287,402 | $454,109 |
|
| ||||||||
| Food and beverage | $405 | $472 | $523 | $536 | $240 | $245 | $232 | $235 |
|
| ||||||||
| Compensationb | $1,517 | $1,869 | $2,234 | $2,520 | $109 | $144 | $121 | $122 |
|
| ||||||||
| Consulting fee | $950 | $1,291 | $1,551 | $1,773 | $35 | $41 | $43 | $49 |
|
| ||||||||
| Honoraria | $108 | $65 | $140 | $96 | $11 | $7 | $3 | $3 |
|
| ||||||||
| Travel and lodging | $618 | $716 | $784 | $806 | $31 | $34 | $30 | $31 |
|
| ||||||||
| All other | $213 | $285 | $321 | $124 | $18 | $20 | $14 | $10 |
All figures represent annual per-physician amounts.
The median among the subset of physicians who received any industry payments in that calendar year.
The Open Payments category of “Compensation for services other than consulting, including serving as faculty or as a speaker at an event other than a continuing education program.”
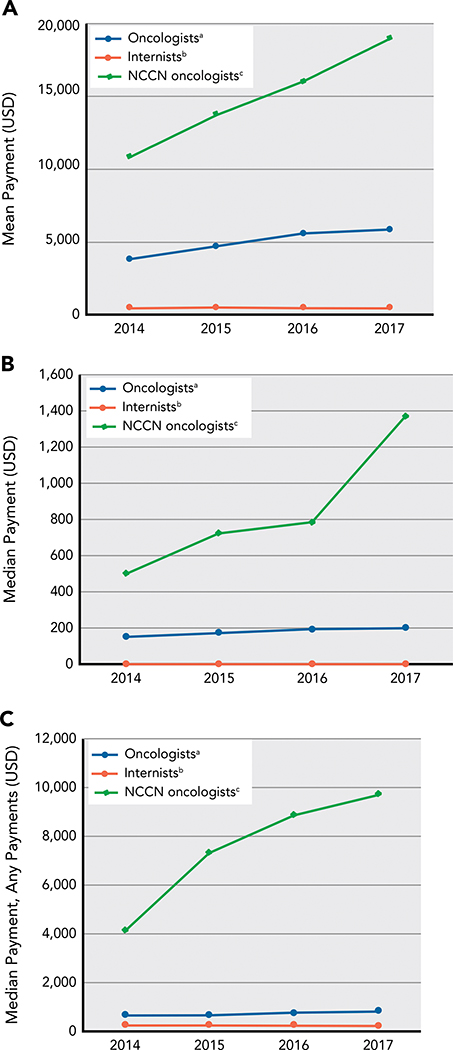
The subset of oncologists who served on NCCN Guidelines Panels received greater dollar amounts in industry payments compared with oncologists overall (Figure 1). These oncologists also experienced a greater relative increase in payments during the study period:median payments increased from $500 in 2014 to $1,366 in 2017 (173% increase) for oncologists serving on NCCN Guidelines Panels compared with $152 to $199 (31% increase) for all oncologists, and mean payments increased from $10,820 to $18,977 (75% increase) compared with $3,811 to $5,854 (54% increase).
Figure 1.
Annual per-physician (A) mean, (B) median, and (C) median dollar value of industry payments to physicians in each category within each calendar year.
aPhysicians who had a subspecialty related to medical oncology, including those who served as a panelist on an NCCN Guidelines committee (n=13,087).
bPhysicians who had a specialty type of internal medicine and no other subspecialty (n=85,640).
cThe subset of oncologists who served as a panelist on 1 (or more) of the NCCN Guidelines committees for the 30 most prevalent cancer types in the United States during the 2014–2017 study period (n=308).
Among most pharmaceutical companies marketing a PD-1 or PD-L1 drug, payments to oncologists increased during the study period (Figure 2). Payments from each company specifically associated with its PD-1/PD-L1 drug began during the year of initial FDA approval. Across all companies marketing PD-1/PD-L1 drugs, mean total payments to oncologists increased from $667 in 2014 to $1,767 in 2017, a 165% increase, whereas the subset of payments specifically associated with PD-1/PD-L1 drugs increased from $28 to $773. In comparison, payments to oncologists from large pharmaceutical companies that marketed anticancer drugs but not PD-1/PD-L1 drugs increased from a per-physician, per-company mean of $139 in 2014 to $182 in 2017, a 31% increase (supplemental eTable 1).
