Abstract
Hidradenitis suppurativa is a common recurrent inflammatory skin disease. It is associated with multiple comorbidities whose temporal relationships are unknown due to long diagnostic delays. This study of otherwise healthy blood donors with self-reported symptoms of hidradenitis suppurativa investigated the temporal relationships of comorbidities. A prospective survival analysis on a nationwide cohort of blood donors, using registry data on drug prescription, was used to calculate the hazard ratio of time until first prescription of medical treatment for the following hidradenitis suppurativa-related comorbidities: heart disease, diabetes, depression, thyroid disease and pain. Hidradenitis suppurativa status was determined by a validated questionnaire, and the survival analysis was adjusted for age, sex, body mass index, smoking status and having an International Classification of Diseases Version 10 (ICD-10) diagnosis of hidradenitis suppurativa. Of the participants, 1,012 reported hidradenitis suppurativa symptoms, and these symptoms increased the hazard ratio of antidepressants (1.73, 95% confidence interval 1.17–2.56, p ≈ 0.006) and analgesics (hazard ratio 1.24, 95% confidence interval 1.11–1.39, p < 0.001). Pain and depression are the first comorbidities to present in hidradenitis suppurativa pathogenesis.
Key words: hidradenitis suppurativa, survival analysis, pre-scription, comorbidity, analgesia, antidepressants
Hidradenitis suppurativa (HS) is a chronic skin disease, which affects 1–2% of the population of western contries (1, 2). In HS, recurrent and inflamed painful nodules develop in intertriginous areas of the skin. These nodules progress to abscesses and/or tunnels (sinus tracts), which may suppurate with malodourous discharge (3, 4), causing non-specialists to often mistake early signs of HS for acne, folliculitis or staphylococcal abscesses (5).
SIGNIFICANCE
Hidradenitis suppurativa is a common recurrent inflammatory skin disease, which is underdiagnosed by physicians. Severe disease is associated with multiple comorbidities, but mild disease with fewer symptoms has been poorly investigated. This study of otherwise healthy blood donors with self-reported hidradenitis suppurativa symptoms and their registry data on drug prescription, found that people with symptoms of mild hidradenitis suppurativa had 1.73 times the risk of depression and 1.24 times the risk of needing pain medication compared with healthy controls. As people with even mild hidradenitis suppurativa are at risk of pain and depression, clinical practice should increase its focus on diagnosis of this disease.
HS is associated with multiple comorbidities: metabolic syndrome (MetS), disorders of the lipoprotein metabolism, diabetes, hyperthyroidism, obesity, smoking, depression, anxiety and Crohn’s disease (6–11). The mean worldwide diagnostic delay of HS is 7.2–10.2 years (12, 13), and studies indicate that up to 90% of people with HS symptoms remain undiagnosed (2, 14). Consequently, diagnosed cases are those with the most severe disease (15), as HS progresses during the long period of diagnostic delay, and knowledge of HS-related comorbidities therefore originates from cross-sectional studies of patients with severe and long-term disease. Currently, very little is known about the development of HS-related comorbidities during the long diagnostic delay between disease manifestation and diagnosis. We hypothesize that even mild cases of HS will have increased risk of developing comorbidities.
To examine this hypothesis, filled prescriptions were used as a surrogate for the diseases they treat, and multiple survival analyses were conducted amongst otherwise healthy blood donors screened for HS through a validated screening questionnaire (16). The endpoints were different groups of medication, as defined by which disease they were used to treat (based on Anatomical Therapeutic Chemical [ATC]) code). This study population experience a healthy donor effect (healthy worker bias) (17), which will have a mitigating influence on prescription frequency. The results are therefore representative of comorbidities that develop in individuals with a mild HS phenotype.
MATERIALS AND METHODS
Study design and population
In this study, the hazard ratio (HR) of filled prescriptions used to treat known HS-related comorbidities was compared between HS screen-positives and healthy controls. The study is part of the Danish Blood Donor Study (DBDS), which is a prospective cohort of healthy blood donors (2, 18–20). DBDS began in March 2010 as a Danish multicentre, public-health study and biobank (www.dbds.dk), and now includes more than 127,000 blood donors aged 18–67 years. Fewer than 5% of invited donors have declined to participate. At inclusion into the DBDS, participants receive a questionnaire pertaining to health-related items, such as age, body mass index (BMI), current smoking status, Major Depression Inventory (MDI), etc. (18).
