Abstract
Paraneoplastic autoimmune multiorgan syndrome is a complex and deadly disease. We retrospectively reviewed the clinical features and risk factors for para-neoplastic autoimmune multiorgan syndrome in 145 Chinese patients. The most common neoplasm was Castleman disease (56%), and patients with Castle-man disease tended to be younger (≤42 years old: 83% vs. 29%) and to have a greater proportions of lichen planus-like lesions (47% vs. 27%) and bronchiolitis obliterans (49% vs. 29%), compared to other neoplasm-associated patients. Among all 145 patients in the study, the survival rates were 84% at 1 year, 65% at 3 years, and 54% at 5 years. Kaplan-Meier curve analysis revealed that mortality was associated with older age (>42 years), neoplasm type, labial lesions, and larger skin lesion area (>17.5% of the body surface area). However, only older age and larger skin lesion area were independent factors associated with mortality in multivariate analysis. We suggest that patients with Castleman disease and paraneoplastic autoimmune multiorgan syndrome have many unique characteristics and the underlying risk factors for death require further exploration.
Key words: paraneoplastic autoimmune multiorgan syndrome, Castleman disease, bronchiolitis obliterans, survival rate, risk factors
Paraneoplastic autoimmune multiorgan syndrome (PAMS), which is also described as paraneoplastic pemphigus, was first defined by Anhalt et al. in 1990 (1, 2). This autoimmune disease involves polymorphous mucocutaneous lesions and various underlying neoplasms (1, 3, 4). Reports have indicated that non-Hodgkin lymphoma (NHL) is the most common neoplasm, followed by chronic lymphocytic leukaemia (CLL) (5, 6), although Castleman disease (CD) is the most common neoplasm in young patients and Chinese patients with PAMS (7–9). Geographical and racial differences may explain the diverse clinical features of PAMS (4, 8, 10–12). Furthermore, PAMS has a high mortality rate that can reach 90% (11, 13, 14), and death is typically related to infection, respiratory failure, associated neoplasms, and multiorgan failure (4, 9, 15). However, there is limited research regarding the risk factors for death. Therefore, we performed a large single-centre retrospective study of Chinese PAMS patients to explore their clinical features, prognosis, and risk factors.
SIGNIFICANCE
Paraneoplastic autoimmune multiorgan syndrome is a rare autoimmune bullous disease with complex features and fatal prognosis. We retrospectively studied patients with paraneoplastic autoimmune multiorgan syndrome from Peking University First Hospital (Beijing, China). Of 145 patients enrolled, Castleman disease was the most common tumour (56%), followed by thymoma (19%). Patients with Castleman disease had higher incidence of lichen planus-like lesions and bronchiolitis obliterans. The one-, three-, and five-year survival rates were 84%, 65%, and 54%, respectively. Respiratory failure (38%) and infection (22%) were the most common causes of death. Patients with the age of >42 years and skin lesions >17.5% body surface area may have a worse prognosis.
MATERIALS AND METHODS
Study design
This retrospective study included 145 patients with PAMS who visited the Peking University First Hospital between June 16, 1999 and January 1, 2019. The study protocol complied with the Declaration of Helsinki guidelines and was approved by the hospital’s ethics committee. The diagnosis of PAMS was based on a modified version of the published diagnostic criteria (1, 16, 17): (i) severe mucosal involvement with or without cutaneous polymorphic eruptions; (ii) the presence of associated neoplasms, especially lymphoproliferative tumours; (iii) histological features of skin or mucosal eruptions, including acantholysis, lichenoid/interface dermatitis, and necrotic keratinocytes; (iv) a positive result for indirect immunofluorescence on a rat bladder; and (v) immunoblot assay bands at 210 kDa (envoplakin) or 190 kDa (periplakin). For the present study, the diagnosis of PAMS required the case to fulfil criteria i and ii and at least two other criteria. Bronchiolitis obliterans (BO) was identified based on respiratory symptoms, severe airflow obstruction during pulmonary function testing, and/or signs of trapped air during computed tomography. Survival times were calculated from the date of disease onset to the date of death or the last follow-up.
Laboratory test
Serum and skin specimens were collected and assessed at the Peking University First Hospital laboratory. Indirect immunofluorescence testing using a rat bladder was performed according to a published standard method (ZSGB-BIO, Beijing, China) (18). Rabbit anti-human immunoglobulin antibodies (ZSGB-BIO, Beijing, China) were used for immunoblotting to detect bands at 210 kDa (envoplakin) and 190 kDa (periplakin).