Figure 2.
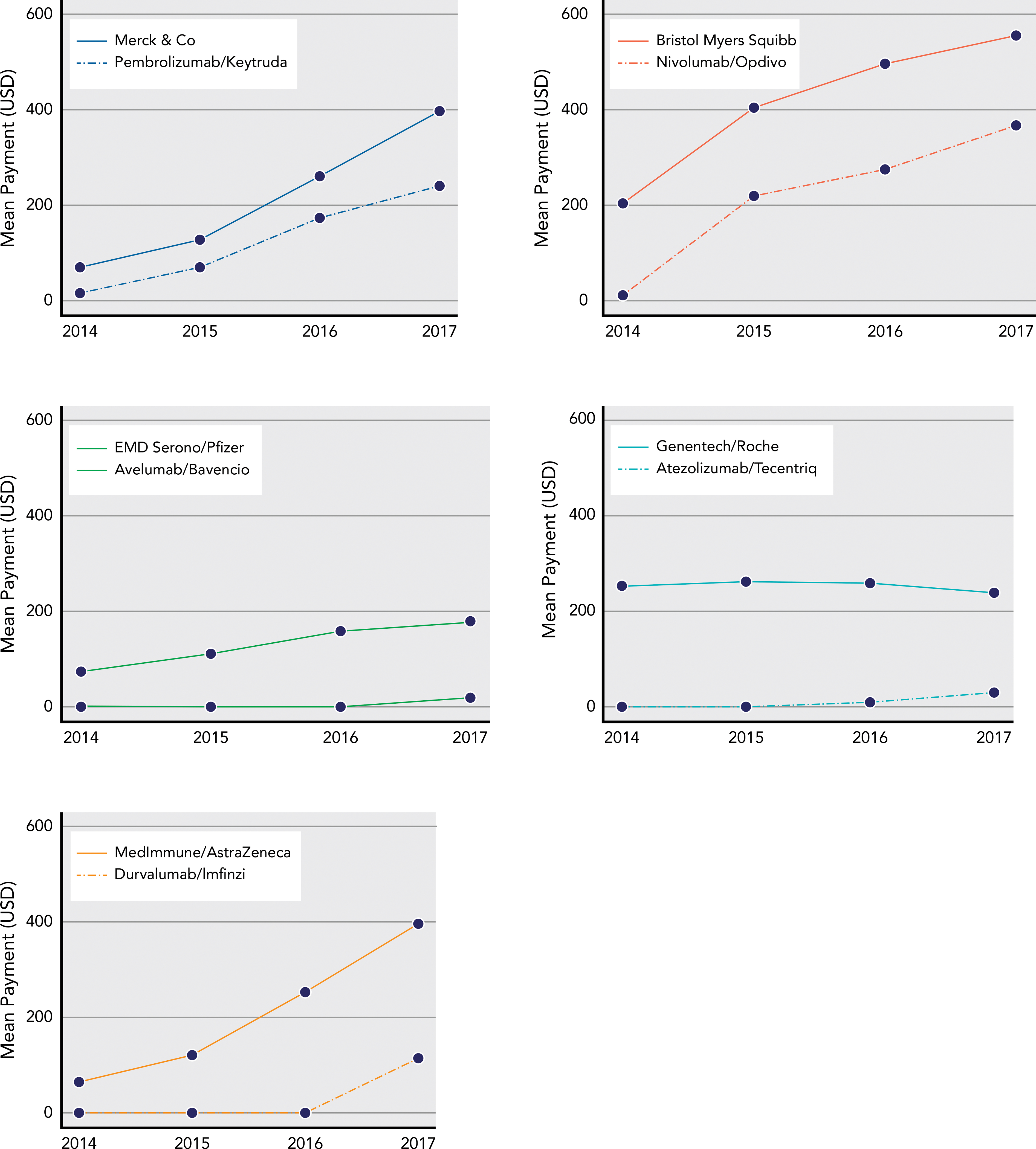
Mean annual per-physician dollar value of general payments to oncologists from pharmaceutical companies that manufactured a PD-1/PD-L1 agent (solid line) and the subset of those payments from that company associated with its PD-1/PD-L1 drug (dashed line). In cases where a single PD-1/PD-L1 drug was comarketed by >1 pharmaceutical company, total payments across both companies are included. The initial FDA approval for each drug occurred in the following years: pembrolizumab, 2014; nivolumab, 2014; atezolizumab, 2016; avelumab, 2017; durvalumab, 2017.