Questionnaire and hidradenitis suppurativa phenotype
In July 2015, a validated HS screening questionnaire (16) was incorporated into the DBDS questionnaire (21). Screen-positives were those who reported a minimum of 2 HS lesions (boils) in an intertriginous skin area during the last 6 months. MDI is a validated Danish questionnaire for depression that covers both diagnostic criteria according to the International Classification of Diseases Version 10 (ICD-10) and the Statistical Manual of Mental Disorders IV classification (22). The current study classified an MDI result as indicative of depression if at least 2 major and 2 minor depressive symptoms were present (ICD-10 criteria).
Registries
Shortly after birth all Danish inhabitants are assigned a unique 10-digit Civil Personal Register (CPR) number, which makes easy record-linkage on a personal level possible (23). These registries were used to identify all drugs prescribed during the study period, as well as to identify all patients diagnosed with HS between 1995 and 2017. For further explanation see Appendix S11.
ATC codes and drug types investigated
All types of medicine are assigned an ATC code, which describes the active substance, and on which organ or system it acts in humans (24). In this study, ATC codes were used as a surrogate for the diseases they treat, and the following drugs relevant for HS-related comorbidities were analysed: heart medication, antidiabetics, antidepressants, thyroid drugs, and pain medication. Heart and pain medication were further subdivided. See supplemental material online for further explanation.
Statistical and survival analyses
Statistical analyses were performed using R-3.5.1 for Windows (The R Foundation for Statistical Computing, Vienna, Austria; GNU General Public license). For descriptive statistics (25) means and standard deviations (SD) are provided. Differences between groups were calculated, with t-tests or Mann–Whitney U tests, depending on normality. All participants with missing information on BMI or smoking status were included in the descriptive analysis, but excluded from the survival analyses.
Several Cox’s proportional hazards regression models (26) were calculated, with the time of filled prescriptions (see above) as primary outcomes. All analyses were adjusted for age, sex, BMI, and smoking status (yes/no); all factors known to be associated with HS (10). Sub-analyses with men and women as separate groups, and sub-analyses for those subgroups of heart and analgesics medication, with at least 5 participants in each group, were performed.
Participants entered into the analyses the date they completed a DBDS questionnaire with the HS screening questionnaire, and were censored at the time they attained the prescription examined for in the individual analysis, or by July 1, 2018 (latest date for current The Danish National Patient Registry (DNPR) edition). Finally, subgroup analyses incorporating all diagnosed HS cases from the DNPR were performed to distinguish between participants with HS symptoms and participants diagnosed with HS.
The Benjamini–Hochberg procedure for multiple testing, applying a false discovery rate of 0.05, was used to correct the survival analyses.
RESULTS
Participants and prescription demographics
A total of 53,173 blood donors completed the DBDS questionnaire between July 1, 2015 and July 2018. In this period, they accrued 284,882 prescriptions over the course of 83,178.7 person years. Descriptive statistics are shown in Table I.
Table I.
Descriptive statistics
| Hidradenitisa n = 1,012 (1.90%) | Healthy n = 52,161 (98.1%) | cp-value | |
|---|---|---|---|
| Days from DBDS inclusion to July 1, | 558 (283) | 572 (282) | 0.48 |
| 2018, mean (SD) | |||
| Sex, n (%) | |||
| Female | 618 (61.1) | 25,240 (48.4) | 1.7×10 –15 |
| Male | 394 (38,9) | 26,921 (51.6) | |
| Smoker, n (%) | |||
| Yes | 267 (26.4) | 6,768 (13.0) | |
| No | 741 (73.2) | 45,263 (86,8) | < 2.2×10 –16 |
| NA | 4 (0.4) | 130 (0.2) | |
| Body mass index, kg/m2, mean (SD) | 27.7 (5.5) | 25.7 (4.2) | < 2.2×10 –16 |
| NA, n (%) | 3 (0.3) | 153 (0.3) | |
| Age at enrolment, years, mean (SD) | 37.0 (11.8) | 40.4 (13.1) | 5.1×10 –16 |
| Diagnosed with HS according to the | < 2.2×10 –16 | ||
| DNPR, n (%) | 153 (15.1) | 130 (0.25) | |
| Demographics on prescriptions | |||
| Number who received prescription, n (%) | 761 (75.2) | 35,959 (68.9) | 2.3×10 –5 |
| Prescriptions (mean per person (SD))b | 6,848 (6.77) | 278,034 (5.33) | 1.32×10 –4 |
| Time to first prescription, mean (SD) | 203.5 (232.4) | 256.3 (257.9) | 6.8×10 –14 |
Based on whether they fulfil the questionnaire definition for HS.
