Abstract
Bullous pemphigoid constitutes a rare dermatological immune-related adverse event of programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) inhibitors. Herein, we review all published cases of anti-PD-1/PD-L1 related bullous pemphigoid and discuss current knowledge on this condition. Clinical and diagnostic findings were found to resemble those of classic bullous pemphigoid. A delayed onset of bullous pemphigoid after commencement of immunotherapy as well as a frequent precendence of a refractory pruritic eruption prior to blister development was oberved, both posing diagnostic challenges. In addition to topical and systemic treatment, most patients required either discontinuation or permanent interruption of immunotherapy. Assessment of tumour outcome did not reveal improved survival in patients developing bullous pemphigoid during immunotherapy, as suggested for other types of skin toxicity, including vitiligo. Better understanding of the pathogenetic mechanism and prognostic implications of this increasingly-reported adverse event is essential in order to establish optimal diagnostic and therapeutic management of these patients.
Key words: autoimmune bullous disorder, bullous pemphigoid, PD-1 inhibitor, PD-L1 inhibitor, checkpoint inhibitor, immune-related adverse event
Bullous pemphigoid (BP) is a common autoimmune bullous skin disorder, characterized by pruritus and urticarial or eczematous eruption followed by bullae formation in most cases. However, up to 20% of patients present with morphologically atypical variants of BP, misleading even experienced dermatologists (1). The hemidesmosomal proteins BP180 (BP antigen 2, collagen XVII) and BP230 (BP antigen 1) have been identified as the main implicated autoantigens in BP pathogenesis (2). In addition, an increasing number of medications have been associated with the development of BP, providing new insights into the complex pathogenesis of this condition (3, 4). The term “drug-induced pemphigoid” has been introduced to describe cases of BP with clinical, histological and immunopathological features identical or similar to those of classic BP, occurring after systemic ingestion or topical use of certain drugs (5). To date, 89 drugs have been correlated with development of BP, while the strongest evidence was observed with gliptins, loop diuretics, penicillin or its derivatives and, lately, inhibitors of the programmed cell death protein-1 (PD-1) or its ligand, programmed death ligand-1 (PD-L1) (6).
SIGNIFICANCE
Bullous pemphigoid has gained increasing recognition among cutaneous adverse events of programmed cell death protein 1/programmed cell death ligand 1 inhibitors. Its clinical presentation may vary. It is mainly characterized by the development of atypical pruritic eruption prior to blistering. Therapeutic management of bullous pemphigoid may pose challenges and may negatively affect ongoing oncological treatment. Dermatological referral, correct grading and establishment of an appropriate therapeutic algorithm may limit the unnecessary modifications of immunotherapy. Further studies may elucidate the underlying immunogenetic mechanisms of this condition and ascertain efficacy and safety of existing therapeutic agents in terms of their potential to control symptoms without affecting the antitumour effect of immunotherapy.
PD-1 inhibitors (nivolumab, pembrolizumab, cemiplimab) and PD-L1 inhibitors (durvalumab, atezolizumab) have emerged as frontline treatment for a growing list of malignancies. Due to their unique mechanism of action they have shown sustained antitumour efficacy, but have also given rise to numerous immune-related adverse events (irAEs). Cutaneous irAEs may occur in up to 49% of patients treated with PD-1/PD-L1 inhibitors, and usually manifest as lichenoid reactions, eczema or vitiligo (7–9). Recently, BP has been described in a subset of patients under anti-PD-1/PD-L1 immunotherapy, while the exact pathogenetic mechanism and prognostic implications of this condition are yet to be clarified (10, 11). Since this uncommon skin toxicity may influence further therapeutic management and oncological outcome to a great extent, improved understanding and management are essential. The aim of this review is to summarize all reported cases of BP related to anti-PD-1/PD-L1 immunotherapy and critically discuss the current pathophysiological, clinical, diagnostic and therapeutic knowledge about this condition.
MATERIALS AND METHODS
Literature search
English medical literature was reviewed for patients who developed BP during or shortly after anti-PD-1 or anti-PD-L1 therapy, using the databases MEDLINE, Embase, and Scopus up to April 2020. The search involved all fields including title, abstract, keywords, and full text and identified all article types with the various terms, combinations and synonyms for “bullous pemphigoid”, “PD-1 inhibitor” and “PD-L1 inhibitor”. All references from the primary articles were manually reviewed to identify any additional relevant articles. Articles without accessible histopathological findings supporting the diagnosis of BP, as well as cases reporting anti-PD-1/PD-L1-related rare BP variants (e.g. mucous membrane pemphigoid, lichen planus pemphigoid) or blistering dermatoses other than BP were excluded. Also, patients undergoing combination systemic cancer therapy were excluded.
