Abstract
The hydrolysis and fermentation of insoluble cellulose were investigated using continuous cultures of Clostridium cellulolyticum with increasing amounts of carbon substrate. At a dilution rate (D) of 0.048 h−1, biomass formation increased proportionately to the cellulose concentration provided by the feed reservoir, but at and above 7.6 g of cellulose liter−1 the cell density at steady state leveled off. The percentage of cellulose degradation declined from 32.3 to 8.3 with 1.9 and 27.0 g of cellulose liter−1, respectively, while cellodextrin accumulation rose and represented up to 4.0% of the original carbon consumed. The shift from cellulose-limited to cellulose-sufficient conditions was accompanied by an increase of both the acetate/ethanol ratio and lactate biosynthesis. A kinetics study of C. cellulolyticum metabolism in cellulose saturation was performed by varying D with 18.1 g of cellulose liter−1. Compared to cellulose limitation (M. Desvaux, E. Guedon, and H. Petitdemange, J. Bacteriol. 183:119–130, 2001), in cellulose-sufficient continuous culture (i) the ATP/ADP, NADH/NAD+, and qNADH produced/qNADH used ratios were higher and were related to a more active catabolism, (ii) the acetate/ethanol ratio increased while the lactate production decreased as D rose, and (iii) the maximum growth yield (Y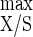 ) (40.6 g of biomass per mol of hexose equivalent) and the maximum energetic yield (Y
) (40.6 g of biomass per mol of hexose equivalent) and the maximum energetic yield (Y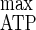 ) (19.4 g of biomass per mol of ATP) were lowered. C. cellulolyticum was then able to regulate and optimize carbon metabolism under cellulose-saturated conditions. However, the facts that some catabolized hexose and hence ATP were no longer associated with biomass production with a cellulose excess and that concomitantly lactate production and pyruvate leakage rose suggest the accumulation of an intracellular inhibitory compound(s), which could further explain the establishment of steady-state continuous cultures under conditions of excesses of all nutrients. The following differences were found between growth on cellulose in this study and growth under cellobiose-sufficient conditions (E. Guedon, S. Payot, M. Desvaux, and H. Petitdemange, Biotechnol. Bioeng. 67:327–335, 2000): (i) while with cellobiose, a carbon flow into the cell of as high as 5.14 mmol of hexose equivalent g of cells−1 h−1 could be reached, the maximum entering carbon flow obtained here on cellulose was 2.91 mmol of hexose equivalent g of cells−1 h−1; (ii) while the NADH/NAD+ ratio could reach 1.51 on cellobiose, it was always lower than 1 on cellulose; and (iii) while a high proportion of cellobiose was directed towards exopolysaccharide, extracellular protein, and free amino acid excretions, these overflows were more limited under cellulose-excess conditions. Such differences were related to the carbon consumption rate, which was higher on cellobiose than on cellulose.
) (19.4 g of biomass per mol of ATP) were lowered. C. cellulolyticum was then able to regulate and optimize carbon metabolism under cellulose-saturated conditions. However, the facts that some catabolized hexose and hence ATP were no longer associated with biomass production with a cellulose excess and that concomitantly lactate production and pyruvate leakage rose suggest the accumulation of an intracellular inhibitory compound(s), which could further explain the establishment of steady-state continuous cultures under conditions of excesses of all nutrients. The following differences were found between growth on cellulose in this study and growth under cellobiose-sufficient conditions (E. Guedon, S. Payot, M. Desvaux, and H. Petitdemange, Biotechnol. Bioeng. 67:327–335, 2000): (i) while with cellobiose, a carbon flow into the cell of as high as 5.14 mmol of hexose equivalent g of cells−1 h−1 could be reached, the maximum entering carbon flow obtained here on cellulose was 2.91 mmol of hexose equivalent g of cells−1 h−1; (ii) while the NADH/NAD+ ratio could reach 1.51 on cellobiose, it was always lower than 1 on cellulose; and (iii) while a high proportion of cellobiose was directed towards exopolysaccharide, extracellular protein, and free amino acid excretions, these overflows were more limited under cellulose-excess conditions. Such differences were related to the carbon consumption rate, which was higher on cellobiose than on cellulose.
Cellulose is the most abundantly produced biopolymer on earth (5, 28). Due to its recalcitrant, durable nature, cellulose accumulates in terrestrial environments, where a variety of cellulolytic microorganism, existing in virtually every niche and clime, decompose it (4, 28, 29). Around 5 to 10% of cellulasic materials are degraded anaerobically, and the final products released during fermentation are methane and carbon dioxide (28, 51); among cellulolytic bacteria, clostridia play an important role in such processes (28).
Clostridium cellulolyticum, a nonruminal, strictly anaerobic, cellulolytic bacterium (45), digests cellulose through the cellulosome (43). This extracellular multienzymatic complex is composed of a variety of cellulases organized around a scaffolding protein called CipC (6, 41, 42). The cellulosomes are found at the surface of the cells and allow both adhesion and efficient degradative activity against the cellulose fibers (3, 8).
Using cellobiose, which is one of the soluble cellodextrins released during cellulolysis, major differences in the regulation of the carbon flow between carbon-limited and carbon-sufficient continuous cultures have been reported (19, 20). As the dilution rate increases, in cellobiose-limited chemostats the metabolic pathways towards ethanol and lactate contribute to balance the reducing equivalents supplied by acetate formation (19), while under cellobiose-saturated conditions (20) the redox balance is essentially maintained by NADH-ferredoxin (NADH-Fd) reductase-hydrogenase and ethanol dehydrogenase activities and the carbon flow is equilibrated by three overflows, i.e., exopolysaccharide, extracellular protein, and amino acid excretions.
Using a substrate more closely related to the natural ecosystem of the bacterium, recent investigations with cellulose-limited chemostats (11) have indicated that there is neither a shift from an acetate-ethanol fermentation to a lactate-ethanol fermentation nor pyruvate overflow at high catabolic rates as previously observed on cellobiose (18, 19). Thus, with this culture condition, C. cellulolyticum appeared well adapted and even restricted to a cellulolytic lifestyle (11, 12). In its natural biota, however, growth under carbon-sufficient conditions is undoubtedly experienced by bacteria and probably more frequently than carbon limitation, since cellulose accumulates in environments (4, 5).
The aim of the present study, then, was to investigate kinetically the C. cellulolyticum fermentation under carbon-saturated conditions by using continuous culture analysis with cellulose and a mineral salt-based medium, which are more closely related to the natural ecosystem of the bacterium (11, 19).
MATERIALS AND METHODS
Organism and growth conditions.
C. cellulolyticum ATCC 35319 (45) was cultured as previously reported (10) on a defined medium (19) containing cellulose MN301 (Macherey-Nagel, Düren, Germany) at various concentrations as specified in Results. All experiments in segmented gas-liquid continuous culture (49) were performed in a 1.5-liter-working-volume fermentor (LSL Biolafitte, St. Germain en Laye, France) at 34°C and pH 7.2 and monitored as previously indicated (11).
Analytical procedures.
Biomass, cellulose concentration, gas analysis, extracellular protein, amino acid, glucose, soluble cellodextrins, glycogen, acetate, ethanol, lactate, and extracellular pyruvate were determined as described previously (10, 11, 17).
