Abstract
An investigation of cellulose degradation by the nonruminal, cellulolytic, mesophilic bacterium Clostridium cellulolyticum was performed in cellulose-fed chemostat cultures with ammonium as the growth-limiting nutrient. At any dilution rate (D), acetate was always the main product of the catabolism, with a yield of product from substrate ranging between 37.7 and 51.5 g per mol of hexose equivalent fermented and an acetate/ethanol ratio always higher than 1. As D rose, the acetyl coenzyme A was rerouted in favor of ethanol pathways, and ethanol production could represent up to 17.7% of the carbon consumed. Lactate was significantly produced, but with increasing D, the specific lactate production rate declined, as did the specific rate of production of extracellular pyruvate. The proportion of the original carbon directed towards phosphoglucomutase remained constant, and the carbon surplus was balanced mainly by exopolysaccharide and glycogen biosyntheses at high D values, while cellodextrin excretion occurred mainly at lower ones. With increasing D, the specific rate of carbon flowing down catabolites increased as well, but when expressed as a percentage of carbon it declined, while the percentage of carbon directed through biosynthesis pathways was enhanced. The maximum growth and energetic yields were lower than those obtained in cellulose-limited chemostats and were related to an uncoupling between catabolism and anabolism leading to an excess of energy. Compared to growth on cellobiose in ammonium-limited chemostats (E. Guedon, M. Desvaux, and H. Petitdemange, J. Bacteriol. 182:2010–2017, 2000), (i) a specific consumption rate of carbon of as high as 26.72 mmol of hexose equivalent g of cells−1 h−1 could not be reached and (ii) the proportions of carbon directed towards cellodextrin, glycogen, and exopolysaccharide pathways were not as high as first determined on cellobiose. While the use of cellobiose allows highlighting of metabolic limitation and regulation of C. cellulolyticum under ammonium-limited conditions, some of these events should then rather be interpreted as distortions of the metabolism. Growth of cellulolytic bacteria on easily available carbon and nitrogen sources represents conditions far different from those of the natural lignocellulosic compounds.
Clostridium cellulolyticum, a nonruminal, cellulolytic, mesophilic bacterium isolated from decayed grass (22), has enabled the catabolization of cellulosic materials. Lignocellulosic compounds usually contain high levels of carbon and low levels of nitrogen (10). Thus, in microbiota where cellulose degradation has occurred, a nitrogen-limited condition is most probably encountered by bacteria (1, 15, 16, 18, 22). A recent growth study of C. cellulolyticum under ammonium limitation indicated the importance of glucose-1-phosphate (G1P) and glucose-6-phosphate (G6P) metabolic nodes for the distribution of carbon flow inside and outside the bacterial cell (10). Yet, as was the case for most of the first investigations carried out with C. cellulolyticum, that study was performed with cellobiose, a soluble cellodextrin, which obviated the need for metabolic analysis on cellulose, where most difficulties in culture monitoring lay.
Recent investigations have shown that bypassing the cellulosome when C. cellulolyticum is grown on soluble glucide induces metabolic deregulation compared to growth on insoluble cellulose (4, 5, 7). Thus, some of the metabolic events previously observed on cellobiose would rather be interpreted as laboratory artifacts due to the use of a soluble substrate far different physically from cellulose (7); in the same way, the cultivation of C. cellulolyticum in a complex medium or under unregulated pH conditions appeared to be deleterious for optimum growth (6, 9, 11, 21) and aberrant compared to the natural bacterial ecosystem (14, 22).
The aim of the present work was thus to investigate, using the chemostat technique, how the fluxes of carbon metabolism were modified when C. cellulolyticum was grown in ammonium limitation with cellulose as the sole carbon and energy source.
MATERIALS AND METHODS
Organism and growth.
C. cellulolyticum ATCC 35319 (22) was grown in a defined medium (11) containing various amount of cellulose MN301 (Macherey-Nagel, Düren, Germany) and ammonium as specified in Results. All experiments were performed in a segmented gas-liquid chemostat (5).
Analytical procedures.
Biomass, cellulose concentration, ammonium, gas analysis, extracellular protein, amino acid, glucose, soluble cellodextrins, glycogen, acetate, ethanol, lactate, and extracellular pyruvate were determined as previously described (4–7, 10, 11). The intracellular compounds NAD+, NADH, ATP, ADP, and AMP and the enzymes pyruvate-ferredoxin (pyruvate-Fd) oxidoreductase (PFO) (EC 1.2.7.1), lactate dehydrogenase (EC 1.1.1.27), acetate kinase (EC 2.7.2.1), and alcohol dehydrogenase (EC 1.1.1.1) were extracted and assayed as described previously (4–7).
Calculations and mapping of carbon flow.
The distribution of the carbon flow within the central metabolic pathways of C. cellulolyticum when grown under cellulose-sufficient conditions was previously described (5–7).
The calculation of the specific rates qcellulose, qacetate, qethanol, qextracellular pyruvate, qlactate, qpyruvate, qNADH produced, qNADH used, qNADH-Fd, and qATP was described previously (7). YAce/S, YEth/S, and YLac/S are the yields of acetate, ethanol, and lactate from substrate, respectively, expressed in grams per mole of hexose equivalent (hexose eq) fermented. The molar yields of growth (YX/S) and energy (YATP) and the maintenance coefficient (m) were determined as already reported (7). The global carbon balance, the energetic charge (EC), the oxidation/reduction index (O/R), the catabolic reduction charge (CRC), the energetic efficiency, the pool turnover, and the ratio of specific enzyme activity to metabolic flux (R) were calculated as indicated previously (5–7).
