Abstract
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare subset of Hodgkin lymphoma (HL). It has a distinct clinical and pathological presentation. Unlike classic HL, where the predominant malignant cells are Reed Sternberg cells, the malignant cells in NLPHL are known as lymphocyte predominant (LP) cells, with their own unique immunohistochemistry antigen expression and staining pattern. Based on risk stratification and staging of the disease, treatment can range from active surveillance in asymptomatic patients with no organ compromise or bulky disease, to aggressive chemotherapeutic agents in advanced disease. Guidelines on which of these chemotherapy regimens would offer the most benefit to our patients are limited due to lack of randomized-controlled studies. Majority of the current prospective data on treatment were inclusive of both HL and NLPHL. Thus, the regimens employed in treatment of NLPHL are similar to the ones used in HL, though NLPHL is often viewed as its own distinct entity. This article aims to review the current literature and future advances on treatment of this rare disease.
Keywords: nodular lymphocyte predominant Hodgkin lymphoma, NLPHL, Hodgkin lymphoma, popcorn cells, ABVD, RCHOP, lymphocyte predominant cells, ofatumumab
Introduction
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a rare Hodgkin lymphoma subtype, accounting for only 5% of all Hodgkin lymphoma cases. 1 As compared to classic Hodgkin lymphoma (cHL), NLPHL has a uniquely different clinical and pathological presentation. Thus, the oncological management of such patients also differs compared to the cHL. NLPHL is notable for the presence of distinct malignant cells termed lymphocyte-predominant (LP) cells. These cells express B-cell-associated antigens such as CD20, but unlike classical Hodgkin lymphoma, they do not typically express CD15 nor CD30. There is a lack of significant randomized controlled studies to guide medical management of this rare disease and management options could consist of aggressive combined modality treatment approaches to observation with close surveillance. We present data from 2 cases managed at our institution and their respective outcomes. We also aim to review the current published literature on the various treatment options for this rare disease.
Epidemiology
NLPHL affects males more than females with a 3:1 predominance. 2 The age distribution is bimodal, occurring in children and adults with a median age of 40. 3 There is a predilection among first degree family members 4 as well as African Americans. 2
Pathology
Both cHL and NLPHL are germinal center type neoplasias. In cHL, the malignant Hodgkin/Reed-Sternberg (HRS) are typically present among a background of acute and chronic inflammatory cells. Malignant LP cells of NLPHL present among a backdrop of follicular dendritic cells, small B lymphocytes, and follicular T lymphocytes. 5 Clusters of epithelioid histiocytes are also sometimes seen around the periphery of the tumor. Plasma cells, eosinophils, and neutrophils are not typically observed. Epstein-Barr virus (EBV) is rarely detected in NLPHL, whereas EBV has been strongly associated in cases of cHL. 6 Architecture and growth pattern of NLPHL is usually nodular, although cases of diffuse architectural patterns or mixed patterns may be observed. 7 LP cells histologically have a large, irregular, polypoid nuclear morphology that is often described as “popcorn cells” (Image 1). The nucleus resembles an exploded popcorn kernel. 8 Distinct histopathological variants have been described by Fan et al 9 based on the immunoarchitectural patterns of the disease. These include (1) classic nodular B cell rich, (2) serpinginous/interconnected nodular types, (3) nodular with predominance of extranodular LP cells, (4) T-cell rich nodular, (5) diffuse with T cell rich backbround (T-cell-rich B-cell lymphoma), and (6) diffuse B-cell pattern. Among these, classic nodular B cell rich and serpinginous/interconnected nodular types have LP cells located within the nodules, while the other patterns have LP cells located outside the nodules. Clinical significance of these types was also assessed in this study. Diffuse T-cell-rich pattern and diffuse patterns in general were shown to have more recurrences and early progression to diffuse large B-cell lymphoma. This was, however, a retrospective analysis and further prospective studies are warranted to evaluate the clinical impact of these patterns.
Image 1.