Statistical analysis
Data regarding different variables were frequently missing, and thus the results for each variable were reported as percentages calculated using the number of patients with available data as the denominator. Receiver operating characteristic curves and the maximum Youden index (sensitivity – [1 – specificity]) were used to determine the optimal cut-off values for categorizing age (42.5 years) and body surface area (BSA, 17.5%). Categorical variables were compared using the chi-squared test or Fisher’s exact test, as appropriate. Patients who were lost to follow-up were excluded from the analyses of milestone survival rate. Overall survival was performed using the Kaplan-Meier method and log-rank test. Univariate Cox proportional hazards regression analyses were initially performed for each variable, with the results reported as hazard ratio (HR) and 95% confidence interval (CI). Variables with a p-value of < 0.1 in the univariate analyses were entered into a multivariate Cox proportional hazards regression model. Listwise deletion was used to manage missing data. All tests were two-sided, and p-values of <0.05 were considered statistically significant. The analyses were performed using IBM SPSS software (version 24.0; IBM Corp., Armonk, NY, USA) and GraphPad Prism software (version 7.0; GraphPad Inc., La Jolla, CA, USA).
RESULTS
Clinical features
The study evaluated data from 145 patients, including 70 male patients (48%) and 75 female patients (52%). Age was recorded for 144 of the 145 patients (99%) and the median age at onset was 37 years (range: 11–75 years), although 17 patients (12%, 17/144) were <18 years old. The optimal cut-off value for age was defined as 42.5 years, and 56 patients (39%, 56/144) were >42 years old. The underlying neoplasms were not identified because of missing histopathological data in 15 patients (10%, 15/145). The most common neoplasm was CD (81/145, 56%), which was followed by thymoma (28/145, 19%) (Fig. 1a), and patients who were ≤18 years old had an increased proportion of CD (13/17, 76%). Patients who were >42 years old had an increased proportion of thymoma (36%, 20/56) (Fig. 1b). Location data for the CD were recorded for 80 patients (99%, 80/81), and only 3 patients (4%, 3/80) had multicentric CD.
Fig. 1.
Neoplasms in patients with paraneoplastic autoimmune multiorgan syndrome (PAMS). (a) The numbers of each neoplasm type in the 145 patients and (b) their distributions according to age group. CD: Castleman disease; CLL: chronic lymphoma leukaemia; NHL: non-Hodgkin lymphoma; FDCS: follicular dendritic cell sarcoma.
Reliable data were available regarding cutaneous lesion morphology for 130 patients (90%, 130/145). The most common morphologies were lichen planus-like lesions (LP-like: 37%, 48/130) and pemphigus-like lesions (20%, 26/130). Severe mucosal lesions without skin lesions were observed for 20 patients (15%, 20/130). Detailed information regarding mucosal lesions were available for 100 patients (69%, 100/145), which frequently involved the buccal mucosa (92%, 92/100), lingual mucosa (91%, 91/100), and labial mucosa (81%, 81/100). Detailed information regarding the lesions is shown in Table I. Data regarding skin lesion extent were available for 66 patients (46%, 66/145), and the median lesion extent corresponded to 39% of the patient’s BSA. The optimal cut-off value for skin lesion extent was defined as 17.5% of the BSA, and 44 patients (67%, 44/66) had skin lesions that covered >17.5% of their BSA.
Table I.
