Abstract
Hidradenitis suppurativa is a chronic, inflammatory skin disorder that affects the pilosebaceous unit of the intertriginous body areas. Pain is one of the most important problems in patients with hidradenitis suppurativa. The aim of this study, which included 1,795 patients, was to evaluate the prevalence and characteristics of pain. The intensity of pain was assessed with a numerical rating scale. In addition, pain intensity was correlated with various clinical features. Pain was reported by 83.6% of subjects. The majority of patients (77.6%) experienced mild pain; women and smokers tended to experience more intense pain. Pain intensity was greater in patients with multiple affected skin areas and correlated positively with the number of those affected areas (r = 0.151, p < 0.001). There was no difference in pain intensity between affected locations. The worst pain was observed in the patients with the most severe disease and it would weaken significantly along with the severity of hidradenitis suppurativa (assessed using the Hurley staging system and the International Hidradenitis Suppurativa Severity Score System).
Key words: pain, hidradenitis suppurativa, acne inversa, numerical rating scale
Hidradenitis suppurativa (HS), or acne inversa, is a chronic, multifactorial recurrent, debilitating inflammatory skin disorder that affects the pilosebaceous unit (1). The reported prevalence of HS varies widely; from 0.03% to 0.09% (2, 3) in Germany and 4% in young women (4). The pathogenesis of HS is not fully understood; possible mechanisms include follicular plugging, inflammation, genetic predisposition and bacterial propagation (1). The disease usually begins in early adulthood, commonly after puberty, and predominantly affects the skin folds of axillary, inguinal, gluteal and perianal areas of the body. HS is characterized by the formation of multiple inflamed nodules, abscesses, draining sinus tracts, fistulas, and scars (5). Due to the extent of the disease, foul smell, purulent discharge and pain, HS has a negative influence on patients’ health-related quality of life and sexual activity (6, 7). Moreover, HS correlates with a higher incidence of depression and increased level of stigmatization (8, 9).
SIGNIFICANCE
This cross-sectional study assessed the prevalence and characteristics of hidradenitis suppurativa-related pain in 1,795 patients with confirmed hidradenitis suppurativa. Most patients (83.6%) reported pain in the last 24 h. In most subjects the pain was mild; female sex and smoking were found to correlate with higher intensity of pain. Moreover, patients with multiple affected areas of skin experienced more pain, and their pain intensity correlated with the number of affected areas. In addition, the intensity of pain was found to depend on the severity of hidradenitis suppurativa, and the pain had a negative influence on patients’ quality of life. The results of this large study provide an important insight into hidradenitis suppurativarelated pain, a topic that requires further research to be completely understood.
Pain is one of the most important burdening symptoms of HS. It is usually linked to inflammatory lesions located deep within and under the skin. The sensation varies among patients and is described as hot, pressing, cutting, sharp, gnawing, sore, or aching (1). HS-associated pain has been reported to have a negative influence on patients’ quality of life (10, 11). However, the available literature on pain in HS in a representative population is limited (10–14). The aim of this study was to evaluate HS-related pain in a large sample of patients with HS in order to broaden our knowledge on this topic and to understand the relevance of pain management in the holistic approach to patients with HS.
MATERIALS AND METHODS
The study included all patients who were selected for LAight® therapy (a combination of intense pulsed light with radio-frequency) (14, 15) in multiple dermatology outpatients’ centres in Germany between April 2017 and February 2020. Patients were only included if they gave informed written consent to the collection, storage and scientific evaluation of their data in an electronic database. All included patients were examined by a doctor specifically trained to determine the Hurley stage in patients with HS. The overall contraindications for LAight therapy included pregnancy, epilepsy, and implanted electrical devices. Moreover, the treatment was initiated in patients with extreme photosensitivity, implants within 10 cm of the lesioned skin, tattoos, piercings, skin neoplasms, infection, or previous treatment with botulinum toxin in the treated HS area. In addition, baseline data, including sex, age, body mass index (BMI), Dermatology Life Quality Index (DLQI) scores, smoking habits and affected body areas, were collected.
