Abstract
Early response to treatment with biologics might be important for the stability of psoriasis and long-term outcome. The aim of this study was therefore to assess whether risk of flares and drug survival are associated with disease activity in the first 6 months of treatment of psoriasis with biologics. Biologic-naïve patients from the Danish nationwide registry, DERMBIO, were grouped based on absolute Psoriasis Area and Severity Index (PASI) during the first 6 months of treatment, as: PASI = 0, PASI > 0–≤2, PASI > 2–≤ 4, and PASI > 4. Among 1,684 patients, 746 achieved PASI= 0, 485 PASI > 0–≤2, 246 PASI > 2–≤4, and 207 PASI > 4. Longer flare-free period and drug survival were observed for patients with lower PASI in the first 6 months of treatment (adjusted hazard ratios for flares (95% confidence interval) with PASI= 0 as reference: PASI > 0–≤2 (1.35 (1.11–1.72]), PASI > 2–≤ 4 (2.32 [1.80–2.99]), and PASI > 4 (2.38 [1.80–3.15])). In conclusion, a low PASI in the first 6 months of treatment with biologics in biologic-naïve patients with psoriasis was associated with a more stable disease course, lower risk of flares, and longer drug survival.
Key words: psoriasis, biologics, drug survival, flares, PASI90, PASI100
In most cases of psoriasis, treatment with biologics is reserved for patients with severe psoriasis. The initiation and evaluation of treatment with biologics are often based on severity measured with the Psoriasis Area and Severity Index (PASI), in which a relative reduction in PASI of 75% (PASI75) has historically been considered an acceptable response (1). With the introduction of newer biologics, PASI90 has been suggested as a possible and more relevant response (2). Indeed, patients with greater PASI reductions report lower impact on quality of life (3, 4) and patients with PASI100 have significantly lower impact on quality of life than those with a response between PASI75 and PASI<100 (5). Also, PASI values early in treatment have been shown to be important for the length of remission time (6–8) and subsequent response (9–12). It has even been hypothesized, that early intensive treatment after initial disease-onset can prevent and minimize recurrences (13). Data from clinical trials have shown that treatment response at weeks 4–8 can predict response at weeks 12–16 (9–12). In addition, 2 small real-world studies of patients treated with secukinumab and infliximab have shown that PASI90 or PASI100 are predictors of overall drug survival (7, 8). Therefore, we hypothesized that patients with more complete clearance during the first 6 months of therapy have more stable psoriasis with longer drug survival. In clinical trials, the primary endpoints are often PASI90 or PASI100. However, response criteria based on relative reductions might not be suitable in real-world situations, since washout periods are not used (14, 15). Thus, a treat-to-target approach, with response criteria based on absolute PASI, might be better in the real-world (15). Two recent studies have shown that an absolute PASI≤2 corresponds to PASI90 and absolute PASI≤4 or PASI≤5 correspond to PASI75, depending on the setting (15, 16).
SIGNIFICANCE
Treatment of psoriasis with biologics is often initiated and evaluated based on objective disease severity measures, such as the Psoriasis Area and Severity Index (PASI). The optimal response criteria have been widely discussed and, currently, a PASI≤2 is considered a good response. However, limited knowledge exists about the long-term consequences of different response criteria. This study found that patients who respond better to treatment in the first 6 months of therapy have a lower risk of disease flaring and a lower risk of discontinuing treatment.
The effect of biologics for psoriasis is often based on short-term response criteria. However, most patients are treated long-term, and knowledge about long-term outcomes of different responses is limited. Therefore, using response criteria based on absolute PASI, this study investigated the long-term consequences of response early in the treatment, by assessing risk of flares and overall drug survival.
MATERIALS AND METHODS
The study was approved by the Danish Data Protection Agency (ref. VD-2018-286). In Denmark, ethics committee approval is not required for register studies. The study was carried out following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations (17).
All patients in DERMBIO were assessed for eligibility. DERMBIO (4, 18–26) is a Danish nationwide clinical registry, where all treatments with biologics (including biosimilars), and novel small-molecule agents for psoriasis are registered since 2007. Patients recorded in DERMBIO are often seen at baseline, after 3 months, after 6 months and then once every 6–12 months, but with some individual differences in schedules. Patient data are collected routinely and included: sex; weight and body mass index; age at baseline and at diagnosis; DLQI at baseline and PASI at baseline and at each subsequent visit; adverse events; psoriatic arthritis; concomitant methotrexate treatment; drug name; hospital; and reason for drug discontinuation in the current study. Data are reported from all academic hospital centres (85% of all treatments), with an estimated coverage of 100% of the treatment series (25). To maximize data integrity, only patients treated in hospital clinics were included (26).