Morphology of cutaneous and mucosal lesions in patients with paraneoplastic autoimmune multiorgan syndrome
| Clinical features | Total (n = 145) | CD (n = 81) | Thymoma (n = 28) | CLL/NHL (n = 14) | FDCS (n = 7) | Undefined (n = 15) |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Cutaneous lesions (n = 130 patients; missing: 15 patients) | ||||||
| No cutaneous lesions | 20/130 (15) | 6/74 (8) | 7/26 (27) | 3/12 (25) | 0/7 (0) | 4/11 (36) |
| Lichen planus-like | 48/130 (37) | 35/74 (47) | 8/26 (31) | 1/12 (8) | 3/7 (43) | 1/11 (9) |
| Pemphigus-like | 26/130 (20) | 13/74 (18) | 5/26 (19) | 4/12 (33) | 2/7 (29) | 2/11 (18) |
| Erythema multiforme-like | 17/130 (13) | 11/74 (15) | 2/26 (8) | 0/12 (0) | 1/7 (14) | 3/11 (27) |
| Bullous pemphigoid-like | 6/130 (5) | 3/74 (4) | 1/26 (4) | 1/12 (8) | 0/7 (0) | 1/11 (9) |
| Others | 13/130 (10) | 6/74 (8) | 3/26 (12) | 3/12 (25) | 1/7 (14) | 0/11 (0) |
| Mucosal lesions (n = 100 patients; missing: 45 patients) | ||||||
| Buccal | 92/100 (92) | 55/57 (97) | 17/21 (81) | 5/7 (71) | 4/4 (100) | 11/11 (100) |
| Lingual | 91/100 (91) | 55/57 (97) | 19/21 (91) | 6/7 (86) | 4/4 (100) | 7/11 (64) |
| Labial | 81/100 (81) | 47/57 (83) | 14/21 (67) | 7/7 (100) | 4/4 (100) | 9/11 (82) |
| Ocular | 60/100 (60) | 40/57 (70) | 10/21 (48) | 1/7 (14) | 3/4 (75) | 6/11 (55) |
| Genital | 55/100 (55) | 39/57 (68) | 9/21 (43) | 0/7 (0) | 2/2 (50) | 5/11 (46) |
| Palatal | 55/100 (55) | 36/57 (63) | 9/21 (43) | 2/7 (29) | 4/4 (100) | 4/11 (36) |
| Gingival | 48/100 (48) | 28/57 (49) | 8/21 (38) | 3/7 (43) | 4/4 (100) | 5/11 (46) |
| Pharyngeal | 30/100 (30) | 21/57 (37) | 4/21 (19) | 2/7 (29) | 2/2 (50) | 1/11 (9) |
CD: Castleman disease; CLL: chronic lymphoma leukaemia; NHL: non-Hodgkin lymphoma; FDCS: follicular dendritic cell sarcoma.
Castleman disease
In comparison with the other neoplasms (including thymoma, CLL, NHL, and follicular dendritic cell sarcoma), patients with CD were more likely to have various clinical features. Features that were significantly more common in CD cases included age of ≤42 years (83% vs. 29%, p < 0.001), presence of LP-like lesions (47% vs. 27%, p = 0.03), ocular involvement (70% vs. 44%, p = 0.01), genital involvement (68% vs. 34%, p = 0.002), buccal involvement (97% vs. 81%, p = 0.02), presence of BO (49% vs. 29%, p = 0.02), respiratory failure (69% vs. 27%, p = 0.03), and indirect immunofluorescence positivity on a rat bladder (82% vs. 55%, p = 0.002). The detailed results are shown in Table II.
Table II.
Features associated with Castleman disease (CD)
| Variablesa | CD (n = 81) | Without CD (n = 49)b | p-value |
|---|---|---|---|
| n/N (%) | n/N (%) | ||
| Age | < 0.001 | ||
| ≤42 years | 67/81 (83) | 14/48 (29) | |
| >42 years | 14/81 (17) | 34/48 (71) | |
| Skin lesions | 0.03 | ||
| Yes | 68/74 (92) | 35/45 (78) | |
| No | 6/74 (8) | 10/45 (22) | |
| Lichen planus-like lesions | 0.03 | ||
| Yes | 35/74 (47) | 12/45 (27) | |
| No | 39/74 (53) | 33/45 (73) | |
| Ocular lesions | 0.01 | ||
| Yes | 40/57 (70) | 14/32 (44) | |
| No | 17/57 (30) | 18/32 (56) | |
| Genital lesions | 0.002 | ||
| Yes | 39/57 (68) | 11/32 (34) | |
| No | 18/57 (32) | 21/32 (66) | |
| Buccal lesions | 0.02 | ||
| Yes | 55/57 (97) | 26/32 (81) | |
| No | 2/57 (4) | 6/32 (19) | |
| Bronchiolitis obliterans | 0.02 | ||
| Yes | 40/81 (49) | 14/ 49 (29) | |
| No | 41/81 (51) | 35/49 (71) | |
| Indirect immunofluorescence on rat bladder | 0.002 | ||
| Positive | 60/73 (82) | 23/42 (55) | |
| Negative | 13/73 (10) | 19/42 (45) | |
| Cause of death | 0.03 | ||
| Respiratory failurec | |||
| Yes | 11/16 (69) | 3/11 (27) | |
| No | 5/16 (31) | 8/11 (27) | |
Detailed information was not available for many variables, which led to inconsistency in the denominators.
Cases without CD including thymoma (n = 28), non-Hodgkin lymphoma (n = 7), chronic lymphocytic leukaemia (n = 7), and follicular dendritic cell sarcoma (n = 7), although undefined neoplasms were not included.
Two patients who died because of respiratory and infection, thus we included 14 cases of respiratory failure.