In line with guidelines for human studies and the World Medical Association Declaration of Helsinki, the anonymized data was then transferred for evaluation.
Demographic characteristics
A total of 1,795 patients (1,152 females and 643 males; mean ± standard deviation (SD) age 40.0 ± 11.8 years) with confirmed diagnosis of HS were included in the study. The majority of subjects were active smokers (55.6%), and 10.5% smoked more than 25 cigarettes a day (heavy smokers). According to the estimated mean ± SD BMI of participants (28.1 ± 6.2 kg/m2), the population was categorized as overweight (Table I).
Table I.
Patients’ characteristics
| Characteristics | All patients (n=1,795) |
|---|---|
| Sex, n (%) | |
| Men | 643 (35.8) |
| Women | 1,152 (64.2) |
| Age, years, mean ± SD | 40.03 ± 11.81 |
| Body mass index, kg/m2, mean ± SD | 28.08 ± 6.17 |
| Smoking, n (%) | |
| Smokers | 998 (55.6) |
| Non-smokers | 797 (44.4) |
| Cigarettes/day), mean ± SD | 8.07 ± 9.22 |
| Hurley stage, n (%) | |
| I | 425 (23.7) |
| II | 1,061 (59.1) |
| III | 309 (17.2) |
| IHS4, n, mean ± SD (range 0-281 points) | 634, 14.9 ± 26 |
| Mild, n (%) | 180 (28.4) |
| Moderate, n (%) | 222 (35) |
| Severe, n (%) | 232 (36.6) |
| WP-NRS (last 24 h) (points), mean ± SD (range 0-10 points) | 3.89 ± 2.89 |
| Mild, n (%) | 1,393 (77.6) |
| Moderate, n (%) | 285 (15.9) |
| Severe, n (%) | 117 (6.5) |
| Dermatology Life Quality Index (points), mean ± SD | 13.2 ± 8.1 |
IHS4: International Hidradenitis Suppurativa Severity Score System; WP-NRS: Worst Pain Numeric Rating Scale; SD: standard deviation.
Hidradenitis suppurativa assessments
Disease severity stage was evaluated in all patients during dermatological examination, using the Hurley staging system. Patients were then divided into groups, from Hurley stage I to III (15). In addition, after establishment of the International Hidradenitis Suppurativa Severity Score System (IHS4) as a validated severity assessment tool (17), the subjects (n = 634) were assessed accordingly and were subsequently divided into 3 groups (mild, moderate, and severe disease). The most intense pain that occurred in the last 24 h was assessed with a numeral rating scale (WP-NRS), where 0 = no pain and 10 = worst imaginable pain. Cut-off points were employed for mild (≤ 6 points), moderate (> 6 and ≤ 8 points) and severe pain (> 8 points) (16).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics v. 26 (SPSS Inc., Chicago, IL, USA) software. All data were assessed for parametric or non-parametric distribution and minimum, maximum, mean and SD numbers were calculated. Analysed quantitative variables were evaluated using Mann–Whitney U test, Spearman’s and Pearson’s correlations, while for qualitative data test χ2 was used. Changes between patients with a Hurley score of I–III and IHS4 mild, moderate and severe were assessed by Kruskal–Wallis 1-way analysis of variance on ranks. A 2-sided p-value ≤ 0.05 was considered statistically significant.
RESULTS
A total of 1,500 patients with HS (83.6%) reported pain in the last 24 h. Mean WP-NRS for the whole HS population was 3.9 ± 2.9 points. There was a statistically significant difference (p < 0.001) between mean pain intensity in women and men (4.1 ± 2.9 and 3.5 ± 2.8 points, respectively). Pain was classified as mild in 77.6% of patients, moderate in 15.9%, and severe in 6.5% (Fig. 1). In addition, pain was of higher intensity (p < 0.02) in smokers (4.04 ± 2.9 points) than in non-smoking patients (3.7 ± 2.8 points). There was no correlation between pain intensity and age, BMI and number of cigarettes smoked per day (detailed data not shown).
Fig. 1.