The percentage of cells that were nonadherent to cellulose fibers was determined by vacuum filtration through 3-μm-pore-size polycarbonate membrane (Millipore, Molsheim, France) as described by Wells et al. (50).
The intracellular compounds NAD+, NADH, ATP, ADP, AMP, glucose-1-phosphate (G1P), and glucose-6-phosphate (G6P) and the enzymes glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (EC 1.2.1.12), pyruvate-Fd oxidoreductase (PFO) (EC 1.2.7.1), lactate dehydrogenase (LDH) (EC 1.1.1.27), acetate kinase (AK) (EC 2.7.2.1), and alcohol dehydrogenase (ADH) (EC 1.1.1.1) were extracted and assayed as reported previously (11, 17).
Calculations.
The metabolic pathways and equations for cellulose fermentation by C. cellulolyticum (expressed as n hexose equivalents [hexose eq], corresponding to n glucose residues of the cellulose chain) were reported previously (10, 11).
The specific rate of hexose residue fermentation (qcellulose) and the specific rates of product formation (qacetate, qethanol, qextracellular pyruvate, qlactate, and qpyruvate) are expressed in millimoles per gram of cells per hour and were calculated as indicated previously (11). qNADH produced and qNADH used are the specific rates of NADH production and NADH consumption, respectively, in millimoles per gram of cells per hour and were calculated as follows: qNADH produced = qpyruvate, and qNADH used = 2 qethanol + qlactate. qNADH-Fd was the specific rate of H2 production via the NADH-Fd-H2 path and corresponded to qNADH produced − qNADH used.
The molar growth yield (YX/S) was expressed in grams of cells per mole of hexose eq fermented. The energetic yield of biomass (YATP) was expressed in grams of cells per mole of ATP produced and calculated as described previously (11): YATP = concentrationbiomass/(1.94 concentrationacetate + 0.94 concentrationethanol + 0.94 concentrationlactate + 0.94 concentrationextracellular pyruvate). The specific rate of ATP generation (qATP) was expressed in millimoles per gram of cells per hour and calculated by the following equation (11): qATP = 1.94 qacetate + 0.94 qethanol + 0.94 qlactate+ 0.94 qextracellular pyruvate. The energetic efficiency (ATP-Eff) corresponding to the ATP generation in cellulose catabolism is given by the ratio of qATP to qcellulose (11).
A Pirt plot was used for the determination of the maximum yield (Ymax) and the maintenance coefficient (m) (46). The energetic charge and oxidation/reduction index (O/R) were calculated as described by Gottschalk (22). The first-order rate constant of cellulose removal was determined with the equation established by Pavlostathis et al. (44) as described previously (11).
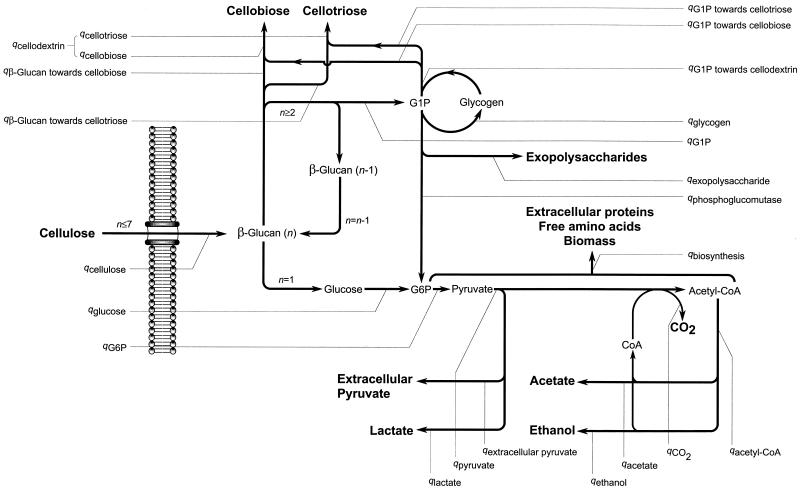
Determination of the distribution of the carbon flow by stoichiometric flux analysis (9) was done by adapting the model developed by Holms (26) to C. cellulolyticum metabolism as depicted in Fig. 1. For further direct calculation of the carbon flow at steady state through each enzyme of the known metabolic pathways, the fluxes were expressed in milliequivalents of carbon (meqC) per gram of cells per hour and calculated as indicated in Table 1.
FIG. 1.
Carbon flow within the central metabolic pathways of C. cellulolyticum grown in cellulose excess. n, number of hexose residues inside the biopolymer. The specific consumption and production rates (q) correspond to the equations given in Table 1.
TABLE 1.
Calculations for flux analysis during cellulose-excess fermentation by C. cellulolyticum in chemostat culture
| Carbon flowa | Equationb |
|---|---|
| qcellulose | (Ccellulose/X) × D |
| qcellobiose | (Ccellobiose/X) × D |
| qcellotriose | (Ccellotriose/X) × D |
| qlactate | (Clactate/X) × D |
| qacetate | (Cacetate/X) × D |
| qethanol | (Cethanol/X) × D |
| qextracellular pyruvate | (Cextracellular pyruvate/X) × D |
| qbiosynthesis | [(Cbiomass − Cglycogen + Camino acid + Cprotein)/X] × D |
| qcarbon dioxide | 1/2 × (qacetate + qethanol) |
| qpyruvate | qacetate + qethanol + qlactate + qextracellular pyruvate + qcarbon dioxide |
| qacetyl-CoA | qacetate + qethanol |
| qG1P | 0.63 × qcellulose |
| qG1P towards cellobiose | 1/2 × qcellobiose |
| qG1P towards cellotriose | 2/3 × qcellotriose |
| qG1P towards cellodextrin | qG1P towards cellobiose + qG1P towards cellotriose |
| qglucose | 0.37 × qcellulose |
| qcellodextrin | qcellobiose + qcellotriose |
| qβ-glucan towards cellobiose | 1/2 × qcellobiose |
| qβ-glucan towards cellotriose | 1/3 × qcellotriose |
| qβ-glucan towards cellodextrin | qβ-glucan towards cellobiose + qβ-glucan towards cellotriose |
| qG6P | qbiosynthesis + qpyruvate |
| qphosphoglucomutase | qG6P − qglucose |
| qglycogen | (Cglycogen/X) × D |
| qexopolysaccharide | qG1P − qphosphoglucomutase − qglycogen − qcellodextrin |
Carbon flows are diagrammed in Fig. 1.
C is the concentration in meqC liter−1 of compound produced or consumed; X is the biomass concentration expressed as grams liter−1; D is the dilution rate in hour−1; and q is the specific metabolic rate in meqC gram of cells−1 hour−1.
The turnover of a pool (hours−1) corresponded to the rate of input or output divided by the pool size, which is then the number of times that the pool turns over every hour (27). R is the ratio of the specific enzyme activity to metabolic flux (11, 27).
RESULTS
C. cellulolyticum continuous culture with increasing concentrations of cellulose.
C. cellulolyticum was grown on cellulose in independent runs using a segmented gas-liquid continuous culture device at a dilution rate of 0.048 h−1 with substrate concentrations ranging from 1.9 to 27.0 g liter−1 (Table 2). With increasing amounts of substrate, the concentration of consumed cellulose rose, but at above 7.6 g liter−1 (i.e., 47.0 mM hexose eq) the concentration of consumed cellulose stagnated at 13.9 to 14.3 mM hexose eq (Table 2). The biomass concentration at each steady state increased with the cellulose concentration in the feed medium reservoir, but at above 7.6 g liter−1 it remained quite constant at around 0.296 g liter−1 (Table 2), and thus the production of biomass paralleled the amount of digested cellulose. Microscopic examination indicated that at low cellulose concentrations unattached cells were observable and that almost all of the cellulose fibers were colonized by bacteria. All of these results indicated that at above 7.6 g of cellulose liter−1, continuous cultures were carried out under cellulose-sufficient conditions.