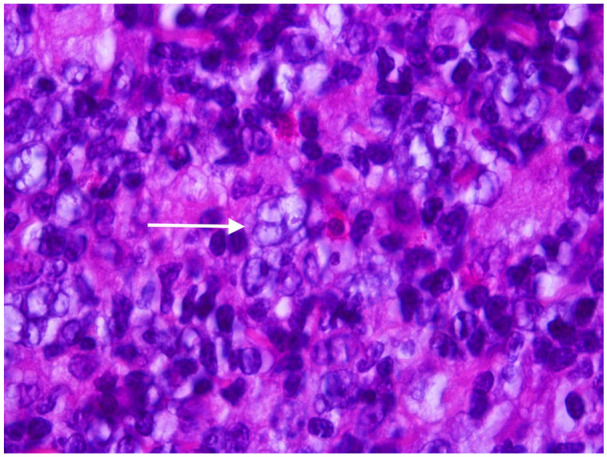
Hematoxylin and Eosin stained section from involved lymph node showing a typical “popcorn” cell characteristic of nodular lymphocyte predominant Hodgkin lymphoma. Note the multilobated nuclei and vesicular chromatin which is typically seen.
Immunophenotype
Immunohistochemistry (IHC) is an integral part of the diagnostic workup of NLPHL as the findings are distinct from cHL. LP cells frequently express CD20, BLC6, CD79a, and other B cell markers. 10 Unlike cHL, they do not express CD15 and CD30 markers. Other IHC stains that are useful in diagnosis are pax5 (Image 2), oct2 (Image 3), and epithelial membra antigen (Image 4). The background cells consist of small B lymphocytes, CD3+, CD4+, CD57+ T cells and CD21+, and CD23+ follicular dendritic cells. 11 Another useful diagnostic feature is the characteristic of PD1/CD279+ T-cells which form rosettes around the “popcorn” cells (Image 5). In all, 50% of cases of NLPHL have been described with having a BCL6 translocation. 12
Image 2.
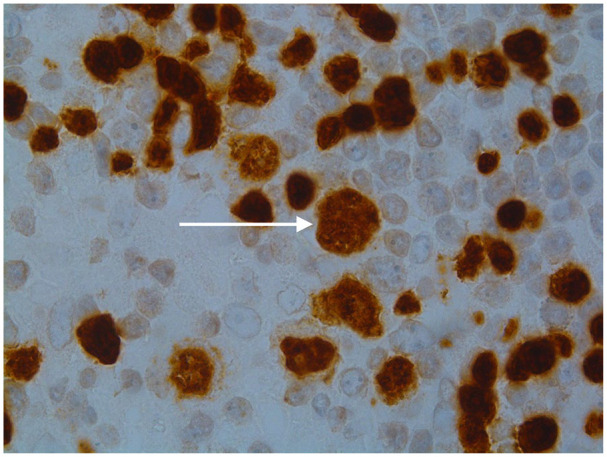
The tumor cells in nodular lymphocyte predominant Hodgkin lymphoma are strongly positive for several B-cell markers including CD20 and Pax-5 (seen here). In contrast Reed-Sternberg cells show very weak Pax-5 expression and are usually negative for CD20.
Image 3.
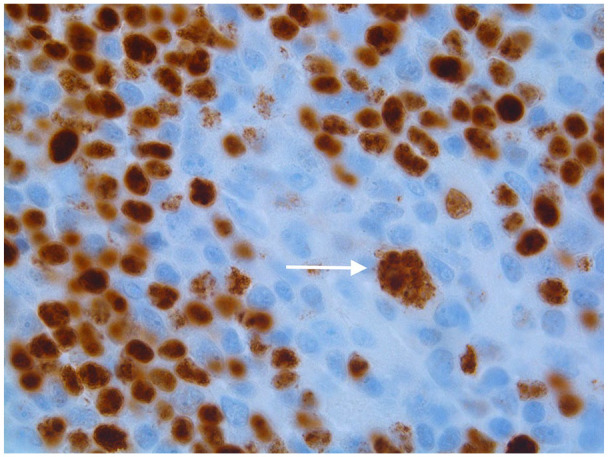
A large popcorn cell nuclei is positive for Oct-2 immunostain, this is a confirmatory finding in NLPHD (Nodular Lymphocyte Predominant Hodgkin Lymphoma).