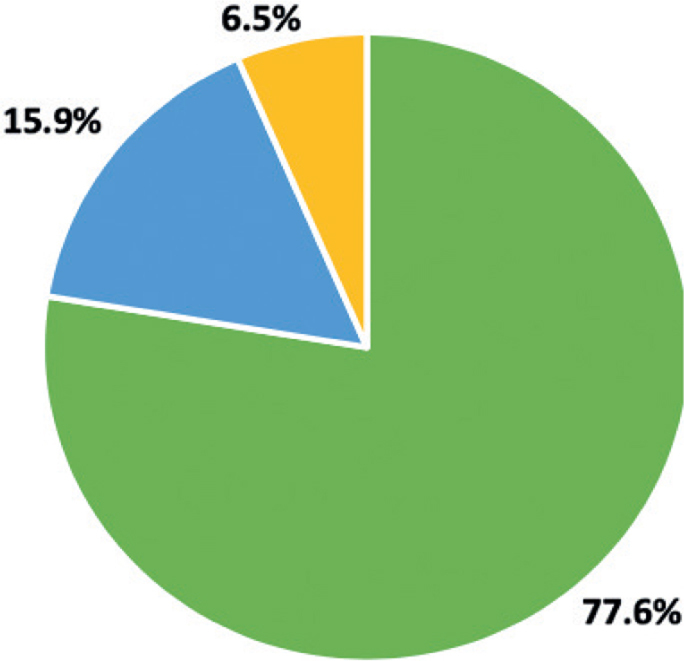
Hidradenitis suppurativa-related pain intensity among patients, based on the numerical rating scale cut-offs.
The severity of HS in the majority of patients (59.1%) was assessed as Hurley stage II, in 23.7% as Hurley stage I, and in 17.2% as Hurley stage III. As for IHS4, 36.6% of patients presented with severe HS, followed by moderate and mild HS (35.0% and 28.4%, respectively). A positive, statistically significant, but weak, correlation (r = 0.277, p < 0.001) was found between WP-NRS and IHS4 scores. Moreover, significant differences were observed in pain intensity between HS severity groups (for both Hurley and IHS4) (Fig. 2a, b). The most severe pain was observed in the Hurley stage III group (4.9 ± 2.8 points), and pain intensity reduced significantly with HS severity, being 3.9 ± 2.9 points for Hurley stage II group and 2.9 ± 2.7 points for Hurley stage I patients (Fig. 1a). Similar findings were noted for the IHS4 scoring system. Patients with severe HS reported pain of highest intensity (4.4 ± 2.8 points), followed by those with moderate HS (3.7 ± 2.9 points) and mild HS (2.6 ± 2.5 points) (Fig. 2b).
Fig. 2.
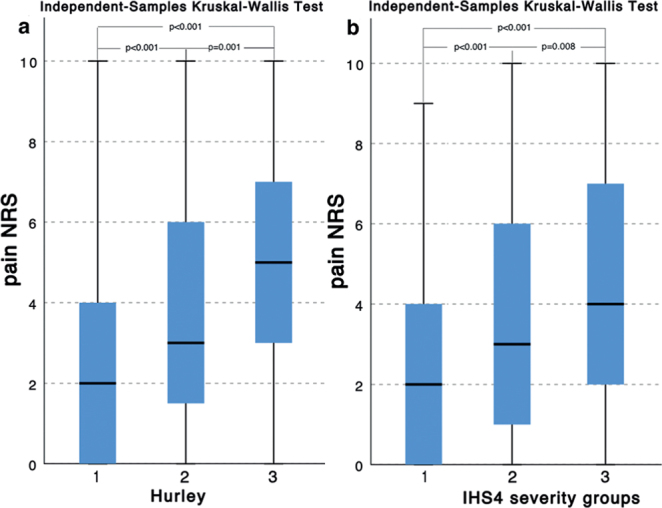
Pain numerical rating scale (NRS) points differences: (a) between Hurley severity groups; and (b) between International Hidradenitis Suppurativa Severity Score System (IHS4) severity groups.