Data extraction and analysis
The following parameters were extracted: patients’ characteristics (age, sex), underlying malignancy, prior systemic cancer therapy, period of time when PD-1/PD-L1 inhibitor was commenced, treatment setting, morphology and distribution of skin lesions, interval between initiation of PD-1/PD-L1 inhibitor and onset of symptoms, findings of histopathology, direct immunofluorescence (DIF) and indirect immunofluorescence (IIF), serum autoantibodies, total IgE, serum eosinophils, BP treatment, BP outcome and tumour response. Descriptive statistical analysis of the collected data was performed using the statistical package for Windows SPSS (version 22.0 IBM Corp: Armonk, NY, USA). Quantitative variables were described as median values and range, while categorical variables were expressed as frequencies and percentages.
RESULTS
The literature search yielded 41 articles, published between 2015 and 2020, reporting a total of 58 cases of BP associated with anti-PD-1/PD-L1 immunotherapy (Table SI1). The median age of patients was 71 years (range 38–90 years), and there was a marked male predominance. The exact frequencies regarding patient characteristics, tumour types and systemic cancer therapy are summarized in Table I.
Table I.
Patient characteristics, tumour types and cancer treatment of bullous pemphigoid cases associated with antiprogrammed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) immunotherapy
| Variables | n/ntotal (%) |
|---|---|
| Patient characteristics | |
| Age, years, median (range) | 71 (38–90) |
| Sex | |
| Male | 43/58 (74.1) |
| Female | 15/58 (25.9) |
| Tumour types | |
| Cutaneous melanoma | 31/58 (53.5) |
| Male | 24/31 (77.4) |
| Female | 7/31 (22.6) |
| NSCLC | 17/58 (29.3) |
| Male | 11/17 (64.7) |
| Female | 6/17 (35.3) |
| Renal cell carcinoma | 4/58 (6.9) |
| Male | 4/4 (100) |
| Female | 0/4 (0) |
| Urothelial carcinoma | 2/58 (3.5) |
| Male | 1/2 (50) |
| Female | 1/2 (50) |
| Oesophageal adenocarcinoma | 1/58 (1.7) |
| Male | 1/1 (100) |
| Female | 0/1 (0) |
| Endometrial adenocarcinoma | 1/58 (1.7) |
| Male | 0/1 (0) |
| Female | 1/1 (100) |
| Cutaneous SCC | 1/58 (1.7) |
| Male | 1/1 (100) |
| Female | 0/1 (0) |
| Clear cell carcinoma | 1/58 (1.7) |
| Male | 1/1 (100) |
| Female | 0/1 (0) |
| Offending checkpoint inhibitor | |
| Anti-PD-1 | |
| Nivolumab | 28/58 (48.3) |
| Pembrolizumab | 25/58 (43.1) |
| Cemiplimab | 1/58 (1.7) |
| Anti-PD-L1 | 3/58 (5.2) |
| Atezolizumab | 1/58 (1.7) |
| Durvalumab | |
| Treatment setting | |
| Palliative | 55/58 (94.8) |
| Adjuvant | 2/58 (3.5) |
| Neo-adjuvant | 1/58 (1.7) |
| Prior systemic cancer treatment | |
| None | 35/58 (60.3) |
| Chemotherapy | 12/58 (20.7) |
| Ipilimumab | 10/58 (17.2) |
| Dabrafenib+trametinib | 1/58 (1.7) |
| Other | 4/58 (6.9) |
NSCLC: non-small cell lung cancer; SCC: squamous cell carcinoma.
