Abstract
Background:
Administrative health care databases can be efficiently analyzed to describe the degree to which patients with end-stage kidney disease (ESKD) have access to kidney transplantation. Measures of access to transplantation are better represented when restricting to only those patients eligible to receive a kidney transplant. The way administrative data can be used to assess kidney transplant eligibility in the absence of clinical data has not been well described.
Objective:
To demonstrate a method that uses administrative health care databases to identify patients with ESKD who have no recorded contraindication to receiving a kidney transplant.
Design and setting:
Population-based cohort study using linked administrative health care databases in Ontario, Canada.
Patients:
Adult patients with ESKD approaching the need for dialysis (predialysis) or receiving maintenance dialysis between January 1, 2013 and March 31, 2015 in Ontario, Canada.
Measurements:
Recipient of a kidney-only or kidney-pancreas transplant.
Methods:
We assessed more than 80 baseline characteristics, including demographic information, comorbidities, kidney-specific characteristics, and referral and listing criteria for kidney transplantation. We compared these characteristics between patients who did and did not receive a kidney transplant.
Results:
We included 23 642 patients with ESKD (11 195 who were predialysis and 12 447 receiving maintenance dialysis). Over a median follow-up of 3.2 years (25th, 75th percentile: 1.3, 5.6), 3215 (13.6%) received a kidney-only or kidney-pancreas transplant. Of the studied characteristics available in administrative databases, >97% of patients with one or more of these characteristics did not receive a kidney transplant during follow-up: ESKD-modified Charlson Comorbidity Index score ≥7 (a higher score represents greater comorbidity), home oxygen use, age above 75 years, dementia, living in a long-term care facility, receiving at least one physician house call in the past year, and a combination of select malignancies (ie, lung, lymphoma, cervical, colorectal, liver, active multiple myeloma, and bladder cancer). Using these combined criteria reduced the total number of patients from 23 642 to 12 539 with no recorded contraindications to transplant (a 47% reduction), while the proportion who received a kidney transplant changed from 13.6% (denominator of 23 642) to 24.9% (denominator of 12 539).
Limitations:
Administrative databases are unable to capture all the complexities of determining transplant eligibility.
Conclusion:
We identified several criteria available within administrative health care databases that can be used to identify patients with ESKD who have no recorded contraindications to kidney transplant. These criteria could be applied when reporting measures of access to kidney transplantation that require knowledge of transplant eligibility.
Keywords: kidney transplant eligibility, health care databases
Abrégé
Contexte:
Les bases de données administratives en santé peuvent être analysées efficacement pour décrire le degré d’accès des patients atteints d’insuffisance rénale terminale (IRT) à une transplantation. Les mesures de l’accès à la transplantation sont mieux représentées lorsqu’on se limite aux patients admissibles pour recevoir une greffe rénale. On manque toutefois d’information sur la façon dont les données administratives peuvent être utilisées, en l’absence de données cliniques, pour évaluer l’admissibilité à une greffe rénale.
Objectif:
Démontrer une méthode utilisant les bases de données administratives en santé pour identifier les patients atteints d’IRT sans contre-indication à une greffe rénale.
Type d’étude:
Étude de cohorte représentative d’une population réalisée en Ontario (Canada) à partir des données administratives en santé.
Sujets:
Des patients ontariens (Canada) atteints d’IRT qui approchaient le besoin de dialyse (prédialyse) ou qui recevaient des traitements de dialyse d’entretien entre le 1er janvier 2013 et le 31 mars 2015.
Mesures:
Les receveurs d’une greffe de rein seulement ou de rein-pancréas.
Méthodologie:
Nous avons évalué plus de 80 caractéristiques initiales, notamment les données démographiques et les comorbidités des patients, et les caractéristiques particulières du rein; en plus des critères d’aiguillage et d’inscription pour une greffe rénale. Ces caractéristiques ont été comparées entre les patients greffés et ceux qui n’avaient pas reçu une greffe.
Résultats:
Nous avons inclus 23 642 patients atteints d’IRT (11 195 en prédialyse et 12 447 sous dialyse d’entretien). Pendant un suivi médian de 3,2 ans (25e percentile: 1,3 an; 75e percentile: 5,6 ans), 3 215 patients (13,6 %) ont reçu une greffe (rein seulement ou rein-pancréas). Plus de 97 % des patients présentant une ou plusieurs des caractéristiques suivantes, disponibles dans les bases de données, n’avaient pas reçu de greffe rénale pendant le suivi: avoir un score d’au moins 7 à l’indice de Charlson ajusté pour l’IRT (un score élevé représente une plus grande comorbidité), consommer de l’oxygène à domicile, avoir plus de 75 ans, souffrir de démence, vivre dans un établissement de soins de longue durée, avoir reçu au moins un appel du médecin au cours de la dernière année et présenter une combinaison de certaines tumeurs malignes (poumons, lymphome, col de l’utérus, colon, rectum, foie, vessie et myélome multiple actif). L’utilisation de ces critères combinés a réduit le nombre total de patients sans contre-indications à la transplantation de 23 642 à 12 539 (réduction de 47 %), faisant ainsi passer la proportion de patients ayant reçu une transplantation rénale de 13,6 % (dénominateur de 23 642) à 24,9 % (dénominateur de 12 539).
Limites:
Les bases de données administratives ne sont pas en mesure de saisir toutes les complexités liées à la détermination de l’admissibilité à une transplantation.
Conclusion:
Nous avons répertorié plusieurs critères disponibles dans les bases de données administratives en santé qui permettent d’identifier les patients atteints d’IRT sans contre-indications à la transplantation rénale. Ces critères pourraient être appliqués lors de la communication de mesures de l’accès à la transplantation rénale qui exigent de connaître l’admissibilité du patient à une transplantation.
Introduction
Kidney transplantation offers improved survival and quality of life to most patients with end-stage kidney disease (ESKD) compared with dialysis. 1 For example, patients who receive a deceased donor kidney transplant realize a 66% to 70% lower risk of mortality at 18 months post-transplant compared with if they had remained on the waitlist. 2 Transplantation is also cost-effective; in Canada, the average annual cost of maintenance dialysis is $110 992 compared with $46 328 in the second year after transplant (values updated to 2021 Canadian dollars). 3 Although kidney transplant is the preferred treatment for ESKD, there are many barriers, including a shortage of transplantable kidneys, a lack of transplant training among health care professionals, a lack of communication between patient care teams, and a complex process of determining transplant eligibility.4-10
To be eligible to receive a kidney transplant, patients must be healthy enough to undergo the kidney transplant surgery and be expected to have a reasonable survival after transplant. In Ontario, transplant eligibility consists of more than 25 criteria that are assessed clinically (summary of criteria provided in Table 1). 11 In brief, patients must have irreversible ESKD defined by the receipt of maintenance dialysis, or a high likelihood of requiring kidney replacement therapy within 12 months of assessment. 11 Patients are also assessed for the presence of absolute (eg, active malignancy) and relative listing contraindications (eg, poor functional capacity). Some of the transplant eligibility criteria are straightforward (eg, patients who are receiving home oxygen therapy are not eligible to receive a transplant), while many criteria require clinical judgment (eg, clinician expects patient likelihood of surviving 5 years post-transplant is below 50%). 11 Transplant eligibility assessment is complex and health care professionals often face competing responsibilities. 12
Table 1.