Survival analysis
The median follow-up time was 23 months (range: 1–204 months) and 43 of the 145 patients (30%) were lost to follow-up. The survival rates were 84% at 1 year, 65% at 3 years, and 54% at 4 years. The 5-year survival rates were 53% for CD, 75% for thymoma, 33% for CLL/NHL, and 40% for follicular dendritic cell sarcoma. Thirty-two patients died within 5 years after disease onset and their median survival time was 9.5 months (range: 1–54 months, 95% CI: 4.9–17.0 months). The most common causes of death were respiratory failure (38%,12/32) and infection (22%, 7/32) (Table III). Although the number of patients with multicentric CD was too small to analyse, it did not appear to influence their survival.
Table III.
Survival rates and causes of death among the patients with paraneoplastic autoimmune multiorgan syndrome
| Total (n = 145) | CD (n = 81) | Thymoma (n = 28) | NHL/CLL (n = 14) | FDCS (n = 7) | Undefined (n = 15) | |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Survival rates, n/N (%)a | ||||||
| 1 year | 98/116 (84) | 60/68 (88) | 20/22 (91) | 8/9 (89) | 3/6 (50) | 7/11 (64) |
| 2 years | 72/97 (74) | 42/55 (76) | 17/20 (85) | 5/7 (71) | 3/6 (50) | 5/9 (56) |
| 3 years | 51/79 (65) | 26/41 (63) | 14/17 (82) | 4/7 (57) | 3/6 (50) | 4/8 (50) |
| 4 years | 46/75 (61) | 22/37 (59) | 13/17 (76) | 4/7 (57) | 3/6 (50) | 4/8 (50) |
| 5 years | 37/69 (54) | 18/34 (53) | 12/16 (75) | 2/6 (33) | 2/5 (40) | 3/8 (38) |
| Causes of death, n/N (%) | ||||||
| Respiratory failure | 12/32 (38) | 10/16(63) | 1/4 (25) | 0/4 (0) | 1/3 (33) | 0/5 (0) |
| Infectious | 7/32 (22) | 4/16 (25) | 1/4 (25) | 0/4 (0) | 1/3 (33) | 1/5 (25) |
| Respiratory failure and infection | 2/32 (6) | 1/16 (6) | 1/4 (25) | 0/4 (0) | 0/3 (0) | 0/5 (0) |
| Neoplasm | 2/32 (6) | 0/16 (0) | 0/4 (0) | 2/4 (50) | 0/3 (0) | 0/5 (0) |
| Other/undefined causesb | 9/32 (28) | 1/16 (6) | 1/4 (25) | 2/4 (50) | 1/3 (33) | 4/5 (75) |
We excluded patients who were lost to follow-up from the survival rate calculations. The denominator was the sum of the patients who died or survived during the corresponding period and the numerator was the number of surviving patients.
One patient died because of cardiopulmonary arrest during surgery to treat the underlying tumour.
CD: Castleman disease; CLL: chronic lymphoma leukaemia; NHL: non-Hodgkin lymphoma; FDCS: follicular dendritic cell sarcoma.
Risk factors
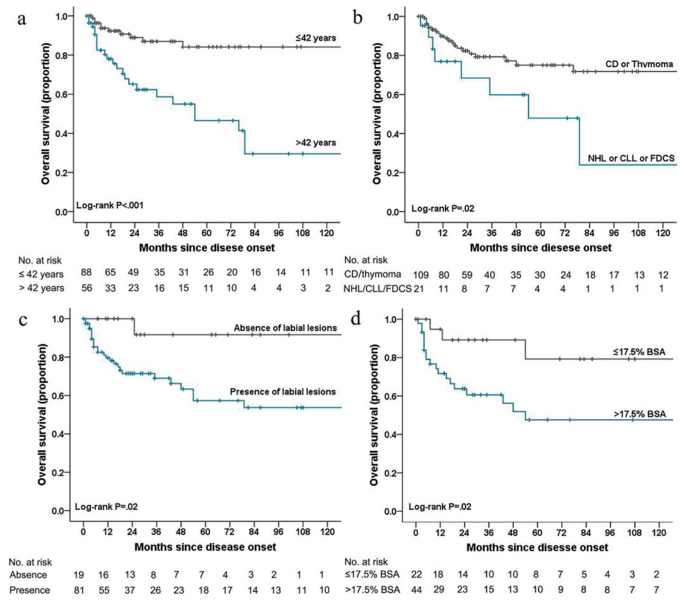
The Kaplan-Meier curves revealed significant differences in overall survival according to age (χ2 = 19.82, p< 0.001) (Fig. 2a), neoplasm type (χ2 = 5.15, p = 0.02) (Fig. 2b), labial involvement (χ2 = 5.53, p = 0.02) (Fig. 2c), and area of skin involvement (χ2 = 5.22, p = 0.02) (Fig. 2d). The results of the univariate Cox proportional hazards regression analyses are shown in Table SI1, and factors with p-values of < 0.1 were entered into the multivariate model. Mortality was only independently associated with older age (>42 years; HR: 9.81, 95% CI: 3.48–27.62) and larger skin lesions (> 17.5% of BSA; HR: 5.90, 95% CI: 1.32–26.42) (Table IV).