With regards to clinical findings, 34.5% of patients (20/58) experienced non-specific cutaneous eruption, mostly pruritic eczematous dermatitis with papules and/or plaques, prior to the development of blisters. The median time between therapy initiation and development of initial skin lesions was 21 weeks (range 1–88 weeks). Pruritus was observed in 67.2% of patients (39/58), occurring with an median delay of 26 weeks (range 1–104 weeks) after the onset of therapy. Fifty-one patients (87.9%) developed blisters. The median time to the appearance of blisters was 27.5 weeks (range 3–104 weeks) from anti-PD-1/PD-L1 commencement. In 5 patients, blisters developed after discontinuation of the PD-1/PD-L1 inhibitor. If present, blisters were distributed in 1, 2 or more than 3 anatomical sites in 13.7% (7/51), 31.4% (16/51) and 54.9% (28/51) of patients, respectively. An exact grading of BP according to common terminology criteria for adverse events (CTCAE) was not provided. Mucosal involvement was found in 15.5% (9/58) of patients, occurring between 10 and 80 weeks (median 52 weeks) after anti-PD-1/PD-L1 initiation. The incidence of further anti-PD-1/PD-L1-related irAEs was low [12.1% (7/58)], with vitiligo (6.9%), arthralgias (3.4%) and hypothyroidism (1.7%) being the only reported findings.
Regarding histopathology, subepidermal bulla and eosinophils were present in 70.7% (41/58) and 81% (47/58) of patients, respectively, while the presence of neutrophils was rare [6.9% (4/58)]. In the majority of cases with reported DIF results [86.8% (46/53)], linear disposition of IgG and/or C3 was observed along the dermo–epidermal junction (DEJ). The IIF was performed in a total of 20 patients and revealed presence of IgG in 85% (17/20) of them. The presence of serum autoantibodies was evaluated in 42 patients, either by enzyme-linked immunosorbent assay (ELISA) or immunoblotting, revealing a clearly higher incidence of BP180 compared with BP230 autoantibodies (73.8% vs 7.1%). Six patients [14.3% (6/42)] also exhibited auto-antibodies against other epitopes, including desmoplakin 1/2, desmoglein 1/3, LAD-1 and 190kDa periplakin, and 7 patients [16.7% (7/42)] exhibited no autoantibodies. Serum eosinophils and total IgE were evaluated and found to be increased in 11 and 7 cases, respectively.
All patients received topical therapy with corticos-teroids. The great majority of them [91.3% (54/58)] required additional systemic therapy with corticosteroids (per os or intravenously) prevailing as first-line treatment [86.2% (50/58)]. Further treatment modalities included tetracyclines (mostly doxycycline) with or without niacinamide [35.5% (20/58)], rituximab [12.1% (7/58)], omalizumab [8.6% (5/58)], methotrexate [5.2% (3/58)], dapsone (3.4% (2/58)), plasma exchange [1.7% (1/58)] and intravenous immunoglobulin [1.7% (1/58)]. Antibiotics were administered with a median delay of 28 weeks (range 4–104 weeks) after immunotherapy start, while 90% (18/20) of patients who received antibiotics had extensive disease (2 or more anatomical sites). The incidence of treatment discontinuation due to BP was evaluated utilizing the group of patients developing BP during, and not after, immunotherapy (n = 53). In nearly three-quarters of them [73.6% (39/53)] BP made the interruption or discontinuation of immunotherapy necessary. In 17 [43.6% (17/39)] of them, BP was diagnosed and treated with a median delay of 20 weeks (range 3–63 weeks) after initial skin toxicity. Four patients were re-challenged with the same PD-1 inhibitor and 4 with another PD-1 inhibitor and/or ipilimumab after BP stabilization. Re-challenging with the same PD-1 inhibitor led to BP recurrence in 1 out of 4 patients, while administration of another checkpoint inhibitor led to BP recurrence in 2 out of 4 patients. Two patients experiencing recurrent disease were sufficiently managed with topical steroids only and one had to discontinue immunotherapy again.
Tumour outcome was documented for 39 patients. Complete response (CR) or partial response (PR) was achieved in 53.8% (21/39) of patients, 7.7% (3/39) of them experienced stable disease (SD) and 38.8% (15/39) had progressive disease (PD). The percentages of nivolumab-and pembrolizumab-associated BP were equal between these groups. All patients who developed vitiligo achieved CR or PR. In patients with CR or PR, the median time to blisters was 24 weeks (or 17 cycles), while in patients with SD or PD, the median time to blisters was 17 weeks (or 9 cycles). Among patients developing BP lesions in 1 body site, 42.8% (3/7) had CR or PR, 28.6% (2/7) had SD or PD, and 28.6% (2/7) had no documented tumour outcome. Among patients who developed BP lesions in 2 body sites, 31.3% (5/16) had CR or PR, 31.3% (5/16) had SD or PD, and 37.4% (6/16) had no documented tumour outcome. Among patients who developed BP lesions in 3 or more body sites, 35.8% (10/28) had CR or PR, 32.1% (9/28) had SD or PD, and 32.1% (9/28) had no documented tumour outcome. Evaluation of the 2 most common systemic BP treatments between responders (CR or PR) and non-responders (PD) identified nearly same incidence regarding corticosteroid administration [85.7% (18/21) vs 80% (12/15)], but notably higher incidence in administration of antibiotic therapy in non-responders [19% (4/21) vs 53.3% (8/15)].