For stoichiometric modeling of C. cellulolyticum metabolism, the calculations of flux through each enzyme of the known metabolic pathway, expressed in milliequivalents of carbon (meqC) per gram of cells per hour, were done as previously described (5–7).
RESULTS
Cellulose degradation and biomass formation under ammonium-limited conditions.
Preliminary results with cellulose-fed continuous cultures indicated that at above 8 g of cellulose liter−1, biomass formation did not further increase even when the concentrations of ammonium or other nutrients were increased (data not shown), indicating that continuous cultures were then under carbon excess (31). With 4.0 mM ammonium, the nitrogen source was limited (10), since the residual ammonium concentration was 0.09 mM at the lowest D tested and reached 3.02 mM at a D value of 0.085 h−1 (Table 1); such data are typical of continuous cultures performed under limitation of a selected nutrient (31). C. cellulolyticum was then cultured under cellulose-sufficient conditions (around 18.7 g of cellulose liter−1) with ammonium as the growth-limiting nutrient (4.0 mM) (Table 1).
TABLE 1.
Cellulose fermentation parameters from continuous steady-state culturesa of C. cellulolyticum under ammonium-limited conditions
| Parameter (unit) | Resultsb obtained at a D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.027 | 0.047 | 0.064 | 0.085 | |
| Biomass (g liter−1) | 0.187 ± 0.021 | 0.167 ± 0.015 | 0.154 ± 0.017 | 0.062 ± 0.008 |
| Consumed cellulose (mM hexose eq) | 11.0 ± 0.6 | 7.5 ± 0.4 | 6.1 ± 0.3 | 2.1 ± 0.1 |
| Residual ammonium (mM) | 0.09 ± 0.01 | 0.80 ± 0.09 | 1.25 ± 0.13 | 3.02 ± 0.27 |
| Acetate (mM) | 12.29 ± 0.63 | 7.68 ± 0.38 | 5.51 ± 0.28 | 1.73 ± 0.09 |
| Ethanol (mM) | 2.19 ± 0.11 | 2.45 ± 0.13 | 2.60 ± 0.15 | 1.12 ± 0.07 |
| Lactate (mM) | 1.58 ± 0.09 | 0.54 ± 0.04 | 0.32 ± 0.02 | 0.05 ± 0.01 |
| Extracellular pyruvate (μM) | 257.1 ± 13.1 | 102.8 ± 5.5 | 54.2 ± 2.9 | NDc |
| H2CO2 ratio | 1.65 | 1.57 | 1.46 | 1.24 |
| Glycogen (mg g of cells−1) | 147.9 ± 4.7 | 164.0 ± 5.3 | 151.2 ± 5.1 | 137.6 ± 4.4 |
| Cellobiose (mM) | 0.35 ± 0.13 | 0.17 ± 0.07 | 0.09 ± 0.04 | ND |
| Cellotriose (mM) | 0.11 ± 0.05 | 0.08 ± 0.02 | 0.05 ± 0.01 | ND |
| Extracellular proteins (mg liter−1) | 10.4 ± 0.7 | 4.6 ± 0.4 | 4.4 ± 0.3 | 0.9 ± 0.1 |
| Free amino acids (mg liter−1) | 102.6 ± 4.1 | 63.3 ± 2.5 | 50.0 ± 2.7 | 15.4 ± 0.8 |
| Carbon recovery (%) | 92.5 | 93.0 | 92.8 | 93.1 |
The cellulose input was around 1.87% (wt/vol) (i.e., 115.4 mM hexose eq) and ammonium was at 4.00 mM.
Values are the averages for samples at steady state ± standard deviations. Values without standard deviations were determined with an average accuracy of ±10%.
ND, not detectable.
At the steady state of the chemostat, biomass was maximum at the lowest D tested, i.e., 0.187 g liter−1, and declined further as D rose, to reach 0.062 g liter−1 at a D value of 0.085 h−1 (Table 1). Under these culture conditions, some undigested cellulose was always left by C. cellulolyticum (Table 1) and represented a high proportion of the original cellulose provided by the feed reservoir; the percentage of remaining cellulose ranged between 90.5 and 98.2%. In all of the runs, microscopic examination of the culture revealed the presence of exopolysaccharides and that most bacteria adhered to the cellulose fibers and few bacteria were found free in the supernatant. Substantial amounts of cellodextrins, namely, cellobiose and cellotriose were detected in the supernatant (Table 1). Intracellular glycogen was produced at all D values and ranged from 137.6 to 164.0 mg g of cells−1 (Table 1); cell growth under carbon excess and nitrogen limitation is usually the best condition for glycogen storage (24–26). The global carbon balance, taking into account acetate, ethanol, lactate, extracellular pyruvate, free amino acids, extracellular proteins, cellodextrins, and biomass, ranged between 92.5 and 93.1% (Table 1).
Impact of ammonium limitation on bacterial cellulose conversion.