Fig. 2.
Kaplan-Meier curves for overall survival among patients with paraneoplastic autoimmune multiorgan syndrome. (a) Kaplan-Meier curves are shown for age grouping (>42 years vs. ≤42 years), (b) the different types of neoplasms, (c) the presence or absence of labial lesions, and (d) for lesions according to proportion of BSA (≤17.5% vs. >17.5%). CD: Castleman disease; CLL: chronic lymphoma leukaemia; NHL: non-Hodgkin lymphoma; FDCS: follicular dendritic cell sarcoma; BSA: body surface area.
Table IV.
Multivariate Cox proportional hazard regression models for death
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Age | ||
| ≤ 42 years | 1 [Reference] | NA |
| > 42 years | 9.81 (3.48–27.62) | < 0.001 |
| Neoplasm | ||
| Castleman disease or thymoma | 1 [Reference] | NA |
| NHL or CLL or FDCS | 0.78 (0.20–2.98) | 0.71 |
| Labial lesions | ||
| No | 1 [Reference] | NA |
| Yes | 3.65 (0.47–28.29) | 0.22 |
| Disease severity | ||
| ≤ 17.5% body surface area | 1 [Reference] | NA |
| > 17.5% body surface area | 5.90 (1.32–26.42) | 0.02 |
HR: hazard ratio; CI: confidence interval; NA: not applicable; CLL: chronic lymphoma leukaemia; NHL: non-Hodgkin lymphoma; FDCS: follicular dendritic cell sarcoma. Bold indicates a statistically significant difference.
DISCUSSION
Since the first report regarding PAMS, several retrospective studies and case series have revealed heterogeneous features and variable prognoses (8, 9, 11, 12, 19–21). Kaplan et al. reported that NHL was the most common neoplasm (38.6%), which was followed by CLL (18.4%) and CD (18.4%) (5), and two other reports have described similar findings (6, 19). However, a Russian study revealed a higher incidence of solid neoplasms (87.5%, 7/8), including rectal, renal, and bladder cancers (11). A study of French patients with PAMS also revealed that a large proportion had carcinomas (77.8%, 7/9), which included renal papillary carcinoma, renal adenocarcinoma, squamous cell carcinoma of the piriform sinus, breast cancer, prostatic carcinoma, and lung carcinoma (22). Another study identified PAMS in 3.8% of Chinese patients with CD (23). We observed that CD was the most common neoplasm in our patients, especially in children. Similar results have also been reported for Chinese Taiwanese patients (8) and Korean patients (12). Interestingly, Zhang et al. reported that the predisposing allele to PAMS was different between Chinese patients (HLACw*14) and Caucasian patients (HLADRB1*03) (10, 24). Mimouni et al. (7) have also reported that Hispanic children were more frequently affected by CD. Based on these findings, we speculated that the type of underlying neoplasm might be related to race and age. Moreover, when PAMS is diagnosed in Chinese patients, a thorough investigation is warranted to identify CD and thymoma.
We observed a large proportion of LP-like lesions (37%), and similar results have been reported among American, Korean, Chinese, and mixed-race patients (6, 7, 12, 25). In contrast, studies from France (26) and Japan (4) have indicated that greater proportions of lesions were pemphigus-like (58%) and erythema multiforme-like (56%). Our high incidence of LP-like lesions might be related to the high prevalence of CD, and this theory is supported by a study that examined American patients with CD and revealed a large proportion of LP-like lesions (68%) (27).
Several theories have been proposed regarding the pathogenesis of PAMS, which include epitope spreading, antigen mimicry, cytotoxicity, autoantibodies, and interleukin-6 (28). Our centre (25) have suggested that autoantibodies were directly generated by the cells involved in CD, and this phenomenon was also observed in vitro (29, 30). We observed that CD patients had a higher positivity rate for indirect immunofluorescence on a rat bladder, which might also support humoral immune mechanisms exerting significant pathogenic effects in patients with CD.