Summary of Ontario Referral and Listing Criteria for Kidney Transplant.
| Criteria for kidney transplant assessment | |
| Criteria | Description |
| Severity of kidney disease | (a) Progressive CKD with anticipated requirement for kidney replacement therapy within next 12 months. |
| (b) ESKD: patients already on maintenance kidney replacement therapy. | |
| Absence of these contraindications | (a) Active malignancy and metastatic cancer. |
| (b) Inoperable critical valve disease. | |
| (c) Active irreversible progressive ischemic heart disease. | |
| (d) Severe left ventricular dysfunction, LVEF <20%, with exception of uremic cardiomyopathy. | |
| Patient consents to kidney transplant | |
| Criteria for waitlisting for kidney transplant | |
| Criteria | Description |
| Absence of contraindications | — |
| ESKD on kidney replacement therapy | — |
| CKD | GFR <15 mL/min on 2 separate measurements |
| Irreversible and progressive deterioration of kidney function over 6-12months | |
| Absolute contraindications | |
| Criteria | Description |
| Comorbidities | Any comorbidity associated with <50% 5-year survival post-transplant or unacceptable high perioperative risk |
| Consent | Patient declined transplant |
| Post-transplant care | Unsafe or inadequate care after transplant |
| Psychosocial | Acute or untreated psychosis or history projecting nonadherence to therapy (Delay kidney transplant until ≥6 mo of demonstrated adherence) |
| Malignancy Cancer-free time interval prior to transplant eligibility varies by cancer type. |
Active malignancy |
| Stage III or IV breast cancer | |
| Liver cancer (unless concurrent liver transplant) | |
| Active multiple myeloma | |
| Pulmonary disease | Home oxygen therapy |
| Uncontrolled asthma | |
| Severe cor pulmonale | |
| Severe COPD | |
| Cardiac disease | Inoperable critical valvular disease |
| Severe nonuremic irreversible cardiomyopathy | |
| Progressive angina | |
| Myocardial infarction <6 mo ago | |
| Incomplete cardiac investigations | |
| Severe diffuse IHD not amenable to intervention and limited survival | |
| Peripheral vascular disease | Large abdominal aortic aneurysm not amenable to surgery |
| Severe occlusive common iliac disease | |
| Active gangrene | |
| Recent atheroembolic event | |
| Gastrointestinal disease | Acute pancreatitis <6 mo ago |
| Active inflammatory bowel disease | |
| Active peptic ulcer disease | |
| Relative contraindications | |
| Criteria | Description |
| Advanced age, poor functional capacity | Advanced age alone is not a contraindication but functional capacity, comorbidities, and risk factors that limit duration of survival to less than transplant wait time |
| Weight | Body weight <10 kg |
| BMI >36 kg/m2 | |
| Underlying kidney disease | Post-transplant recurrence to precipitate rapid, progressive graft loss |
| Psychosocial | Cognitive impairment: cannot obtain informed consent, lack social supports to ensure therapy adherence |
| Delay kidney transplant ≥6 mo abstinence from substance abuse | |
| Extrarenal systemic illnesses | |
| Criteria | Description |
| Infection | Active infection |
| Chronic infected wounds | |
| Pulmonary disease | Moderate COPD |
| Cardiac disease | IHD that does not meet specified criteria: (a) Asymptomatic low-risk patients (b) Asymptomatic patients with negative noninvasive test (c) Status-post successful intervention (d) Appropriate medical therapy for noncritical disease |
| Cerebral vascular disease | ≥6 mo after stroke or TIA on risk-reduction therapy |
| Peripheral vascular disease | PVD considered in presence of other comorbidities |
| Gastrointestinal disease | Chronic pancreatitis <1 y in remission |
| Liver disease | Severe cirrhosis unless simultaneous liver-kidney transplant |
| Genitourinary disease | Urologic precipitant of kidney disease without adequate urinary tract drainage |
| Hematologic disease | Require comprehensive investigations for hypercoagulable states, cytopenias, thrombophilias prior to transplant |
| Hyperparathyroidism | Consider parathyroidectomy for complications arising from severe persistent hyperparathyroidism |
Source. Adapted from Trillium Gift of Life Network. Kidney Donation and Transplant. https://www.giftoflife.on.ca/resources/pdf/transplant/ON_Adult%20Kidney_Tx_Referral_and_Listing_Criteria_4.0.pdf.
Note. BMI = body mass index; CKD = chronic kidney disease; COPD = chronic obstructive pulmonary disease; ESKD = end-stage kidney disease; GFR = glomerular filtration rate; IHD = ischemic heart disease; LVEF = left ventricular ejection fraction; PVD = peripheral vascular disease; TIA = transient ischemic attack.
Improving access to kidney transplant is a priority in Ontario, Canada, 13 and in many other jurisdictions. In this process, quality indicators (eg, rate of kidney transplantation) have been developed to measure access to kidney transplant in Ontario’s 27 Regional Renal Programs that deliver care at any given time to more than 10 000 patients with ESKD with no recorded contraindications to transplant across the province (which includes those who are approaching the need to start maintenance dialysis). 13 The total number of patients with ESKD can be used as the “denominator” for such indicators and is readily available in health care administrative databases. However, a more accurate estimate of the rate of transplant would be to restrict this denominator to transplant-eligible patients. The 2 ways of reporting results often do not correlate. For example, for 9 American transplant programs, performance on transplant rate metrics based on waitlist-based measurements (based on a denominator of patients already deemed eligible to receive a transplant) correlated poorly with rates based on transplant referral rates (based on a denominator of patients who may or may not be eligible to receive a transplant). 14 Unfortunately, registries of kidney programs do not routinely collect information on whether a patient is transplant-eligible, and in clinical practice there is some subjectivity in applying the criteria to assess eligibility.
We conducted this study to examine whether information available in administrative health care databases can be used to identify patients with ESKD who have no recorded contraindications to receiving a kidney transplant. Specifically, we assessed more than 80 baseline characteristics recorded in administrative data and examined how well they helped distinguish between patients who received a kidney transplant from those who did not. If some characteristics operate well, they can be considered for use in measures of access to kidney transplantation and facilitate more accurate comparisons of this metric across transplant programs.
Methods
Design and Setting
We conducted this study using administrative health care databases from Ontario, Canada, held at ICES (ices.on.ca/). These data sets were linked using unique encoded identifiers and analyzed at ICES. The use of data in this project was authorized under section 45 of Ontario’s Personal Health Information Protection Act, which does not require review by a Research Ethics Board. The reporting of this study follows the Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement (Supplemental Table S1). 15
Administrative Database Sources
We obtained the study population, baseline characteristics, and outcome using several linked administrative databases. Supplemental Table S2 provides details of all the databases that were used. In brief, we identified patients on maintenance dialysis using the Ontario Renal Reporting System (ORRS) and used serum creatinine and urine albumin-to-creatinine ratio information recorded in the Ontario Laboratories Information System to calculate the estimated glomerular filtration rate (eGFR) and the Kidney Failure Risk Equation (KFRE).