TABLE 2.
Fermentation parameters from continuous culture of C. cellulolyticum with increasing concentrations of cellulose at a D value of 0.048 h−1
| Parameter (unit) | Resultsa obtained with a cellulose concn (g liter−1) of:
|
||||
|---|---|---|---|---|---|
| 1.9 | 3.8 | 7.6 | 14.4 | 27.0 | |
| Biomass (g liter−1) | 0.095 ± 0.008 | 0.195 ± 0.017 | 0.212 ± 0.023 | 0.297 ± 0.031 | 0.294 ± 0.033 |
| Consumed cellulose (mM hexose eq) | 3.7 ± 0.2 | 7.7 ± 0.3 | 9.7 ± 0.5 | 14.3 ± 0.8 | 13.9 ± 0.7 |
| qcellulose (mmol g of cells−1 h−1) | 1.87 | 1.89 | 2.21 | 2.31 | 2.26 |
| Acetate (mM) | 3.50 ± 0.13 | 7.59 ± 0.21 | 10.59 ± 0.37 | 17.03 ± 0.55 | 16.04 ± 0.57 |
| Ethanol (mM) | 1.73 ± 0.07 | 3.34 ± 0.11 | 3.64 ± 0.14 | 4.29 ± 0.17 | 4.15 ± 0.13 |
| Lactate (mM) | 0.05 ± 0.01 | 0.13 ± 0.01 | 0.62 ± 0.03 | 0.56 ± 0.02 | 0.75 ± 0.03 |
| Extracellular pyruvate (μM) | 11.4 ± 0.7 | 33.3 ± 1.8 | 161.6 ± 9.1 | 179.2 ± 8.5 | 194.6 ± 10.2 |
| Glycogen (mg g of cells−1) | 99.1 ± 3.7 | 102.3 ± 3.1 | 55.6 ± 1.9 | 61.4 ± 2.5 | 62.7 ± 2.2 |
| Cellobiose (mM) | NDb | ND | 0.08 ± 0.02 | 0.15 ± 0.02 | 0.17 ± 0.05 |
| Cellotriose (mM) | ND | ND | 0.04 ± 0.01 | 0.06 ± 0.02 | 0.07 ± 0.01 |
| Extracellular proteins (mg liter−1) | 8.4 ± 0.5 | 14.1 ± 0.7 | 9.9 ± 0.4 | 6.1 ± 0.3 | 7.6 ± 0.3 |
| Free amino acids (mg liter−1) | 33.0 ± 1.7 | 62.4 ± 3.9 | 77.7 ± 4.2 | 118.1 ± 5.7 | 112.4 ± 6.1 |
| YX/S (g of cells mol of hexose eq−1) | 25.6 | 25.4 | 21.8 | 20.8 | 21.2 |
| YATP (g of cells mol of ATP eq−1) | 11.2 | 10.8 | 8.6 | 7.9 | 8.2 |
| qATP (mmol g of cells−1 h−1) | 4.27 | 4.43 | 5.56 | 6.08 | 5.84 |
| Carbon recovery (%) | 95.3 | 95.5 | 97.4 | 96.8 | 96.1 |
Values are the averages for samples at steady state ± standard deviations. Values without standard deviations were determined with an average accuracy of ±10%.
ND, not detectable.
Shifting from cellulose-limited to cellulose-excess conditions was accompanied by a drop of both YX/S and YATP, whereas qATP increased (Table 2), showing that an uncoupling growth phenomenon occurred. The shift from cellulose limitation to cellulose saturation was accompanied by an increase of lactate biosynthesis (Table 2) as well as the acetate/ethanol ratio, which increased from 2.02 to 3.86 with 1.9 and 27.0 g of cellulose liter−1, respectively. As lactate production rose, extracellular pyruvate production increased as well (Table 2). The decrease in ethanol production in favor of acetate production was associated with additional ATP, explaining the fact that the qATP increased during the shift to cellulose saturation (Table 2).
On a synthetic medium, cellulose was converted into cell mass, fermentative catabolites, extracellular amino acids, and proteins (Table 2). Exopolysaccharides were observable by microscopic examination but could not be measured due to significant interference, as already explained (11). While cellodextrins were not present in cellulose limitation, cellobiose and cellotriose were detected in the supernatant under cellulose-excess conditions (Table 2). However, neither glucose nor cellodextrins with longer chains than cellotriose could be assayed by enzymatic, high-pressure liquid chromatography, and thin-layer chromatography techniques. Taking these compounds into account, the global carbon balance was found to be in the range of 95.3 to 97.4% (Table 2).
Cellulose degradation in continuous culture at high substrate concentrations.
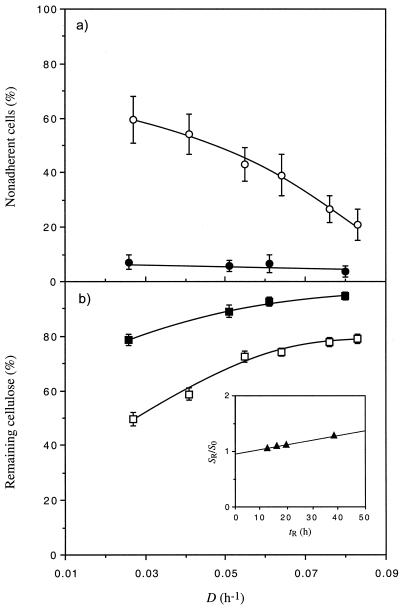
C. cellulolyticum was cultivated in cellulose excess with 18.1 g of cellulose liter−1 at different D values, which ranged from 0.026 to 0.080 h−1 (Table 3). From the lowest to the highest D value tested, the cell density at steady state decreased while the observed cell yield (YX/S) increased (Table 3). The Pirt plot of these data (r2 = 0.992) permitted determination of a Y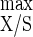 of 40.6 g of biomass mol of hexose eq consumed−1 and a maintenance coefficient (m) of 1.0 mmol of hexose eq g of cells−1 h−1. The percentage of nonadherent cells remained very low, ranging from 7.2 to 2.7% (Fig. 2a). In a cellulose-limited chemostat (i.e., 3.7 g of cellulose liter−1), however, the percentage of planktonic cells decreased from 59.6 to 21.0% as D increased from 0.027 to 0.083 h−1 and was always much higher than in cellulose saturation (Fig. 2a). The proportion of undegraded cellulose was much lower in cellulose limitation than under cellulose-sufficient conditions; with increasing D it rose from 49.6 to 79.0% and from 78.6 to 94.7%, respectively (Fig. 2b). With this culture condition, C. cellulolyticum always left undigested cellulose. Plots of Sr/S0 versus tR (tR = 1/D) were linear, with a first-order rate constant of 0.008 h−1 determined from linear regression of the data (r2 = 0.996) (Fig. 2b).
of 40.6 g of biomass mol of hexose eq consumed−1 and a maintenance coefficient (m) of 1.0 mmol of hexose eq g of cells−1 h−1. The percentage of nonadherent cells remained very low, ranging from 7.2 to 2.7% (Fig. 2a). In a cellulose-limited chemostat (i.e., 3.7 g of cellulose liter−1), however, the percentage of planktonic cells decreased from 59.6 to 21.0% as D increased from 0.027 to 0.083 h−1 and was always much higher than in cellulose saturation (Fig. 2a). The proportion of undegraded cellulose was much lower in cellulose limitation than under cellulose-sufficient conditions; with increasing D it rose from 49.6 to 79.0% and from 78.6 to 94.7%, respectively (Fig. 2b). With this culture condition, C. cellulolyticum always left undigested cellulose. Plots of Sr/S0 versus tR (tR = 1/D) were linear, with a first-order rate constant of 0.008 h−1 determined from linear regression of the data (r2 = 0.996) (Fig. 2b).