The prognosis of PAMS has improved slightly during recent years, relative to previously reported mortality rates of 64–90% (8, 11, 13, 14, 20, 26, 31), although the mortality rate remains high. Ouedraogo et al. (6) analysed 144 patients with PAMS and reported a 1-year survival rate of 62.4% and an overall mortality rate of 57%, with CLL/NHL being the most common neoplasm (75.7%). Our patients had slightly better prognoses, although the survival rates were lower for patients with CLL/NHL than for patients with CD and thymoma. The Kaplan-Meier analyses also confirmed that CD and thymoma were associated with better prognoses. Thus, the better survival rates in our study might be related to the relatively high incidences of CD and thymoma in our patients.
Patients with PAMS often die because of complications, rather than because of the disease itself (10, 16, 31). Variable incidences of respiratory failure or BO have been reported (19.2–92.4%) (4, 6, 10, 16, 25, 27, 32). The main cause of death in our population was respiratory failure (38%), which is consistent with previously reported findings (7, 12, 16, 27, 33). Nevertheless, BO was not significantly associated with a poor prognosis, which conflicts with previously reported findings (6, 7, 15, 27, 31). The pathological involvement of BO might be related to lung tissue containing plakins or desmoglein 3 autoantigens that could be attacked by autoimmune cells during some phases (1, 4), which could lead to sloughing of epithelial cells, evolving fibrosis, and obstruction of small airways that could lead to respiratory failure (31). In patients with CD and thymoma, antibodies can disappear after tumour resection (9, 29) and it is possible that progression to respiratory failure might cease after several critical weeks, during which time patients with BO should receive respiratory support. Lee et al. (34) systemically reviewed 68 patients from 26 articles and reported that patients with BO had an increased occurrence of CD (odds ratio: 3.86, 95% CI: 2.2–6.7). In our study, patients with CD also had higher rates of BO and respiratory failure, although the prognoses of CD were not worse than that of other neoplasms. In addition, respiratory failure was only slightly more common among patients with BO and CD (28%, 11/40), relative to patients with BO and other neoplasms (21%, 3/14). Thus, the high incidence of respiratory failure in CD cases might be related to the high prevalence of BO, rather than a poor outcome of BO in CD-associated PAMS cases. Nevertheless, these findings might have been influenced by the high rate of loss to follow-up, the short follow-up duration for some patients, and the low incidence of NHL/CLL.
Our Kaplan-Meier analysis revealed that CD and thymoma had better prognoses than NHL, CLL, and FDCS, which is consistent with most previous studies (9, 10, 12, 14, 16, 27, 35). Patients who experience complete neoplasm resection have a mortality rate of only 25% and the mucocutaneous lesions gradually improved over a period of 5–10 months (9, 29). These patients also tend to have better response to therapy after neoplasm resection (25). However, Nikolskaia et al. (27) have reported that 10 patients with CD (35.7%, 10/28) died in the postoperative period after complete tumour resection, usually from respiratory failure. These results did not conflict with the better long-term survival rate for patients with CD, although they do highlight the importance of perioperative care and therapy (36). The association was also not significant in our multivariate analysis, which is consistent with the results reported by Leger et al. (26). Nevertheless, the small sample of malignant neoplasms in our study might explain this discrepancy, and further investigations are needed.
Based on our receiver operating characteristic curve analysis, we propose that an age of > 42 years might be a risk factor for poor outcomes. Leger et al. (26) have reported that age was not significantly related to prognosis, although they noted that younger patients tended to experience better outcomes. Ouedraogo et al. (6) have suggested that bullous pemphigoid-like lesions and toxic epidermal necrolysis-like lesions were associated with the decreased survival of patients with PAMS. Leger et al. (26) have proposed that erythema multiforme-like lesions were a risk factor for a poor prognosis. We did not detect a significant relationship between cutaneous lesion morphology and mortality, although it is important to note that skin lesions covering >17.5% of the patient’s BSA might be a risk factor for poor outcomes. This could explain the poor outcomes in cases of erythema multiforme-like lesions and extensive mucocutaneous lesions that were reported by Leger et al. (26).
Limitations
This study has several limitations that should be considered. First, the retrospective analysis of patients from a single Chinese centre is prone to bias, and the findings may not generalize to other populations (e.g., because of genetic differences). Second, there was a relatively high rate of loss to follow-up, which might also be a source of bias. Third, incomplete data for many variables limited further explorations of clinical features and risk factors.