Cohort
We included adults from Ontario, Canada, approaching the need for dialysis (ie, predialysis) and individuals receiving maintenance dialysis between January 1, 2013 to March 31, 2015. We selected this timeframe due to data availability and to allow individuals the potential to be followed for at least 5 years after cohort entry, allowing for adequate time to receive a transplant (average wait time in Ontario for a deceased donor transplant is 5 years). 16
Predialysis
We defined predialysis using laboratory criteria: (1) eGFR ≤15 mL/min per 1.73 m2, 17 or (2) ≥50% estimated chance of receiving kidney replacement therapy within the next 2 years, as assessed with the KFRE. 18 We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration equation. Since race information was not available in our data sources, all patients were assumed to not be of black race in the equation. Black Ontarians represent 4.7% of the Ontario population. 19 To ensure stable kidney failure, at least 2 eGFR or 2 KFRE values were required and had to be separated by at least 2 weeks but no more than 12 months. We selected a KFRE ≥50% to ensure the patient’s kidney function was low enough to reasonably be listed for a transplant and receive a transplant during the follow-up period. We excluded individuals who were <18 years of age (eligibility criteria for kidney transplant differs for pediatric patients), and those with a prior history of receiving maintenance dialysis or any solid organ transplant (including kidney). Because at least 2 measurements of eGFR or KFRE were required for cohort entry, the later date at which the individual satisfied cohort entry was set as the date that the predialysis definition was met. Specifically, the cohort entry date (ie, index date) for patients who met the predialysis definition prior to or on January 1, 2013 was set as January 1, 2013, whereas the index date for individuals who met the laboratory criteria between January 2, 2013 and March 31, 2015 was defined as the later laboratory date of the pair that satisfied the eGFR or KFRE criteria.
Maintenance dialysis
Maintenance dialysis was defined by the use of chronic hemodialysis or peritoneal dialysis in ORRS. Similar to the predialysis cohort, we excluded individuals who were <18 years of age or had evidence of a prior solid organ transplant (including kidney). To ensure we were not capturing patients receiving acute dialysis, patients who received dialysis for the first time had to remain on dialysis for at least 30 days to meet the criteria for maintenance dialysis. The index date for patients active on maintenance dialysis on or prior to the beginning of the study was January 1, 2013, while individuals who more recently initiated maintenance dialysis had an index date between January 2, 2013 and March 31, 2015.
We combined the predialysis and maintenance dialysis cohorts, creating one independent group of patients with ESKD. The cohorts were followed for at least 5 years, to assess which baseline characteristics on cohort entry were associated with very low likelihood of receiving a kidney-only or kidney-pancreas transplant in follow-up.
Characteristics Assessed to Identify Patients With No Recorded Contraindication to Kidney Transplant
To operationalize predefined criteria for transplant eligibility using administrative health care databases, we used several strategies to identify patients with no recorded contraindications to kidney transplant. We considered more than 80 baseline characteristics (Table 2), including demographic information (eg, older age), comorbidities (eg, different types of cancer), and other characteristics used by clinicians to assess transplant eligibility as defined by Ontario’s referral and listing criteria for adult kidney transplantation. 11 Using clinical expertise, we also selected medical conditions, such as living in a long-term care facility, that are not explicitly listed in the referral and listing criteria, but may influence a clinician’s decision regarding a patient’s transplant eligibility. We assessed most comorbidities in the 5 years prior to index date. We used a shorter lookback window for some conditions (eg, substance abuse) to align with Ontario’s referral and listing criteria. Further details on baseline characteristics can be found in Supplemental Table S3. The potential for measurement error in some variables was also considered. While many of the reported characteristics are validated measures, no variable has 100% accuracy. Thus, we expected a small proportion of patients who underwent transplantation during follow-up would have conditions recorded in administrative databases that are absolute contraindications to transplantation.
Table 2.
Baseline Characteristics for Patients With End-Stage Kidney Disease, Presented by Kidney Transplant Status.
| Kidney transplant in follow-up | |||||
|---|---|---|---|---|---|
| Characteristics | Yes (N = 3215) |
No (N = 20 427) |
Entire cohort (N = 23 642) | Percentage of individuals with no transplant | Standardized difference a |
| Demographics | |||||
| Age, y b | 54 (44, 62) | 73 (63, 81) | 70 (59, 80) | 1.54 | |
| Age categories, y b | |||||
| <50 | 1241 (38.6) | 1590 (7.8) | 2831 (12.0) | 56.2 | 0.78 |
| 50-60 | 1051 (32.7) | 2675 (13.1) | 3726 (15.8) | 71.8 | 0.48 |
| >60-65 | 458 (14.2) | 2059 (10.1) | 2517 (10.6) | 81.8 | 0.13 |
| >65-70 | 310 (9.6) | 2452 (12.0) | 2762 (11.7) | 88.8 | 0.08 |
| >70-75 | 128 (4.0) | 2772 (13.6) | 2900 (12.3) | 95.6 | 0.34 |
| >75 | 27 (0.8) | 8879 (43.5) | 8906 (37.7) | 99.7 | 1.20 |
| Female | 1201 (37.4) | 9217 (45.1) | 10 418 (44.1) | 88.5 | 0.16 |
| Income quintilec,d | |||||
| 1 (low) | 764 (23.8) | 5361 (26.2) | 6125 (25.9) | 87.5 | 0.06 |
| 2 | 688 (21.4) | 4567 (22.4) | 5255 (22.2) | 86.9 | 0.02 |
| 3 (middle) | 665 (20.7) | 4021 (19.7) | 4686 (19.8) | 85.8 | 0.02 |
| 4 | 600 (18.7) | 3563 (17.4) | 4163 (17.6) | 85.6 | 0.03 |
| 5 (high) | 498 (15.5) | 2915 (14.3) | 3413 (14.4) | 85.4 | 0.03 |
| Rural residencee,f | 290 (9.0) | 2384 (11.7) | 2674 (11.3) | 89.2 | 0.09 |
| Year of cohort entry (index year) | |||||
| 2013 | 2640 (82.1) | 14 735 (72.1) | 17 375 (73.5) | 84.8 | 0.24 |
| 2014 | 468 (14.6) | 4613 (22.6) | 5081 (21.5) | 90.8 | 0.21 |
| 2015 | 107 (3.3) | 1079 (5.3) | 1186 (5.0) | 91.0 | 0.10 |
| Kidney-specific characteristics | |||||