In 350 patients (19.5%) lesions were limited to a single localization, while the remainder (80.5%) had multiple affected skin areas. WP-NRS was significantly higher (p < 0.001) in patients with multiple HS lesion locations (4 ± 2.9 and 3.3 ± 2.9 points, respectively) Moreover, intensity of pain correlated significantly (r = 0.151, p < 0.001) with the number of affected skin areas. Among the most commonly involved single localizations were the armpits, anogenital area, groins and buttocks. Analysis of variance did not reveal any difference in pain intensity between those locations (detailed data not shown). Mean DLQI for the whole studied population was 13.2 ± 8.1 points. The WP-NRS for the last 24 h showed significant strong correlation (r = 0.581, p < 0.001) with impairment in quality of life (DLQI).
DISCUSSION
HS-associated pain is the most burdensome symptom of HS, affecting up to 97% of patients during the course of the disease (11). The pain is frequently described as sharp, acute, gnawing, tenderness, or throbbing (17, 18). Although the pain appears to be mainly nociceptive, recent studies suggested an important role of the associated neuropathic component (19). The researchers indicate that overexpression of IL-1β may play a significant role in the pathogenesis of HS-associated pain (20, 21).
To the best of our knowledge, HS-related pain has not been evaluated previously in such a large population. The results of the current study confirm that pain is reported by most patients with HS (83.6%). The prevalence of pain in the current group was slightly higher than in a study by Matusiak et al. (11) (77.5%), which was one of the first studies to assess the clinical characteristics of pain and pruritus in a group of 103 patients with HS. Similarly, in the study by Kaaz et al. (10), on the influence of itch and pain on quality of sleep in patients with HS, fewer people experienced pain (79.6%) than in the current study population. Furthermore, in the above-mentioned studies, the authors assessed the prevalence of pain over longer periods of time (the previous 7 and 3 days, respectively), which may complicate the interpretation in direct comparison with our results. Due to the recurrent course of HS, it was not surprising that the estimated prevalence of pain during the whole course of the disease in these 2 studies was higher (97.1% and 86%, respectively) (10, 11).
Pain intensity assessed with a NRS did not differ greatly from previous reports (10–12, 22). Similar to the results of studies mentioned above, most subjects had mild pain symptoms (77.6%). However, the current study also found that pain in women was reported as significantly more severe than in men. Another finding of the current study, which was in accordance with the study by Matusiak et al. (11) is that the intensity of pain was significantly higher for more advanced HS. The results were independent of the severity assessment method (Hurley staging system or IHS4). Moreover, pain intensity correlated positively with IHS4 scores. Similar results were reported previously for Hurley staging, Hidradenitis Suppurativa Score, and Hidradenitis Suppurativa Severity Index (10).
HS characteristically begins as follicular plugging in a single area, which later progresses to inflammatory nodules. Nevertheless, during its course, HS tends to affect multiple body areas (23). Among the most frequently affected body zones in the current study population were the armpits, groins, buttocks and anogenital region. Involvement of multiple skin areas was proven to have stronger negative influence on patients’ quality of life (7). In addition, it has been shown that involvement of the anogenital region has the strongest impact on patients’ quality of life and psychological status (7). Similarly, in the current study, patients with multiple involved skin areas had statistically more severe pain. However, the current study did not find any differences in pain perception between patients with different body areas affected.
A significant role in the pathogenesis of HS is attributed to smoking and obesity (5). A connection was established between disease severity and both smoking and high BMI (24). The mechanism of influence of nicotine on the pathogenesis of HS is yet to be determined; however, nicotine receptors are highly expressed on follicular epithelium (25). Obesity may contribute to the pathogenesis, since obesity itself is considered a state of inflammation (26). In addition, obesity may increase friction in the skin folds, which plays a role in the pathophysiology of the disease. Due to increased knowledge of the possible role of mechanical stress in the pathogenesis of HS, patients should be educated regarding the best fabrics and styles of undergarments (27). The correlations between these risk factors and pain have not been studied until now. Although the current study population was overweight (mean BMI > 25 kg/m2) no correlation was found between BMI and pain intensity. More than half of the patients were active smokers, and they reported significantly higher pain levels than non-smokers. Nevertheless, no correlation was found between pain and number of cigarettes smoked per day.