Patient selection
Only biologic-naïve patients (i.e. patients not previously treated with biologics) with a treatment series of biologics (either originator compounds or biosimilar versions) were included. Patients were divided into 4 response groups, based on absolute PASI: PASI= 0, PASI > 0–≤ 2, PASI > 2–≤ 4, and PASI > 4. The response had to be achieved after the first month and at least once during the first 6 months of therapy, the first time the response was achieved was categorized as the index date. If a patient had PASI values corresponding to more than one response group during the first 6 months of treatment, the response with lowest the PASI was chosen, e.g. if a patient had a PASI= 5 after 3 months of therapy and PASI = 1 after 6 months, the patient was allocated to the PASI>0–≤2 with index date at the 6 months visit, whereas a patient with a PASI= 1 after 3 months and a PASI= 5 after 6 months were allocated to the PASI > 0–≤ 2 with index date at the 3 months’ visit and the visit at 6 months was considered a flare. Sensitivity analyses, where patients were allocated based on the highest, last, and first PASI were conducted. Patients had to be considered responders by the treating physician at index date and continue treatment thereafter with at least one subsequent visit with an available PASI after the index date. For patients with more than 14 months between visits, the patient was considered lost to follow-up and the treatment was censored at the last visit. Patients were followed for up to 5 years or until data were censored at 20 October 2019. Two different definitions of flares were assessed; an increase in absolute PASI of minimum 3 and an increase in absolute PASI of minimum 5 between visits. As a sensitivity analysis, flares were defined as an increase in the area categories of PASI. All patients with PASI subcategories available were included and a flare was defined as an absolute increase of ≥ 2 in one of the areas predefined for calculating PASI (head, arms, legs or trunk). Another sensitivity analysis for the PASI0, PASI > 0–≤ 2, and PASI > 2–≤ 4 was carried out, where a flare was defined as a visit with a PASI > 5.
Statistical analysis
For continuous data, means with standard deviations (SD) and medians with interquartile ranges (IQR) were reported. For categorical outcomes, numbers with percentages were presented. For normally distributed data 1-way analysis of variance (ANOVA) was used to assess differences between groups and for non-parametric data Kruskal–Wallis test was used. For categorical outcomes χ2 tests were used to assess differences between groups.
For flares, a discrete survival time model was constructed with a complementary log-log link model to estimate hazard ratios (HR) with 95% confidence intervals (95% CI) (27). The discrete survival time model was constructed by separating the follow-up period into 6-months intervals. Patients who stopped treatment without experiencing a flare was censored at discontinuation date. Hazard ratios are presented as crude, partially adjusted and fully adjusted. The partly adjusted model included adjustment for age, sex, and drug, and the fully adjusted model included additional adjustments for methotrexate, psoriatic arthritis, body weight, and hospital. In a sensitivity analysis, pretreatment PASI was included in the model. In addition, sub-analyses with the individual drugs and in accordance with introduction of ustekinumab (1 March 2009) and secukinumab (1 April 2015) to the Danish market were conducted.
Survival analyses of time in treatment for each of the response groups were performed using Cox regression. Analyses were conducted with reason for discontinuation due to all causes, lack of efficacy, and adverse events. Patients were censored if they stopped treatment due to remission or if there were more than 14 months between visits in all analyses. Patients stopping treatment due to all other reasons were considered an event in the analysis regarding discontinuation due to all causes. The analysis was conducted with all treatments combined and sub-analysis with the individual drugs was conducted.
Missing data in the covariates were imputed with a Markov Chain Monte Carlo-based multiple imputation approach with 100 imputations (28). A 2-sided p-value < 0.05 was considered statistically significant in all instances.
All data management and statistical analyses were performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA) and Stata software version 15.0 (StataCorp, College Station, TX, USA).
RESULTS
Patient characteristics
In total 1,684 patients were included in the study; of these, 746 achieved PASI = 0, 485 had PASI > 0–≤ 2, 246 had PASI > 2–≤ 4, and 207 had PASI > 4 at minimum one visit during the first 6 months of treatment (Fig. 1). The median time until response was 112 (IQR 91–154) days with 95.4% of the patients achieving response after 3 or 6 months (Table I). Of the included patients 65.7% were male and 31.9% had a diagnosis of psoriatic arthritis (PsA) with no differences across groups (Table I). More patients in the higher absolute PASI groups were treated with etanercept and more patients were treated with concomitant methotrexate than in the lower absolute PASI groups. Patients with lower absolute PASI had lower mean body weight and were younger than patients with higher absolute PASI and were more often treated with secukinumab (Table I).
Fig. 1.
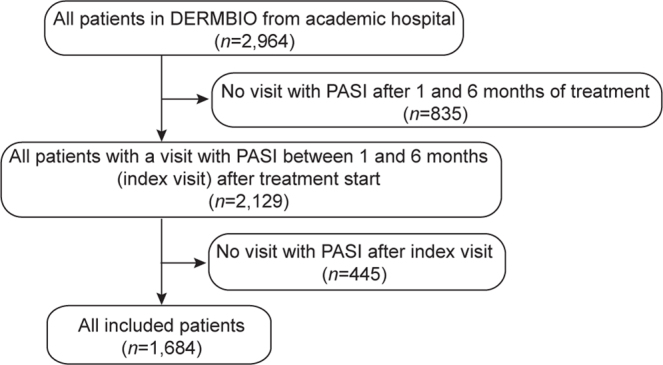
Flowchart of patient selection. PASI: Psoriasis Area and Severity Index.
Table I.