Conclusions
We found that CD was the most common neoplasm among Chinese patients with PAMS, and this neoplasm was associated with greater proportions of LP-like lesions, BO, and indirect immunofluorescence positivity on a rat bladder. Age and race might be associated with the type of underlying neoplasms in these cases. Better survival rates at various milestones were observed for CD and thymoma, although tumour type was not independently associated with mortality in the multi-variate analyses. Unexpectedly, we found that BO was not a significant risk factor for mortality, although poor survival might be associated with older age (>42 years) and larger skin lesions (>17.5% of BSA). Further studies are needed to help clarify the clinical features and risk factors in cases of PAMS.
ACKNOWLEDGEMENTS
Funding: The study was supported by the National Natural Science Foundation of China [81130030 and 81000694] and the Beijing Natural Science Foundation [7172214]. The funders did not take part in the study design, data collection, data analysis, manuscript preparation, or the decision to submit the manuscript.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med 1990; 323: 1729–1735. [DOI] [PubMed] [Google Scholar]
- 2.Amber KT, Valdebran M, Grando SA. Paraneoplastic autoimmune multiorgan syndrome (PAMS): Beyond the single phenotype of paraneoplastic pemphigus. Autoimmun Rev 2018; 17: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 3.Czernik A, Camilleri M, Pittelkow MR, Grando SA. Paraneoplastic autoimmune multiorgan syndrome 20 years after. Int J Dermatol 2011; 50: 905–914. [DOI] [PubMed] [Google Scholar]
- 4.Ohzono A, Sogame R, Li X, Teye K, Tsuchisaka A, Numata S, et al. Clinical and immunological findings in 104 cases of para-neoplastic pemphigus. Br J Dermatol 2015; 173: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan I, Hodak E, Ackerman L, Mimouni D, Anhalt GJ, Calderon S. Neoplasms associated with paraneoplastic pemphigus: a review with emphasis on non-hematologic malignancy and oral mucosal manifestations. Oral Oncol 2004; 40: 553–562. [DOI] [PubMed] [Google Scholar]
- 6.Ouedraogo E, Gottlieb J, de Masson A, Lepelletier C, Jachiet M, Salle de Chou C, et al. Risk factors for death and survival in paraneoplastic pemphigus associated with hematologic malignancies in adults. J Am Acad Dermatol 2019; 80: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 7.Mimouni D, Anhalt GJ, Lazarova Z, Aho S, Kazerounian S, Kouba DJ, et al. Paraneoplastic pemphigus in children and adolescents. Br J Dermatol 2002; 147: 725–732. [DOI] [PubMed] [Google Scholar]
- 8.Cho YT, Kao JT, Chen HJ, Wang LF, Chu CY. Paraneoplastic pemphigus: A retrospective case series in a referral center in northern Taiwan. Dermatologica Sinica 2014; 32: 1–6. [Google Scholar]
- 9.Zhang J, Qiao QL, Chen XX, Liu P, Qiu JX, Zhao H, et al. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: a single-center experience of 22 cases. J Cancer Res Clin Oncol 2011; 137: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol 2013; 54: 241–250. [DOI] [PubMed] [Google Scholar]
- 11.Allenova A, Lepekhova A, Olisova O, Teplyuk N, Kolkhir P. Paraneoplastic pemphigus in Russian patients: a single center case series. Int J Dermatol 2018; 57: e44–e46. [DOI] [PubMed] [Google Scholar]
- 12.Choi Y, Nam KH, Lee JB, Lee JY, Ihm CW, Lee SE, et al. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol 2012; 39: 973–981. [DOI] [PubMed] [Google Scholar]
- 13.Nousari HC, Deterding R, Wojtczack H, Aho S, Uitto J, Hashimoto T, et al. The mechanism of respiratory failure in para-neoplastic pemphigus. N Engl J Med 1999; 340: 1406–1410. [DOI] [PubMed] [Google Scholar]
- 14.Mascaró JM Jr, Ferrando J, Solé MT, Alsina M, Nousari HC, Anhalt GJ, et al. Paraneoplastic pemphigus: a case of long-term survival associated with systemic lupus erythematosus and polymyositis. Dermatology 1999; 199: 63–66. [DOI] [PubMed] [Google Scholar]
- 15.Kartan S, Shi VY, Clark AK, Chan LS. Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment. Am J Clin Dermatol 2017; 18: 105–126. [DOI] [PubMed] [Google Scholar]
- 16.Anhalt GJ. Paraneoplastic pemphigus. J Investig Dermatol Symp Proc 2004; 9: 29–33. [DOI] [PubMed] [Google Scholar]