Number of prescriptions from the time of inclusion to 1 July 2018.
Pearson’s X2, Wilcoxon signed-rank test (age) or Welch’s 2-sample t-test (body mass index).
HS: hidradenitis suppurativa; SD: standard deviation; NA: not available; DBDS: Danish Blood Donor Study; DNPR: The Danish National Patient Registry. Bold numbers indicate all statistically significant tests.
In short, 1.9% (1,012/53,173) were HS screen-positives. Compared with healthy controls, these were primarily female, younger, more overweight, more were active smokers, and a higher proportion had been diagnosed with HS (for all p < 2×10–15).
During the study period, 69.1% (36,720/53,173) of the participants received one or more prescriptions. 75.2% (761/1,012) of the screen-positives filled a prescription, and they were responsible for 2.4% (6,848/284,882) of all prescriptions. Amongst the controls, 68.9% (35,959/52,161) filled a prescription. Screen-positives were more likely to fill at least one prescription within the study period (p = 2.3×10–5), on average they filled a higher number of prescriptions 6.8 vs 5.3 (p = 1.3×10–4), and filled the first prescription earlier than the controls (p = 6.8×10–14).
Survival analyses on prescriptions
As shown in Table II, survival analysis for time to filled prescription revealed that HS screen-positives had a shorter time to filled prescription for antidepressant (HR 1.73, 95% confidence interval (CI) 1.17–2.56, p = 0.006) (Fig. 1a). Similar, but not significant, results were found when analyses were stratified by sex. Screen-positives also had a shorter time to filled prescription on all kinds of pain medication (HR 1.24, 95% CI 1.11–1.39, p < 0.001) (Fig. 1b), and for non-steroidal anti-inflammatory drugs (NSAID) specifically (HR 1.20, 95% CI 1.09–1.42, p = 0.001) (Fig. 1c). For all kinds of pain medication and for NSAID specifically positive results were found for females (all kinds of pain medication (HR 1.25, 95% CI 1.10–1.43, p < 0.001), and NSAID (HR 1.31, 95% CI 1.12– 1.53, p < 0.001)).
Table II.
Effect of hidradenitis suppurativa (HS) disease status on hazard ratio (HR) of receiving medicinal prescriptions
| HS events (event rate) | Healthy events (event rate) | HR (95% CI) | p-value | |
|---|---|---|---|---|
| All heart medication, n = 1,745 | 40 (0.04) | 1,705 (0.03) | 1.33 (0.97–1.82) | 0.08 |
| Men, n= 916 | 17 (0.04) | 899 (0.03) | 1.54 (0.95–2.49) | 0.08 |
| Women, n = 818 | 23 (0.04) | 795 (0.03) | 1.20 (0.79–1.82) | 0.40 |
| Subgroup analysis for all groups with n >5 | ||||
| Diuretics, n = 256 | 9 (0.009) | 247 (0.005) | 1.44 (0.74–2.83) | 0.29 |
| Men, n= 65 | One group <5 | One group <5 | ||
| Women, n = 188 | One group <5 | One group <5 | ||
| Beta-blocker, n = 321 | 10 (0.01) | 311 (0.006) | 1.51 (0.80–2.84) | 0.21 |
| Men, n= 134 | One group <5 | One group <5 | ||
| Women, n = 168 | One group <5 | One group <5 | ||
| Calcium antagonist, n = 302 | 7 (0.007) | 295 (0.006) | 1.39 (0.65–2.96) | 0.39 |