| Cause of end-stage kidney disease g | |||||
| Glomerulonephritis | 522 (25.7) | 1125 (10.8) | 1647 (13.2) | 68.3 | 0.39 |
| Cystic kidney disease | 180 (8.9) | 308 (3.0) | 488 (3.9) | 63.1 | 0.25 |
| Diabetes | 590 (29.0) | 3909 (37.5) | 4499 (36.1) | 86.9 | 0.18 |
| Renal vascular disease | 270 (13.3) | 1818 (17.5) | 2088 (16.8) | 87.1 | 0.12 |
| Other | 270 (13.3) | 2091 (20.1) | 2361 (19.0) | 88.6 | 0.18 |
| Missing/Unknown | 199 (9.8) | 1165 (11.2) | 1364 (11.0) | 85.4 | 0.05 |
| Estimated glomerular filtration rate h | 13.32 (10.74, 15.70) | 13.23 (11.22, 14.50) | 13.23 (11.17, 14.55) | 0.10 | |
| Kidney Failure Risk Equation i | 0.63 (0.51, 0.80) | 0.46 (0.20, 0.62) | 0.50 (0.22, 0.64) | 0.79 | |
| Multicare kidney clinic visit j | 715 (60.4) | 4900 (48.9) | 5615 (50.2) | 87.3 | 0.23 |
| Dialysis modality k | |||||
| In-center hemodialysis | 1339 (65.9) | 8745 (84.0) | 10 084 (81.0) | 86.7 | 0.43 |
| Received dialysis at a nursing home | 0 (0.0) | 16 (0.2) | 16 (0.1) | 100.0 | 0.06 |
| Peritoneal dialysis/home hemodialysis | 692 (34.1) | 1655 (15.9) | 2347 (18.9) | 70.5 | 0.43 |
| Comorbidity indices | |||||
| Charlson Comorbidity Index | 2 (1, 4) | 3 (2, 5) | 3 (2, 5) | 0.44 | |
| Elixhauser score | 5 (0, 9) | 6 (0, 16) | 6 (0, 15) | 0.35 | |
| ESKD-modified Charlson Comorbidity Index | 0 (0-1) | 2 (0-3) | 1 (0-3) | 0.73 | |
| ESKD-modified Charlson Comorbidity Index categories | |||||
| 0 | 1690 (52.6) | 5669 (27.8) | 7359 (31.1) | 77.0 | 0.52 |
| 1 | 810 (25.2) | 3901 (19.1) | 4711 (19.9) | 82.8 | 0.15 |
| 2 | 305 (9.5) | 2884 (14.1) | 3189 (13.5) | 90.4 | 0.14 |
| 3 | 259 (8.1) | 3061 (15.0) | 3320 (14.0) | 92.2 | 0.22 |
| 4 | 49 (1.5) | 1597 (7.8) | 1646 (7.0) | 97.0 | 0.30 |
| 5 | 72 (2.2) | 1460 (7.1) | 1532 (6.5) | 95.3 | 0.23 |
| 6 | 18 (0.6) | 814 (4.0) | 832 (3.5) | 97.8 | 0.23 |
| ≥7 | 12 (0.4) | 1041 (5.1) | 1053 (4.5) | 98.9 | 0.29 |
| The Johns Hopkins ACG System Aggregated Diagnosis Groups l | 13 (11, 16) | 15 (12, 18) | 15 (12, 18) | 0.39 | |
| Psychosocial considerations | |||||
| History of medical noncompliance in the last 1 y | 32 (1.0) | 299 (1.5) | 331 (1.4) | 90.3 | 0.04 |
| Hospitalization for any mental illness in the last 6 mo * | * | * | * | <98.0* | * |
| Cancer | |||||
| Any cancer diagnosis | 131 (4.1) | 2037 (10.0) | 2168 (9.2) | 94.0 | 0.23 |
| Combination of select cancers (lung, lymphoma, cervical, colorectal, liver, multiple myeloma, and bladder cancer)* | 20-24 (0.6-0.7) | 888 (4.3) | 908-912 (3.8) | 97.3-97.7 | 0.23-0.24 |
| Cancer free m | 3120 (97.0) | 18 520 (90.7) | 21 640 (91.5) | 85.6 | 0.27 |
| Pulmonary disease | |||||
| Home oxygen therapy n | 8 (0.2) | 1075 (5.3) | 1083 (4.6) | 99.3 | 0.31 |
| Chronic obstructive pulmonary disease (sensitive definition) n | 309 (9.6) | 6122 (30.0) | 6431 (27.2) | 95.2 | 0.53 |
| Asthma | 38 (1.2) | 286 (1.4) | 324 (1.4) | 88.3 | 0.02 |
| Cor pulmonale | 0 (0.0) | 35 (0.2) | 35 (0.1) | 100.0 | 0.06 |
| Cardiac disease | |||||
| Ischemic heart disease | 452 (14.1) | 5299 (25.9) | 5751 (24.3) | 92.1 | 0.30 |
| Myocardial infarction (last 6 mo) | 20 (0.6) | 444 (2.2) | 464 (2.0) | 95.7 | 0.13 |
| Myocardial infarction (last 1 y) | 31 (1.0) | 682 (3.3) | 713 (3.0) | 95.7 | 0.16 |
| Angina | 124 (3.9) | 1750 (8.6) | 1874 (7.9) | 93.4 | 0.20 |
| Peripheral vascular disease | |||||
| Abdominal aneurysm repair | 9 (0.3) | 138 (0.7) | 147 (0.6) | 93.9 | 0.06 |
| Hospitalization for active gangrene in the last 6 mo * | * | * | * | ≥98.0* | * |
| Peripheral vascular disease | 201 (6.3) | 1811 (8.9) | 2012 (8.5) | 90.0 | 0.10 |
| Atheroembolic events | |||||
| Stroke/ transient ischemic attack in the last 1 y | 100 (3.1) | 1319 (6.5) | 1419 (6.0) | 93.0 | 0.16 |
| Ischemic stroke in the last 1 y | 8 (0.2) | 197 (1.0) | 205 (0.9) | 96.1 | 0.09 |
| Gastrointestinal disease | |||||
| Hospitalization for inflammatory bowel disease in the last 6 mo | 9 (0.3) | 60 (0.3) | 69 (0.3) | 87.0 | 0.00 |
| Hospitalization for acute pancreatitis in the last 6 mo | 10 (0.3) | 90 (0.4) | 100 (0.4) | 90.0 | 0.02 |
| Hospitalization for peptic ulcer in the last 6 mo | 22 (0.7) | 224 (1.1) | 246 (1.0) | 91.1 | 0.04 |
| TGLN relative listing contraindications | |||||
| BMI, o kg/m2 | 26.96 (23.56, 31.43) | 27.62 (23.91, 32.17) | 27.52 (23.86, 32.04) | 0.09 | |
| Obesity identified using International Classification of Disease codes (denominator restricted to individuals with missing a BMI) | 32 (3.3) | 374 (4.8) | 406 (4.7) | 92.1 | 0.08 |
| Psychosocial considerations | |||||
| Dementia n | 9 (0.3) | 2079 (10.2) | 2088 (8.8) | 99.6 | 0.46 |
| Alcoholism in the last 6 mo | 6 (0.2) | 156 (0.8) | 162 (0.7) | 96.3 | 0.08 |
| Substance abuse in the last 6 mo | 33 (1.0) | 514 (2.5) | 547 (2.3) | 94.0 | 0.11 |
| Chronic pancreatitis in the last 1 y* | 1-5 (0.0-0.2) | 33 (0.2) | 34-38 (0.1-0.2) | 86.8-97.1 | 0.00-0.04 |
| Liver disease (cirrhosis) in the last 1 y | 6 (0.2) | 238 (1.2) | 244 (1.0) | 97.5 | 0.12 |
| Hyperparathyroidism | 54 (1.7) | 263 (1.3) | 317 (1.3) | 83.0 | 0.03 |
| Other potential contraindications not explicitly listed in TGLN referral and listing criteria | |||||
| Long-term care residence n ,* | 1-5 (0.0-0.2) | 1478 (7.2) | 1479-1483 (6.3) | 99.7-99.9 | 0.38-0.39 |
| Healthy p | 1339 (41.6) | 1567 (7.7) | 2906 (12.3) | 53.9 | 0.86 |
| Congestive heart failure n | 437 (13.6) | 8798 (43.1) | 9235 (39.1) | 95.3 | 0.69 |
| Hypertension n | 2704 (84.1) | 18 932 (92.7) | 21 636 (91.5) | 87.5 | 0.27 |
| Diabetes n | 1418 (44.1) | 12 959 (63.4) | 14 377 (60.8) | 90.1 | 0.40 |
| Venous thromboembolism | 112 (3.5) | 1046 (5.1) | 1158 (4.9) | 90.3 | 0.08 |
| Coronary artery disease without angina | 979 (30.5) | 8624 (42.2) | 9603 (40.6) | 89.8 | 0.25 |
| Severe cardiac disease q | 299 (9.3) | 4063 (19.9) | 4362 (18.5) | 93.1 | 0.30 |
| Major amputation (ankle, below/above knee) | 22 (0.7) | 496 (2.4) | 518 (2.2) | 95.8 | 0.14 |
| Minor amputation (toe, partial foot) | 30 (0.9) | 492 (2.4) | 522 (2.2) | 94.3 | 0.12 |
| Health care utilization in the last 1 y | |||||
| At least one intensive care unit visit | 301 (9.4) | 3900 (19.1) | 4201 (17.8) | 92.8 | 0.28 |
| At least one palliative care service in the last year | 77 (2.4) | 1876 (9.2) | 1953 (8.3) | 96.1 | 0.29 |
| At least one home visit by a health care professional in the last year | 549 (17.1) | 9460 (46.3) | 10 009 (42.3) | 94.5 | 0.66 |