The percentage of carbon flowing toward fermentative metabolites, given by the ratio qpyruvate/qcellulose (Table 2), indicated that 68.8 to 74.4% of the consumed cellulose was converted to extracellular pyruvate, lactate, CO2, acetate, and ethanol. Acetate always remained the predominant fermentation end product, since it represented between 59.3 and 75.3% of the carbon flowing down the catabolite. As D rose, qacetate and qethanol increased 1.3- and 4.8-fold, respectively, but the acetate/ethanol ratio then decreased from 5.61 to 1.54 (Table 2). The specific lactate production rate, however, decreased with D. The NADH balance (qNADH produced/qNADH used) was calculated from the catabolic pathways producing and consuming reducing equivalents. Both qNADH produced and qNADH used increased with D from 2.36 to 3.98 mmol g of cells−1 h−1 and from 0.86 to 3.14 mmol g of cells−1 h−1, respectively, whereas the qNADH produced/qNADH used ratio declined from 2.74 to 1.27 (Table 2). NAD+ is reduced during the biosynthesis of acetate, lactate, and ethanol by glyceraldehyde-3-phosphate dehydrogenase, but the regeneration of the NAD+ pool can occur only during lactate and ethanol production via dehydrogenase activities; NADH is then really oxidized by ethanol and lactate metabolic pathways, while acetate biosynthesis only generates reducing equivalents. Despite this apparent imbalance when only catabolic pathways were taken into account, the intracellular NADH/NAD+ ratio was always lower than 1 and the CRC remained constant at ca. 0.35 (Table 3). As previously observed (7), an efficient reoxidation of NADH via H2 production in addition to carbon fermentative pathways was underlined by O/R, H2/CO2, and qNADH produced/qNADH used variation.
TABLE 2.
Specific rates and yields of product formation in cellulose chemostats with ammonium as a limiting nutrient
| Parameter (unit) | Resultsa obtained at D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.027 | 0.047 | 0.064 | 0.085 | |
| Specific rates | ||||
| qcellulose (mmol g of cells−1 h−1) | 1.58 | 2.10 | 2.52 | 2.90 |
| qpyruvate (mmol g of cells−1 h−1) | 2.36 | 3.03 | 3.53 | 3.98 |
| qacetate (mmol g of cells−1 h−1) | 1.77 | 2.16 | 2.29 | 2.38 |
| qethanol (mmol g of cells−1 h−1) | 0.32 | 0.69 | 1.08 | 1.54 |
| qlactate (mmol g of cells−1 h−1) | 0.23 | 0.15 | 0.13 | 0.07 |
| qNADH produced (mmol g of cells−1 h−1) | 2.36 | 3.03 | 3.53 | 3.98 |
| qNADH used (mmol g of cells−1 h−1) | 0.86 | 1.53 | 2.29 | 3.14 |
| qNADH-Fd (mmol g of cells−1 h−1) | 1.50 | 1.49 | 1.23 | 0.84 |
| qATP (mmol g of cells−1 h−1) | 3.96 | 4.99 | 5.59 | 6.12 |
| Yields: | ||||
| YAce/S (g mol of hexose eq−1) | 51.5 | 47.4 | 41.8 | 37.7 |
| YEth/S (g mol of hexose eq−1) | 9.2 | 15.1 | 19.7 | 24.4 |
| YLac/S (g mol of hexose eq−1) | 6.6 | 3.4 | 2.4 | 1.0 |
| YX/S (g of cells mol of hexose eq−1) | 17.1 | 22.4 | 25.4 | 29.3 |
| YATP (g of cells mol of ATP−1) | 6.8 | 9.4 | 11.4 | 13.9 |
| O/R | 1.02 | 0.97 | 0.95 | 0.99 |
| qNADH producedqNADH used | 2.74 | 1.98 | 1.54 | 1.27 |
| ATP-Effb | 2.50 | 2.37 | 2.22 | 2.11 |
Values calculated were determined with an average accuracy of ±10%.
ATP-Eff, energetic efficiency.
TABLE 3.
Adenylate and pyridine nucleotide contents of C. cellulolyticum cells at steady state
| Parameter (unit) | Resultsa obtained at D value (h−1) of:
|
|||
|---|---|---|---|---|
| 0.027 | 0.047 | 0.064 | 0.085 | |
| ATP (μmol g of cells−1) | 3.72 ± 0.19 | 4.16 ± 0.22 | 4.12 ± 0.20 | 3.24 ± 0.15 |
| ADP (μmol g of cells−1) | 1.54 ± 0.09 | 1.78 ± 0.08 | 2.42 ± 0.13 | 2.61 ± 0.14 |
| AMP (μmol g of cells−1) | 0.22 ± 0.02 | 0.45 ± 0.05 | 0.31 ± 0.04 | 0.23 ± 0.03 |
| EC | 0.82 | 0.79 | 0.78 | 0.75 |
| NADH (μmol g of cells−1) | 5.37 ± 1.06 | 4.23 ± 0.92 | 6.41 ± 1.33 | 5.77 ± 1.24 |
| NAD+ (μmol g of cells−1) | 10.33 ± 2.09 | 8.52 ± 1.83 | 11.47 ± 2.34 | 9.78 ± 2.01 |
| NADH/NAD+ ratio | 0.52 | 0.50 | 0.56 | 0.59 |
| CRC | 0.34 | 0.33 | 0.36 | 0.37 |
Values are the averages for samples at steady state ± standard deviations. Values without standard deviations calculated were determined with an average accuracy of ±10%.