Image 4.
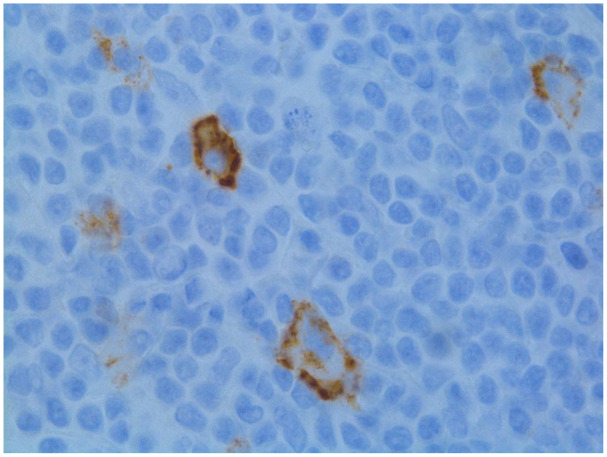
Epithelial membrane antigen (EMA) immunostain is a useful stain in supporting the diagnosis of nodular lymphocyte predominant Hodgkin lymphoma. The neoplastic cells show membraneous and dot-like paranuclear staining in the Golgi region.
Image 5.
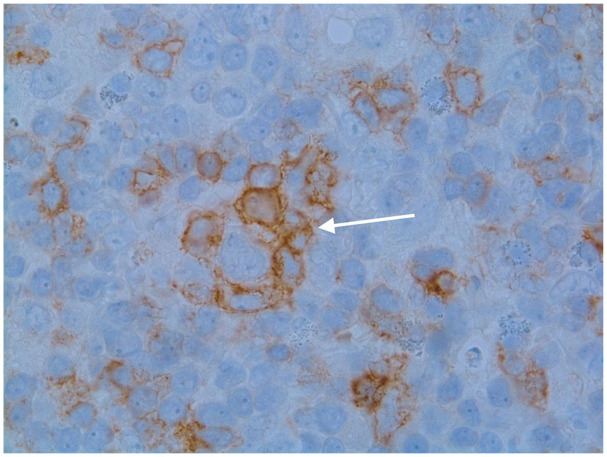
A useful diagnostic feature is the characteristic of PD1/CD279+ T-cells to form rosettes around the “popcorn” cells that characterize nodular lymphocyte predominant Hodgkin lymphoma. In this image the large atypical cell can be seen in the center surrounded by PD1 (+) T-lymphocytes.
Clinical Presentations
The vast majority of patients with NLPHL present with stage I or II disease. It typically presents with painless peripheral lymphadenopathy, most commonly in the neck or axilla. It oftentimes presents only in a single regional lymph node station. 6 The involvement of central lymph nodes is also not common. Constitutional B symptoms such as fever, night sweats, weight loss are also rare. This is likely due to the fact that LP cells are not a rich source of pro-inflammatory cytokines. Only 6% to 15% of cases have reported B symptoms. 3 Organ involvement (liver, spleen) and bone marrow involvement is rare.3,13 If bone marrow is involved, it usually indicates an aggressive variant.
NLPHL regularly has an indolent course; however, there is a risk of transformation into aggressive non-Hodgkins lymphoma (NHL) and or late illness relapse.14,15 While transformation to aggressive NHL do occur in cHL, with reported rates of transformation upto 3%, 16 rates of transformation seem to be substantially more in NLPHL with data showing transformation risk up to 17%. 17
Staging
The staging of NLPHL is conducted by utilizing the Ann Arbor staging system with Cotswolds modifications. 18 This staging system includes clinical evaluation (history and physical examination), laboratory studies, and positron emission tomography (PET) imaging. Since bone marrow involvement is rare, a bone marrow biopsy is not routinely performed for staging purposes, although persistent cytopenias and clinical suspicion may prompt an evaluation of the marrow space.