| Men, n= 182 | One group <5 | One group <5 | ||
| Women, n = 117 | One group <5 | One group <5 | ||
| Drugs affecting the RAS system, n = 588 17 (0.017) | 571 (0.011) | 1.82 (1.12–2.96) | 0.02 | |
| Men, n=354 | 7 (0.018) | 347 (0.013) | 1.55 (0.73–3.30) | 0.25 |
| Women, n = 228 | 10 (0.016) | 218 (0.009) | 2.05 (1.08–3.90) | 0.03 |
| Lipid modifying drugs, n = 672 | 11 (0.011) | 661 (0.013) | 1.15 (0.63–2.09) | 0.65 |
| Men, n= 439 | 6 (0.015) | 433 (0.016) | 1.17 (0.52–2.63) | 0.70 |
| Women, n = 231 | 5 (0.008) | 226 (0.009) | 1.13 (0.46–2.76) | 0.79 |
| Antidiabetics, n= 160 | 9 (0.009) | 151 (0.003) | 1.83 (0.92–3.64) | 0.084 |
| Men, n= 78 | One group <5 | One group <5 | ||
| Women, n = 81 | One group <5 | One group <5 | ||
| Antidepressants, n = 683 | 28 (0.028) | 655 (0.013) | 1.73 (1.17–2.56) | 0.006 a |
| Men, n= 269 | 8 (0.020) | 261 (0.010) | 1.66 (0.78–3.54) | 0.19 |
| Women, n = 406 | 20 (0.032) | 386 (0.015) | 1.74 (1.10–2.74) | 0.02 |
| Thyroid drugs, n = 606 | 16 (0.016) | 590 (0.011) | 1.27 (0.77–2.10) | 0.35 |
| Men, n = 92 | One group <5 | One group <5 | ||
| Women, n = 512 | One group <5 | One group <5 | ||
| All pain medication, n = 13,394 | 325 (0.32) | 13,069 (0.25) | 1.24 (1.11–1.39) | <0.001 a |
| Men, n= 5,948 | 102 (0.26) | 5,846 (0.22) | 1.22 (1.00–1.48) | 0.053 |
| Women, n = 7,369 | 223 (0.36) | 7,146 (0.28) | 1.25 (1.10–1.43) | < 0.001 a |
| Subgroup analysis for all groups with n ≥ 5 | ||||
| NSAID/anti-rheumatic, n = 9,665 | 240 (0.24) | 9,425 (0.18) | 1.20 (1.09–1.42) | 0.001 a |
| Men, n= 4,531 | 73 (0.19) | 4,458 (0.17) | 1.11 (0.88–1.41) | 0.37 |
| Women, n = 5,080 | 167 (0.27) | 4,913 (0.19) | 1.31 (1.12–1.53) | <0.001 a |
| Common antipyretics, n = 8,441 | 194 (0.19) | 8,247 (0.16) | 1.10 (0.95–1.27) | 0.19 |
| Men, n= 3,622 | 64 (0.16) | 3,558 (0.13) | 1.25 (0.98–1.60) | 0.08 |
| Women, n = 4,772 | 130 (0.21) | 4,642 (0.18) | 1.04 (0.87–1.24) | 0.67 |
| Opioid analgesic, n = 2,166 | 54 (0.053) | 2,112 (0.040) | 1.17 (0.89–1.54) | 0.27 |
| Men, n= 1,086 | 15 (0.038) | 1,071 (0.040) | 0.85 (0.50–1.44) | 0.54 |
| Women, n = 1,067 | 39 (0.063) | 1,028 (0.041) | 1.36 (0.98–1.89) | 0.06 |
| Migraine medication, n = 1,065 | 32 (0.032) | 1,033 (0.020) | 1.49 (1.04–2.12) | 0.03 |
| Men, n =214 | 6 (0.015) | 208 (0.008) | 1.97 (0.87–4.47) | 0.10 |
| Women, n = 841 | 26 (0.042) | 815 (0.032) | 1.41 (0.95–2.09) | 0.09 |
All survival analysis were corrected for body mass index (BMI), age, sex and smoking status. Analyses were not conducted for groups with fewer than 5 participants.
Significance after Benjamini-Hochberg correction for multiple testing. Inconsistencies for subgroup analysis are due the fact that people can have multiple prescriptions (e.g. heart medication), and thus they can be enrolled in multiple subgroup analyses.
NSAID: non-steroid anti-inflammatory drug; RAS: Renin-Angiotensin system. Bold numbers indicate all statistically significant tests.
Fig. 1.