In terms of the yield of product from substrate, both acetate and lactate decreased with increasing D, from 51.5 to 37.7 g mol of hexose eq−1 and from 6.6 to 1.0 g mol of hexose eq−1, respectively, while YEth/S was enhanced from 9.2 to 24.4 g mol of hexose eq−1 (Table 2). From the lowest to the highest D tested, the observed cell yields (YX/S) increased from 17.1 to 29.3 g of cells per mol of hexose eq consumed (Table 2); the YX/S is affected by both the microbial endogenous metabolism and maintenance energy requirements. From a Pirt plot of the data (r2 = 0.995), a true growth yield (Y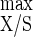 ) of 41.8 g of biomass mol of hexose eq consumed−1 and an m value of 0.9 mmol of hexose eq g of cells−1 h−1 were determined. The apparent energetic yield (YATP) increased from 6.8 to 13.9 g of cells mol of ATP−1 as D rose (Table 2). From a Pirt plot (r2 = 0.992), a Y
) of 41.8 g of biomass mol of hexose eq consumed−1 and an m value of 0.9 mmol of hexose eq g of cells−1 h−1 were determined. The apparent energetic yield (YATP) increased from 6.8 to 13.9 g of cells mol of ATP−1 as D rose (Table 2). From a Pirt plot (r2 = 0.992), a Y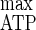 of 24.6 g of cells mol of ATP−1 and an mATP of 2.9 mmol of ATP g of cells−1 h−1 were determined. A mean value of 0.79 could be maintained for the adenylate EC for all of the dilution rates tested (Table 3).
of 24.6 g of cells mol of ATP−1 and an mATP of 2.9 mmol of ATP g of cells−1 h−1 were determined. A mean value of 0.79 could be maintained for the adenylate EC for all of the dilution rates tested (Table 3).
Metabolic flux analysis of cellulose utilization in ammonium-limited chemostats.
The modification of the carbon flow distribution in the central metabolic pathway of C. cellulolyticum when grown under ammonium-limited conditions with cellulose as the sole carbon and energy source is shown in Table 4. The rate of cellulose consumption varied from 9.50 to 17.39 meqC g of cells−1 h−1 from the lowest to the highest D tested (Table 4). With increasing D, qpyruvate increased as well, but in terms of the percentage of the original carbon uptake, it represented from 74.4 to 68.8%, while carbon through biosynthesis pathways varied from 18.2 to 24.5% (Table 4). Regardless of D, most of the carbon directed toward biosynthesis was attributed to biomass of between 11.3 and 19.4%, while both extracellular proteins and free amino acids represented a proportion of the original carbon of between 5.2 and 6.9%.
TABLE 4.
Carbon fluxes in cellulose-fed chemostats of C. cellulolyticum under ammonium-limited conditions
| Carbon flowa | Results obtained at a D value (h−1) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.027
|
0.047
|
0.064
|
0.085
|
|||||
| meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | meqC g of cells−1 h−1 | % | |
| qcellulose | 9.50 | 100.0 | 12.61 | 100.0 | 15.11 | 100.0 | 17.39 | 100.0 |
| qGIP | 5.98 | 68.0 | 7.94 | 63.0 | 9.51 | 63.0 | 10.95 | 63.0 |
| qcellodextrin | 0.89 | 9.3 | 1.04 | 8.0 | 0.81 | 5.4 | NDb | ND |
| qGIP towards cellodextrin | 0.49 | 5.2 | 0.58 | 4.6 | 0.46 | 3.1 | ND | ND |
| qβ-glucan towards cellodextrin | 0.40 | 4.2 | 0.43 | 3.4 | 0.34 | 2.3 | ND | ND |
| qglycogen | 0.15 | 1.6 | 0.29 | 2.3 | 0.36 | 2.4 | 0.43 | 2.5 |
| qexopolysaccharide | 0.07 | 0.7 | 0.02 | 0.1 | 0.26 | 1.7 | 0.76 | 4.4 |
| qglucose | 3.52 | 37.0 | 4.67 | 37.0 | 5.60 | 37.0 | 6.44 | 37.0 |
| qphosphoglucomutase | 5.27 | 55.5 | 7.06 | 56.0 | 8.43 | 55.8 | 9.79 | 56.3 |
| qG6P | 8.79 | 92.5 | 11.73 | 93.0 | 14.02 | 92.8 | 16.20 | 93.2 |
| qbiosynthesis | 1.73 | 18.2 | 2.63 | 20.9 | 3.45 | 22.8 | 4.26 | 24.5 |
| qpyruvate | 7.07 | 74.4 | 9.10 | 72.2 | 10.58 | 70.0 | 11.94 | 68.6 |
| qacetyl-CoA | 4.18 | 44.0 | 5.70 | 45.2 | 6.74 | 44.6 | 7.83 | 45.0 |
| qlactate | 0.68 | 7.2 | 0.46 | 3.6 | 0.40 | 2.6 | 0.20 | 1.1 |
| qextracellular pyruvate | 0.11 | 1.2 | 0.09 | 0.7 | 0.07 | 0.4 | ND | ND |
| qcarbon dioxide | 2.09 | 22.0 | 2.85 | 22.6 | 3.37 | 22.3 | 3.91 | 22.5 |
| qethanol | 0.63 | 6.7 | 1.38 | 10.9 | 2.16 | 14.3 | 3.08 | 17.7 |
| qacetate | 3.55 | 37.3 | 4.32 | 34.3 | 4.58 | 30.3 | 4.75 | 27.3 |
Carbon flows were calculated as specified in Materials and Methods.