In analyzing the seasonality of payments, we found substantial variation in the value of payments received during each calendar week for both oncologists and internists (supplemental eFigure 2). Oncologists received more in every calendar week compared with internists, and there was greater variation in payments to oncologists. Weekly payments were lower in the summer (27th week) and the beginning/end of the year (1st and 52nd weeks) compared with the rest of the year. After accounting for seasonal variation, a consistent increase across the study period in annual payments to oncologists was observed, and no increase was observed for internists (supplemental eFigure 3).
Oncologists at a later career stage (those with earlier medical school graduation years) tended to receive payments with a greater value than did oncologists with more recent medical school graduation years (Figure 3). Consistent with the trend observed for oncologists in aggregate, most (43/44) medical school graduation year cohorts received payments with a greater mean, per-physician dollar value in 2017 versus 2014. However, consistent year-to-year increases were less common among cohorts with graduation years before 1980. Because most graduation year cohorts comprised several hundred oncologists, means were subject to variation driven by individuals receiving large dollar-value payments. Similar relative trends were seen for internists, albeit at lower absolute dollar values (supplemental eFigure 4).
Figure 3.
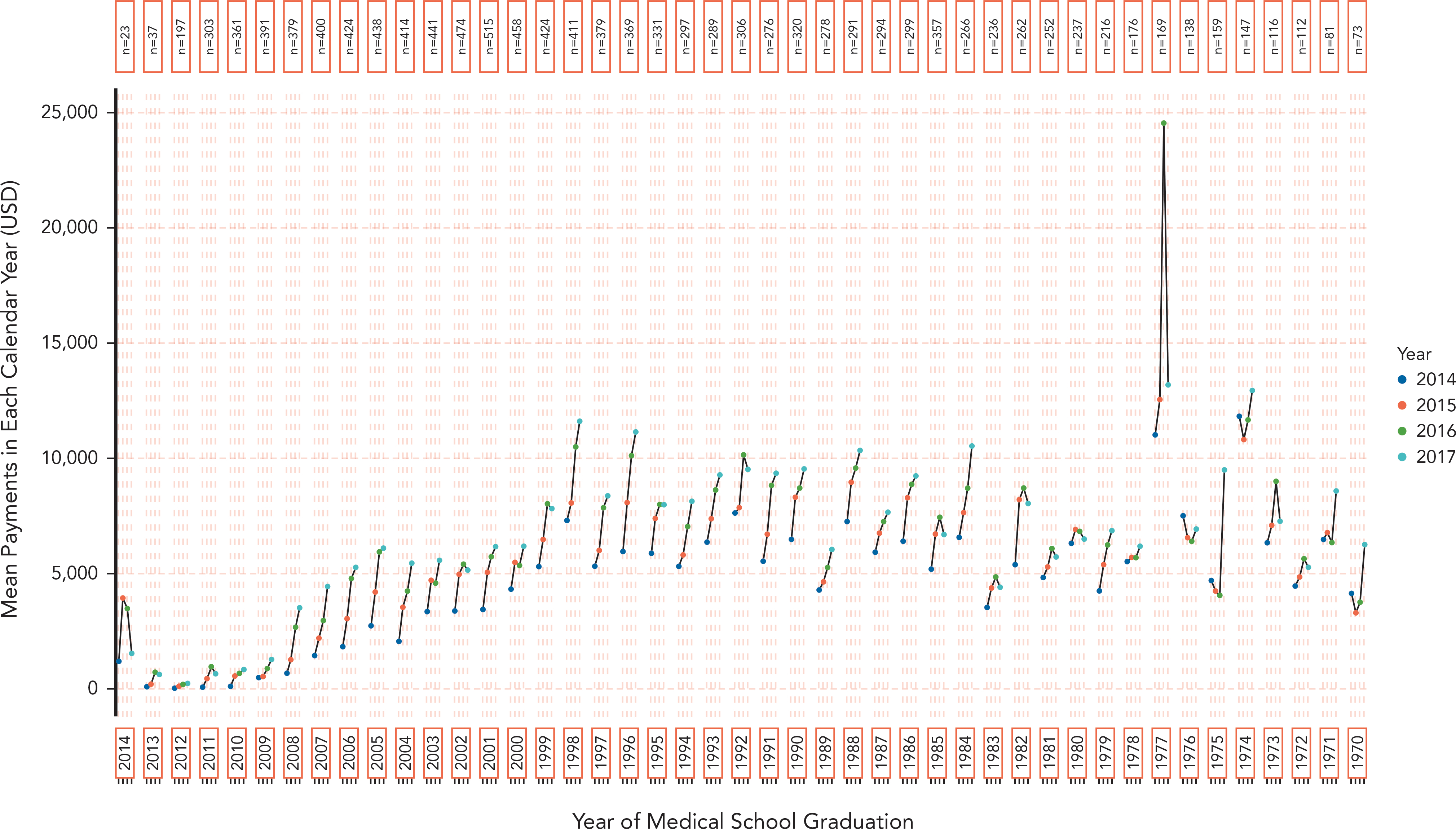
Mean annual per-physician dollar value of general industry payments to oncologists over career trajectory, grouped by medical school graduation year(1970–2014). Individual cohorts of physicians were created based on shared graduation year, and payments over the 2014–2017 study period are shown for each cohort. The 27 oncologists who graduated from medical school after 2014 are not shown.
Discussion
Our work is in line with prior findings that industry payments to physicians are highly concentrated within a relatively small proportion of physicians.25–27 Notably, our findings suggest that payments are becoming more concentrated over time, with mean per-oncologist payments increasing substantially more than median payments, and the group of the highest-earning oncologists (those accepting >$10,000 per year) increasing from 7.0% of oncologists in 2014% to 10.2% of oncologists in 2017. Most of this increase was accounted for by higher consulting fees and compensation for services (which includes speaking fees).2