DISCUSSION
Bullous dermatoses constitute rare irAEs of checkpoint inhibitors, with a frequency ranging from 1% to 8% among cutaneous irAEs according to retrospective studies (12–15). With regards to BP, it appears to be a class effect of anti-PD-1 immunotherapy, since it has not yet been observed in the context of therapy with cytotoxic T-lymphocyte-associated protein 4 inhibitors (15). Its frequency is rather underestimated, most likely due to publication bias and possible misdiagnosis of mild or atypical cases, leading to insufficient documentation in oncology reports. In line with previous observations, we found melanoma to be the most frequent underlying malignancy, a median patients’ age of 71 years and a significant male predominance, as opposed to classic BP, which favours female population (16). A higher incidence of melanoma has been reported for male populations between 70 and 74 years of age, a fact that may be partially related to the observed higher incidence of BP in male patients receiving anti-PD1/PD-L1 immunotherapy for melanoma (17). Patient age has not been correlated with the likelihood of developing irAEs (including skin toxicity) during immunotherapy, and thus may not predict the possibility of BP development (18, 19). Finally, induction of irAEs from PD-1/PD-L1 inhibitors has been found to be 2.2 times more likely in patients with melanoma compared with those with lung cancer (p = 0.013), a result that is also reflected in the current findings (19).
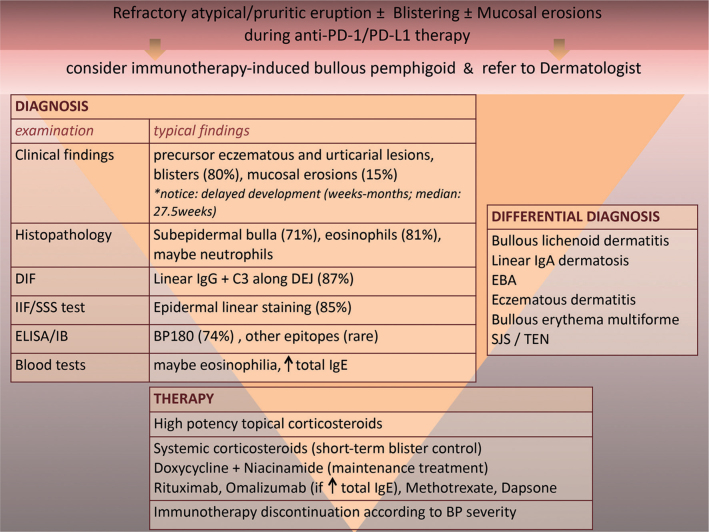
The clinical findings of drug-associated BP resembled those of classic disease, including a prodromal, pruritic, papular or eczematous eruption followed by moderate-to-severe skin blistering in 87.9% and mucosal involvement in 15.5% of cases. It is worth noting that BP occurred with a median latency of 27.5 weeks after the beginning of immunotherapy while some patients developed BP after completing the second year of treatment or shortly after its discontinuation. A delayed onset of cutaneous irAEs (≥ 3 months) is common and has also been described for other dermatological irAEs, including granulomatous reactions and erythema multiforme (20, 21). Diagnostic assessment should include histopathological examination and DIF, which typically reveal a subepidermal bulla together with eosinophilic infiltration and linear IgG and C3 along DEJ, respectively. Additional IIF and salt-split-skin testing may also show a positive epidermal linear staining. When available, implementation of ELISA or immunoblotting should be considered, enabling the detection of circulating autoantibodies that typically target BP180 or less common hemidesmosomal antigens (Table II). Given the broad spectrum and latency of clinical manifestations, it has been proposed that diagnostic criteria should focus on the aforementioned diagnostic findings, giving only a minor role to the clinical presentation (22). The clinical, diagnostic and therapeutic observations concerning anti-PD-1/PD-L1 related BP are summarized in Fig. 1.
Table II.