Patients’ characteristics
| PASI = 0 (n = 746) | PASI > 0-< 2 (n = 485) | PASI > 2-< 4 (n = 246) | PASI > 4 (n = 207) | p-value | |
|---|---|---|---|---|---|
| Male, n (%) | 476 (64.0) | 316 (65.3) | 168 (68.9) | 145 (70.7) | 0.26 |
| Psoriatic arthritis, n (%) | 250 (33.5) | 140 (28.9) | 83 (33.7) | 64 (30.9) | 0.33 |
| Methotrexate, n (%) | 65 (8.7) | 39 (8.0) | 28 (11.4) | 33 (15.9) | 0.0066 |
| Age, years, mean (SD) | 43.1 (14.7) | 43.9 (14.7) | 46.5 (14.4) | 46.2 (14.0) | 0.0028 |
| Weight, kg, mean (SD) | 87.1 (20.4) | 90.2 (19.8) | 91.2 (20.5) | 95.0 (22.8) | <0.0001 |
| Missing: 86 | Missing: 66 | Missing: 42 | Missing: 64 | ||
| Body max index, mean (SD) | 28.5 (6.7) | 30.1 (6.8) | 29.8 (6.4) | 31.4 (7.0) | <0.0001 |
| Missing: 190 | Missing: 132 | Missing: 64 | Missing: 83 | ||
| Disease duration, years, median (IQR) | 19.0 (11.5-28.0) | 19.8 (11.4-28.8) | 19.4 (11.6-27.0) | 19.5 (11.9-29.5) | 0.87* |
| Missing: 273 | Missing: 165 | Missing: 68 | Missing: 50 | ||
| Pretreatment PASI, median (IQR) | 11.0 (8.0-15.0) | 10.0 (6.8-14.0) | 12.0 (9.0-16.0) | 13.0 (10.0-19.0) | <0.0001* |
| Missing: 108 | Missing: 65 | Missing: 39 | Missing: 42 | ||
| Pretreatment DLQI, median (IQR) | 13.0 (8.0-18.0) | 12.0 (8.0-17.0) | 14.0 (8.0-19.0) | 12.0 (7.0-17.0) | 0.36* |
| Missing: 231 | Missing: 133 | Missing: 69 | Missing: 78 | ||
| Drugs, n (%) | |||||
| Adalimumab | 260 (34.9) | 182 (37.5) | 87 (35.4) | 70 (33.8) | 0.59 |
| Etanercept | 49 (6.6) | 55 (11.3) | 51 (20.7) | 61 (29.5) | <0.0001 |
| Infliximab | NS | NS | NS | NS | 0.46 |
| Ustekinumab | 227 (30.4) | 172 (35.5) | 90 (36.6) | 56 (27.0) | 0.045 |
| Secukinumab | 169 (22.7) | 47 (9.7) | 8 (3.3) | 6 (2.9) | <0.0001 |
| Other | NS | NS | NS | NS | 0.83 |
| Time to response, days, median (IQR) | 112 (91-158) | 112 (91-161) | 119 (91-175) | 97 (83-126) | <0.0001* |
| Achieving response after 3 months, n (%) | 493 (66.1) | 326 (67.2) | 136 (55.3) | 148 (71.5) | 0.0015 |
| Achieving response after 6 months, n (%) | 228 (30.6) | 144 (29.7) | 99 (40.2) | 36 (17.4) | <0.0001 |
| Flare (PASI > 3), n (%) | |||||
| Flare | 157 (21.1) | 142 (29.3) | 111 (45.1) | 87 (42.0) | |
| Stop treatment | 21 (14.8) | 22 (16.5) | 25 (24.8) | 29 (35.8) | 0.0010 |
| Return of PASI | 46 (32.4) | 43 (32.3) | 38 (37.6) | 18 (22.2) | 0.17 |
| Flare (PASI > 5), n (%) | |||||
| Flare | 64 (8.6) | 59 (12.2) | 55 (22.4) | 45 (21.7) | |
| Stop treatment | 10 (17.5) | 11 (20.0) | 15 (28.9) | 15 (36.6) | 0.12 |
| Return of PASI | 19 (33.3) | 17 (30.9) | 18 (34.6) | 10 (24.4) | 0.73 |
Outcomes are not shown where one group had < 3 patients.
Kruskal–Wallis test was used to assess differences in the groups.
PASI: Psoriasis Area and Severity Index; DLQI: Dermatology Life Quality Index; IQR: interquartile range; SD: standard deviation; NS: not shown due to data security requirements.
Drug survival
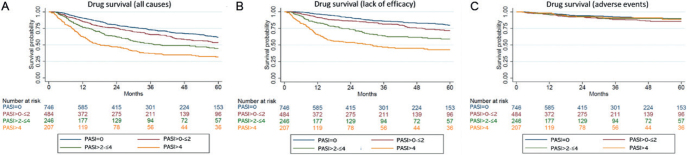
The median length of time from response to discontinuation of treatment (drug survival) for all causes of discontinuation was 448 days (IQR 238–846). For all causes of treatment discontinuation, drug survival was better for patients with PASI ≤ 2 compared with those who never had a PASI below 2 in the first 6 months of treatment (Fig. 2a). Similar results were seen for discontinuation due to lack of efficacy (Fig. 2b). No differences were seen for discontinuation due to adverse events across response groups (Fig. 2c).
Fig. 2.
Kaplan–Meier curves illustrating the time from achieving response to discontinuation of treatment (drug survival) for (A) all causes of treatment cessation; (B) lack of efficacy; and (C) adverse events. PASI: Psoriasis Area and Severity Index.
For all causes of discontinuation, HRs were 1.35 (95% CI 1.10–1.66) for PASI > 0–≤ 2, 1.93 (95% CI 1.53–2.42) for PASI > 2–≤ 4, and 3.09 (95% CI 2.47–3.87) for PASI > 4 when having PASI = 0 as reference (Table SI1). In general, similar results were observed for all drugs except etanercept where a trend towards a lower drug survival for PASI = 0 compared with PASI > 0–≤ 2 (0.65 (95% CI 0.38–1.09)) was observed (Fig. S11).