Case 1
A 61-year-old African American female, with a past medical history of mitral regurgitation, hypertension, hyperlipidemia, iron deficiency anemia, and anxiety, presented for incidental lymphadenopathy found on a routine mammogram. She denied experiencing any fevers, night sweats, or excessive fatigue on evaluation. Notably, she did report a 70-pound weight loss over 2 years. On physical examination, left axillary adenopathy was noted. It was a painless, fluctuant, rubbery, 4 cm mass. Another 3 cm infraclavicular/pectoral nodal mass was also palpated. The patient underwent a chest/abdomen/pelvis computed tomography (CT) which demonstrated left axillary and infraclavicular lymphadenopathy. A follow-up PET/CT showed hypermetabolic lesions in the left axillary and infraclavicular regions concerning for malignancy. The patient then underwent an excisional biopsy of the left axillary lymph node with pathological review confirming the diagnosis of NLPHL. Given the PET/CT findings and the presence of B symptoms (unintentional weight loss), the patient was staged as IIb disease. After discussion with the multidisciplinary team, it was felt that the patient had wide-field/borderline bulky disease, hence a combined modality approach including radiation and CD20 directed therapy was utilized in management. The patient had significant mitral regurgitation, so a doxorubicin-based regimen was not utilized due to concerns for precipitating heart failure. Weekly Rituximab was administered for 4 weeks. Repeat PET showed complete resolution (nodes were still present but not PET avid). The patient subsequently completed consolidation radiotherapy. The patient has remained in remission since.
Case 2
A 59-year-old female with a past medical history of restrictive lung disease, chronic venous insufficiency, and arthritis presented for evaluation of non-tender peripheral lymphadenopathy. On presentation, she stated that she had felt “tiny knots” in her neck over the past year and that they had been enlarging only intermittently and non-bothersome. She denied any other associated symptoms, such as fevers, night sweats, or chills. On physical examination, multiple mobile 2 cm lymph nodes were palpated in the right and left supraclavicular area. Laboratory parameters did not indicate any cytopenias. An excisional biopsy of the right supraclavicular lymph node was performed. PET and CT scans were also performed, which demonstrated hypermetabolic lymphadenopathy most pronounced within the right neck, mediastinal, and bilateral hilar areas. The biopsy and imaging results were consistent with Stage II NLPHL. Upon further discussion with the patient, a decision was made to observe the patient and to not initiate chemotherapy. A bone marrow biopsy was not performed at this time, as it would not have changed the management approach and patient wanted to avoid any unnecessary procedures. A repeat CT chest/abdomen/pelvis showed slightly enlarging neck lymphadenopathy (right >left). However, patient continued to deny any B symptoms, and there were no significant concerns for organ compromise. Other regions including the hilar and mediastinal lymphadenopathy had remained stable. The patient continues to remain asymptomatic and follow-up visits, laboratory parameters, and imaging scans have also indicated stable disease. We continue to actively observe the patient.
Treatment
The overall data of various chemotherapeutic agents for NLPHL are limited as there are no randomized-controlled studies and very few prospective studies to guide management. Majority of the larger studies such as the ones conducted by German Hodgkin Study Group (GHSG) included both NLPHL and cHL in their trials. It was not until 1993, that a separate registry was created for NLPHL. Given the presence of B cell markers, some thought leaders in the field feel that NLPHL behaves more similar to follicular lymphoma than Hodgkins. 19 There is also some controversy whether currently employed standard treatment regimens with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) is adequate for treatment of NLPHL. 20 Risk stratification is also essential in helping select patients who may benefit from treatment with chemotherapy. In early stage, non-symptomatic patients (stage IA and stage IIA), a bulky mass (≥10 cm), non-contiguous stage IIA, and threatened organ compromise confers a more adverse risk and thus treatment with chemotherapy in combination with radiation may be indicated. According to National Comprehensive Cancer Network (NCCN) recommendations, treatment is also indicated for all symptomatic stages I to IV disease.