TABLE 3.
Fermentation parameters from continuous steady-state culturesa of C. cellulolyticum under cellulose-sufficient conditions
| Parameter (unit) | Resultsb obtained at a D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.026 | 0.051 | 0.061 | 0.080 | |
| Biomass (g liter−1) | 0.367 ± 0.034 | 0.266 ± 0.023 | 0.205 ± 0.019 | 0.161 ± 0.012 |
| Consumed cellulose (mM hexose eq) | 23.3 ± 1.1 | 12.2 ± 0.6 | 8.5 ± 0.4 | 5.95 ± 0.3 |
| qcellulose (mmol g of cells−1 h−1) | 1.65 | 2.33 | 2.53 | 2.91 |
| Product yield (%) of qpyruvate | ||||
| Acetate | 70.8 | 75.5 | 76.6 | 78.0 |
| Ethanol | 24.1 | 21.2 | 20.8 | 20.5 |
| Lactate | 5.2 | 3.3 | 2.6 | 1.5 |
| Extracellular pyruvate | 0.8 | 0.5 | 0.4 | 0.3 |
| qpyruvate (mmol g of cells−1 h−1) | 2.68 | 3.55 | 3.67 | 4.02 |
| Glycogen (mg g of cells−1) | 52.3 ± 1.7 | 63.6 ± 2.3 | 95.4 ± 3.5 | 81.2 ± 2.8 |
| Cellobiose (mM) | 0.24 ± 0.09 | 0.10 ± 0.04 | 0.07 ± 0.02 | NDc |
| Cellotriose (mM) | 0.11 ± 0.05 | 0.05 ± 0.02 | 0.03 ± 0.01 | ND |
| Extracellular proteins (mg liter−1) | 13.9 ± 0.7 | 6.2 ± 0.4 | 4.4 ± 0.3 | 2.2 ± 0.1 |
| Free amino acids (mg liter−1) | 190.6 ± 8.5 | 104.9 ± 5.9 | 62.9 ± 3.7 | 47.1 ± 2.1 |
| YX/S (g of cells mol of hexose eq−1) | 15.8 | 21.9 | 24.1 | 27.5 |
| Carbon recovery (%) | 97.4 | 96.7 | 93.5 | 92.9 |
The cellulose input was 1.81% (wt/vol), and ammonium was at 15.13 mM.
Values are the averages for samples at steady state ± standard deviations. Values without standard deviations were determined with an average accuracy of ±10%.
ND, not detectable.
FIG. 2.
Percentage of nonadherent cells (a) and proportion of undigested cellulose (b) in cellulose-limited (i.e., 3.7 g liter−1) (○, □) and in cellulose-excess (i.e., 18.1 g liter−1) (●, ▪) continuous culture of C. cellulolyticum. Error bars indicated standard deviations. Inset, correlation between SR/S0 and tR under cellulose-sufficient conditions.
Acetate was always the predominant fermentation end product (Table 3), with the ratio of acetate to ethanol increasing from 2.94 to 3.80. Lactate was also significantly produced, while extracellular pyruvate did not exceed 0.8% of the qpyruvate. Another part of the carbon was oriented towards amino acid, protein, and biomass (Table 3). These compounds were taken into account in addition to fermentative end products and cellodextrins for calculation of carbon recovery, which then ranged between 92.9 and 97.4%.
Kinetics analysis of microbial cellulose conversion under cellulose-sufficient conditions.
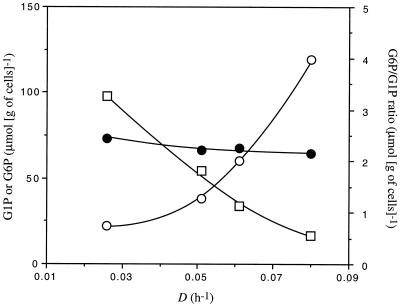
The carbon flow in the central metabolic pathway of C. cellulolyticum (Fig. 1) grown in cellulose-sufficient continuous culture is compiled in Table 4. With increasing D, the proportion of carbon flowing down the catabolite declined from 81.2 to 69.1%, while it was enhanced through biosynthesis pathways from 16.3 to 23.8%. In parallel, qG6P gradually rose, but this increase represented a decreasing proportion of the original carbon. As a result, the G6P pool slowly declined (Fig. 3) as D rose, and when expressed in term of turnover this pool increased from 22.1 to 41.7 h−1. The proportion of carbon directed towards exopolysaccharide and glycogen was low at a D of 0.026 h−1 and reached 5.7 and 1.4%, respectively, with the highest D tested (Table 4). In contrast, the qG1P towards cellodextrin declined from 2.0% to nil at a D of 0.080 h−1, since no cellodextrin could then be detected (Table 4). The G1P flux through phosphoglucomutase varied from 60.4 to 55.9% (Table 4). G1P then accumulated with increasing D (Fig. 3), which resulted in the turnover decreasing from 46.4 to 15.4 h−1. The proportion of the carbon flux towards phosphoglucomutase declined, and the G1P, which was directed towards cellodextrin at low D values, was rerouted towards glycogen and exopolysaccharide at higher D values. The percentage of carbon directed towards the fermentative end products declined as D rose (Table 4). One part of the flux was converted to acetyl coenzyme A (acetyl-CoA), i.e., from 50.9 to 45.2%. In the same time, qacetate and qethanol increased, but when expressed as a percentage of qcellulose, the two fluxes declined (Table 4). Another part of the carbon flowing down glycolysis was oriented towards the lactate production pathway. As D was enhanced, lactate production decreased, as did the pyruvate leak.
TABLE 4.