Risk of flare: PASI increase ≥ 3
Overall, 497 (29.5%) patients experienced a flare, defined as an increase in PASI of minimum 3 between visits, of these 29.2% continued treatment and returned to pre-flare PASI in the subsequent visit and 19.5% of the patients stopped treatment as a consequence of the flare (Table I).
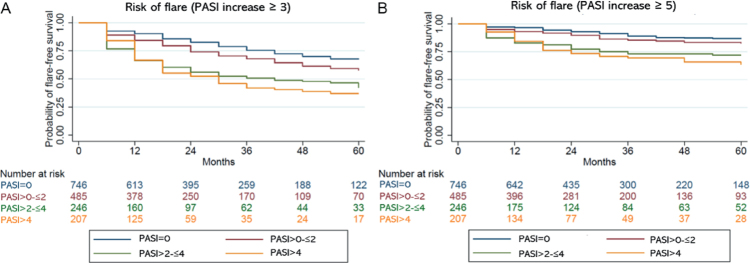
The median time for all groups from response to a flare (flare-free survival) was 326 days (IQR 133–686) for those who experienced a flare defined as an increase in PASI ≥ 3. Overall, the PASI = 0 and PASI > 0–≤ 2 groups had longer flare-free survival than the PASI > 2–≤ 4 and PASI > 4 groups when defining flare as an increase in PASI ≥ 3 (Fig. 3).
Fig. 3.
Kaplan–Meier curves illustrating time from achieving response to flare ( flare-free survival) when defining flares as an increase of (A) Psoriasis Area and Severity Index (PASI) ≥ 3 or (B) PASI ≥ 5.
With the PASI = 0 group as the reference group, crude HR for flaring (PASI ≥ 3) for the PASI > 0–≤ 2 group were 1.46 (95% CI 1.16–1.83), 2.70 (95% CI 2.12–3.45) for the PASI > 2–≤ 4, and 2.92 (95% CI 2.24–3.80) for the PASI > 4 group (Fig. 4). When adjusting for drug, sex, and age, HR decreased to 1.37 (95% CI 1.09–1.73) for PASI > 0–2 group, 2.39 (95% CI 1.86–3.07) for PASI > 2–≤ 4 group, and 2.51 (95% CI 1.92–3.30) for PASI > 4 group. Further adjusting (fully adjusted) for methotrexate, PsA, hospital, and body weight did not alter the results and neither did including pretreatment PASI as a covariate in the model (Table SII1). In addition, similar results were observed for the individual drugs, except for etanercept for which, no differences were observed across groups (Table SIII1).
Fig. 4.
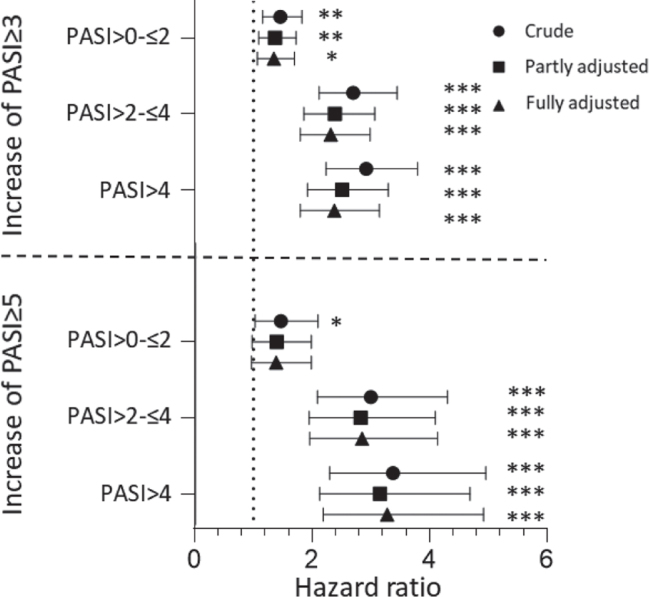
Hazard ratios of flares defined as an increase of Psoriasis Area and Severity Index (PASI) ≥ 3 or PASI ≥ 5 for different response groups compared with PASI = 0. The partly adjusted model included adjustment for age, sex, and the drug, and the fully adjusted model included additional adjustments for methotrexate, psoriatic arthritis, body weight, and hospital. *p < 0.05; **p < 0.01; ***p < 0.001.
With the PASI > 0–≤ 2 group as the reference, the fully adjusted model revealed a HR of 1.72 (95% CI 1.34–2.22) for PASI > 2–≤ 4 and HR of 1.77 (95% CI 1.34–2.332) for PASI > 4 group (Table SIV1).
Risk of flare: PASI increase ≥ 5
When defining a flare as an increase in PASI of minimum 5, 224 patients (13.3%) experienced a flare, after which 28.6% continued treatment and returned to pre-flare PASI and 22.8% of patients stopped treatment (Table I).
For patients who experienced a flare defined as an increase in PASI ≥ 5, the median time from achieving response to a flare was 335 days (IQR 117–700). Longer flare-free survival rates were seen for the PASI = 0 and PASI > 0–≤ 2 groups compared with the PASI > 2–≤ 4 and PASI > 4 groups when defining a flare as an increase in PASI ≥ 5 (Fig. 3).