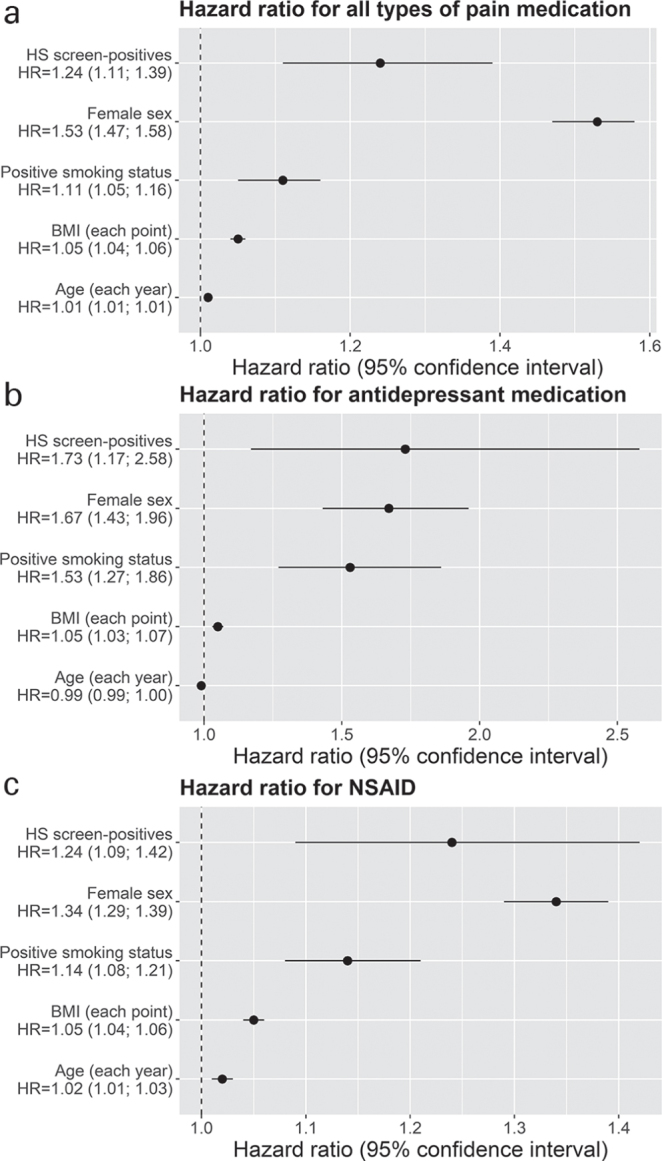
Forrest plots for the hazard ratios of prescription of (a) antidepressant medication, (b) all types of pain medication, (c) non-steroid anti-inflammatory drugs (NSAIDs). HS: hidradenitis suppurativa; BMI: body mass index: age.enroll: years of age at enrolment.
When correcting for multiple testing, HS symptoms did not affect the time to filled prescription for antidiabetics, heart or thyroid medication.
Incorporating diagnosed HS status into the survival analyses as a variable resulted in no noteworthy changes in the effect of HS symptoms on HR of filled prescriptions. However, being diagnosed with HS in the DNPR additionally increased the HR of time to filled prescription for all kinds of pain medication (HR 1.6, p = 0.004), common antipyretics (HR 1.8, p = 0.002) and for antidepressants (HR 1.6, p = 0.004).
DISCUSSION
This study found that HS screenpositives had a significantly higher HR of filling prescriptions on antidepressant medication (HR 1.73), pain medication (HR 1.24) and NSAIDs (HR 1.20). As the study population was not sampled from a dermatological department/specialized care unit, they include undiagnosed cases with, probably, a milder phenotype (15). The likelihood of inclusion of people with a milder phenotype in this study is further increased by the fact the study population comprises blood donors. Pain and depression are evidently the first HS-related comorbidities to manifest and, as HRs are not markedly changed after adjusting for diagnosed HS, these comorbidities manifest even in people with a mild HS phenotype/in early stages of the disease.
Pain is often reported as the most disabling factor in HS (27). Pain is experienced by 77.5% of patients during a period of 7 days (28). However, even the highest rated treatment modalities are only moderately effective for HS-related pain (29). A US registry study of 254,000 annual HS-related healthcare visits found that analgesics were prescribed in 25.1% of HS-related consultations (30). Surprisingly, in the US study, 82.1% of analgesics prescribed were for opioids (30), but only 16.2% (2,166/13,394) of analgesic prescriptions in the current study were for opiods. This discrepancy can be partly explained by 3 factors: Danish physicians follow the analgesic algorithm created by the WHO (31), which has the use of opioids as the last step; US patients with HS have been shown to have an increased risk of substance abuse (odds ratio 1.5), with opioids ranking second amongst substances abused (32); and, as the US study was a hospital-based registry study, the patients probably had a more severe HS phenotype (15) and thus were more likely to need opioids. Interestingly, a study investigating the type of pain experienced by patients with HS showed that they most often experience both nociceptive and neuropathic pain (33). NSAIDs are typically used to treat nociceptive pain, whereas neuropathic pain can be treated with antidepressants. The fact that participants with a HS diagnosis in the DNPR have a further increased HR of time to filled prescription of pain medication supports the statement that these participants have a more severe phenotype.