Detected autoantibodies in patients with anti-programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1)-associated bullous pemphigoid (BP)
| Autoantibody | Patients (n) |
|---|---|
| Anti-BP180, NC16A domain | 31 |
| Anti-BP230 | 3 |
| Anti-BP180, c-terminal domain | 2 |
| Anti-LAD-1 | 2 |
| Anti-Desmoglein 1/3 | 2 |
| Anti-Desmoplakin 1/2 | 1 |
| Anti-BMZ | 1 |
| Anti-190kDa periplakin | 1 |
| No autoantibodies | 7 |
| Not documented/assessed | 16 |
Fig. 1.
Clinical, diagnostic and therapeutic characteristics of anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1)-related bullous pemphigoid (BP) based on published cases. DIF: direct immunofluorescence; IIF: indirect immunofluorescence; SSS test: salt-split skin test; DEJ: dermo-epidermal junction; IB: immunoblotting; EBA: epidermolysis bullosa acquisita; SJS: Stevens-Johnson syndrome; TEN: toxic epidermal necrolysis; IgG: immunoglobulin G; IgE: immunoglobulin E; ELISA: enzyme-linked immunoassay.
It is known that, except for DEJ, BP180 is expressed on the surface of malignant melanocytic tumours, as well as on non-small cell lung cancer cells and urothelial epithelium (23). This finding initially raised suspicion about a possible paraneoplastic nature of BP in tumour patients, particularly in patients with renal cancer, laryngeal cancer, and lymphoid leukaemia (p < 0.05). (24). However, accumulating reports on BP resolving after immunotherapy cessation even in non-responders, rationalized the causal relationship between PD-1/PD-L1 inhibitors and BP. To date, the “same-antigen-theory” is gaining acceptance suggesting that targeting of BP180 on tumour cells activates a cross-reactive immunogenicity against the basal membrane of the skin and vice versa (11). Interestingly, a recent study analysed the expression of skin antigens in NSCLC cancer tissue, assuming that they could trigger the production of autoantibodies that might affect therapy response. Among them, higher levels of anti-BP180 IgG at baseline were found to be significantly associated with better therapy response (p = 0.01), prolonged overall survival (p = 0.04) and higher probability to develop skin irAEs (p = 0.04) during anti-PD-1/PD-L1 treatment, suggesting that anti-BP180 IgG may be considered as a biomarker (25). Likewise, the presence of collagen XVII-positive melanoma cells in a metastatic lymph node, but not in the primary tumour, has been demonstrated in one patient with melanoma and BP who responded completely to pembrolizumab (26). Future studies on immunohistochemical characteristics of both primary and metastatic tumour cells may help us better understand how BP180 overexpression is related to such outcomes.
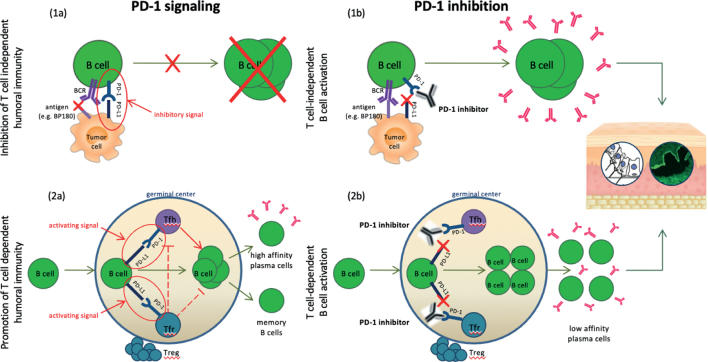
In addition to cross-reactivity, the presence of auto-antibodies against different epitopes found in anti-PD-/PD-L1 associated BP may be attributed to epitopespreading phenomena, as seen in dipeptidyl peptidase-4-inhibitor-associated BP (27). It has been proposed that autoantibodies may even develop secondary to a lichenoid process, a unique cutaneous adverse event of PD-1 inhibitors, which often manifests early after therapy initiation and may lead to exposure of the hemidesmosomal proteins to the immune system (28). It must be highlighted that commercial ELISA, the most frequently implemented diagnostic method, detects autoantibodies only against the NC16A portion of BP180. Hence, diagnostic assessment should include methods such as Western blotting, which enables the investigation of the major autoantibodies involved in the pathogenesis of anti-PD-1/PD-L1-associated BP. Irrespective of the implicating antigen, it is widely believed that autoimmune phenomena caused by PD-1/PD-L1 inhibitors involve both B- and T-cell dysregulation. This is responsible for the aberrant production of autoantibodies, which consecutively target BMZ-antigens, as illustrated in Fig. 2 (29–31).