With PASI = 0 group as reference group, crude HRs for flares (PASI ≥45) were 1.47 (95% CI 1.03-2.10) for the PASI>0-≤ 2, 3.00 (95% CI 2.09–4.31) for the PASI > 2–≤ 4, and 3.38 (95% CI 2.30–4.96) for the PASI > 4 group (Fig. 4). When adjusting for drug, sex, and age HR decreased to 1.40 (95% CI 0.98–1.99) for PASI > 0–≤ 2 group, 2.83 (95% CI 1.95–4.10) for PASI > 2–≤ 4 group, and 3.16 (95% CI 2.13–4.69) for PASI > 4 group. Further adjusting (fully adjusted) for methotrexate, PsA, hospital, and body weight did not alter the results, and neither did including pretreatment PASI as a covariate in the model (Table SII1).
When using PASI > 0–≤ 2 as the reference, the fully adjusted model revealed HR of 2.05 (95% CI 1.42–2.98) for PASI > 2–≤ 4 and 2.36 (95% CI 1.58–3.53) for the PASI > 4 group (Table SIV1).
Sensitivity analyses
As sensitivity analyses, the inclusion period was restricted based on 2 time periods; introduction of, respectively, us-tekinumab in March 2009 and secukinumab in April 2015, which revealed no significant differences in the results (Table SII1). In addition, the risk of flares was assessed, with 2 other differences in flares, based on subcategories of PASI and having a visit with a registered PASI > 5. In total, 1,012 patients had available subcategories of PASI, and 8.9% of these experienced a flare of ≥ 2 in one of the areas of PASI (head, arms, legs or trunk). Similar results as for flares, defined as an increase of PASI ≥ 3 or PASI ≥ 5, were observed, with the PASI = 0 and PASI > 0–≤ 2 groups having better flare-free survivals than the PASI > 2–≤ 4 and PASI > 4 groups (Table SII1). Likewise, the risk of having a visit with a registered PASI>5 was lower in the PASI = 0 group than in the PASI > 0–≤ 2 and PASI > 2–≤ 4 groups (Table SII1).
Previously, PASI ≤ 5 has been considered an important cut-off (1), and this study therefore investigated the risk of flares in groups where response criteria were defined as PASI = 0, PASI > 0–≤ 2, and PASI >2–≤ 5, respectively. However, changing the definition of response criteria did not yield any significant changes compared with the primary findings of this study (Table SV1). In addition, this study assessed 3 different ways of allocating patients to the response groups, based on first visit, last visit, and visit with the highest PASI. Changing the way in which patients were allocated to response groups did not, in general, yield any significant changes compared with the primary findings of this study (Table SV1). However, the differences between risk of flares in the PASI = 0 and PASI > 0–≤ 2 groups were no longer significant in these sensitivity analyses.
DISCUSSION
Among Danish biologic-naïve patients treated with biologics for psoriasis, a low absolute PASI within the first 6 months of treatment was associated with a more stable disease course and lower risk of flares. Notably, patients who achieved an absolute PASI ≤ 2 within the first 6 months of therapy had a lower risk of flares compared with those with PASI > 2. In addition, these patients presented a more favourable drug survival.
Previous studies have suggested that early response is important for long-term outcomes. A small study of 46 patients treated with topical drugs or phototherapy found that PASI at week 12 was associated with length of remission (6), and smaller retrospective cohort studies of patients treated with secukinumab and infliximab showed that PASI90 or PASI100 clearance was associated with longer drug survival (7, 8). However, it is well established that biologics lose efficacy over time (29). A retrospective chart review study of patients with complete or near-complete clearance investigated recurrences of psoriasis (30). Here, recurrences were seen in 48 of 60 patients, with mean reappearance time of 782 days for adalimumab and 697 days for etanercept. Another study, comprising 270 patients treated with tumour necrosis factor (TNF)-targeting drugs, who discontinued treatment, showed that patients with PASI = 0 experienced disease worsening and/or relapse less frequently compared with patients with PASI > 0 (31). In line with these results, the current study found that patients with psoriasis, who achieved PASI = 0 during the first 6 months of treatment, experienced more stable psoriasis compared with patients with PASI > 0. In addition, patients with PASI ≤ 2 showed more stable psoriasis than those with PASI > 2, with a lower risk of flares and longer drug survival. The current study could not assess whether this association is because patients who do not achieve low PASI in the first 6 months of treatment have difficult-to-treat disease and therefore flare more easily, or that the better course for patients with low PASI in the first 6 months is due to more optimized treatment with less chronic inflammation, and thus a lesser risk of flares. Pretreatment PASI might be considered a confounder, as it can influence both the risk of flares and allocation to PASI groups. However, we did not include this in the main analysis, as the pretreatment PASI is dependent on washout period and previous treatment. Nevertheless, including pretreatment PASI in the model did not influence the interpretation of the results, although the effect size for the risk of flares in the PASI > 2–≤ 4 and PASI >4 groups diminished slightly.
Patients with active psoriasis have a generalized inflammatory state with a hyperactive immune system, marked by increased cytokine production, dendritic cell and T-cell activation (29). Thus, patients with higher PASI might have an activated immune system, with preactivated tissue-resident T-cells, which can easily lead to flares (13). Other factors influencing the risk of flares include non-compliance, adverse events, normal fluctuation in psoriatic disease severity, and inability to tolerate the medication (32). However, no differences were seen for discontinuation due to adverse events across response groups. Another important factor might be drug concentration. Drug concentrations early in treatment have shown to be important for response to ustekinumab and adalimumab (33, 34). Thus, patients with worse response and higher PASI might be undertreated, and flares might be a consequence of lower serum concentrations. Importantly, as we observed many patients who stopped treatment after flares, drug concentration measurement and dose escalation might be a way to handle flares (35).