The association between depression and HS is well established (7, 11, 34), and recently a Danish nationwide registry study revealed that diagnosed depression most often temporally precedes the diagnosis of HS (35). The results of the current study are in agreement with this, but further indicate that depression is one of the first 2 HS-related comorbidities to manifest. A post hoc subgroup analysis further revealed that 27.1% (112/414) of participants whose MDI was indicative of depression at the time of enrollment later received an antidepressant prescription, with only 8.9% (4,664/52,580) of those who did not fulfil the MDI criteria later receiving an antidepressant prescription (p < 2.2×10–16). This indicates that antidepressants were prescribed for depression and not for pain.
The current study did not find a higher HR of antidiabetics, heart or thyroid medication despite the fact that HS is related to diabetes (6, 35), MetS (6, 35) and hyperthyroidism (9). However, only 15.1% of the HS screen-positives had also been diagnosed with HS. As the majority of screen-positives therefore have a milder HS phenotype than seen in the clinic (15), this indicates that these comorbidities develop either primarily in those with more severe disease or later in the disease course. This theory is supported by recent findings that demonstrate that, temporally, hypercholesterolaemia is diagnosed after a patient has been diagnosed with HS, whereas diabetes often precedes the HS diagnosis by as little as 6 months (35). While the results of the current study regarding hyperthyroidism do not match the association with HS found in the literature (9), too little is known about the temporal association of these diseases to draw conclusions.
Study strengths and limitations
An important strength of this study is that the DBDS enabled us to collect community-based, rather than hospital-based, data. Hereby, all participants with symptoms compatible with HS diagnostic criteria are identified, and sampling is not skewed towards severe ases from specialized clinics (15). The results of the current study therefore better depict the comorbidities of mild HS. Likewise, by using prescriptions as a surrogate for disease, the current study was able to capture those participants who are treated by their general physicians for mild phenotypes of disease. A further strength of the current study is that the DBDS collects information on BMI and smoking status, both of which are associated with HS (10), and with the development of comorbidities. Furthermore, the Danish National Prescription Registry has an excellent capture rate for all medicinal groups investigated in this study (84.6% for the lowest group) (36).
A limitation of the current study is that, while it is intuitive that the study population must have milder HS symptoms than those treated at specialized clinics (15), the current study cannot determine the actual HS severity of the participants.
Another limitation is the healthy donor effect inherent to this study, which increases the risk of type II errors, as it less likely to sample those who will later fill a prescription than it is to sample people who will not. Consequently, the HRs presented here are conservative. An additional limitation exists for depression, as only moderate or severe levels of depression are treated with antidepressants, and as antidepressants are also used for related conditions, such as anxiety and neuropathic pain. Some degree of uncertainty concerning prescription reasons therefore exist; however, the post hoc subgroup analysis using the MDI showed that antidepressant prescriptions were most often prescribed to those experiencing symptoms of depression. Likewise, mild phenotypes of hypertension and/or diabetes treated solely with exercise and dietary restrictions will not be revealed in the current study. It should still be pointed out, however, that as Danish general physicians are not obligated to report diagnoses to the DNPR (only hospitals are), identifying diseases through their ICD-10 code would also not have revealed these cases.
In addition, without access to chart information, weight loss or cessation of smoking is not known, and over a number of years changes will affect disease development, and thus prescription necessity. This therefore means there is a risk of creating residual confounding, which may, in turn, affect our results in a way that cannot be predicted. However, logic dictates that this residual confounding will be less than the confounding introduced by not controlling for BMI and smoking status.
Conclusion
Undiagnosed cases with a probably mild HS phenotype have an increased HR of filling a prescription for antidepressants (HR 1.73) analgesics (HR 1.24) and anti-inflammatory analgesics (HR 1.2), but not of antidiabetics, heart or thyroid medication. The fact that even mild HS increases the risk of depression is important, as depression is estimated to be the leading cause of global burden of disease by 2030 (37), and as pain has previously been shown to be the most disabling factor in HS (27). The current study shows that pain and depression are the first HS-related comorbidities to manifest, and that they appear even in milder cases of HS.
ACKNOWLEDGEMENTS
This paper received funding from the Leo Foundation (reference number LF 18002). The funding source was not involved in the planning, execution or reporting of this study.