- 17.Paolino G, Didona D, Magliulo G, Iannella G, Didona B, Mercuri SR, et al. Paraneoplastic pemphigus: insight into the autoimmune pathogenesis, clinical features and therapy. Int J Mol Sci 2017; 18: 2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helou J, Allbritton J, Anhalt GJ. Accuracy of indirect immunofluorescence testing in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol 1995; 32: 441–447. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Wang M, Nong L, Wang L, Cen X, Liu W, et al. Clinical and laboratory characterization of 114 cases of Castleman disease patients from a single centre: paraneoplastic pemphigus is an unfavourable prognostic factor. Br J Haematol 2015; 169: 834–842. [DOI] [PubMed] [Google Scholar]
- 20.Han SP, Fu LS, Chen LJ. Masked pemphigus among pediatric patients with Castleman’s disease. Int J Rheum Dis 2019; 22: 121–131. [DOI] [PubMed] [Google Scholar]
- 21.Wang R, Li J, Wang M, Hao H, Chen X, Li R, et al. Prevalence of myasthenia gravis and associated autoantibodies in para-neoplastic pemphigus and their correlations with symptoms and prognosis. Br J Dermatol 2015; 172: 968–975. [DOI] [PubMed] [Google Scholar]
- 22.Fournet M, Roblot P, Levillain P, Guillet G, Machet L, Misery L. Étude rétrospective d’une série de pemphigus paranéoplasiques. Ann Dermatol Venereol 2018; 145: 564–571. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Rao H, Xu X, Li Z, Liao B, Wu H, et al. Clinical characteristics and outcomes of Castleman disease: A multicenter study of 185 Chinese patients. Cancer Sci 2018; 109: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Bu DF, Li D, Zhu XJ. Genotyping of HLA-I and HLA-II alleles in Chinese patients with paraneoplastic pemphigus. Br J Dermatol 2008; 158: 587–591. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Zhu X, Li R, Tu P, Wang R, Zhang L, et al. Para-neoplastic pemphigus associated with Castleman tumor: a commonly reported subtype of paraneoplastic pemphigus in China. Arch Dermatol 2005; 141: 1285–1293. [DOI] [PubMed] [Google Scholar]
- 26.Leger S, Picard D, Ingen-Housz-Oro S, Arnault JP, Aubin F, Carsuzaa F, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol 2012; 148: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 27.Nikolskaia OV, Nousari CH, GJ. A. Paraneoplastic pemphigus in association with Castleman’s disease. Br J Dermatol 2003; 149: 1143–1151. [DOI] [PubMed] [Google Scholar]
- 28.Frew JW, Murrell DF. Paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome): clinical presentations and pathogenesis. Dermatol Clin 2011; 29: 419–425. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Bu D, Yang Y, Chen X, Zhu X. Castleman’s tumours and production of autoantibody in paraneoplastic pemphigus. Lancet 2004; 363: 525–531. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Bu DF, Huang YC, Zhu XJ. Role of autoantibodies against the linker subdomains of envoplakin and periplakin in the pathogenesis of paraneoplastic pemphigus. Chin Med J (Engl) 2009; 122: 486–495. [PubMed] [Google Scholar]
- 31.Nguyen VT, Ndoye A, Bassler KD, Shultz LD, Shields MC, Ruben BS, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome. Arch Dermatol 2001; 137: 193–206. [PubMed] [Google Scholar]
- 32.Zhen JF, Zhang L, Cao XX, Feng J, Zhou DB, Lin SB, et al. Clinical analysis of unicentric Castleman’s disease with para-neoplastic pemphigus and bronchiolitis obliterans. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2017; 39: 492–498. [DOI] [PubMed] [Google Scholar]
- 33.Raza HA, Nokes BT, Rosenthal AC, Mangold AR, Kelemen K, Jokerst CE, et al. Unicentric castleman disease complicated by paraneoplastic bronchiolitis obliterans and pemphigus. Respir Med Case Rep 2018; 25: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Bloom R, Amber KT. A systematic review of patients with mucocutaneous and respiratory complications in para-neoplastic autoimmune multiorgan syndrome: Castleman’s disease is the predominant malignancy. Lung 2015; 193: 593–596. [DOI] [PubMed] [Google Scholar]
- 35.Zimmermann J, Bahmer F, Rose C, Zillikens D, Schmidt E. Clinical and immunopathological spectrum of paraneoplastic pemphigus. J Dtsch Dermatol Ges 2010; 8: 598–606. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Zhang B. Paraneoplastic pemphigus. J Dermatol 2007; 34: 503–511. [DOI] [PubMed] [Google Scholar]