One important question regarding pharmaceutical industry strategy is whether higher priority is placed on clinical practitioners, who may directly prescribe the greatest drug volumes, or on academic physicians, who are more likely to be involved in clinical research and clinical practice guideline creation. Our results suggest the latter. Oncologists who served on an NCCN Guidelines Panels received more than twice the mean dollar value in general payments in 2014 compared with all oncologists, a gap that increased to more than 3 times the mean dollar value by 2017; this gap was even more pronounced when comparing median payments. This finding may reflect the greater involvement of academic oncologists in clinical research and industry consulting, which is often associated with payments for consulting fees and travel expenses.
Our work also highlights the stark interspecialty difference in payments between medical oncology and internal medicine. Interspecialty differences in industry payments are well documented26,27; our work adds the new observation of equally stark differences in trends between specialties, with substantial growth in industry payments among oncologists, but payments to internists remaining largely un-changed. This finding is not surprising in light of the increasing number of high-priced anticancer drugs28–30 and the resulting higher financial return to pharmaceutical companies for convincing oncologists to use them.
We observed a rapid increase in the value of payments related to immunotherapy drugs during the study period. Among the companies that marketed 1 of the 5 PD-1 or PD-L1 drugs approved during the study period, payments to oncologists increased by a relatively greater amount compared with industry payments overall or compared with payments from other large drug companies that do not have PD-1 or PD-L1 drugs. Increases across the whole study period were apparent for the companies marketing pembrolizumab and nivolumab, which were first approved in 2014, and for the companies marketing atezolizumab, avelumab, and durvalumab, which were first approved in 2016, 2017, and 2017, respectively; increases in the 2017 calendar year suggest that similar patterns may occur in the postapproval period for these drugs as well. A rapid increase in payments associated with nivolumab was reported previously,31 and our results suggest that this trend may be consistent across immunotherapy agents.
Payments associated with each company’s PD-1/PD-L1 drug accounted for most of the aggregate increase across these companies, and accounted for all of the increase in payments from the manufacturer of nivolumab in particular. With mean payments associated with PD-1/PD-L1 drugs increasing from $28 in 2014 to $733 in 2017, these drugs alone accounted for more than one-third of the increase observed across all drug manufacturers ($2,043) during the study period. This finding suggests that the introduction of new drugs may contribute substantially to the overall increase in payments to oncologists, as opposed to increasing payments for older drugs.