Carbon fluxes under cellulose-saturated conditions with C. cellulolyticum
| Carbon flowa | Results obtained at a D value (h−1) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.026
|
0.051
|
0.061
|
0.080
|
|||||
| meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | |
| qcellulose | 9.91 | 100.0 | 13.98 | 100.0 | 15.20 | 100.0 | 17.44 | 100.0 |
| qG1P | 6.24 | 63.0 | 8.80 | 63.0 | 9.57 | 63.0 | 10.98 | 63.0 |
| qcellodextrin | 0.34 | 3.5 | 0.41 | 3.0 | 0.37 | 2.6 | NDb | ND |
| qG1P towards cellodextrin | 0.20 | 2.0 | 0.24 | 1.7 | 0.21 | 1.5 | ND | ND |
| qβ-glucan towards cellodextrin | 0.15 | 1.5 | 0.18 | 1.3 | 0.16 | 1.1 | ND | ND |
| qglycogen | 0.05 | 0.5 | 0.12 | 0.9 | 0.22 | 1.4 | 0.24 | 1.4 |
| qexopolysaccharide | 0.01 | 0.1 | 0.10 | 0.7 | 0.55 | 3.6 | 0.99 | 5.7 |
| qglucose | 3.67 | 37.0 | 5.18 | 37.0 | 5.63 | 37.0 | 6.46 | 37.0 |
| qphosphoglucomutase | 5.99 | 60.4 | 8.34 | 59.7 | 8.59 | 56.5 | 9.75 | 55.9 |
| qG6P | 9.66 | 97.5 | 13.52 | 96.7 | 14.22 | 93.5 | 16.21 | 92.9 |
| qbiosynthesis | 1.61 | 16.3 | 2.88 | 20.6 | 3.22 | 21.2 | 4.16 | 23.8 |
| qpyruvate | 8.04 | 81.2 | 10.64 | 76.1 | 11.00 | 72.3 | 12.05 | 69.1 |
| qacetyl-CoA | 5.05 | 50.9 | 6.82 | 48.8 | 7.11 | 46.8 | 7.89 | 45.2 |
| qlactate | 0.41 | 4.2 | 0.35 | 2.5 | 0.29 | 1.9 | 0.18 | 1.0 |
| qextracellular pyruvate | 0.06 | 0.6 | 0.05 | 0.4 | 0.05 | 0.3 | 0.04 | 0.2 |
| qcarbon dioxide | 2.52 | 25.5 | 3.41 | 24.4 | 3.56 | 23.4 | 3.95 | 22.6 |
| qethanol | 1.28 | 12.9 | 1.50 | 10.7 | 1.52 | 10.0 | 1.64 | 9.4 |
| qacetate | 3.77 | 38.0 | 5.33 | 38.1 | 5.59 | 36.8 | 6.25 | 35.8 |
Carbon flows were calculated as specified in Materials and Methods and are diagrammed in Fig. 1.
ND, not determined.
FIG. 3.
G1P (○), G6P (●), and G6P/G1P ratio (□) as a function of dilution rate in cellulose-sufficient continuous culture of C. cellulolyticum.
Relationships between carbon flow and enzymatic activities, energetic balance, and redox balance.
In vitro GAPDH, PFO, ADH, and AK activities were higher under growth conditions giving higher in vivo specific production rates (Table 5). For LDH, however, the specific enzyme activities decreased with D, which was correlated with the in vivo lactate production rate. A ratio of specific enzyme activity to metabolic flux (R) (11, 27) was then calculated; R was higher than 1 for all enzymes tested. Therefore, these enzymes were not limiting with respect to the carbon flow, and thus fluxes were determined more by the concentration of substrate available than by the enzyme activity (12, 26).
TABLE 5.
Specific enzymatic activities in C. cellulolyticum cell extract at steady-state growth under cellulose-excess conditions
| Enzyme | Results obtained at D value (h−1) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.026
|
0.051
|
0.061
|
0.080
|
|||||
| Sp acta | Rb | Sp act | R | Sp act | R | Sp act | R | |
| GAPDH (EC 1.2.1.12) | 1.47 ± 0.22 | 2.87 ± 0.38 | 3.24 ± 0.41 | 3.91 ± 0.47 | ||||
| LDH (EC 1.1.1.27) | 0.25 ± 0.06 | 80.8 | 0.22 ± 0.03 | 82.8 | 0.16 ± 0.04 | 74.6 | 0.11 ± 0.05 | 81.9 |
| PFO (EC 1.2.7.1) | 0.62 ± 0.07 | 10.9 | 0.72 ± 0.06 | 9.4 | 0.89 ± 0.09 | 11.1 | 0.95 ± 0.11 | 10.7 |
| AK (EC 2.7.2.1) | 0.31 ± 0.04 | 7.3 | 0.66 ± 0.08 | 11.0 | 1.03 ± 0.12 | 16.4 | 1.33 ± 0.19 | 18.9 |
| ADH (EC 1.1.1.1) | 0.23 ± 0.03 | 16.0 | 0.32 ± 0.04 | 19.0 | 0.39 ± 0.03 | 22.8 | 0.45 ± 0.06 | 24.4 |
Expressed in micromoles minute−1 milligram of protein−1. Results are means and standard deviations.
R, ratio of specific enzymatic activity to metabolic flux through the considered metabolic path; flux was expressed as micromoles milligram of protein−1 minute−1.
As D increased, qATP, was enhanced while the stoichiometry of ATP generated over fermented cellulose, i.e., ATP-Eff, declined from 2.66 to 2.37 (Table 6). Thus, the acetate production could not compensate for the ATP loss per hexose eq fermented due to the decrease of both ethanol and lactate production. A mean value of 0.77 was obtained for the adenylate energy charge (Table 6). The apparent energetic yield increased with D (Table 6), and from a Pirt plot of the data (r2 = 0.988) a Y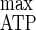 of 19.4 g of cells mol of ATP−1 and an mATP of 3.1 mmol of ATP g of cells−1 h−1 were determined.
of 19.4 g of cells mol of ATP−1 and an mATP of 3.1 mmol of ATP g of cells−1 h−1 were determined.
TABLE 6.
Energetic balance in cellulose-saturated continuous culture of C. cellulolyticum
| Parameter (unit) | Resultsa obtained at a D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.026 | 0.051 | 0.061 | 0.080 | |
| ATP (μmol g of cells−1) | 2.84 ± 0.15 | 2.31 ± 0.11 | 2.17 ± 0.19 | 1.89 ± 0.10 |
| ADP (μmol g of cells−1) | 1.41 ± 0.08 | 1.21 ± 0.06 | 1.29 ± 0.09 | 1.25 ± 0.05 |
| AMP (μmol g of cells−1) | 0.32 ± 0.05 | 0.27 ± 0.03 | 0.11 ± 0.01 | 0.25 ± 0.02 |
| Energetic charge | 0.78 | 0.77 | 0.79 | 0.74 |
| qATP (mmol g of cells−1 h−1) | 4.39 | 5.98 | 6.23 | 6.89 |
| ATP-Eff | 2.66 | 2.57 | 2.46 | 2.37 |
| YATP (g of cells mol of ATP−1) | 5.9 | 8.5 | 9.8 | 11.6 |
Values are the averages for samples at steady-state ± standard deviations. Values without standard deviations were determined with an average accuracy of ±10%.
Calculating the coenzyme balance, it could first be observed that both qNADH produced and qNADH used increased with D, as did the qNADH produced/qNADH used ratio (Table 7). This excess of produced NADH correlated with increases of both the H2/CO2 ratio, which was always higher than 1, and the qNADH-Fd. These results suggested that the intracellular NADH/NAD+ ratio was maintained by the NADH-Fd reductase and hydrogenase, since these interconnected enzymatic activities can oxidize NADH via H2 production (18–20). The O/R, determined from the gas production ratio and fermentative end product concentration, was very close to 1 and indicated an efficient reoxidation of NADH via H2 production in addition to carbon fermentative pathways (11).
TABLE 7.