| At least one physician house call in the last year | 19 (0.6) | 1021 (5.0) | 1040 (4.4) | 98.2 | 0.27 |
| At least one aggressive care service in the last year | 1474 (45.8) | 12 042 (59.0) | 13 516 (57.2) | 89.1 | 0.26 |
| At least one supportive care visit in the last 1 y | 422 (13.1) | 7288 (35.7) | 7710 (32.6) | 94.5 | 0.54 |
Note. All baselines assessed 5 years prior to index date unless otherwise indicated. Data are presented as No. (%) or median (25th, 75th percentile). ACG = Adjusted Clinical Group; BMI = body mass index; ESKD = end-stage kidney disease; TGLN = Trillium Gift of Life Network.
We used standardized differences to compare baseline characteristics between patients who did and did not receive a kidney transplant during follow-up. A meaningful difference is considered as a difference >0.1. Bold text represents a meaningful difference.
Characteristic is a relative listing contraindication in Ontario’s Referral and Listing Criteria for Adult Kidney Transplantation.
Income presented as quintiles of average neighborhood income.
Income quintile 3 was imputed for missing income quintiles; <0.1% missing.
Rural residence defined as living in an area with a population ≤10 000.
Missing rurality was imputed as urban; <0.1% missing.
Restricted to the maintenance dialysis population.
Restricted to the predialysis population. For estimated glomerular filtration rate, the most recent value on or within 1 year before the index date was selected.
Restricted to predialysis patients with a valid Kidney Failure Risk Equation (KFRE) measure. The most recent KFRE calculated on or within 1 year before the index date was selected.
Restricted to the predialysis population. Evidence of being in a multicare kidney clinic was defined as any evidence of attending the clinic prior to the index date.
Dialysis modality is restricted to the maintenance dialysis population. The most recent modality on or before the index date was selected.
The Adjusted Clinical Group (ACG) is a population/patient case mix adjustment system that was applied to score comorbidity. The ACG generates a measurement of an individual’s expected health services consumption. Ambulatory Diagnostic Groups (ADGs) are generated by categorization of ICD-9/ICD-9-CM codes into 32 groups. The ADGs are based on chronicity, disability, clinical similarity and likelihood to require specialty care. (Reference: The Johns Hopkins University Bloomberg School of Public Health, Health Services Research & Development Center. The Johns Hopkins ACG Case-Mix System Version 10 Release Notes. [Editor in Chief: Jonathan P. Weiner]. The Johns Hopkins University. 2011).
Lookback window 2 years from the index date.
Lookback window >5 years from the index date (ie, looked back as far as databases available).
Individuals with missing body mass indices were excluded in the median body mass index calculation.
Healthy defined as no diabetes, no severe cardiovascular disease, age 18 to 65 years, no active malignancy, no major lower limb amputation, and no chronic obstructive pulmonary disease.
Severe cardiac disease defined as a composite of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft surgery, and ischemic stroke.
In accordance with ICES privacy policies, cell sizes less than or equal to 5 cannot be reported.
In Ontario, “any comorbidity that decreases the likelihood of surviving the next 5 years to below 50%” 11 is an absolute listing contraindication to receiving a kidney transplant. In efforts to apply this subjective criterion to administrative databases, we conducted a comprehensive literature search of predictive models to assess mortality (detailed information on our literature review can be found in the Supplemental Appendix). We included several comorbidity indices validated in kidney disease populations including the Charlson Comorbidity Index, ESKD-modified Charlson Comorbidity Index, Elixhauser model, and Johns Hopkins Adjusted Clinical Group scoring system (The Johns Hopkins ACG System Ver 10).20-25
Kidney Transplant
Our outcome of interest was first-time kidney-only or kidney-pancreas transplant. We used a validated definition to define kidney-only transplants, with a sensitivity and positive predictive value >95%, compared with manual chart review (kidney-pancreas transplants have not been validated). 24 We followed all individuals until a kidney transplant, death or end of follow-up (where the last potential follow-up date was March 31, 2020).
Statistical Analysis
We examined baseline characteristics for the entire ESKD cohort (ie, combining predialysis and dialysis) and by kidney transplant status during follow-up (yes versus no). We also examined the proportion of all individuals in the cohort with each characteristic who never received a transplant. We presented continuous variables as medians (25th, 75th percentiles), whereas categorical variables were presented as percentages. We used standardized differences to compare baseline characteristics between patients who did and did not receive a kidney transplant during follow-up. Standardized differences describe differences in mean rankings divided by a pooled estimate of the within-group standard deviation of rankings. 26 A meaningful difference is considered as a difference ≥0.1. 27 All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
We included 23 642 patients with ESKD, of which 11 195 were predialysis and 12 447 were on maintenance dialysis. In the predialysis cohort, 6055 (54.1%) initiated maintenance dialysis during follow-up. Cohort selection is outlined in Supplemental Figures S1a-d. Over a median of 3.2 years (1.3, 5.6) and maximum of 7.2 years follow-up time, 3215 (13.6%) patients received a kidney transplant, including 3050 kidney-only transplants and 165 kidney-pancreas transplants. When examining the number of kidney transplants by ESKD status, 184 (5.7%) transplants occurred in individuals who were predialysis (attributing ESKD status to the status at the time of transplant [ie, an as-treated approach]) and 3031 (94.3%) in individuals receiving maintenance dialysis. A total of 15 051 (63.7%) persons died during the follow-up period, with 4140 (27.5%) individuals who were predialysis and 10 911 (72.5%) individuals receiving dialysis (attributing ESKD status to the status at the time of death).