Optimal response criteria have been discussed widely, currently with focus on absolute values, either single values of PASI, Physician Global Assessment (PGA), or body surface area (BSA) (14, 15, 36–38) or composite outcomes (39). We provide here evidence of the importance of high clearance on stability and long-term outcome of psoriasis. A recent consensus opinion paper proposed that absolute PASI ≤ 2 should be the pursued PASI goal (40). Our data suggest that patients who are completely clear have more stable psoriasis than patients with some remaining psoriasis. However, PASI = 0 might still be a too ambitious response criterion, as less than half of the patients in the current study achieved this. Also having PASI = 0 as a response criterion could lead to multiple switches in patients who are satisfied with their treatment even with a few remaining plaques. Along these lines, patients generally report that obtaining almost complete skin clearance is a more important treatment goal than complete skin clearance (41). Importantly, patients with PASI ≤ 2 showed markedly more stable psoriasis than those with PASI > 2. This underscores the importance of PASI ≤ 2 and, based on these results, PASI ≤ 2 appears to be a reasonable response.
When interpreting the results, some limitations should be discussed. First, no clear definition of a flare exists in the literature, and the use of PASI is exposed to inter-rater variability, which might be more pronounced in the higher PASI response groups. This potential bias might lead to an overestimation of the effect of early PASI on risk of flares. However, using different definitions of flares led to similar results; thus this risk is considered negligible. Another limitation is that the study comprised mainly patients receiving older biologics and might not be generalized to newer biologics, but controlling for the drug and analysing the drugs separately, did not significantly alter the results. The study originates from a Danish healthcare system in which choice of biologic and evaluation of efficacy are dictated by national guidelines, and the results might not be fully generalizable to other healthcare systems with different structures. On the other hand, all patients in Denmark receiving biologics for psoriasis and fulfilling the inclusion criteria were included, thus minimizing the risk of selection bias. Lastly, we cannot measure compliance, and as both response and risk of flares can be influenced by this, we cannot refute that some of the results can be attributed to lack of compliance. This would arguably be a differential bias, that would tend to favour more infrequent dosing schedules (e.g. every 12 weeks, since these are often administered in-office) compared with frequent dosing schedules (e.g. weekly home-administration). However, we observed similar results for drugs with different dosing schedules. Strengths of the current study include the high number of included patients, the prospectively collected data, a validated measurement of disease severity and flares, and clinically relevant cut-offs for response categories. In addition, potential confounding factors are collected routinely, and this study presents risk estimates both unadjusted and adjusted for these confounders. Although, pretreatment PASI might be a potential confounder it was not included in the main model in the current study, as no washout of prior treatment occurred, and patients might initiate biologics due to adverse events or lack of efficacy of methotrexate, and the pretreatment PASI might therefore be influenced by this. Nevertheless, including pretreatment PASI in a sensitivity analysis did not alter the interpretation of the results. However, we cannot refute residual or unmeasured confounders.
With biologic therapy for psoriasis, a low PASI within the first 6 months of treatment in biologic-naïve patients was associated with a more stable disease course, and lower risk of flares and treatment discontinuation. A treatment target of PASI ≤ 2 in the first 6 months of treatment appears to be a reasonable and clinically relevant cut-off for response in the treatment of psoriasis with biologics.
ACKNOWLEDGEMENTS
The DERMBIO registry has entered into agreements with Abbvie, Eli Lilly, Almirall, UCB, Novartis, Janssen, and Leo Pharma. They receive post-marketing data and had no influence on the data collection, statistical analyses, manuscript preparation or decision to submit.
Conflicts of interest. NDL has been an honorary speaker for Eli Lilly and Janssen Cilag. AE has received research funding from Pfizer, Eli Lilly, Novartis, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, Leo Pharma, Samsung Bioepis Co., Ltd, Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. MKR has been a paid speaker for AbbVie, Almirall, and LEO Pharma. Consulting, or serving on expert/advisory boards with AbbVie, Almirall, Janssen Cilag, and Eli Lilly. He served as investigator for Janssen Cilag, and Novartis. CVN has served on an advisory board for Almirall and received educational grants from AbbVie and Janssen. TND has been a paid speaker for Janssen Cilag and has been a consultant or has served on Advisory Boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly and LEO Pharma. LI has been a paid speaker for MSD, Pfizer, AbbVie, Almirall, Janssen Cilag, Eli Lilly, Novartis, LEO Pharma, Samsung and UCB. Consulting or serving on expert/advisory boards with Pfizer, AbbVie, Almirall, BMS, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma and MSD. Served as investigator for MSD, Pfizer, AbbVie, Janssen Cilag, Eli Lilly, Novartis, Amgen and LEO Pharma and received research and educational grant from Pfizer, AbbVie, Novartis, MSD and Leo Pharma. LS has been a paid speaker for AbbVie, Eli Lilly, Novartis, and LEO Pharma, and has been a consultant or has served on Advisory Boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi. She has served as an investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZenica, Eli Lilly, Novartis, Regeneron, and LEO Pharma, and has received research and educational grants from Novartis, Sanofi, Janssen Cilag, and LEO Pharma.