Data in the DNPR & DNPRR is protected by the Danish Act on Processing of Personal Data and can only be accessed following application. Therefore, data sharing for this study is not possible.
This study has been approved by Danish Data Protection Agency, Copenhagen (2012-58-0004, RH-30-0444 / I-suite no.: 00922) and the Committee on Health Research Ethics in the Central Denmark Region (M-20090237).
Conflicts of interest. RKA, ICL, KSB, CE and OB Pedersen have no conflicts of interest to declare. GBEJ has received honoraria from AbbVie, Chemocentryx, Coloplast, Incyte, Inflarx, Novartis, Pierre Fabre and UCB for participation on advisory boards, and grants from Abbvie, Astra-Zeneca, Inflarx, Janssen-Cilag, Leo Pharma, Novartis, Regeneron and Sanofi, for participation as an investigator, and received speaker honoraria from AbbVie, Boehringer-Ingelheim, Galderma and MSD. He has also received unrestricted departmental grants from Abbvie, Leo Pharma and Novartis.
REFERENCES
- 1.Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, et al. Interventions for hidradenitis suppurativa: a Cochrane systematic review incorporating GRADE assessment of evidence quality. Br J Dermatol 2016; 174: 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Theut Riis P, Pedersen OB, Sigsgaard V, Erikstrup C, Paarup HM, Nielsen KR, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol 2019; 180: 774–781. [DOI] [PubMed] [Google Scholar]
- 3.Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012; 366: 158–164. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015; 231: 184–190. [DOI] [PubMed] [Google Scholar]
- 5.Clerc H, Tavernier E, Giraudeau B, Bourdais-Sallot A, Samimi M, Abdo I, et al. Understanding the long diagnostic delay for hidradenitis suppurativa: a national survey among French general practitioners. Eur J Dermatol 2019; 29: 97–99. [DOI] [PubMed] [Google Scholar]
- 6.Tzellos T, Zouboulis CC, Gulliver W, Cohen AD, Wolkenstein P, Jemec GB. Cardiovascular disease risk factors in patients with hidradenitis suppurativa: a systematic review and meta-analysis of observational studies. Br J Dermatol 2015; 173: 1142–1155. [DOI] [PubMed] [Google Scholar]
- 7.Shavit E, Dreiher J, Freud T, Halevy S, Vinker S, Cohen AD. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2015; 29: 371–376. [DOI] [PubMed] [Google Scholar]
- 8.Shalom G, Freud T, Ben Yakov G, Khoury R, Dreiher J, Vardy DA, et al. Hidradenitis suppurativa and inflammatory bowel disease: a cross-sectional study of 3,207 patients. J Invest Dermatol 2016; 136: 1716–1718. [DOI] [PubMed] [Google Scholar]
- 9.Miller IM, Vinding G, Sorensen HA, Rytgaard H, Mogensen UB, Ellervik C, et al. Thyroid function in hidradenitis suppurativa: a population-based cross-sectional study from Denmark. Clin Exp Dermatol 2018; 43: 899–905. [DOI] [PubMed] [Google Scholar]
- 10.Killasli H, Sartorius K, Emtestam L, Svensson Å. Hidradenitis suppurativa in Sweden: a registry-based cross-sectional study of 13,538 patients. Dermatology 2020; 236: 281–288. [DOI] [PubMed] [Google Scholar]
- 11.Tzur Bitan D, Berzin D, Cohen A. Hidradenitis suppurativa and bipolar disorders: a population-based study. Dermatology 2020; 236: 298–304. [DOI] [PubMed] [Google Scholar]
- 12.Garg A, Neuren E, Cha D, Kirby JS, Ingram JR, Jemec GBE, et al. Evaluating patients’ unmet needs in hidradenitis suppurativa: results from the Global Survey Of Impact and Healthcare Needs (VOICE) Project. J Am Acad Dermatol 2020; 82: 366–376. [DOI] [PubMed] [Google Scholar]
- 13.Saunte DM, Boer J, Stratigos A, Szepietowski JC, Hamzavi I, Kim KH, et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173: 1546–1549. [DOI] [PubMed] [Google Scholar]
- 14.Santos JV, Lisboa C, Lanna C, Costa-Pereira A, Freitas A. Is the prevalence of hidradenitis suppurativa being overestimated in Europe? Or is the disease underdiagnosed? Evidence from a nationwide study across Portuguese public hospitals. Int J Dermatol 2017; 56: 1491–1492. [DOI] [PubMed] [Google Scholar]