Baseline characteristics are presented in Table 2 for the entire cohort and by individuals who did and did not receive a kidney transplant during follow-up (n = 3215 and 20 427, respectively). Supplemental Table S4 provides additional baseline characteristics. Patients who received a kidney transplant (versus those who did not) were more likely to be male (62.6% versus 54.9%), have ESKD caused by glomerulonephritis (25.7% versus 10.8%), have KFRE of ≥70% (39.3% versus 15.6%), and received care at a multicare kidney clinic 28 composed of a multidisciplinary team dedicated to the care of patients at high risk of progression to ESKD (60.4% versus 48.9%).
Of the studied characteristics, >98% of patients with each of the following characteristics did not receive a kidney transplant during follow-up: those with an ESKD-modified Charlson Comorbidity Index score ≥7 (a higher score indicates greater comorbidity), home oxygen use, aged >75 years old, dementia, long-term care residence, or receipt of at least one physician house call in the past year (Table 3). Post hoc we combined several malignancies, selecting those for which >97% of patients never received a transplant during follow-up. These malignancies included lung, lymphoma, cervical, colorectal, liver, multiple myeloma, and bladder cancer. While >98% of patients with cor pulmonale or a hospitalization for active gangrene never underwent a transplant, they were not included in our selection criteria due to concerns about the accuracy of these codes in our administrative databases.
Table 3.
Criteria Identified to Help Select a Cohort of Individuals With End-Stage Kidney Disease Who Have No Recorded Contraindications to Kidney Transplant.
| Kidney transplant in follow-up | |||||
|---|---|---|---|---|---|
| Characteristics | Yes (N = 3215) |
No (N = 20 427) |
Entire cohort (N = 23 642) | Percentage of individuals with no transplant | Standardized difference a |
| Age >75 y | 27 (0.8) | 8879 (43.5) | 8906 (37.7) | 99.7 | 1.20 |
| End-stage kidney disease-modified Charlson Comorbidity Index score ≥7 | 12 (0.4) | 1041 (5.1) | 1053 (4.5) | 98.9 | 0.29 |
| Combination of select cancers (lung, lymphoma, cervical, colorectal, liver, multiple myeloma, and bladder cancer) b | 20-24 (0.6-0.7) | 888 (4.3) | 908-912 (3.8) | 97.3-97.7 | 0.23-0.24 |
| Home oxygen therapy c | 8 (0.2) | 1075 (5.3) | 1083 (4.6) | 99.3 | 0.31 |
| Dementia c | 9 (0.3) | 2079 (10.2) | 2088 (8.8) | 99.6 | 0.46 |
| Long-term care residenceb,c | 1-5 (0.0-0.2) | 1478 (7.2) | 1479-1483 (6.3) | 99.7-99.9 | 0.38-0.39 |
| At least one physician house call in the last year | 19 (0.6) | 1021 (5.0) | 1040 (4.4) | 98.2 | 0.27 |
Note. Data are presented as No. (%).
We used standardized differences to compare baseline characteristics between patients who did and did not receive a kidney transplant during follow-up. A meaningful difference is considered as a difference >0.1. Bold text represents a meaningful difference.
In accordance with ICES privacy policies, cell sizes less than or equal to 5 cannot be reported.
Lookback window >5 years from the index date (ie, looked back as far as databases available).
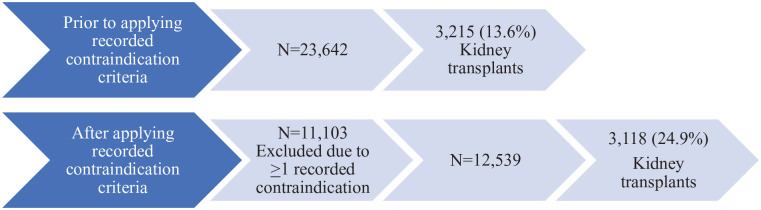
After restricting our cohort to individuals with no recorded contraindications to transplant as outlined above, 11 103 individuals were excluded due to presence of at least one contraindication. In other words, we reduced our cohort from 23 642 patients to 12 539 patients with no recorded contraindication to transplant (a 47% reduction). Of the 11 103 individuals with at least one recorded contraindication, 97 underwent a transplant during the follow-up period, accounting for 3.0% of all transplants, with the remaining 3118 (97.0%) transplants occurring in individuals with no recorded contraindications. After the exclusion of individuals with at least one contraindication to kidney transplant, 24.9% (denominator of 12 539) underwent a kidney transplant in follow-up compared with 13.6% (denominator of 23 642) without the exclusion (Figure 1).
Figure 1.
Flow diagram of end-stage kidney disease cohort and number of kidney transplants prior to and after applying exclusion criteria of ≥1 recorded contraindications to kidney transplant.
Discussion
We identified several criteria available within administrative health care databases that can be used to identify patients with no recorded contraindications to kidney transplant with a reasonable degree of confidence. In this study, an ESKD-modified Charlson Comorbidity Index score ≥7, home oxygen use, age greater than 75 years old, dementia, residence at a long-term care facility, receipt of at least one physician house call in the past year, and presence of a set of malignancies identified persons who were very unlikely to receive a kidney transplant in follow-up. We also illustrated how application of these criteria substantially changed the denominator of patients with ESKD and subsequent proportion of kidney transplants. These criteria may be considered when creating a cohort of patients with ESKD and could also be used as censoring events when calculating the rate of kidney transplantation.
Previous studies have demonstrated regional variations in rates of kidney transplantation and transplant referral.29-31 In Canada, a 3-fold disparity in transplant referral rates was noted between Atlantic provinces and Manitoba. 29 Similarly, transplant rates ranged from 0% to 75% across 308 dialysis facilities in Georgia. 31 However, these rates should be interpreted with caution, as the denominator used included all patients with ESKD rather than only transplant-eligible patients. When a previous study restricted the cohort to “healthy patients” (ie, no overt contraindications to kidney transplant such as age 18-50 years, absence of diabetes, coronary artery disease or cancer), the median 10-year cumulative incidence of kidney transplantation in Ontario was 65.8%, compared with 17.2% when all patients receiving dialysis were included. 32 Our study results can be used to calculate new quality indicators for access to kidney transplantation, allowing for more objective comparisons of rates within and between programs over time.