ND, not determined.
Carbon flux was distributed differently over the known catabolic routes (acetate, ethanol, carbon dioxide, extracellular pyruvate, and lactate) as a function of D. One part of the flux was converted to acetyl coenzyme A (acetyl-CoA) via PFO. As D rose, qacetyl-CoA increased, while the proportion of carbon flowing through PFO remained quite constant at around 44.7% (Table 4). qacetate and qethanol increased with D, but, expressed as a percentage of qcellulose, it appeared that the carbon fluxes split differently at this metabolic branch point; qacetate production declined from 37.3 to 27.3% of the carbon uptake, while ethanol increased from 6.7 to 17.7% of the cellulose fermented (Table 4). Another part of the carbon flowing down glycolysis was oriented towards the lactate metabolic pathway, where lactate production dropped from 7.2 to 1.1%, as did the pyruvate leak, which decreased from 1.2 to be nil at the highest D tested (Table 4). The ratio of specific enzymatic activity to specific metabolic production rate (R) was always higher than 1 with the enzymes tested (Table 5). At each step in the central metabolic pathways, the intracellular concentrations of substrates, products, and cofactor and effector molecules, as well as intracellular ionic strength, redox potential, or pH, can influence the partitioning and regulation of the carbon flux (12). Nevertheless, the fact that fluxes were much less than the available enzyme activity indicated that the carbon flows were determined by the concentration of substrate available more than by the enzyme activity (13).
TABLE 5.
Specific enzymatic activities in C. cellulolyticum cell extract at steady state in cellulose-fed chemostats with ammonium limitation
| Enzymea | Results obtained at D value (h−1) of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.027
|
0.047
|
0.064
|
0.085
|
|||||
| Sp actb | Rc | Sp act | R | Sp act | R | Sp act | R | |
| LDH (EC 1.1.1.27) | 0.38 ± 0.08 | 74.4 | 0.25 ± 0.04 | 73.2 | 0.21 ± 0.05 | 68.4 | 0.12 ± 0.03 | 77.7 |
| PFO (EC 1.2.7.1) | 0.60 ± 0.05 | 12.8 | 0.76 ± 0.09 | 11.9 | 0.92 ± 0.14 | 12.1 | 1.02 ± 0.11 | 11.6 |
| AK (EC 2.7.2.1) | 0.78 ± 0.09 | 19.6 | 0.76 ± 0.08 | 15.6 | 0.69 ± 0.07 | 13.4 | 0.63 ± 0.05 | 11.8 |
| ADH (EC 1.1.1.1) | 0.18 ± 0.03 | 25.1 | 0.31 ± 0.05 | 19.9 | 0.38 ± 0.04 | 15.7 | 0.49 ± 0.06 | 14.2 |
LDH, lactate dehydrogenase; AK, acetate kinase; ADH, alcohol dehydrogenase.
Expressed in micromoles minute−1 milligram of proteins−1. Results are means and standard deviations.
R, ratio of specific enzymatic activity to metabolic flux through the considered metabolic path; flux was expressed as micromoles miligram of protein−1 minute−1.
The G6P pool was fueled by the carbon flowing from glucokinase and phosphoglucomutase activities and was further metabolized by glycolysis. The qG6P increased from 8.79 to 16.23 meqC g of cells−1 h−1 with higher D and represented a mean of 92.3% of the carbon uptake (Table 4). The G1P came from cellodextrin phosphorylase activity. From this metabolic node and under these culture conditions, G1P could either be stored as glycogen, be converted to exopolysaccharide or cellodextrin, or flow down the glycolysis via phosphoglucomutase. The proportion of G1P directed towards cellodextrin declined from 5.2% at a D of 0.027 h−1 to nil at a D of 0.085 h−1, since no cellodextrin could then be detected (Tables 1 and 5). At low D, the percentages of carbon metabolized as exopolysaccharide and glycogen were low, and they increased to reach 4.4 and 2.5%, respectively, with the highest D tested (Table 4). The proportion of the carbon flux which was converted to cellodextrins at low D was then rerouted towards glycogen and exopolysaccharide at higher D values. The carbon flow via phosphoglucomutase increased from 5.27 to 9.79 meqC g of cells−1 h−1, but the proportion of the original carbon flowing through this metabolic pathway remained quite constant, ranging from 55.5 to 56.3% (Table 4).
DISCUSSION
In ammonium-limited chemostats with cellulose, the main product of cellulose catabolism was acetate. The proportion of the carbon flowing down PFO remained quite constant, but acetyl-CoA split differently as D rose. At low D, acetate production was favored, but acetyl-CoA was reoriented towards ethanol metabolic pathways as D rose, increasing the proportion of the carbon flux 2.6-fold towards ethanol from the highest to the lowest D tested. The specific lactate production rate as well as the pyruvate leak decreased with increasing D. A study of the interaction between carbon and nitrogen metabolisms in Fibrobacter succinogenes revealed modification of carbon fluxes (17), since addition of ammonium to resting cells metabolizing glucose induced acetate production.