Redox balance of C. cellulolyticum at steady state under cellulose-sufficient conditions
| Parameter (unit) | Resultsa obtained at a D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.026 | 0.051 | 0.061 | 0.080 | |
| NADH (μmol g of cells−1) | 6.67 ± 1.36 | 7.56 ± 1.59 | 5.16 ± 1.09 | 8.12 ± 1.62 |
| NAD+ (μmol g of cells−1) | 11.31 ± 2.33 | 12.01 ± 2.43 | 11.40 ± 2.25 | 12.32 ± 2.44 |
| NADH/NAD+ ratio | 0.59 | 0.63 | 0.45 | 0.66 |
| qNADH produced (mmol g of cells−1 h−1) | 2.68 | 3.55 | 3.67 | 4.02 |
| qNADH used (mmol g of cells−1 h−1) | 1.42 | 1.61 | 1.61 | 1.70 |
| qNADH-Fd (mmol g of cells−1 h−1) | 1.26 | 1.93 | 2.05 | 2.32 |
| qNADH produced/qNADH used ratio | 1.89 | 2.20 | 2.27 | 2.36 |
| H2/CO2 ratio | 1.43 | 1.55 | 1.61 | 1.67 |
| O/R | 1.03 | 1.01 | 0.98 | 0.96 |
Values are the averages for samples at steady state ± standard deviations. Values without standard deviations were determined with an average accuracy of ±10%.
DISCUSSION
A continuous culture system in which the feed rate is set externally is generally regarded as a chemostat if cell growth is also limited by a selected nutrient(s) (21, 52). Upon increasing the carbon substrate concentration in the feed reservoir, cellulose was no longer the growth-limiting nutrient at and above 7.6 g of cellulose liter−1, and all other nutrients appeared in excess (10, 20). Growth under substrate excess generally results in oscillations and hysteresis (23, 24, 37, 52), but such phenomena did not occur, since steady states of both residual cellulose concentration and biomass monitored during cell culture could be maintained (39). Then, as previously observed with cellobiose (20), a stable carbon excess continuous culture could be imposed on C. cellulolyticum. From studies of growth of C. cellulolyticum in cellobiose-fed continuous culture in a stepwise fashion (18) and in cellulose batch culture with a reinoculation mode (10), it was demonstrated that growth arrest was not associated with the production of extracellular toxic compounds, and thus it is unlikely that the steady state of the present continuous culture could be maintained by such growth inhibition. Yet, comparing the maximum growth yields and maximum energetic yield obtained under cellulose-sufficient conditions (i.e., Y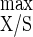 = 40.6 g of biomass mol of hexose eq consumed−1 and Y
= 40.6 g of biomass mol of hexose eq consumed−1 and Y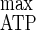 = 19.4 g of cells mol of ATP−1) to those resulting from cellulose limitation (i.e., Y
= 19.4 g of cells mol of ATP−1) to those resulting from cellulose limitation (i.e., Y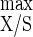 = 50.5 g of biomass mol of hexose eq consumed−1 and Y
= 50.5 g of biomass mol of hexose eq consumed−1 and Y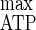 = 30.3 g of cells mol of ATP−1 [11]), it could be observed that both maximum yields were lowered in the presence of a cellulose excess. The decline of these yields indicated that an uncoupling growth phenomenon had occurred; it also took place with the rise in lactate production accompanied by a pyruvate leak. Thus, the growth stagnation was certainly related to an accumulation of an intracellular inhibitory compound(s) (18); intracellular inhibition could furthermore explain the establishment of a steady state under the condition of an excess of all nutrients (52).
= 30.3 g of cells mol of ATP−1 [11]), it could be observed that both maximum yields were lowered in the presence of a cellulose excess. The decline of these yields indicated that an uncoupling growth phenomenon had occurred; it also took place with the rise in lactate production accompanied by a pyruvate leak. Thus, the growth stagnation was certainly related to an accumulation of an intracellular inhibitory compound(s) (18); intracellular inhibition could furthermore explain the establishment of a steady state under the condition of an excess of all nutrients (52).
The understanding of microbial cellulose metabolism is of both ecological and biotechnological interest. Cellulose degradation plays a key role in the global carbon cycle (28, 29, 51) and is a promising strategy in consolidated bioprocessing for the production of biochemical compounds (25, 31–34). So far, however, very few studies have been devoted to cellulose digestion by cellulolytic bacteria under substrate-saturated conditions (38, 40, 48). Under cellulose-sufficient culture conditions, cellulose digestion always follows first-order kinetics, where k (0.008 h−1) was much lower than under cellulose-limited conditions (0.046 h−1) (11). Once cellulose-saturated conditions were attained, approximately the same amount of cellulose was digested, since the biomass concentration at steady state stagnated. Therefore, k will vary for each cellulose concentration used with this growth condition, since cellulose degradation will follow first-order kinetics with respect to the remaining cellulose concentration (44). Opposite to what was observed with cellulose limitation (11), the lactate-ethanol production was lowered as D rose and had to be balanced by dihydrogen production via the NADH-Fd reductase, which led to an increased H2/CO2 ratio. The acetate/ethanol ratio was always higher than 1, but in contrast to the case with cellulose limitation, it increased with D (11). The specific lactate production rate as well as the pyruvate leak decreased with increasing D but always remained higher than in a cellulose-limited chemostat (11). The R values for LDH were around 80.0 and were not as high as with cellulose limitation, where R could reach 434.2 (11). From the G1P metabolic node, cellodextrins were produced and represented up to 3.5% of the carbon uptake at the lowest D value tested. G1P was rerouted towards exopolysaccharide and glycogen as D rose; as in cellulose limitation, these biosyntheses could be adjusted as a function of carbon flux. Such glycogen turnover was recently observed with Fibrobacter succinogenes on cellulose (7, 14). A concomitant decrease of the percentage of carbon flowing through qphosphoglucomutase, qG6P, and qpyruvate in favor of biosynthesis pathways could explain the relative drop in lactate production as D increased. Compared to that in cellulose-limited chemostats, the proportion of unattached cells was low. With cellulose limitation the available surface area is saturated by bacteria, while under cellulose-sufficient conditions the cellulose surface area is largely accessible for bacterial adhesion (10, 13). This was correlated with a higher cellulose digestion rate reflected by higher qcellulose under substrate excess conditions, since most of the cells adhered to cellulose fibers and thus participated directly in cellulose digestion. While cellodextrin was undetectable with cellulose limitation (11), cellobiose and cellotriose were detected here in the supernatant; such a finding was certainly related to a reversible phosphorylase reaction (1, 2, 30, 35, 36, 47, 50). Under cellobiose-sufficient conditions (17, 20), only cellotriose was detected, but the present results suggest that cellobiose could also be synthesized de novo during cell growth on cellobiose.