REFERENCES
- 1.Mrowietz U, De Jong E, Kragballe K, Langley R, Nast A, Puig L, et al. A consensus report on appropriate treatment optimization and transitioning in the management of moderate-to-severe plaque psoriasis. J Eur Acad Dermatol Venereol 2014; 28: 438–453. [DOI] [PubMed] [Google Scholar]
- 2.Puig L. PASI 90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol 2015; 29: 645–648. [DOI] [PubMed] [Google Scholar]
- 3.Loft ND, Egeberg A, Rasmussen MK, Bryld LE, Gniadecki R, Dam TN, et al. Patient-reported outcomes during treatment in patients with moderate-to-severe psoriasis: a Danish nationwide study. Acta Derm Venereol 2019; 99: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 4.Hesselvig JH, Egeberg A, Loft ND, Zachariae C, Kofoed K, Skov L. Correlation between dermatology life quality index and psoriasis area and severity index in patients with psoriasis treated with ustekinumab. Acta Derm Venereol 2018; 98: 335–339. [DOI] [PubMed] [Google Scholar]
- 5.Strober B, Papp KA, Lebwohl M, Reich K, Paul C, Blauvelt A, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol, 2016; 75: 77–82.e77. [DOI] [PubMed] [Google Scholar]
- 6.Coimbra S, Oliveira H, Belo L, Figueiredo A, Rocha-Pereira P, Santos-Silva A. Principal determinants of the length of remission of psoriasis vulgaris after topical, NB-UVB, and PUVA therapy. Am J Clin Dermatol 2013; 14: 49–53. [DOI] [PubMed] [Google Scholar]
- 7.Ferrières L, Konstantinou M, Bulai Livideanu C, Hegazy S, Tauber M, Amelot F, et al. Long-term continuation with secukinumab in psoriasis: association with patient profile and initial psoriasis clearance. Clin Exp Dermatol 2019; 44: e230–e234. [DOI] [PubMed] [Google Scholar]
- 8.Magis Q, Jullien D, Gaudy-Marqueste C, Baumstark K, Viguier M, Bachelez H, et al. Predictors of long-term drug survival for infliximab in psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 96–101. [DOI] [PubMed] [Google Scholar]
- 9.Gordon KB, Betts KA, Sundaram M, Signorovitch JE, Li J, Xie M, et al. Poor early response to methotrexate portends inadequate long-term outcomes in patients with moderate-to-severe psoriasis: evidence from 2 phase 3 clinical trials. J Am Acad Dermatol 2017; 77: 1030–1037. [DOI] [PubMed] [Google Scholar]
- 10.Tan H, Valdez H, Griffiths CE, Mrowietz U, Tallman A, Wolk R, et al. Early clinical response to tofacitinib treatment as a predictor of subsequent efficacy: results from two phase 3 studies of patients with moderate-to-severe plaque psoriasis. J Dermatolog Treat 2017; 28: 3–7. [DOI] [PubMed] [Google Scholar]
- 11.Zhu B, Edson-Heredia E, Cameron G, Shen W, Erickson J, Shrom D, et al. Early clinical response as a predictor of subsequent response to ixekizumab treatment: results from a phase II study of patients with moderate-to-severe plaque psoriasis. Br J Dermatol 2013; 169: 1337–1341. [DOI] [PubMed] [Google Scholar]
- 12.Pinter A, Gerdes S, Papavassilis C, Reinhardt M. Characterization of responder groups to secukinumab treatment in moderate to severe plaque psoriasis. J Dermatolog Treat 2020; 31: 769–775. [DOI] [PubMed] [Google Scholar]
- 13.Iversen L, Eidsmo L, Austad J, De Rie M, Osmancevic A, Skov L, et al. Secukinumab treatment in new-onset psoriasis: aiming to understand the potential for disease modification–rationale and design of the randomized, multicenter STEPI n study. J Eur Acad Dermatol Venereol 2018; 32: 1930–1939. [DOI] [PubMed] [Google Scholar]
- 14.Norlin J, Nilsson K, Persson U, Schmitt-Egenolf M. Complete skin clearance and Psoriasis Area and Severity Index response rates in clinical practice: predictors, health-related quality of life improvements and implications for treatment goals. Br J Dermatol 2020; 182: 965–973. [DOI] [PubMed] [Google Scholar]
- 15.Mahil S, Wilson N, Dand N, Reynolds N, Griffiths C, Emsley R, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol 2020; 182: 1158–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig L, Dossenbach M, Berggren L, Ljungberg A, Zachariae C. Absolute and relative Psoriasis Area and Severity Indices (PASI) for comparison of the efficacy of ixekizumab to etanercept and placebo in patients with moderate-to-severe plaque psoriasis: an integrated analysis of UNCOVER-2 and UNCOVER-3 outcomes. Acta Derm Venereol 2019; 99: 971–977. [DOI] [PubMed] [Google Scholar]
- 17.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 2007; 4: e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gniadecki R, Kragballe K, Dam T, Skov L. Comparison of drug survival rates for adalimumab, etanercept and infliximab in patients with psoriasis vulgaris. Br J Dermatol 2011; 164: 1091–1096. [DOI] [PubMed] [Google Scholar]