Fig. 2.
Suggested pathogenesis of anti-programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) associated bullous pemphigoid (BP) involving both B- and T-cell dysregulation. (1a) PD-1 signalling in T-cell-independent humoral immunity: PD-1 signalling inhibits the binding of the tumour antigen (e.g. BP180) to its receptor on B cells (BCR), suppressing further B cell expansion. (1b) T cell independent B cell activation after PD-1 inhibition: PD-1 blockade enhances the BCR response and initiates B cell expansion with subsequent antibody production, responsible for a cross-reactive immunogenicity against the basal membrane of the skin. (2a) PD-1 signalling in the T-cell-dependent humoral immunity: PD-1 signalling acts as a promoter in T-cell-dependent humoral immunity for both follicular helper T (Tfh)-B cell and follicular regulatory T (Tfr)-B cell interaction within the germinal centres. Tfh cells mediate the selection and survival of B cells, enabling their differentiation into either high-affinity antibody-producing plasma cells or memory B cells. On the other hand, Tfr cells suppress both Tfh cells and B cells and thus control unwarranted germinal centre reactions. (2b) T-cell-dependent B cell activation after PD-1 inhibition: PD-1 inhibition negatively impacts both the selection of potentially mutated B cells by Tfh cells and the inhibitory function of Tfr, enhancing an aberrant production of low-affinity plasma cells, that can drive antibody-mediated autoimmune phenomena, including BP. Treg: regulatory T cell.
Combination of topical and systemic corticosteroids constitutes the mainstay of treatment for short-term blister control in anti-PD-1/PD-L1-associated BP. If maintenance therapy is required, tetracyclines may be prioritized, given their proven efficacy and favourable safety profile for long-term blister control in classic BP (32). Rituximab has been successfully used to treat corticosteroid refractory anti-PD-1/PD-L1-associated BP (Table SI1). It has been suggested that this agent should rather be preferred over T-cell-depleting immunosuppressants (azathioprine, mycophenolate mofetil), due to its targeted inhibition of pathogenic B cells (14). Likewise, omalizumab has also been proven to be efficient and should be considered in patients with elevated total IgE (33). As observed in the current study, most patients will probably require discontinuation of immunotherapy, a decision that depends mainly on the severity of BP (i.e. CTCAE Grade ≥ 2). Notably, 43.6% of patients who discontinued immunotherapy were diagnosed with BP with a median delay of 20 weeks after initial skin toxicity. Hence, atypical eruptions should warrant prompt dermatological referral, extensive diagnostic evaluation and appropriate treatment, to prevent further BP progression and prolonged interruption of immunotherapy. Given the small number of patients undergoing re-challenge, it is not possible to draw conclusions regarding potential recurrence after recommencing immunotherapy, and this should depend on blistering control and perceived benefits. Future studies may identify biomarkers to predict an increased risk of relapse, such as increased levels of total serum IgE, eosinophils, BP180 and CD19 in classic BP treated with rituximab (34).
Accumulating evidence associates certain dermatological irAEs, such as vitiligo, lichenoid and spongiotic dermatitis with a robust immune response (35, 36). However, data concerning BP are missing and, to date, only one retrospective study (n = 12) associates the development of BP with improved tumour response after anti-PD-1 therapy (13). Our results could not support this correlation. However, they should be interpreted with caution due to the retrospective analysis of the data, which cannot assess the effect of confounding factors (e.g. prolonged systemic immunosuppression, withdrawal of immunotherapy) on tumour outcome. In addition, tumour outcome was not found to be correlated with BP severity. Interestingly, a higher incidence of tetracycline administration was observed in non-responders than in responders. Recent studies have demonstrated a detrimental effect of antibiotics in overall and progression-free survival during cancer immunotherapy, especially if antibiotic exposure occurs shortly before therapy start (37–39). This effect has been principally attributed to alterations in gut microbiome accounting for a subsequent dampened immune response (40). However, data determining the critical window after initiation of immunotherapy are still lacking (37). Therefore, we could not definitely attribute the worse outcome to antibiotic exposure, considering the long latency of antibiotic administration after anti-PD-1/PD-L1 commencement (median delay of 28 weeks), the concomitant or sequential use of immunosuppressants, and the insufficient baseline data provided, especially regarding tumour burden and performance status. Nevertheless, further research is needed in order to investigate this important finding.