The negative influence of HS on quality of life of patients is well documented (9). In a qualitative study by Esmann et al. (28) responders indicated pain as one of the significant factors in impaired daily functioning and poor quality of life. Moreover, multiple studies have found pain to be one of the most important contributors to decreased quality of life (4, 22, 29, 30). More than one-third of patients in the current study reported a very large effect on their quality of life. Moreover, comparable to the study by Matusiak et al. (11), pain intensity correlated positively with DLQI scores, indicating the importance of pain perception in patients’ well-being.
This study has some limitations. Only basic evaluation of the patients selected for LAight® therapy treatment was performed. Since this did not include all necessary questions about HS-related pain, this study was unable to fully characterize the pain. However, due to the large sample size of the study, which markedly exceeds the representative number of patients in the HS population in Germany, the results provide an important insight into HS-related pain.
In conclusion, to the best of our knowledge, this is the largest study to date to assess intensity of pain in patients with HS, and its relationships with demographic data and clinical parameters. The results clearly show that pain is an important and frequent burden for patients with HS. Although published data on the characteristics and prevalence of pain in HS is scarce, the impact of HS-related pain on quality of life was well documented. During the diagnosis and treatment of HS, clinicians should pay close attention to the management of accompanying pain.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Zouboulis CC, Desai N, Emtestam L, Hunger RE, Ioannides D, Juhasz I, et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29: 619–644. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Kwon HS, Jung HM, Kim GM, Bae JM. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J Eur Acad Dermatol Venereol 2018; 32: 1784–1790. [DOI] [PubMed] [Google Scholar]
- 3.Schneider-Burrus S, Lux G, van der Linde K, Barbus S, Huss-Marp J, Tsaousi A, et al. Hidradenitis suppurativa – prevalence analyses of German statutory health insurance data. J Eur Acad Dermatol Venereol 2020. Jun 24. [Epub ahead of print]. [DOI] [PubMed]
- 4.Jemec GB, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35: 191–194. [DOI] [PubMed] [Google Scholar]
- 5.Sabat R, Jemec GBE, Matusiak L, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers 2020; 6: 18. [DOI] [PubMed] [Google Scholar]
- 6.Cuenca-Barrales C, Molina-Leyva A. Risk factors of sexual dysfunction in patients with hidradenitis suppurativa: a cross-sectional study. Dermatology 2020; 236: 37–45. [DOI] [PubMed] [Google Scholar]
- 7.Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol 2010; 90: 264–268. [DOI] [PubMed] [Google Scholar]
- 8.Vazquez BG, Alikhan A, Weaver AL, Wetter DA, Davis MD. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol 2013; 133: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matusiak L, Bieniek A, Szepietowski JC. Hidradenitis suppurativa markedly decreases quality of life and professional activity. J Am Acad Dermatol 2010; 62: 706–708, 8 e1. [DOI] [PubMed] [Google Scholar]
- 10.Kaaz K, Szepietowski JC, Matusiak L. Influence of itch and pain on sleep quality in patients with hidradenitis suppurativa. Acta Derm Venereol 2018; 98: 757–761. [DOI] [PubMed] [Google Scholar]
- 11.Matusiak L, Szczech J, Kaaz K, Lelonek E, Szepietowski JC. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol 2018; 98: 191–194. [DOI] [PubMed] [Google Scholar]
- 12.Ring HC, Sorensen H, Miller IM, List EK, Saunte DM, Jemec GB. Pain in hidradenitis suppurativa: a pilot study. Acta Derm Venereol 2016; 96: 554–556. [DOI] [PubMed] [Google Scholar]