Previous reports on experiments with cellobiose stated that the adhesion-colonization phase of the process of cellulose digestion by C. cellulolyticum (15, 16) corresponded to a carbon-sufficient period (20). It was thus argued that a carbon flow of as high as 5.14 mmol of hexose eq g of cells−1 could be attained with cellulose as a substrate (20). With cellulose saturation, however, the entering carbon flow remained lower than expected, i.e., 2.91 mmol of hexose eq g of cells−1 h−1. The NADH/NAD+ ratio was always lower than 1 on cellulose, whereas a ratio of as high as 1.51 was obtained with cellobiose excess (20); this result was most probably related to a higher carbon consumption rate which led to rate-limiting fluxes through ethanol and dihydrogen production pathways on cellobiose. Thus, the proper NADH/NAD+ ratio was maintained only when ethanol and lactate production complemented the path towards H2 production via NADH-Fd reductase activity. With a carbon excess, free amino acid could account for 15.4% of the cellobiose fermented (20), against a maximum of 5.8% on cellulose, while exopolysaccharide represented up to 38.1% of the cellobiose consumed (20) and there was a maximum of only 5.7% with cellulose as the substrate. It thus appeared that some of the general metabolic trends associated with carbon-sufficient conditions, such as (i) the ATP/ADP ratio always being higher than 1, (ii) the elevated production of lactate at a low D, and (iii) the concomitant increase of qethanol, qNADH-Fd, qNADH produced/qNADH used, NADH/NAD+, and H2/CO2 as D rose and some other regulations of bacterial metabolism, were not observed on cellulose compared to cellobiose. Even if cellulose degradation must be considered as a microbial process rather than a purely enzymatic event, the strong influence of the cellulosome on the entering carbon flow must be taken into account (10, 11). The study of C. cellulolyticum catabolism with soluble glucide allowed the demonstration of bacterial metabolic limitation, but this response should be interpreted as deregulation of the metabolism. All of these results demonstrate that C. cellulolyticum was able to correctly regulate and optimize carbon metabolism in limited and saturated conditions using a substrate more representative of its natural environment, i.e., cellulose (12).
ACKNOWLEDGMENTS
This work was supported by the Commission of European Communities FAIR program (contract CT950191 [DG12SSMA]) and by the program Agrice (contract 9701041).
We thank Anne-Cécile Aubry and Guy Raval for excellent technical assistance and Edward McRae for correcting the English and for critical reading of the manuscript.
REFERENCES
- 1.Alexander J K. Cellobiose phosphorylase from Clostridium thermocellum. Methods Enzymol. 1972;28:944–948. [Google Scholar]
- 2.Alexander J K. Cellodextrin phosphorylase from Clostridium thermocellum. Methods Enzymol. 1972;28:948–953. [Google Scholar]
- 3.Bayer E A, Chanzy H, Lamed R, Shoham Y. Cellulose, cellulases and cellulosomes. Curr Opin Struct Biol. 1998;8:548–557. doi: 10.1016/s0959-440x(98)80143-7. [DOI] [PubMed] [Google Scholar]
- 4.Bayer E A, Lamed R. The cellulose paradox: pollutant par excellence and/or a reclaimable natural resource? Biodegradation. 1992;3:171–188. doi: 10.1007/BF00129082. [DOI] [PubMed] [Google Scholar]
- 5.Béguin P, Aubert J P. The biological degradation of cellulose. FEMS Microbiol Rev. 1996;13:25–58. doi: 10.1111/j.1574-6976.1994.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 6.Belaich J P, Tardiff C, Belaich A, Gaudin C. The cellulolytic system of Clostridium cellulolyticum. J Biotechnol. 1997;57:3–14. doi: 10.1016/s0168-1656(97)00085-0. [DOI] [PubMed] [Google Scholar]
- 7.Bibollet X, Bosc N, Matulova M, Delort A M, Gaudet G, Forano E. 13C and 1H NMR study of cellulose metabolism by Fibrobacter succinogenes S85. J Biotechnol. 2000;77:37–47. doi: 10.1016/s0168-1656(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 8.Boisset C, Chanzy H, Henrissat B, Lamed R, Shoham Y, Bayer E A. Digestion of crystalline cellulose substrates by Clostridium thermocellum cellulosome: structural and morphological aspects. Biochem J. 1999;340:829–835. [PMC free article] [PubMed] [Google Scholar]
- 9.Desai R P, Nielsen L K, Papoutsakis E T. Stoichiometric modeling of Clostridium acetobutylicum fermentations with non-linear constraints. J Biotechnol. 1999;71:191–205. doi: 10.1016/s0168-1656(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 10.Desvaux M, Guedon E, Petitdemange H. Cellulose catabolism by Clostridium cellulolyticum growing in batch culture on defined medium. Appl Environ Microbiol. 2000;66:2461–2470. doi: 10.1128/aem.66.6.2461-2470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desvaux M, Guedon E, Petitdemange H. Carbon flux distribution and kinetics of cellulose fermentation in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. J Bacteriol. 2001;183:119–130. doi: 10.1128/JB.183.1.119-130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desvaux M, Petitdemange H. Flux analysis of the metabolism of Clostridium cellulolyticum grown in cellulose-fed continuous culture on a chemically defined medium under ammonium-limited conditions. Appl Environ Microbiol. 2001;67:3846–3851. doi: 10.1128/AEM.67.9.3846-3851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fields M W, Russell J B. Fibrobacter succinogenes S85 ferments ball-milled cellulose as fast as cellobiose until cellulose surface area is limiting. Appl Microbiol Biotechnol. 2000;54:570–574. doi: 10.1007/s002530000426. [DOI] [PubMed] [Google Scholar]
- 14.Gaudet G, Forano E, Dauphin G, Delort A M. Futile cycling of glycogen in Fibrobacter succinogenes as shown by in situ 1H-NMR and 13C-NMR investigation. Eur J Biochem. 1992;207:155–162. doi: 10.1111/j.1432-1033.1992.tb17032.x. [DOI] [PubMed] [Google Scholar]
- 15.Gelhaye E, Gehin A, Petitdemange H. Colonization of crystalline cellulose by Clostridium cellulolyticum ATCC 35319. Appl Environ Microbiol. 1993;59:3154–3156. doi: 10.1128/aem.59.9.3154-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelhaye E, Petitdemange H, Gay R. Adhesion and growth rate of Clostridium cellulolyticum ATCC 35319 on crystalline cellulose. J Bacteriol. 1993;175:3452–3458. doi: 10.1128/jb.175.11.3452-3458.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guedon E, Desvaux M, Petitdemange H. Kinetic analysis of Clostridium cellulolyticum carbohydrate metabolism: importance of glucose 1-phosphate and glucose 6-phosphate branch points for distribution of carbon fluxes inside and outside cells as revealed by steady-state continuous culture. J Bacteriol. 2000;182:2010–2017. doi: 10.1128/jb.182.7.2010-2017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guedon E, Desvaux M, Payot S, Petitdemange H. Growth inhibition of Clostridium cellulolyticum by an inefficiently regulated carbon flow. Microbiology. 1999;145:1831–1838. doi: 10.1099/13500872-145-8-1831. [DOI] [PubMed] [Google Scholar]