Active Surveillance
In patients who have no significant symptoms, bulky disease or threatened organ compromise, active surveillance may be acceptable if the patient is comfortable deferring treatment. Active surveillance has not been directly compared to treatment in randomized controlled studies, but retrospective studies indicate that surveillance is associated with comparable long-term survival with no associated toxicities incurred with chemotherapeutic agents. One retrospective study of 163 patients performed at Memorial Sloan Kettering evaluated active surveillance versus treatment in patients who were diagnosed with NLPHL. Patients were treated with radiotherapy alone (46%), active surveillance (23%), chemotherapy (16%), combined modality treatment (CMT) (12%), or rituximab monotherapy (4%). The median follow-up was 69 months. Those who underwent active surveillance had a shorter 5-year PFS of 77% (95% confidence interval [CI]: 56-89) versus 87% (95% CI: 79-92; P = .017) for patients who underwent treatment. Of note, there was no improvement in overall survival (OS) as it was 99% for all cohorts. 21
Stage 1A and Contiguous Stage IIA
Involved site radiation therapy (ISRT) alone is adequate for treatment of most stage IA and contiguous stage IIA NLPHL. The largest study showing that ISRT is equally effective with a better toxicity profile compared to CMT came from the German Hodgkin Study Group. In this study, 229 patients either received CMT (n = 72), extended-field RT (n = 49), or involved-field RT (n = 108). Progression-free survival PFS and OS rates were compared at 8 years and results were: CMT—PFS 88.5%, OS 98.6%; extended-field RT—PFS 84.3%, OS 95.7%; and involved-field RT—PFS 91.9%, OS 99.0%. Grade III/IV acute toxicities were more often observed in patients who received CMT. 22
A U.S. study also observed the long-term outcomes of patients treated for NLPHL. In this study, the 10-year PFS and OS rates for stage I patients were 89% and 96%, respectively. PFS and OS between involved-field RT and RT to extended field were also similar. 23
Stage IB, Stage IIB, Bulky Stage IA or Stage IIA, and Non-Contiguous Stage IIA
Patients with early-stage disease with B symptoms, tumor size ≥10 cm or non-contiguous nodes are generally categorized as intermediate stages and are usually treated with CMT. A retrospective study using the British Columbia Cancer Agency (BCCA) database involved 88 patients with early-stage (stage 1A/1B or 2A, non-bulky disease < 10 cm) NLPHL who had received RT alone (n = 32) or CMT (which consisted of ABVD) (n = 56). Patients who received ABVD in the CMT group compared with the RT alone had an improvement in progression-free survival (91% vs 65% P = .0024) and OS (93% vs 84%, P = .074). Analysis from the GHSG also showed similar findings. In this study, most patients received 2 or 4 cycles of ABVD or ABVD-based regimens followed by consolidation RT. 20
Treatment of NLPHL in Advanced Stages (Stage III and IV)
When treatment for NLPHL is indicated, conventional chemotherapy +/- anti-CD20 antibody is most often utilized. Several factors including performance status, disease burden, histopathological patterns, and sites of disease should be taken into consideration prior to initiating treatment. There is no consensus regarding a preferred chemotherapy regimen for NLPHL as there have been no head-to-head comparisons. CD20-targeted regimens such as rituximab is generally employed in combination with chemotherapy as most cases of NLPHL express high levels of CD20 antigen. NCCN guidelines list ABVD, cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP), cyclophosphamide, vinblastine, prednisolone (CVbP), and single agent rituximab as chemotherapy regimens used at most member institutions. Escalated BEACOPP has also been utilized in patients with robust performance status or relapsed disease. 24