When soluble β-glucans enter into the bacterial cell, they are first converted into G1P and G6P (4–7). Under ammonium-limited conditions, the proportion of the carbon flowing via phosphoglucomutase was quite constant at around 55.9%. The remaining G1P flowed in favor of cellodextrin biosynthesis at low D and towards exopolysaccharide and glycogen as D rose. On cellobiose, cellotriose production could represent up to 16.7% of the carbon uptake, while a maximum of 3.3% was obtained on cellulose. On cellobiose, as with cellulose, no cellodextrin longer than cellotriose was detected extracellularly (10). In addition, exopolysaccharide biosynthesis could account for 16.0% of the cellobiose fermented (10), against a maximum of 4.2% with cellulose as the carbon substrate. The stoichiometric model equation for exopolysaccharide formation gave consistent results when data from ammonium-limited chemostats with cellobiose (10) were applied to the equations developed in the present investigation. For example, at D = 0.013 h−1 a qexopolysaccharide of 0.41 meqC g of cells−1 h−1 was obtained, against 0.54 meqC g of cells−1 h−1 using our equations, and at D = 0.115 h−1 a qexopolysaccharide of 4.43 meqC g of cells−1 h−1 was obtained, against 4.65 meqC g of cells−1 h−1 using the equations developed in the present work.
In ammonium-limited chemostats with cellulose as the sole carbon and energy source, both Y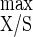 , i.e., 41.8 g of biomass mol of hexose eq consumed−1, and Y
, i.e., 41.8 g of biomass mol of hexose eq consumed−1, and Y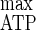 , i.e., 24.6 g of cells mol of ATP−1, were lower than those under cellulose-limited conditions, where Y
, i.e., 24.6 g of cells mol of ATP−1, were lower than those under cellulose-limited conditions, where Y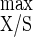 = 50.5 g of biomass mol of hexose eq consumed−1and Y
= 50.5 g of biomass mol of hexose eq consumed−1and Y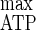 = 30.3 g of cells mol of ATP−1 (5), while m and mATP did not vary compared to those obtained with cellulose limitation (5). On the basis of the Y
= 30.3 g of cells mol of ATP−1 (5), while m and mATP did not vary compared to those obtained with cellulose limitation (5). On the basis of the Y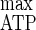 (29, 30), the chemostat cultures with ammonium limitation used ATP inefficiently, and the calculated rate of spilling of ATP (qATP) was higher than that under cellulose-limited conditions (5). Such a decline of growth and energetic yields was related to the uncoupling between anabolism, which is limited by the nitrogen source, and catabolism, which is not limited by the carbon source, leading to an excess of energy. Such a phenomenon is generally encountered under such culture conditions and is not eliminated by the use of insoluble cellulose, where the entering carbon flow is nevertheless limited by the depolymerization rate of the cellulosome cellulases. Some continuous cultures performed under limitation of nutrients other than the carbon source gave higher rates of carbon substrate utilization when the carbon was in excess than when the carbon was limited; these cultures had a greatly increased maintenance energy requirement and used the remaining energy more efficiently than in carbon-limited chemostats (19, 20, 28). Since maintenance energy, which corresponds to the expenditure of energy towards functions that are not directly growth related, did not increase, additional maintenance of growth potential which involved slip reactions was not taken into account when C. cellulolyticum was cultivated in ammonium limitation with cellulose as the sole carbon and energy source (20, 28) and the wasting of energy associated with maintenance function did not occur (3).
(29, 30), the chemostat cultures with ammonium limitation used ATP inefficiently, and the calculated rate of spilling of ATP (qATP) was higher than that under cellulose-limited conditions (5). Such a decline of growth and energetic yields was related to the uncoupling between anabolism, which is limited by the nitrogen source, and catabolism, which is not limited by the carbon source, leading to an excess of energy. Such a phenomenon is generally encountered under such culture conditions and is not eliminated by the use of insoluble cellulose, where the entering carbon flow is nevertheless limited by the depolymerization rate of the cellulosome cellulases. Some continuous cultures performed under limitation of nutrients other than the carbon source gave higher rates of carbon substrate utilization when the carbon was in excess than when the carbon was limited; these cultures had a greatly increased maintenance energy requirement and used the remaining energy more efficiently than in carbon-limited chemostats (19, 20, 28). Since maintenance energy, which corresponds to the expenditure of energy towards functions that are not directly growth related, did not increase, additional maintenance of growth potential which involved slip reactions was not taken into account when C. cellulolyticum was cultivated in ammonium limitation with cellulose as the sole carbon and energy source (20, 28) and the wasting of energy associated with maintenance function did not occur (3).