We hypothesized an annual spike in payments coinciding with the ASCO Annual Meeting, which occurs in late May or early June each year, potentially resulting from the physician–industry contact facilitated by this meeting. Our analysis of calendar year trends in payments found no evidence of such an increase. We did observe relative troughs in payments, among both oncologists and internists, occurring at year-end and in the summer months, which may coincide with periods of reduced activity on the part of academic clinicians and/or pharmaceutical companies.
Our findings are consistent with prior reports that later-career physicians receive more industry payments than early-career physicians.26 Our results further suggest that at least within oncology, this trend has not resulted from a decreasing willingness to accept industry payments among younger physicians. Rather, those oncologists who would have finished training soon before our study period (roughly, those graduating medical school in 2004–2008) experienced rapid increases in payments during the study period, approaching payment rates similar to older oncologists. Whether these patterns will change among future oncologists who were still in training for part or all of our study period (most of those graduating in 2009 or later) remains to be seen. Oncologists in earlier graduation cohorts saw less consistent growth in industry payments during the study period, suggesting that industry may engage less with oncologists who are nearing retirement (those graduating medical school in 1970 would have been approximately 70 years old at the beginning of the study period). Physician sex26,32–35 and specialty26,27 have been identified as key physician characteristics associated with receipt of industry payments, and our study suggests career stage is an important characteristic as well.
This study does not address the consequences of industry payments. Industry payments commonly occur in venues where physicians receive information from manufacturers about new drugs. In cases where the drugs being promoted offer increased therapeutic benefit, it is likely that industry payments would lead to improved patient outcomes. Prior research has found that industry promotional activity, in aggregate, focuses most heavily on drugs with lower therapeutic benefit and that industry information downplays drug toxicity,36–39 which may contribute to the observed association between industry contact and lower-quality prescribing.5,38,40,41
The datasets used in this study have limitations. Open Payments data have been found to contain inaccuracies,42 and they include types of payments that some many not consider to signify a true conflict of interest between the company and the recipient physician, such as funding of research through philanthropic donations.43 Still, Open Payments data undergo a rigorous validation process, and one report on Open Payments found the data to be highly accurate overall.42,44 Our analytic cohort of oncologists serving on NCCN Guidelines Panels did not include NCCN Guidelines for all cancer types and did not differentiate between oncologists with less or more committee service time; however, the selected cohort is still likely to be generally reflective of payment patterns among NCCN Guidelines Panel Members and of the differences between this group and other oncologists. Notably, this study cannot establish the causality of the observed associations between oncologist characteristics and industry payments—whether pharmaceutical companies are increasingly seeking to make payments to oncologists, or to those who serve on NCCN Guidelines Panels, or whether these groups of physicians more actively seek out financial relationships with companies.
Conclusions
Industry influence in medical research and practice remains a concern. In terms of the dollar value of payments to oncologists, the basis for this concern continues to grow. Ideally, medical practice and clinical practice guidelines should be based on the scientific evidence alone. Personal payments, information, and funding from the pharmaceutical industry are not needed to achieve this goal and may detract from it. The medical community should therefore act to quickly reduce, and eventually to end entirely, the practice of accepting personal payments from industry.
Supplementary Material
Funding:
Research reported in this article was supported by the NCI of the NIH/Memorial Sloan Kettering Cancer Center under support grant P30-CA008748.