ABVD regimen was retrospectively analyzed from the BCCA database. Forty-two newly diagnosed advanced NLPHL patients had received chemotherapy with ABVD or ABVD-like protocols. Twenty-seven of whom received ABVD achieved 84% 10-year OS and 75% 10-year FFTF. Among those treated, the lymphoma recurrence rate was ∼40% at 10 years. 25 This indicates a higher rate of recurrence compared to BEACOPP based regimens. This fact, however, must be carefully weighed against the significant acute and late onset toxicities associated with aggressive regimens such as BEACOPP. 24 The largest study for supporting BEACOPP comes from a retrospective GHSG study of 144 patients with advanced NLPHL who mainly had received escalated BEACOPP. Ten-year PFS and OS were 69.8% and 87.4%, respectively. 14
R-CHOP based regimens has also showed promise and was retrospectively studied from a database of 59 patients between 1995 and 2015. Twenty-seven of these patients were treated with R-CHOP. Overall response rate (ORR) was 100% and complete response (CR) was 89%. The 5- and 10-year PFS were 88.5% and 59.3%, respectively. 26 No patients treated with R-CHOP experienced histologic transformation at 5-year follow-up.
Rituximab has also shown good response rates with a reduced overall toxicity profile, but survival data have shown to be inferior compared to other regimens described. A Stanford group phase 2 study initially conducted a study of 22 patients who received 4 weekly doses of rituximab. Due to high recurrence rates, a maintenance phase with rituximab every 6 months for 2 years was also utilized. ORR was 100%, CR was 57%, with a 5-year PFS of 41.7% in patients with only 4 initial doses and 51.9% in patients with the added maintenance phase. In all, 29% of patients developed transformation to aggressive NHL. 27
Relapses are observed in approximately 15% of patients with a median time to recurrence of 3.7 years. 28 Transformation to a more aggressive form with B cell immunophenotype are also frequently observed in the relapsed cases. Therefore, all patients who do not respond to initial therapy or develop recurrent disease, a repeat biopsy is necessary. Treatment of relapsed disease is based on biopsy findings, burden of disease, and prior therapy. Localized non-transformed relapses can be treated with ISRT while extensive disease requires systemic treatment with utilization of a different chemotherapeutic regimen other than the one previously employed. Subsequent relapses are often treated with high dose chemotherapy and autologous hematopoietic cell transplantation (HCT). Newer clinical trials, such as the ones being conducted by Cologne University hospital in Germany, are looking to analyze the effectiveness of ibrutinib and ofatumumab in relapsed cases. We are still awaiting data from these trials. There is also a lack of high-quality data regarding optimal treatment in cases with transformation to diffuse large B cell lymphoma. Majority of the cases are treated with high dose chemotherapy followed by autologous HCT.
Conclusion
NLPHL remains a rare but important subcategory of Hodgkin lymphoma. Pathological and immunohistochemical markers denote this to be a different disease process compared to cHL. Treatment modalities range from watchful waiting to aggressive chemotherapy regimens. There continues to be lack of randomized controlled studies dedicated to solely studying the treatment outcomes for NLPHL. Current guidelines suggest utilization of cHL based regimens as well as regimens used for treatment of B cell-NHL. Prospective studies should be conducted to better optimize treatment and outcomes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Verbal informed consent was obtained from the patients for their anonymized information to be published in this article.