- 13.Ring HC, Theut Riis P, Miller IM, Saunte DM, Jemec GB. Self-reported pain management in hidradenitis suppurativa. Br J Dermatol 2016; 174: 909–911. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen RM, Lindso Andersen P, Sigsgaard V, Theut Riis P, Jemec GB. Pain perception in patients with hidradenitis suppurativa. Br J Dermatol 2020; 182: 166–174. [DOI] [PubMed] [Google Scholar]
- 15.Zouboulis CC, Del Marmol V, Mrowietz U, Prens EP, Tzellos T, Jemec GB. Hidradenitis suppurativa/acne inversa: criteria for diagnosis, severity assessment, classification and disease evaluation. Dermatology 2015; 231: 184–190. [DOI] [PubMed] [Google Scholar]
- 16.Chien CW, Bagraith KS, Khan A, Deen M, Syu JJ, Strong J. Establishment of cutpoints to categorize the severity of chronic pain using composite ratings with Rasch analysis. Eur J Pain 2017; 21: 82–91. [DOI] [PubMed] [Google Scholar]
- 17.Horvath B, Janse IC, Sibbald GR. Pain management in patients with hidradenitis suppurativa. J Am Acad Dermatol 2015; 73: S47–S51. [DOI] [PubMed] [Google Scholar]
- 18.Patel ZS, Hoffman LK, Buse DC, Grinberg AS, Afifi L, Cohen SR, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa [corrected]. Curr Pain Headache Rep 2017; 21: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huilaja L, Hirvonen MJ, Lipitsa T, Vihervaara A, Harvima R, Sintonen H, et al. Patients with hidradenitis suppurativa may suffer from neuropathic pain: a Finnish multicenter study. J Am Acad Dermatol 2020; 82: 1232–1234. [DOI] [PubMed] [Google Scholar]
- 20.Mailhot B, Christin M, Tessandier N, Sotoudeh C, Bretheau F, Turmel R, et al. Neuronal interleukin-1 receptors mediate pain in chronic inflammatory diseases. J Exp Med 2020; 217: e20191430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witte-Handel E, Wolk K, Tsaousi A, Irmer ML, Mossner R, Shomroni O, et al. The IL-1 Pathway is hyperactive in hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol 2019; 139: 1294–1305. [DOI] [PubMed] [Google Scholar]
- 22.Onderdijk AJ, van der Zee HH, Esmann S, Lophaven S, Dufour DN, Jemec GB, et al. Depression in patients with hidradenitis suppurativa. J Eur Acad Dermatol Venereol 2013; 27: 473–478. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman LK, Ghias MH, Lowes MA. Pathophysiology of hidradenitis suppurativa. Semin Cutan Med Surg 2017; 36: 47–54. [DOI] [PubMed] [Google Scholar]
- 24.Sartorius K, Emtestam L, Jemec GB, Lapins J. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol 2009; 161: 831–839. [DOI] [PubMed] [Google Scholar]
- 25.Hana A, Booken D, Henrich C, Gratchev A, Maas-Szabowski N, Goerdt S, et al. Functional significance of non-neuronal acetylcholine in skin epithelia. Life Sci 2007; 80: 2214–2220. [DOI] [PubMed] [Google Scholar]
- 26.Gerner RR, Wieser V, Moschen AR, Tilg H. Metabolic inflammation: role of cytokines in the crosstalk between adipose tissue and liver. Can J Physiol Pharmacol 2013; 91: 867–872. [DOI] [PubMed] [Google Scholar]
- 27.Loh TY, Hendricks AJ, Hsiao JL, Shi VY. Undergarment and fabric selection in the management of hidradenitis suppurativa. Dermatology 2019. Aug 29 [Online ahead of print]. [DOI] [PubMed]
- 28.Esmann S, Jemec GB. Psychosocial impact of hidradenitis suppurativa: a qualitative study. Acta Derm Venereol 2011; 91: 328–332. [DOI] [PubMed] [Google Scholar]
- 29.von der Werth JM, Jemec GB. Morbidity in patients with hidradenitis suppurativa. Br J Dermatol 2001; 144: 809–813. [DOI] [PubMed] [Google Scholar]
- 30.Wolkenstein P, Loundou A, Barrau K, Auquier P, Revuz J; Quality of Life Group of the French Society of Dermatology. Quality of life impairment in hidradenitis suppurativa: a study of 61 cases. J Am Acad Dermatol 2007; 56: 621–623. [DOI] [PubMed] [Google Scholar]