- 19.Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld L, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol 2015; 29: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 20.Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, et al. Pharmacological undertreatment of coronary risk factors in patients with psoriasis: observational study of the Danish nationwide registries. PLoS One 2012; 7: e36342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egeberg A, Iversen L, Gniadecki R, Hvid L, Dam T, Bryld L, et al. Characteristics of patients receiving ustekinumab compared with secukinumab for treatment of moderate-to-severe plaque psoriasis – nationwide results from the DERMBIO registry. J Eur Acad Dermatol Venereol 2017; 31: 1183–1187. [DOI] [PubMed] [Google Scholar]
- 22.Loft N, Skov L, Bryld L, Gislason G, Egeberg A. Treatment history of patients receiving biologic therapy for psoriasis – a Danish nationwide study. J Eur Acad Dermatol Venereol 2017; 31: e362–e363. [DOI] [PubMed] [Google Scholar]
- 23.Loft ND, Skov L, Rasmussen MK, Gniadecki R, Dam TN, Brandslund I, et al. Genetic polymorphisms associated with psoriasis and development of psoriatic arthritis in patients with psoriasis. PloS One 2018; 13: e0192010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loft N, Skov L, Iversen L, Gniadecki R, Dam T, Brandslund I, et al. Associations between functional polymorphisms and response to biological treatment in Danish patients with psoriasis. Pharmacogenomics J 2018; 18: 494. [DOI] [PubMed] [Google Scholar]
- 25.Gniadecki R, Bang B, Bryld L, Iversen L, Lasthein S, Skov L. Comparison of long-term drug survival and safety of biologic agents in patients with psoriasis vulgaris. Br J Dermatol 2015; 172: 244–252. [DOI] [PubMed] [Google Scholar]
- 26.Egeberg A, Ottosen M, Gniadecki R, Broesby-Olsen S, Dam T, Bryld L, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol 2018; 178: 509–519. [DOI] [PubMed] [Google Scholar]
- 27.Lin J-H, Lee W-C. Complementary log regression for sufficient-cause modeling of epidemiologic data. Sci Rep 2016; 6: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall, CRC Press, 1997. [Google Scholar]
- 29.Levin EC, Gupta R, Brown G, Malakouti M, Koo J. Biologic fatigue in psoriasis. J Dermatolog Treat 2014; 25: 78–82. [DOI] [PubMed] [Google Scholar]
- 30.No D, Amin M, Reddy S, Egeberg A, Wu J. Sites of recurrence in patients following clearance of psoriasis with biologic therapy. J Eur Acad Dermatol Venereol 2017; 31: e297–e298. [DOI] [PubMed] [Google Scholar]
- 31.Stinco G, Balato N, Buligan C, Campanati A, Dastoli S, Di NM, et al. A multicentre retrospective case-control study on suspension of TNF-inhibitors and outcomes in psoriatic patients (STOP study). G Ital Dermatol Venereol 2019; 154: 392–399. [DOI] [PubMed] [Google Scholar]
- 32.Tyring S, Gordon KB, Poulin Y, Langley RG, Gottlieb AB, Dunn M, et al. Long-term safety and efficacy of 50 mg of etanercept twice weekly in patients with psoriasis. Arch Dermatol 2007; 143: 719–726. [DOI] [PubMed] [Google Scholar]
- 33.Tsakok T, Wilson N, Dand N, Loeff FC, Bloem K, Baudry D, et al. Association of serum ustekinumab levels with clinical response in psoriasis. JAMA Dermatol 2019; 155: 1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson N, Tsakok T, Dand N, Bloem K, Duckworth M, Baudry D, et al. Defining the therapeutic range for adalimumab and predicting response in psoriasis: a multicenter prospective observational cohort study. J Invest Dermatol 2019; 139: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gniadecki R, Leonardi C, Gordon K, Gu Y, Geng Z, Nader A, et al. Long-term optimization of outcomes with flexible adalimumab dosing in patients with moderate to severe plaque psoriasis. J Eur Acad Dermatol Venereol 2018; 32: 1297–1304. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol 2017; 76: 290–298. [DOI] [PubMed] [Google Scholar]
- 37.Puig L, Carrascosa J, Carretero G, De La Cueva P, Lafuente-Urrez R, Belinchón I, et al. Spanish evidence-based guidelines on the treatment of psoriasis with biologic agents, 2013. Part 1: on efficacy and choice of treatment. Actas Dermosifiliogr 2013; 104: 694–709. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Torres RM, Paradela S, Fonseca E. Long-term response to etanercept monotherapy in moderate to severe psoriasis: assessment in daily practice by the maintenance of low values of PASI and BSA. J Dermatolog Treat 2014; 25: 54–56. [DOI] [PubMed] [Google Scholar]
- 39.Grine L, de la Brassinne M, Ghislain PD, Hillary T, Lambert J, Segaert S, et al. A Belgian consensus on the definition of a treat-to-target outcome set in psoriasis management. J Eur Acad Dermatol Venereol 2020; 34: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carretero G, Puig L, Carrascosa J, Ferrándiz L, Ruiz-Villaverde R, De la Cueva P, et al. Redefining the therapeutic objective in psoriatic patients candidates for biological therapy. J Dermatolog Treat 2018; 29: 334–346. [DOI] [PubMed] [Google Scholar]
- 41.Egeberg A, Thyssen JP. Factors associated with patient-reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol 2019; 81: 943–949. [DOI] [PubMed] [Google Scholar]