References
- 1. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes: 2016 US Lymphoid Malignancy Statistics by World Health Organization Subtypes. CA Cancer J Clin. 2016;66(6):443-459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107(1):265-276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Diehl V, Sextro M, Franklin J, et al. Clinical presentation, course, and prognostic factors in lymphocyte-predominant Hodgkin’s disease and lymphocyte-rich classical Hodgkin’s disease: report from the European Task Force on Lymphoma Project on Lymphocyte-Predominant Hodgkin’s Disease. J Clin Oncol. 1999;17(3):776-783. doi: 10.1200/JCO.1999.17.3.776. [DOI] [PubMed] [Google Scholar]
- 4. Saarinen S, Pukkala E, Vahteristo P, et al. High familial risk in nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol. 2013;31(7):938-943. doi: 10.1200/JCO.2012.43.5958. [DOI] [PubMed] [Google Scholar]
- 5. Nogová L, Rudiger T, Engert A. Biology, clinical course and management of nodular lymphocyte-predominant Hodgkin lymphoma. Hematology. 2006;2006(1):266-272. doi: 10.1182/asheducation-2006.1.266. [DOI] [PubMed] [Google Scholar]
- 6. Weiss LM, Chen YY, Liu XF, Shibata D. Epstein-Barr virus and Hodgkin’s disease. Am J pathol. 1991;139(6):1259-1265. [PMC free article] [PubMed] [Google Scholar]
- 7. Boudová L, Torlakovic E, Delabie J, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/histiocyte-rich B-cell lymphoma. Blood. 2003;102(10):3753-3758. doi: 10.1182/blood-2003-02-0626. [DOI] [PubMed] [Google Scholar]
- 8. Falini B, Bigerna B, Pasqualucci L, et al. Distinctive expression pattern of the BCL-6 protein in nodular lymphocyte predominance Hodgkin’s disease. Blood. 1996;87(2):465-471. doi: 10.1182/blood.V87.2.465.bloodjournal872465. [DOI] [PubMed] [Google Scholar]
- 9. Fan Z, Natkunam Y, Bair E, Tibshirani R, Warnke RA. Characterization of variant patterns of nodular lymphocyte predominant hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol. 2003;27(10):1346-1356. doi: 10.1097/00000478-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 10. Savage KJ, Mottok A, Fanale M. Nodular lymphocyte-predominant Hodgkin lymphoma. Semin Hematol. 2016;53(3):190-202. doi: 10.1053/j.seminhematol.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 11. Hartmann S, Eichenauer DA, Plütschow A, et al. Histopathological features and their prognostic impact in nodular lymphocyte—predominant Hodgkin lymphoma—a matched pair analysis from the German Hodgkin Study Group (GHSG). Br J Haematol. 2014;167(2):238-242. doi: 10.1111/bjh.12997. [DOI] [PubMed] [Google Scholar]
- 12. Wlodarska I, Nooyen P, Maes B, et al. Frequent occurrence of BCL6 rearrangements in nodular lymphocyte predominance Hodgkin lymphoma but not in classical Hodgkin lymphoma. Blood. 2003;101(2):706-710. doi: 10.1182/blood-2002-05-1592. [DOI] [PubMed] [Google Scholar]
- 13. Shimabukuro-Vornhagen A, Haverkamp H, Engert A, et al. Lymphocyte-rich classical Hodgkin’s lymphoma: clinical presentation and treatment outcome in 100 patients treated within German Hodgkin’s Study Group trials. J Clin Oncol. 2005;23(24):5739-5745. doi: 10.1200/JCO.2005.17.970. [DOI] [PubMed] [Google Scholar]
- 14. Eichenauer DA, Plütschow A, Fuchs M, et al. Long-term follow-up of patients with nodular lymphocyte-predominant Hodgkin lymphoma treated in the HD7 to HD15 trials: a report from the German Hodgkin Study Group. J Clin Oncol. 2020;38(7):698-705. doi: 10.1200/JCO.19.00986. [DOI] [PubMed] [Google Scholar]