Our results are not meant to be used clinically to determine the transplant eligibility of any given patient. For example, our study found that only 0.8% of patients aged >75 years received a kidney transplant. However, advanced age is not a contraindication to kidney transplant, and high-functioning older adults (≥65 years old) should be considered for transplant as they still can derive quality of life and mortality benefits.2,33-35 Furthermore, although we are able to map the majority of referral and listing criteria for kidney transplants to administrative databases, contraindications such as inoperable valve disease cannot be reliably captured in these data sources.
Approximately 3% of all transplants observed in our study occurred among patients with at least one recorded contraindication as defined using our methodology with administrative database codes. There are several potential explanations for this finding. First, we did not capture improvements in a patient’s health that would deem a patient transplant-eligible during follow-up. Second, there is potential for misclassification of baseline characteristics with administrative data. Third, clinical decisions in transplant eligibility assessment are complex and are difficult to capture in administrative data. For example, patients with any comorbidity that decreases estimated 5-year survival to less than 50% is listed in Ontario as an absolute contraindication for kidney transplant. 11 We used different comorbidity indices to help apply this criterion, including the Charlson Comorbidity Index, ESKD-modified Charlson Comorbidity Index, and Elixhauser score, although limited by their poor discriminatory capacities to predict 1-year mortality in the chronic kidney disease population. 20
As the 1-year allograft and short-term survival after kidney transplant improves, using these parameters to evaluate the quality of a transplant program is becoming obsolete. 36 Schold and colleagues 37 have urged for reform in kidney transplant quality metrics. Recently, a multiphase framework encompassing a broad range of kidney transplant quality indicators, including timely and equitable access and efficiency measures, has been proposed. 36 Applying the criteria we developed can help ascertain the denominator over which transplant rates can be measured and compared between programs. Limited health care resources can then be deployed to best address gaps in access.
The results of this study should be interpreted in the setting of its limitations. First, all the complexities of transplant eligibility assessment will never be able to be captured in administrative databases. For example, in the absence of a validated post-transplant mortality prediction model, exclusion of candidates based on comorbidities that confer poor post-transplant survival remains at the discretion of the clinicians. The comorbidity indices used in our study have limitations in predicting 1-year mortality in this patient population and were not initially designed to determine transplant eligibility. 20 Although our data showed >98% of patients with Charlson Comorbidity Index score ≥9 or Elixhauser score ≥31 did not receive a kidney transplant in follow-up, these were not included in our final list of criteria due to their lower discriminative ability compared with ESKD-modified Charlson Comorbidity Index. 20 Second, patients had the potential to be followed for at least 5 years but it is possible more patients would have received a kidney transplant if we used a longer follow-up period. Third, our study used the CKD-EPI equation based on creatinine 17 omitting the race coefficients; our analysis predated the 2021 new equation that was built without the race variable. 38 Our equation may have underestimated the eGFR in black individuals and thus overestimated the number of such individuals meeting the eGFR criteria in the predialysis cohort. 38 Fourth, a small number of individuals with acute kidney injury may have been included in our cohort. We minimized this by requiring 2 eGFR or KFRE values to enter within a prespecified time frame in the predialysis cohort and by requiring individuals to remain on dialysis for at least 30 days in the maintenance dialysis cohort. Last, transplant eligibility criteria may vary slightly across provinces and countries; therefore, application of the criteria to other regions may require modifications. However, similar methodology may be used to capture pertinent information pertaining to kidney transplant eligibility in local health care databases.
Conclusion
This study provides a proof of concept of how administrative health care databases can be used to identify a set of patients without recorded contraindications to receiving a kidney transplant. This technique can be used in program performance reporting, a key aspect of quality assessment and improvement in kidney transplantion. 36 Our results serve as an exemplar for future research efforts to better report on the access to kidney transplantation using information available in administrative data.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581221111712 for Using Administrative Health Care Databases to Identify Patients With End-Stage Kidney Disease With No Recorded Contraindication to Receiving a Kidney Transplant by Carol Wang, Kyla L. Naylor, Bin Luo, Sarah E. Bota, Stephanie N. Dixon, Seychelle Yohanna, Darin Treleaven, Lori Elliott and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581221111712 for Using Administrative Health Care Databases to Identify Patients With End-Stage Kidney Disease With No Recorded Contraindication to Receiving a Kidney Transplant by Carol Wang, Kyla L. Naylor, Bin Luo, Sarah E. Bota, Stephanie N. Dixon, Seychelle Yohanna, Darin Treleaven, Lori Elliott and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Acknowledgments
We thank ORN for their content advice. We thank IQVIA Solutions Canada Inc. for using their Drug Information Database. The authors thank Dr Peter Blake, Rebecca Cooper, and Lisa Joya for their support with this activity.
Footnotes
Ethics Approval and Consent to Participate: ICES is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of, the allocation of resources to, or planning for all or part of the health system. Projects conducted under section 45, by definition, do not require review by a Research Ethics Board. This project was conducted under section 45 and approved by ICES’ Privacy and Compliance Office.
Consent for Publication: All authors consent to the publication of this study.
Data Availability Statement: The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access can be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Amit Garg received an investigator-initiated grant from Astellas which featured as partnership funds in CIHR-funded research. The other authors declare no conflicts of interest. The results presented in this article have not been published previously in whole or part.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr Amit Garg was supported by the Dr. Adam Linton Chair in Kidney Health Analytics and a CIHR Clinician Investigator Award. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study was conducted with funding and the support of the Ontario Renal Network (ORN), a division of Ontario Health. The study was completed at the ICES Western site, where core funding is provided by the Academic Medical Organization of Southwestern Ontario, the Schulich School of Medicine and Dentistry, Western University, and the Lawson Health Research Institute. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and information compiled and provided by MOHLTC and CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of MOHLTC and CIHI. This study was conducted with funding and the support of the Ontario Renal Network (ORN), a division of Ontario Health. Parts of this material are based on data and information provided by Ontario Health (OH). The opinions, results, view, and conclusions reported in this article are those of the authors and do not necessarily reflect those of OH. No endorsement by OH is intended or should be inferred.