Footnotes
Disclosures: Dr. Bach has disclosed serving as an advisory board member for EQRx; serving on the Board of Directors for Oncology Analytics; receiving personal fees and nonfinancial support from the American Society for Health-System Pharmacists, Gilead Pharmaceuticals, Vizient, the Hematology Oncology Pharmacy Association, United Rheumatology, Oppenheimer & Co, Oncology Analytics, the Kaiser Permanente Institute for Health Policy, the Congressional Budget Office, America’s Health Insurance Plans, and Geisinger; personal fees from WebMD, Goldman Sachs, Defined Health, JMP Securities, Mercer, Foundation Medicine, Grail, Morgan Stanley, the New York State Rheumatology Society, Cello Health, Anthem, Magellan Health; and grant support from Kaiser Permanente, and Arnold Ventures. Dr. Mitchell has disclosed receiving a research abstract award from the Conquer Cancer Foundation, which was partially funded by Merck. The remaining authors have disclosed that they have not received any financial consideration from any person or organization to support the preparation, analysis, results, or discussion of this article.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Marshall DC, Jackson ME, Hattangadi-Gluth JA. Disclosure of industry payments to physicians: an epidemiologic analysis of early data from the Open Payments program. Mayo Clin Proc 2016;91:84–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall DC, Moy B, Jackson ME, et al. Distribution and patterns of industry-related payments to oncologists in 2014. J Natl Cancer Inst 2016;108:djw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lo B, Field MJ, eds. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 4.Mitchell AP, Basch EM, Dusetzina SB. Financial relationships with industry among National Comprehensive Cancer Network Guideline authors. JAMA Oncol 2016;2:1628–1631. [DOI] [PubMed] [Google Scholar]
- 5.Lexchin J Interactions between physicians and the pharmaceutical industry: what does the literature say? CMAJ 1993;149:1401–1407. [PMC free article] [PubMed] [Google Scholar]
- 6.Lerner TG, Miranda M da C, Lera AT, et al. The prevalence and influence of self-reported conflicts of interest by editorial authors of phase III cancer trials. Contemp Clin Trials 2012;33:1019–1022. [DOI] [PubMed] [Google Scholar]
- 7.Hayes MJ, Prasad V. Association between conflict of interest and published position on tumor-treating fields for the treatment of glioblastoma. J Cancer Policy 2019;21:100189. [Google Scholar]
- 8.Valachis A, Polyzos NP, Nearchou A, et al. Financial relationships in economic analyses of targeted therapies in oncology. J Clin Oncol 2012;30:1316–1320. [DOI] [PubMed] [Google Scholar]
- 9.Perlis RH, Perlis CS. Physician payments from industry are associated with greater Medicare Part D prescribing costs. PLoS One 2016;11:e0155474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh JS, Franklin JM, Avorn J, et al. Association of industry payments to physicians with the prescribing of brand-name statins in Massachusetts. JAMA Intern Med 2016;176:763–768. [DOI] [PubMed] [Google Scholar]
- 11.DeJong C, Aguilar T, Tseng CW, et al. Pharmaceutical industry-sponsored meals and physician prescribing patterns for Medicare beneficiaries. JAMA Intern Med 2016;176:1114–1122. [DOI] [PubMed] [Google Scholar]
- 12.Modi PK, Wang Y, Kirk PS, et al. The receipt of industry payments is associated with prescribing promoted alpha-blockers and overactive bladder medications. Urology 2018;117:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bandari J, Turner RM II, Jacobs BL, et al. The relationship of industry payments to prescribing behavior: a study of degarelix and denosumab. Urol Pract 2017;4:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh P, Forman H, Adamson AS, et al. Impact of industry payments on prescribing patterns for tumor necrosis factor inhibitors among Medicare beneficiaries. J Gen Intern Med 2019;34:176–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell AP, Winn AN, Dusetzina SB. Pharmaceutical industry payments and oncologists’ selection of targeted cancer therapies in Medicare beneficiaries. JAMA Intern Med 2018;178:854–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell AP, Winn AN, Lund JL, et al. Evaluating the strength of the association between industry payments and prescribing practices in oncology. Oncologist 2019;24:632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins NM, Meyer MJ, Bernat JL. Scope and nature of financial conflicts of interest between neurologists and industry: 2013–2016. Neurology 2019;93:438–449. [DOI] [PubMed] [Google Scholar]
- 18.Morse E, Fujiwara RJT, Mehra S. Industry payments to physicians and prescriptions of brand-name proton-pump inhibitors. Otolaryngol Head Neck Surg 2019;160:70–76. [DOI] [PubMed] [Google Scholar]
- 19.Schlager E, Flaten H, St Claire C, et al. Industry payments to dermatologists: updates from the 2016 Open Payments data. Dermatol Online J 2018;24:13030/qt8r74w3c4. [PubMed] [Google Scholar]