Limitations
This review is limited by the quality of the data available in the reports, which largely include case reports and small retrospective studies, given the rarity of this adverse event. Moreover, the heterogeneity of reporting, especially concerning duration of pharmacological immunosuppression, BP duration and grading, as well as tumour outcome, limits the ability to assess the severity of this condition and conduct an in-depth analysis with regards to its prognostic and therapeutic implications.
Conclusion
BP has gained increasing recognition among cutaneous irAEs of PD-1/PD-L1 inhibitors. Distinguishing BP from other cutaneous toxicities or even tumour-induced prurigo is not always straightforward. Refractory, atypical pruritic eruptions, even in the absence of blisters, should warrant dermatological referral and further investigation. Prompt diagnosis and management might decrease the necessity of treatment discontinuation, improving the oncological outcome. Further studies may clarify the underlying immunogenetic mechanisms of this condition, identify patients at risk of development of BP, and ascertain the efficacy and safety of existing therapeutic agents in terms of their potential to control symptoms without affecting the antitumour efficacy of immunotherapy.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Vasiliki Kanellou for English language editing.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: Etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013; 31: 391–399. [DOI] [PubMed] [Google Scholar]
- 2.Nishie W. Update on the pathogenesis of bullous pemphigoid: an autoantibody-mediated blistering disease targeting collagen XVII. J Dermatol Sci 2014; 73: 179–186. [DOI] [PubMed] [Google Scholar]
- 3.Stavropoulos PG, Soura E, Antoniou C. Drug-induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol 2014; 28: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 4.Patsatsi A, Vyzantiadis TA, Chrysomallis F, Devliotou-Panagiotidou D, Sotiriadis D. Medication history of a series of patients with bullous pemphigoid from northern Greece – observations and discussion. Int J Dermatol 2009; 48: 132–135. [DOI] [PubMed] [Google Scholar]
- 5.Vassileva S. Drug-induced pemphigoid: bullous and cicatricial. Clin Dermatol 1998; 16: 379–387. [DOI] [PubMed] [Google Scholar]
- 6.Verheyden M, Bilgic A, Murrell D. A systematic review of drug-induced pemphigoid. Acta Derm Venereol 2020; 100: adv00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibaud V. Dermatologic reactions to immune checkpoint inhibitors. Am J Clin Dermatol 2018; 19: 345–361. [DOI] [PubMed] [Google Scholar]
- 8.Belum VR, Benhuri B, Postow MA, Hellmann MD, Lesokhin AM, Segal NH, et al. Characterization and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer 2016; 60: 12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol 2016; 74: 455–461. [DOI] [PubMed] [Google Scholar]
- 10.Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol 2018; 57: 664–669. [DOI] [PubMed] [Google Scholar]
- 11.Zhao CY, Hwang SJE, Consuegra G, Chou S, Fernandez-Peñas P. Anti-programmed cell death-1 therapy-associated bullous disorders: a systematic review of the literature. Melanoma Res 2018; 28: 491–501. [DOI] [PubMed] [Google Scholar]
- 12.Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy. J Am Acad Dermatol 2019; 80: 990–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson CA, Singer S, Chen T, Puleo AE, Lian CG, Wei EX, et al. Bullous pemphigoid after anti-PD-1 therapy: a retrospective case-control study evaluating impact on tumor response and survival outcomes. J Am Acad Dermatol 2020; Jan 10 [Epub ahead of print]. [Google Scholar]
- 14.Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol 2019; 37: 2746–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol 2017; 44: 158–176. [DOI] [PubMed] [Google Scholar]
- 16.Kridin K, Ludwig RJ. The Growing incidence of bullous pemphigoid: overview and potential explanations. Front Med (Lausanne) 2018; 5: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan TA, Lu Y, Edwards K, Jakowatz J, Meyskens FL, Liu-Smith F. Race-, age-, and anatomic site-specific gender differences in cutaneous melanoma suggest differential mechanisms of early-and late-onset melanoma. Int J Environ Res Public Health 2019; 16: 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ksienski D, Wai ES, Croteau NS, Freeman AT, Chan A, Fiorino L, et al. Association of age with differences in immune related adverse events and survival of patients with advanced nonsmall cell lung cancer receiving pembrolizumab or nivolumab. J Geriatr Oncol 2020; 11: 807–813. [DOI] [PubMed] [Google Scholar]