High intracellular concentrations of glycogen are often found when growth is limited, e.g., by phosphorus, sulfur, and nitrogen and in the presence of an excess of a carbon source (24–26). Here, the intracellular glycogen concentrations, i.e., between 137.6 and 164.0 mg g of cells−1 and representing a proportion of the cellulose uptake by the cell of between 1.6 and 2.5%, were higher than those in cellulose-limited chemostats, which were between 58.8 and 108.7 mg g of cells−1 and represented between 0.4 and 1.7% of the cellulose consumed (5). Compared to those of cellulolytic rumen bacteria, which can accumulate intracellular polysaccharide representing from around 30% and up to 60% of the cell dry weight (27), the glycogen storage capacity of C. cellulolyticum, even under the culture conditions used here, was more limited, since it did not exceed 16% of the cell dry weight. Under these culture conditions, glycogen was present at all dilution rates. Previous investigations with C. cellulolyticum suggested that the glycogen turnover was involved in carbon flow regulation (5, 10). Such observations could be compared with those on F. succinogenes, where glycogen turnover was observed with both cellobiose and cellulose (2, 8) and where study of the interaction of carbon and nitrogen metabolisms indicated that addition of ammonium to resting cells of F. succinogenes decreased the flux through glycogen biosynthesis (17).
In ammonium limitation with cellulose, the carbon flows down cellodextrin, exopolysaccharide, and glycogen were not as high as those with cellobiose. Such a metabolic event seems to be strongly related to the specific rate of consumption of the carbon substrate, which could reach 26.72 meqC g of cells−1 h−1 on cellobiose, against 17.39 meqC g of cells−1 h−1 on cellulose (Table 4). The use of a soluble carbon substrate allows highlighting of bacterial metabolic limitation by triggering deregulation of the metabolism (4–7, 10). On cellulose, a substrate more closely related to its natural ecosystem, C. cellulolyticum was enabled to deal with ammonium limitation. However, it should be pointed out that many of the anaerobic environments in which cellulose is degraded are deficient in combined nitrogen (14, 16, 23), and several previously described cellulolytic clostridia exhibited ammonium-repressible nitrogenase activity (15, 16, 18). Hence, in the same way that growth of C. cellulolyticum with soluble glucide appeared as an aberration when taking into account the natural bacterial ecosystem, cultures with ammonium as a sole and easily available nitrogen source could distort the bacterial metabolism, as suggested by a recent study with Clostridium hungatei (18). This could further explain the presence of free amino acids in the culture supernatant even with ammonium limitation, which suggests that the uptake of nutrients and the generation of biosynthetic precursors occur faster than their utilization for biomass production. In the environment, natural lignocellulosic compounds contain hemicellulose and lignine in addition to cellulose fibers. The proportion and fitting together of these biopolymers and the nature of the nitrogen source certainly influence the degradative capacities of C. cellulolyticum, underlining that much remain to be learned about the N2-fixing ability of and the catabolism of lignocellulose by cellulolytic bacteria.
ACKNOWLEDGMENTS
This work was supported by the Commission of European Communities FAIR program (contract CT950191 [DG12SSMA]) and by the Agrice program (contract 9701041).
We thank Sabine d'Andrea and Guy Raval for excellent technical assistance and Edward McRae for correcting the English and for critical reading of the manuscript.
REFERENCES
- 1.Bayer E A, Lamed R. The cellulose paradox: pollutant par excellence and/or a reclaimable natural resource? Biodegradation. 1992;3:171–188. doi: 10.1007/BF00129082. [DOI] [PubMed] [Google Scholar]
- 2.Bibollet X, Bosc N, Matulova M, Delort A M, Gaudet G, Forano E. 13C and 1H NMR study of cellulose metabolism by Fibrobacter succinogenes S85. J Biotechnol. 2000;77:37–47. doi: 10.1016/s0168-1656(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 3.Bond D R, Russell J B. A role for fructose 1,6-diphosphate in the ATPase-mediated energy-spilling reaction of Streptococcus bovis. Appl Environ Microbiol. 1996;62:2095–2099. doi: 10.1128/aem.62.6.2095-2099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desvaux M, Guedon E, Petitdemange H. Cellulose catabolism by Clostridium cellulolyticum growing in batch culture on defined medium. Appl Environ Microbiol. 2000;66:2461–2470. doi: 10.1128/aem.66.6.2461-2470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desvaux M, Guedon E, Petitdemange H. Carbon flux distribution and kinetics of cellulose fermentation in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. J Bacteriol. 2001;183:119–130. doi: 10.1128/JB.183.1.119-130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desvaux M, Guedon E, Petitdemange H. Metabolic flux in cellulose batch and cellulose-fed continuous cultures of Clostridium cellulolyticum in response to acidic environment. Microbiology. 2001;147:1461–1471. doi: 10.1099/00221287-147-6-1461. [DOI] [PubMed] [Google Scholar]