- 20.Marshall DC, Tarras ES, Rosenzweig K, et al. Trends in financial relationships between industry and individual medical oncologists in the United States from 2014 to 2017: a cohort study [abstract]. J Clin Oncol 2019;37(Suppl):Abstract 6520. [Google Scholar]
- 21.Elsamadicy AA, Freedman IG, Koo AB, et al. Characteristics of reported industry payments to neurosurgeons: a 5-year Open Payments database study. World Neurosurg 2021;145:e90–99. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services. Dataset downloads. Accessed February 8, 2021. Available at: https://www.cms.gov/OpenPayments/Explore-the-Data/Dataset-Downloads.html
- 23.Centers for Medicare & Medicaid Services. Explore and download Medicare provider data. Accessed February 8, 2021. Available at: https://data.cms.gov/provider-data/?redirect=true
- 24.American Cancer Society. Cancer facts & figures 2018. Atlanta, GA: American Cancer Society; 2018. [Google Scholar]
- 25.Samuel AM, Webb ML, Lukasiewicz AM, et al. Orthopaedic surgeons receive the most industry payments to physicians but large disparities are seen in Sunshine Act data. Clin Orthop Relat Res 2015;473:3297–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inoue K, Blumenthal DM, Elashoff D, et al. Association between physician characteristics and payments from industry in 2015–2017: observational study. BMJ Open 2019;9:e031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tringale KR, Marshall D, Mackey TK, et al. Types and distribution of payments from industry to physicians in 2015. JAMA 2017;317:1774–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bach P Price & value of cancer drug. Monthly and median costs of cancer drugs at the time of FDA approval 1965–2019. Accessed February 8, 2021. Available at: https://www.mskcc.org/research-programs/health-policy-outcomes/cost-drugs
- 29.Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000–2014. JAMA Oncol 2016;2:960–961. [DOI] [PubMed] [Google Scholar]
- 30.Dusetzina SB, Huskamp HA, Keating NL. Specialty drug pricing and out-of-pocket spending on orally administered anticancer drugs in Medicare Part D, 2010 to 2019. JAMA 2019;321:2025–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macleod LC, Ayyash OM, Bandari J, et al. Unprecedented marketing for novel systemic kidney cancer therapy: physician-directed money in the Open Payments database. Presented at the 70th NSAUA Annual Meeting; October 2, 2018; Toronto, Canada. [Google Scholar]
- 32.Weiss A, Parina R, Tapia VJ, et al. Assessing the domino effect: female physician industry payments fall short, parallel gender inequalities in medicine. Am J Surg 2018;216:723–729. [DOI] [PubMed] [Google Scholar]
- 33.Weng JK, Valle LF, Nam GE, et al. Evaluation of sex distribution of industry payments among radiation oncologists. JAMA Netw Open 2019;2:e187377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ngaage LM, Harris C, Gao C, et al. Investigating the gender pay gap in industry contributions to academic neurosurgeons. World Neurosurg 2019;130:516–522.e1. [DOI] [PubMed] [Google Scholar]
- 35.Tringale KR, Hattangadi-Gluth JA. Types and distributions of biomedical industry payments to men and women physicians by specialty, 2015. JAMA Intern Med 2018;178:421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenway T, Ross JS. US drug marketing: how does promotion correspond with health value? BMJ 2017;357:j1855. [DOI] [PubMed] [Google Scholar]
- 37.Lexchin J The relation between promotional spending on drugs and their therapeutic gain: a cohort analysis. CMAJ Open 2017;5:E724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010;7:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lexchin J What information do physicians receive from pharmaceutical representatives? Can Fam Physician 1997;43:941–945. [PMC free article] [PubMed] [Google Scholar]
- 40.Brax H, Fadlallah R, Al-Khaled L, et al. Association between physicians’ interaction with pharmaceutical companies and their clinical practices: a systematic review and meta-analysis. PLoS One 2017;12:e0175493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brunt CS. Physician characteristics, industry transfers, and pharmaceutical prescribing: empirical evidence from Medicare and the Physician Payment Sunshine Act. Health Serv Res 2019;54:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murrin S Open Payments data: review of accuracy, precision, and consistency in reporting. Office of the Inspector General, U.S. Department of Health and Human Services. Accessed February 9, 2021. Available at: https://oig.hhs.gov/oei/reports/oei-03-15-00220.pdf [Google Scholar]
- 43.Ratain MJ. Forecasting unanticipated consequences of “The Sunshine Act”: mostly cloudy. J Clin Oncol 2014;32:2293–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal S, Brown D. The Physician Payments Sunshine Act—two years of the Open Payments program. N Engl J Med 2016;374:906–909. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.