ORCID iDs: Carol Wang  https://orcid.org/0000-0003-0225-8307
https://orcid.org/0000-0003-0225-8307
Kyla L. Naylor  https://orcid.org/0000-0002-5304-8038
https://orcid.org/0000-0002-5304-8038
Stephanie N. Dixon  https://orcid.org/0000-0002-7566-6574
https://orcid.org/0000-0002-7566-6574
Seychelle Yohanna  https://orcid.org/0000-0003-0404-7319
https://orcid.org/0000-0003-0404-7319
Amit X. Garg  https://orcid.org/0000-0003-3398-3114
https://orcid.org/0000-0003-3398-3114
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093-2109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 2. Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 3. Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50(1):235-242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 4. Senghor AS. Reasons for dialysis patients choosing or refusing kidney transplantation as renal replacement therapy: a qualitative study. Nephrol Ther. 2019;15(7):511-516. doi: 10.1016/j.nephro.2019.07.327. [DOI] [PubMed] [Google Scholar]
- 5. Kazley AS, Simpson KN, Chavin KD, Baliga P. Barriers facing patients referred for kidney transplant cause loss to follow-up. Kidney Int. 2012;82(9):1018-1023. doi: 10.1038/ki.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garg AX. Helping more patients receive a living donor kidney transplant. Clin J Am Soc Nephrol. 2018;13:1918-1923. doi: 10.2215/CJN.00760118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnieh L, McLaughlin K, Manns BJ, et al. Barriers to living kidney donation identified by eligible candidates with end-stage renal disease. Nephrol Dial Transplant. 2011;26(2):732-738. doi: 10.1093/ndt/gfq388. [DOI] [PubMed] [Google Scholar]
- 8. Knight RJ, Teeter LD, Graviss EA, et al. Barriers to preemptive renal transplantation. Transplantation. 2015;99(3):576-579. doi: 10.1097/TP.0000000000000357. [DOI] [PubMed] [Google Scholar]
- 9. Sandal S, Charlebois K, Fiore JF, Jr, et al. Health professional–identified barriers to living donor kidney transplantation: a qualitative study. Can J Kidney Health Dis. 2019;6. doi: 10.1177/2054358119828389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Getchell LE, McKenzie SQ, Sontrop JM, Hayward JS, McCallum MK, Garg AX. Increasing the rate of living donor kidney transplantation in Ontario: donor- and recipient-identified barriers and solutions. Can J Kidney Health Dis. 2017;4. doi: 10.1177/2054358117698666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Trillium Gift of Life Network. Kidney Donation and Transplant. https://www.giftoflife.on.ca/resources/pdf/transplant/ON_Adult%20Kidney_Tx_Referral_and_Listing_Criteria_4.0.pdf. Accessed July 5, 2022.
- 12. Hanson CS, Chadban SJ, Chapman JR, Craig JC, Wong G, Tong A. Nephrologists’ perspectives on recipient eligibility and access to living kidney donor transplantation. Transplantation. 2016;100(4):943-953. [DOI] [PubMed] [Google Scholar]
- 13. Yohanna S, Naylor KL, Mucsi I, et al. A quality improvement intervention to enhance access to kidney transplantation and living kidney donation (EnAKT LKD) in patients with chronic kidney disease: clinical research protocol of a cluster-randomized clinical trial. Can J Kidney Health Dis. 2021;8. doi: 10.1177/2054358121997266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paul S, Melanson T, Mohan S, et al. Kidney transplant program waitlisting rate as a metric to assess transplant access. Am J Transplant. 2021;21(1):314-321. doi: 10.1111/ajt.16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trillium Gift of Life Network. Kidney Donation and Transplant. https://www.giftoflife.on.ca/resources/pdf/TGLN_Kidney_Brochure_WEB.pdf. Accessed July 5, 2022.
- 17. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604-612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Kidney Failure Risk Equation. https://kidneyfailurerisk.com. Accessed July 5, 2022.
- 19. Statistics Canada. Diversity of the Black Population in Canada: An Overview. 2019. https://www150.statcan.gc.ca/n1/en/pub/89-657-x/89-657-x2019002-eng.pdf?st=w4XVSyj-. Accessed July 5, 2022.
- 20. McArthur E, Bota SE, Sood MM, et al. Comparing five comorbidity indices to predict mortality in chronic kidney disease: a retrospective cohort study. Can J Kidney Health Dis. 2018;5. doi: 10.1177/2054358118805418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22. Hemmelgarn BR, Manns BJ, Quan H, Ghali WA. Adapting the Charlson comorbidity index for use in patients with ESRD. Am J Kidney Dis. 2003;42(1):125-132. doi: 10.1016/S0272-6386(03)00415-3. [DOI] [PubMed] [Google Scholar]
- 23. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8-27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 24. Lam NN, McArthur E, Kim SJ, Knoll GA. Validation of kidney transplantation using administrative data. Can J Kidney Health Dis. 2015;2:20. doi: 10.1186/s40697-015-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiner JP. The Johns Hopkins ACG® Case-Mix System Version 10.0 Release Notes. The Johns Hopkins University Bloomberg School of Public Health, Health Services Research & Development Center. [Google Scholar]
- 26. Yang D, Dalton J. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum. 2012;335:1-6. [Google Scholar]
- 27. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228-1234. doi: 10.1080/03610910902859574. [DOI] [Google Scholar]
- 28. Brimble S, Blake P, Patel M. Multi-Care Kidney Clinic Best Practices. 2019;1-43. [Google Scholar]
- 29. Kim SJ, Gill JS, Knoll G, et al. Referral for kidney transplantation in Canadian Provinces. J Am Soc Nephrol. 2019;30(9):1708-1721. doi: 10.1681/ASN.2019020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Patzer RE, McPherson L. Variation in kidney transplant referral: how much more evidence do we need to justify data collection on early transplant steps. J Am Soc Nephrol. 2019;30(9):1554-1556. doi: 10.1681/ASN.2019070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patzer RE, Plantinga LC, Paul S, et al. Variation in dialysis facility referral for kidney transplantation among patients with end-stage renal disease in Georgia. JAMA. 2015;314:582-594. doi: 10.1001/jama.2015.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Naylor KL, Dixon SN, Garg AX, et al. Variation in access to kidney transplantation across renal programs in Ontario, Canada. Am J Transplant. 2017;17(6):1585-1593. doi: 10.1111/ajt.14133. [DOI] [PubMed] [Google Scholar]
- 33. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:S291-S332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rao PS, Merion RM, Ashby VB, et al. Renal transplantation in elderly patients older than 70 years of age: results from the Scientific Registry of Transplant Recipients. Transplantation. 2007;83:1069-1074. doi: 10.1097/01.tp.0000259621.56861.31. [DOI] [PubMed] [Google Scholar]
- 35. McAdams-DeMarco MA, James N, Salter ML, Walston J, Segev DL. Trends in kidney transplant outcomes in older adults. J Am Geriatr Soc. 2014;62(12):2235-2242. doi: 10.1111/jgs.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knoll GA, Fortin MC, Gill J, et al. Measuring quality in living donation and kidney transplantation: moving beyond survival metrics. Kidney Int. 2020;98(4):860-869. doi: 10.1016/j.kint.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 37. Schold JD, Patzer RE, Pruett TL, Mohan S. Quality metrics in kidney transplantation: current landscape, trials and tribulations, lessons learned, and a call for reform. Am J Kidney Dis. 2019;74(3):382-389. doi: 10.1053/j.ajkd.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 38. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021;385:1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581221111712 for Using Administrative Health Care Databases to Identify Patients With End-Stage Kidney Disease With No Recorded Contraindication to Receiving a Kidney Transplant by Carol Wang, Kyla L. Naylor, Bin Luo, Sarah E. Bota, Stephanie N. Dixon, Seychelle Yohanna, Darin Treleaven, Lori Elliott and Amit X. Garg in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581221111712 for Using Administrative Health Care Databases to Identify Patients With End-Stage Kidney Disease With No Recorded Contraindication to Receiving a Kidney Transplant by Carol Wang, Kyla L. Naylor, Bin Luo, Sarah E. Bota, Stephanie N. Dixon, Seychelle Yohanna, Darin Treleaven, Lori Elliott and Amit X. Garg in Canadian Journal of Kidney Health and Disease