- 7.Desvaux M, Guedon E, Petitdemange H. Kinetics and metabolism of cellulose degradation at high substrate concentrations in steady-state continuous cultures of Clostridium cellulolyticum on a chemically defined medium. Appl Environ Microbiol. 2001;67:3837–3845. doi: 10.1128/AEM.67.9.3837-3845.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudet G, Forano E, Dauphin G, Delort A M. Futile cycling of glycogen in Fibrobacter succinogenes as shown by in situ 1H-NMR and 13C-NMR investigation. Eur J Biochem. 1992;207:155–162. doi: 10.1111/j.1432-1033.1992.tb17032.x. [DOI] [PubMed] [Google Scholar]
- 9.Giallo J, Gaudin C, Belaich J P. Metabolism and solubilization of cellulose by Clostridium cellulolyticum H10. Appl Environ Microbiol. 1985;49:1216–1221. doi: 10.1128/aem.49.5.1216-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guedon E, Desvaux M, Petitdemange H. Kinetic analysis of Clostridium cellulolyticum carbohydrate metabolism: importance of glucose 1-phosphate and glucose 6-phosphate branch points for distribution of carbon fluxes inside and outside cells as revealed by steady-state continuous culture. J Bacteriol. 2000;182:2010–2017. doi: 10.1128/jb.182.7.2010-2017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guedon E, Desvaux M, Payot S, Petitdemange H. Growth inhibition of Clostridium cellulolyticum by an inefficiently regulated carbon flow. Microbiology. 1999;145:1831–1838. doi: 10.1099/13500872-145-8-1831. [DOI] [PubMed] [Google Scholar]
- 12.Holms H. The central metabolic pathways of Escherichia coli: relationship between flux and control at a branch point, efficiency of conversion to biomass, and excretion of acetate. Curr Top Cell Regul. 1986;28:69–105. doi: 10.1016/b978-0-12-152828-7.50004-4. [DOI] [PubMed] [Google Scholar]
- 13.Holms H. Flux analysis and control of the central metabolic pathways in Escherichia coli. FEMS Microbiol Rev. 1996;19:85–116. doi: 10.1111/j.1574-6976.1996.tb00255.x. [DOI] [PubMed] [Google Scholar]
- 14.Leschine S B, Canale-Parola E. Mesophilic cellulolytic clostridia from fresh water environments. Appl Environ Microbiol. 1983;46:728–737. doi: 10.1128/aem.46.3.728-737.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leschine S B, Canale-Parola E. Carbon cycling by cellulose-fermenting nitrogen-fixing bacteria. Adv Space Res. 1989;9:149–152. [Google Scholar]
- 16.Leschine S B, Holwell K, Canale-Parola E. Nitrogen fixation by anaerobic cellulolytic bacteria. Science. 1988;242:1157–1159. doi: 10.1126/science.242.4882.1157. [DOI] [PubMed] [Google Scholar]
- 17.Matheron C, Delort A M, Gaudet G, Liptaj T, Forano E. Interaction between carbon and nitrogen metabolism in Fibrobacter succinogenes S85: a 1H and 13C nuclear magnetic resonance and enzymatic study. Appl Environ Microbiol. 1999;65:1941–1948. doi: 10.1128/aem.65.5.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monserrate E, Leschine S B, Canale-Parola E. Clostridium hungatei sp. nov., a mesophilic N2-fixing cellulolytic bacterium isolated from soil. Int J Syst Evol Microbiol. 2001;51:123–132. doi: 10.1099/00207713-51-1-123. [DOI] [PubMed] [Google Scholar]
- 19.Neijssel O M, Tempest D W. The regulation of carbohydrate metabolism in Klebsiella aerogenes NCTC 418 organisms growing in chemostat culture. Arch Microbiol Physiol. 1975;106:251–258. doi: 10.1007/BF00446531. [DOI] [PubMed] [Google Scholar]
- 20.Neijssel O M, Tempest D W. Bioenergetics aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient culture. Arch Microb Physiol. 1976;107:215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- 21.Payot S, Guedon E, Cailliez C, Gelhaye E, Petitdemange H. Metabolism of cellobiose by Clostridium cellulolyticum growing in continuous culture: evidence for decreased NADH reoxidation as a factor limiting growth. Microbiology. 1998;144:375–384. doi: 10.1099/00221287-144-2-375. [DOI] [PubMed] [Google Scholar]
- 22.Petitdemange E, Caillet F, Giallo J, Gaudin C. Clostridium cellulolyticum sp. nov., a cellulolytic mesophilic species from decayed grass. Int J Syst Bacteriol. 1984;34:155–159. [Google Scholar]
- 23.Postgate J R. The fundamentals of nitrogen fixation. Cambridge, United Kingdom: Cambridge University Press; 1982. [Google Scholar]
- 24.Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- 25.Preiss J, Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–233. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- 26.Preiss J. Regulation of glycogen synthesis. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 1015–1024. [Google Scholar]
- 27.Russell J B. Strategies that ruminal bacteria use to handle excess carbohydrate. J Anim Sci. 1998;76:1955–1963. doi: 10.2527/1998.7671955x. [DOI] [PubMed] [Google Scholar]
- 28.Russell J B, Cook G M. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol Rev. 1995;59:48–62. doi: 10.1128/mr.59.1.48-62.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stouthamer A H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Leeuwenhoek. 1973;39:545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- 30.Stouthamer A H, Bettenhaussen C. Utilization of energy for growth and maintenance in continuous and batch cultures of microorganisms. Biochim Biophys Acta. 1973;301:53–70. doi: 10.1016/0304-4173(73)90012-8. [DOI] [PubMed] [Google Scholar]
- 31.Zeng A P. Continuous culture. In: Demain A L, Davies J E, editors. Manual of industrial microbiology and biotechnology. Washington, D.C.: ASM Press; 1999. pp. 151–164. [Google Scholar]