- 19.Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LL, Patel G, Chiesa-Fuxench ZC, McGettigan S, Schuchter L, Mitchell TC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol 2018; 154: 1057–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornejo CM, Haun P, English J 3rd, Rosenbach M. Immune checkpoint inhibitors and the development of granulomatous reactions. J Am Acad Dermatol 2019; 81: 1165–1175. [DOI] [PubMed] [Google Scholar]
- 22.Cozzani E, Gasparini G, Burlando M, Drago F, Parodi A. Atypical presentations of bullous pemphigoid: clinical and immunopathological aspects. Autoimmun Rev 2015; 14: 438–445. [DOI] [PubMed] [Google Scholar]
- 23.Fairley JA, Heintz PW, Neuburg M, Diaz LA, Giudice GJ. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995; 133: 385–392. [DOI] [PubMed] [Google Scholar]
- 24.Ong E, Goldacre R, Hoang U, Sinclair R, Goldacre M. Associations between bullous pemphigoid and primary malignant cancers: an English national record linkage study, 1999 – 2011. Arch Dermatol Res 2014; 306: 75–80. [DOI] [PubMed] [Google Scholar]
- 25.Ali OH, Bomze D, Ring SS, Berner F, Fässler M, Diem S, et al. BP180-specific IgG is associated with skin adverse events, therapy response, and overall survival in non-small cell lung cancer patients treated with checkpoint inhibitors. J Am Acad Dermatol 2020; 82: 854–861. [DOI] [PubMed] [Google Scholar]
- 26.Wada N, Uchi H, Furue M. Bullous pemphigoid induced by pembrolizumab in a patient with advanced melanoma expressing collagen XVII. J Dermatol 2017; 44: e240–e241. [DOI] [PubMed] [Google Scholar]
- 27.García-Díez I, España A, Iranzo P. Epitope-spreading phenomena in dipeptidyl peptidase-4 inhibitor-associated bullous pemphigoid. Br J Dermatol 2019; 180: 1267–1268. [DOI] [PubMed] [Google Scholar]
- 28.Morris LM, Lewis HA, Cornelius LA, Chen DY, Rosman IS. Neutrophil-predominant bullous pemphigoid induced by checkpoint inhibitors: a case series. J Cutan Pathol 2020; 47: 742–746. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, Yao Z, Zhou X, Zhang W, Zhang X, Zhang F. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol 2020; 213: 108377. [DOI] [PubMed] [Google Scholar]
- 30.Xia Y, Jeffrey Medeiros LJ, Young KH. Signaling pathway and dysregulation of PD1 and its ligands in lymphoid malignancies. Biochim Biophys Acta 2016; 1865: 58–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Liu Z, Dang E, Jin L, He Z, Yang L, et al. Follicular helper T cells (Tfh) and IL-21 involvement in the pathogenesis of bullous pemphigoid. PLoS One 2013; 8: e68145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams HC, Wojnarowska F, Kirtschig G, Mason J, Godec TR, Schmidt E, et al. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet 2017; 389: 1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kremer N, Snast I, Cohen ES, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol 2019; 20: 209–216. [DOI] [PubMed] [Google Scholar]
- 34.Polansky M, Eisenstadt R, DeGrazia T, Zhao X, Liu Y, Feldman R. Rituximab therapy in patients with bullous pemphigoid: a retrospective study of 20 patients. J Am Acad Dermatol 2019; 81: 179–186. [DOI] [PubMed] [Google Scholar]
- 35.Hua C, Boussemart L, Mateus C, Routier E, Boutros C, Cazenave H, et al. Association of vitiligo with tumor response in patients with metastatic melanoma treated with pembrolizumab. JAMA Dermatol 2016; 152: 45–51. [DOI] [PubMed] [Google Scholar]
- 36.Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: a retrospective case-control study. J Am Acad Dermatol 2019; 79: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson BE, Routy B, Nagrial A, Chin VT. The effect of antibiotics on clinical outcomes in immune - checkpoint blockade : a systematic review and meta-analysis of observational studies. Cancer Immunol Immunother 2020; 69: 343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim H, Lee JE, Hong SH, Lee MA, Kang JH, Kim IH. The effect of antibiotics on the clinical outcomes of patients with solid cancers undergoing immune checkpoint inhibitor treatment: a retrospective study. BMC Cancer 2019; 19: 1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinato DJ, Gramenitskaya D, Altmann DM, Boyton RJ, Mullish BH, Marchesi JR, et al. Antibiotic therapy and outcome from immune-checkpoint inhibitors. J Immunother Cancer 2019; 7: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]