- 19.Guedon E, Payot S, Desvaux M, Petitdemange H. Carbon and electron flow in Clostridium cellulolyticum grown in chemostat culture on synthetic medium. J Bacteriol. 1999;181:3262–3269. doi: 10.1128/jb.181.10.3262-3269.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guedon E, Payot S, Desvaux M, Petitdemange H. Relationships between cellobiose catabolism, enzyme levels and metabolic intermediates in Clostridium cellulolyticum grown in a synthetic medium. Biotechnol Bioeng. 2000;67:327–335. doi: 10.1002/(sici)1097-0290(20000205)67:3<327::aid-bit9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Gottschal J C. Continuous culture. In: Lederberg J, editor. Encyclopedia of microbiology. Vol. 1. New York, N.Y: Academic Press; 1992. pp. 559–572. [Google Scholar]
- 22.Gottschalk G. Bacterial metabolism. New York, N.Y: Springer-Verlag; 1985. [Google Scholar]
- 23.Harrison D E F, Topiwala H H. Transient and oscillatory states of continuous culture. In: Ghose T H, Fiechter A, editors. Advanced biochemical engineering. Vol. 3. Berlin, Germany: Springer-Verlag; 1974. pp. 168–219. [Google Scholar]
- 24.Hjortso M A, Nielsen J. A conceptual model of autonomous oscillations in microbial cultures. Chem Eng Sci. 1994;49:1083–1095. [Google Scholar]
- 25.Hogsett D A, Alm H J, Bernardez T D, South C R, Lynd L R. Direct microbial conversion: prospects, progress, and obstacles. Appl Biochem Biotechnol. 1992;34–35:527–541. [Google Scholar]
- 26.Holms H. The central metabolic pathways of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr Top Cell Regul. 1986;28:69–105. doi: 10.1016/b978-0-12-152828-7.50004-4. [DOI] [PubMed] [Google Scholar]
- 27.Holms H. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev. 1996;19:85–116. doi: 10.1111/j.1574-6976.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 28.Leschine S B. Cellulose degradation in anaerobic environments. Annu Rev Microbiol. 1995;49:399–426. doi: 10.1146/annurev.mi.49.100195.002151. [DOI] [PubMed] [Google Scholar]
- 29.Ljungdahl L G, Eriksson K E. Ecology of microbial cellulose degradation. Adv Microb Ecol. 1985;8:237–299. [Google Scholar]
- 30.Lou J, Dawson K A, Strobel H J. Cellobiose and cellodextrin metabolism by the ruminal bacterium Ruminococcus albus. Curr Microbiol. 1997;35:221–227. doi: 10.1007/s002849900242. [DOI] [PubMed] [Google Scholar]
- 31.Lynd L R. Overview and evaluation of fuel ethanol from cellulosic biomass: technology, economics, the environments, and policy. Annu Rev Energy Environ. 1996;21:403–465. [Google Scholar]
- 32.Lynd L R, Grethlein H E, Wolkin R H. Fermentation of cellulosic substrates in batch and continuous culture of Clostridium thermocellum. Appl Environ Microbiol. 1989;55:3131–3139. doi: 10.1128/aem.55.12.3131-3139.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynd L R, Cushman J H, Nichols R J, Wyman C E. Fuel ethanol from cellulosic biomass. Science. 1991;251:1318–1323. doi: 10.1126/science.251.4999.1318. [DOI] [PubMed] [Google Scholar]
- 34.Lynd L R, Wyman C E, Gerngross T U. Biocommodity engineering. Biotechnol Prog. 1999;15:777–793. doi: 10.1021/bp990109e. [DOI] [PubMed] [Google Scholar]
- 35.Matheron C, Delort A M, Gaudet G, Forano E. Simultaneous but differential metabolism of glucose and cellobiose in Fibrobacter succinogenes cells, studied by in vivo 13C NMR. Can J Microbiol. 1996;42:1091–1099. doi: 10.1139/m96-140. [DOI] [PubMed] [Google Scholar]
- 36.Matheron C, Delort A M, Gaudet G, Forano E. In vivo 13C NMR study of glucose and cellobiose metabolism by four cellulolytic strains of the genus Fibrobacter. Biodegradation. 1998;9:451–461. doi: 10.1023/a:1008329814100. [DOI] [PubMed] [Google Scholar]
- 37.Menzel K, Zeng A P, Biebl H, Deckwer W D. Kinetic, dynamic and pathway studies of glycerol metabolism by Klebsiella pneumoniae in anaerobic continuous culture. 1. The phenomena and characterization of oscillation and hysteresis. Biotechnol Bioeng. 1996;52:549–560. doi: 10.1002/(SICI)1097-0290(19961205)52:5<549::AID-BIT2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell W J. Physiology of carbohydrate to solvent conversion by clostridia. Adv Microb Physiol. 1998;39:31–130. doi: 10.1016/s0065-2911(08)60015-6. [DOI] [PubMed] [Google Scholar]
- 39.Monod J. La technique de culture continue théorie et applications. Ann Inst Pasteur. 1950;79:390–410. [Google Scholar]
- 40.Ohmiya K, Nokura K, Shimizu S. Enhancement of cellulose degradation by Ruminococcus albus at high cellulose concentration. J Ferment Technol. 1983;61:25–30. [Google Scholar]
- 41.Pages S, Belaich A, Fierobe H P, Tardif C, Gaudin C, Belaich J P. Sequence analysis of scaffolding protein CipC and ORFXp, a new cohesin-containing protein in Clostridium cellulolyticum: comparison of various cohesin domains and subcellular localization of ORFXp. J Bacteriol. 1999;181:1801–1810. doi: 10.1128/jb.181.6.1801-1810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pages S, Belaich A, Tardif C, Reverbel-Leroy C, Gaudin C, Belaich J P. Interaction between the endoglucanase CelA and the scaffolding protein CipC of the Clostridium cellulolyticum cellulososme. J Bacteriol. 1996;178:2279–2286. doi: 10.1128/jb.178.8.2279-2286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pages S, Gal L, Belaich A, Gaudin C, Tardif C, Belaich J P. Role of scaffolding protein CipC of Clostridium cellulolyticum in cellulose degradation. J Bacteriol. 1997;179:2810–2816. doi: 10.1128/jb.179.9.2810-2816.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pavlostathis S G, Miller T L, Wolin M J. Kinetics of insoluble cellulose fermentation by continuous cultures of Ruminococcus albus. Appl Environ Microbiol. 1988;54:2660–2663. doi: 10.1128/aem.54.11.2660-2663.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petitdemange E, Caillet F, Giallo J, Gaudin C. Clostridium cellulolyticum sp. nov., a cellulolytic mesophilic species from decayed grass. Int J Syst Bacteriol. 1984;34:155–159. [Google Scholar]
- 46.Pirt S J. Principles of microbe and cell cultivation. Oxford, United Kingdom: Blackwell Scientific Publishers; 1975. [Google Scholar]
- 47.Russell J B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985;49:572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russell J B. Strategies that ruminal bacteria use to handle excess carbohydrate. J Anim Sci. 1998;76:1955–1963. doi: 10.2527/1998.7671955x. [DOI] [PubMed] [Google Scholar]
- 49.Weimer P J, Shi Y, Odt C L. A segmented gas/liquid delivery system for continuous culture of microorganisms on insoluble substrates and its use for growth of Ruminococcus flavefaciens on cellulose. Appl Microbiol Biotechnol. 1991;36:178–183. [Google Scholar]
- 50.Wells J E, Russell J B, Shi Y, Weimer P J. Cellodextrin efflux by the cellulolytic ruminal bacterium Fibrobacter succinogenes and its potential role in the growth of nonadherent bacteria. Appl Environ Microbiol. 1995;61:1757–1762. doi: 10.1128/aem.61.5.1757-1762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolin M J, Miller T L. Bioconversion of organic carbon to CH4 and CO2. Geomicrobiol J. 1987;5:239–259. [Google Scholar]
- 52.Zeng A P. Continuous culture. In: Demain A L, Davies J E, editors. Manual of industrial microbiology and biotechnology. Washington, D.C.: ASM Press; 1999. pp. 151–164. [Google Scholar]