- 15. Al-Mansour M, Connors JM, Gascoyne RD, et al. Transformation to aggressive lymphoma in nodular lymphocyte-predominant Hodgkin’s lymphoma. J Clin Oncol. 2010;28(5):793-799. doi: 10.1200/JCO.2009.24.9516. [DOI] [PubMed] [Google Scholar]
- 16. Rueffer U, Josting A, Franklin J, et al. Non-Hodgkin’s lymphoma after primary Hodgkin’s disease in the German Hodgkin’s Lymphoma Study Group: incidence, treatment, and prognosis. J Clin Oncol. 2001;19(7):2026-2032. doi: 10.1200/JCO.2001.19.7.2026. [DOI] [PubMed] [Google Scholar]
- 17. Eyre TA, Gatter K, Collins GP, Hall GW, Watson C, Hatton CS. Incidence, management, and outcome of high-grade transformation of nodular lymphocyte predominant Hodgkin lymphoma: long-term outcomes from a 30-year experience. Am J Hematol. 2015;90(6):E103-E110. doi: 10.1002/ajh.23989. [DOI] [PubMed] [Google Scholar]
- 18. Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630-1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 19. Horwich A, Specht L, Ashley S. Survival analysis of patients with clinical stages I or II Hodgkin’s disease who have relapsed after initial treatment with radiotherapy alone. Eur J Cancer. 1997;33(6):848-853. doi: 10.1016/s0959-8049(96)00518-7. [DOI] [PubMed] [Google Scholar]
- 20. Savage KJ, Skinnider B, Al-Mansour M, et al. Treating limited-stage nodular lymphocyte predominant Hodgkin lymphoma similarly to classical Hodgkin lymphoma with ABVD may improve outcome. Blood. 2011;118(17):4585-4590. doi: 10.1182/blood-2011-07-365932. [DOI] [PubMed] [Google Scholar]
- 21. Borchmann S, Joffe E, Moskowitz CH, et al. Active surveillance for nodular lymphocyte-predominant Hodgkin lymphoma. Blood. 2019;133(20):2121-2129. doi: 10.1182/blood-2018-10-877761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eichenauer DA, Plütschow A, Fuchs M, et al. Long-term course of patients with Stage IA nodular lymphocyte-predominant Hodgkin lymphoma: a report from the German Hodgkin Study Group. J Clin Oncol. 2015;33(26):2857-2862. doi: 10.1200/JCO.2014.60.4363. [DOI] [PubMed] [Google Scholar]
- 23. Chen RC, Chin MS, Ng AK, et al. Early-stage, lymphocyte-predominant Hodgkin’s lymphoma: patient outcomes from a large, single-institution series with long follow-up. J Clin Oncol. 2010;28(1):136-141. doi: 10.1200/JCO.2009.24.0945. [DOI] [PubMed] [Google Scholar]
- 24. Eichenauer DA, Engert A. How I treat nodular lymphocyte-predominant Hodgkin lymphoma. Blood. 2020;136(26):2987-2993. doi: 10.1182/blood.2019004044. [DOI] [PubMed] [Google Scholar]
- 25. Xing KH, Connors JM, Lai A, et al. Advanced-stage nodular lymphocyte predominant Hodgkin lymphoma compared with classical Hodgkin lymphoma: a matched pair outcome analysis. Blood. 2014;123(23):3567-3573. doi: 10.1182/blood-2013-12-541078. [DOI] [PubMed] [Google Scholar]
- 26. Fanale MA, Cheah CY, Rich A, et al. Encouraging activity for R-CHOP in advanced stage nodular lymphocyte–predominant Hodgkin lymphoma. Blood. 2017;130(4):472-477. doi: 10.1182/blood-2017-02-766121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Advani RH, Horning SJ, Hoppe RT, et al. Mature results of a phase II study of rituximab therapy for nodular lymphocyte-predominant Hodgkin lymphoma. J Clin Oncol. 2014;32(9):912-918. doi: 10.1200/JCO.2013.53.2069. [DOI] [PubMed] [Google Scholar]
- 28. Eichenauer DA, Plütschow A, Schröder L, et al. Relapsed and refractory nodular lymphocyte-predominant Hodgkin lymphoma: an analysis from the German Hodgkin Study Group. Blood. 2018;132(14):1519-1525. doi: 10.1182/blood-2018-02-836437. [DOI] [PubMed] [Google Scholar]