- 15.Kokolakis G, Wolk K, Schneider-Burrus S, Kalus S, Barbus S, Gomis-Kleindienst S, et al. Delayed diagnosis of hidradenitis suppurativa and its effect on patients and healthcare system. Dermatology 2020; 236: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinding GR, Miller IM, Zarchi K, Ibler KS, Ellervik C, Jemec GB. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol 2014; 170: 884–889. [DOI] [PubMed] [Google Scholar]
- 17.Ullum H, Rostgaard K, Kamper-Jorgensen M, Reilly M, Melbye M, Nyren O, et al. Blood donation and blood donor mortality after adjustment for a healthy donor effect. Transfusion 2015; 55: 2479–2485. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen OB, Erikstrup C, Kotze SR, Sorensen E, Petersen MS, Grau K, et al. The Danish Blood Donor Study: a large, prospective cohort and biobank for medical research. Vox Sang 2012; 102: 271. [DOI] [PubMed] [Google Scholar]
- 19.Kaspersen KA, Pedersen OB, Petersen MS, Hjalgrim H, Rostgaard K, Moller BK, et al. Obesity and risk of infection: results from the Danish Blood Donor Study. Epidemiology 2015; 26: 580–589. [DOI] [PubMed] [Google Scholar]
- 20.Hansen TF, Banasik K, Erikstrup C, Pedersen OB, Westergaard D, Chmura PJ, et al. DBDS Genomic Cohort, a prospective and comprehensive resource for integrative and temporal analysis of genetic, environmental and lifestyle factors affecting health of blood donors. BMJ Open 2019; 9: e028401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgdorf KS, Felsted N, Mikkelsen S, Nielsen MH, Thorner LW, Pedersen OB, et al. Digital questionnaire platform in the Danish Blood Donor Study. Comput Methods Programs Biomed 2016; 135: 101–104. [DOI] [PubMed] [Google Scholar]
- 22.Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord 2001; 66: 159–164. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organization WH . ATC structure and principles. [accessed May 18, 2020]. Available from: https://www.whocc.no/atc/structure_and_principles/.
- 25.Wickham H. tidyverse: Easily Install and Load the ‘Tidyverse’ 2017 [cited 2019 Sept 24]. Available from: https://CRAN.R-projects.org/package=tidyverse.
- 26.Therneau TM. Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 27.von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 2001; 144: 809–813. [DOI] [PubMed] [Google Scholar]
- 28.Matusiak L, Szczech J, Kaaz K, Lelonek E, Szepietowski JC. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol 2018; 98: 191–194. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez JM, Thompson AM, Borgstrom M, Orenstein LAV, Hsiao JL, Shi VY. Pain management modalities for hidradenitis suppurativa: a patient survey. J Dermatolog Treat 2020. Sep 20. [Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.McMillan K. Hidradenitis suppurativa: number of diagnosed patients, demographic characteristics, and treatment patterns in the United States. Am J Epidemiol 2014; 179: 1477–1483. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) . WHO guidelines for the management of cancer pain. [accessed May 18, 2020]. Available from: https://www.who.int/ncds/management/palliative-care/Infographic-cancer-pain-lowres.pdf.
- 32.Garg A, Papagermanos V, Midura M, Strunk A, Merson J. Opioid, alcohol, and cannabis misuse among patients with hidradenitis suppurativa: a population-based analysis in the United States. J Am Acad Dermatol 2018; 79: 495–500.e491. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen RM, Lindsø Andersen P, Sigsgaard V, Theut Riis P, Jemec GB. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol 2020; 182: 166–174. [DOI] [PubMed] [Google Scholar]
- 34.Machado MO, Stergiopoulos V, Maes M, Kurdyak PA, Lin PY, Wang LJ, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol 2019; 155: 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kjærsgaard Andersen R, Jørgensen IF, Reguant R, Jemec GBE, Brunak S. Disease trajectories for hidradenitis suppurativa in the Danish population. JAMA Dermatol 2020; 156: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt M, Pedersen L, Sørensen HT. Existing data sources for clinical epidemiology: the Danish National Database of Reimbursed Prescriptions. Clin Epidemiol 2012; 4: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lepine JP, Briley M. The increasing burden of depression. Neuropsychiatr Dis Treat 2011; 7: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


