Abstract
Neoadjuvant immune checkpoint blockade represents a novel approach for potentially decreasing the risk of recurrence in patients with nonmetastatic renal cell carcinoma (RCC). In this early phase clincal tiral, we evaluated the safety and tolerability of neoadjuvant treatment with the programmed cell death protein 1 (PD-1) inhibitor nivolumab in patients with nonmetastatic high-risk RCC. Non-primary endpoints included objective radiographic tumor response rate, immune-related pathologic response rate, quality of life alterations, and metastasis-free and overall survival. In total, 17 patients were enrolled in this study and underwent surgery without a delay after receiving three every-2-wk doses of neoadjuvant nivolumab. Adverse events (AEs) of any grade occurred in 14 (82.4%) patients, with two (11.8%) experiencing grade 3 events. Ten (58.8%) patients experienced an AE of any grade potentially attributable to nivolumab (all grade 1–2), and no grade 4–5 AEs occurred regardless of treatment attribution. The most common AEs were grade 1 fatigue (41.2%), grade 1 pruritis (29.4%), and grade 1 rash (29.4%). All evaluable patients had stable disease as per established radiographic criteria, with one (6.7%) demonstrating features of an immune-related pathologic response. Quality of life remained stable during treatment, with improvements relative to baseline noted at ≥6 mo postoperatively. Metastasis-free survival and overall survival were 85.1% and 100% at 2 yr, respectively.
Keywords: Renal cell carcinoma, Immunotherapy, Neoadjuvant therapy, Clinical trial
Patient summary:
In this study, we evaluated the safety and tolerability of preoperative administration of three doses of the immune checkpoint inhibitor nivolumab in patients with clinically localized high-risk renal cell carcinoma. We demonstrated the safety of this approach and found that, although most patients will not experience a radiographic response to treatment, a subset may have features of an immune-related pathologic response.
Multiple randomized trials have demonstrated improved treatment outcomes in patients with metastatic renal cell carcinoma (RCC) treated with inhibitors of the immune checkpoint molecule programmed cell death protein 1 (PD-1) or its ligand PD-L1 (reviewed by Rappold et al [1]). Consequently, there is growing interest in the use of these agents in the neoadjuvant and/or adjuvant settings in patients with nonmetastatic RCC who are at a high risk of disease recurrence [2,3]. Data on the impact of neoadjuvant immune checkpoint blockade on intra- and postoperative complications are relatively limited at the present time. Additionally, the effect of these agents on the primary tumor in cases of nonmetastatic RCC remains an open question, as current experience with prenephrectomy immune checkpoint blockade has been limited to retrospective studies involving mostly patients with metastatic disease [4–6]. Herein, we provide the results of an early phase clinical trial evaluating neoadjuvant administration of the PD-1 inhibitor nivolumab in patients with nonmetastatic high-risk clear cell RCC.
A prospective, open-label, single-arm trial (ClinicalTrials.gov identifier NCT02575222) was conducted to assess the primary endpoint of safety and tolerability of neoadjuvant nivolumab in patients nonmetastatic high-risk RCC. Patients received nivolumab (3 mg/kg) on day 1 of each of three consecutive 14-d cycles of therapy, followed by surgery within 7 d of completion of cycle 3 (Supplementary Fig. 1).
Patients with biopsy-confirmed nonmetastatic high-risk clear cell RCC (T2a-T4Nany M0 or Tany N1M0) planned to undergo radical or partial nephrectomy were assessed for enrollment. Complete inclusion/exclusion criteria are provided in the Supplementary material. A run-in phase of five patients was followed by continuous safety monitoring with stopping rules until 15 patients were enrolled with complete evaluable data for the primary and secondary endpoints.
The primary trial endpoint was safety and tolerability of nivolumab. Adverse events (AEs) were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. Additionally, surgical complications were recorded using the Clavien grading system. Secondary endpoints included objective radiographic tumor response rate as assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and immune-related response criteria (iRC), quality of life alterations using the National Comprehensive Cancer Network/Functional Assessment of Cancer Therapy-Kidney Symptom Index 19 (NCCN-FACT FKSI-19), and metastasis-free and overall survival. Additionally, immune-related pathologic response (irPR) was evaluated as an exploratory endpoint. Complete methodological details of the trial with references to previously described endpoint measures are provided in the Supplementary material.
Patients were monitored for AEs from day 1 of treatment to 3 mo postoperatively. Protocol-mandated clinical follow-up occurred at postoperative months 1, 3, 6, and 12. The trial was designed for an a priori safety endpoint where the risk of the following was <30%: (1) clinically significant drug toxicities resulting in treatment discontinuation and/or (2) grade ≥III postoperative surgical complications.
Between February 2016 and June 2018, 23 patients were assessed for eligibility and 17 enrolled in the study (Supplementary Fig. 2). One patient was enrolled despite papillary RCC on biopsy, constituting a protocol deviation, but was included in the analysis of the primary safety endpoint. Another patient did not complete a posttreatment computed tomography (CT) scan prior to surgery and was excluded from the analysis of radiographic response. Patients had a median long-axis tumor diameter of 7.9 cm (interquartile range [IQR] 6.7–8.5), and six (35.3%), one (5.9%), seven (41.2%), and three (17.6%) patients had clinical T stages of T2a, T2b, T3a and T3b, respectively. All patients had clinical N0M0 disease at the time of enrollment. Additional details of the study cohort can be found in Supplementary Table 1.
All patients completed three doses of neoadjuvant nivolumab and underwent surgery without delay. The median time from study biopsy to surgery was 55 d (IQR 53–62). In total, 49 AEs were observed among 14 (82.4%) patients, with two (11.8%) experiencing grade 3 events (Supplementary Table 2). Ten (58.8%) patients experienced 25 (51.0%) AEs, which were potentially attributable to nivolumab (all grade 1–2). No grade 4–5 AEs occurred regardless of treatment attribution. The most common AEs were grade 1 fatigue (41.2%), grade 1 pruritis (29.4%), and grade 1 rash (29.4%). Only one patient experienced an intraoperative complication. The study’s surgeons reported no changes to the tissue planes (eg, adhesions, edema, or fibrosis) following neoadjuvant nivolumab, and the single intraoperative complication was not felt to be related to drug. No patient experienced a Clavien grade ≥III postoperative complication. Other surgical and pathologic data are presented in Supplementary Table 3.
All 15 evaluable patients with clear cell RCC had stable disease as per the established radiographic response criteria, with mostly minor changes being observed in tumor measurements (Supplementary Table 4 and Fig. 1). One patient, however, experienced a 15.7% decrease in their long axis tumor diameter. Although this change did not meet the radiographic criteria for a clinical response, this patient was found to have features of an irPR within their nephrectomy specimen (Fig. 2). No other irPRs were observed. Quality of life remained stable during treatment with improvements relative to baseline noted at 6 and 12 mo postoperatively (Supplementary Fig. 3 and Supplementary Table 5).
Fig. 1 –
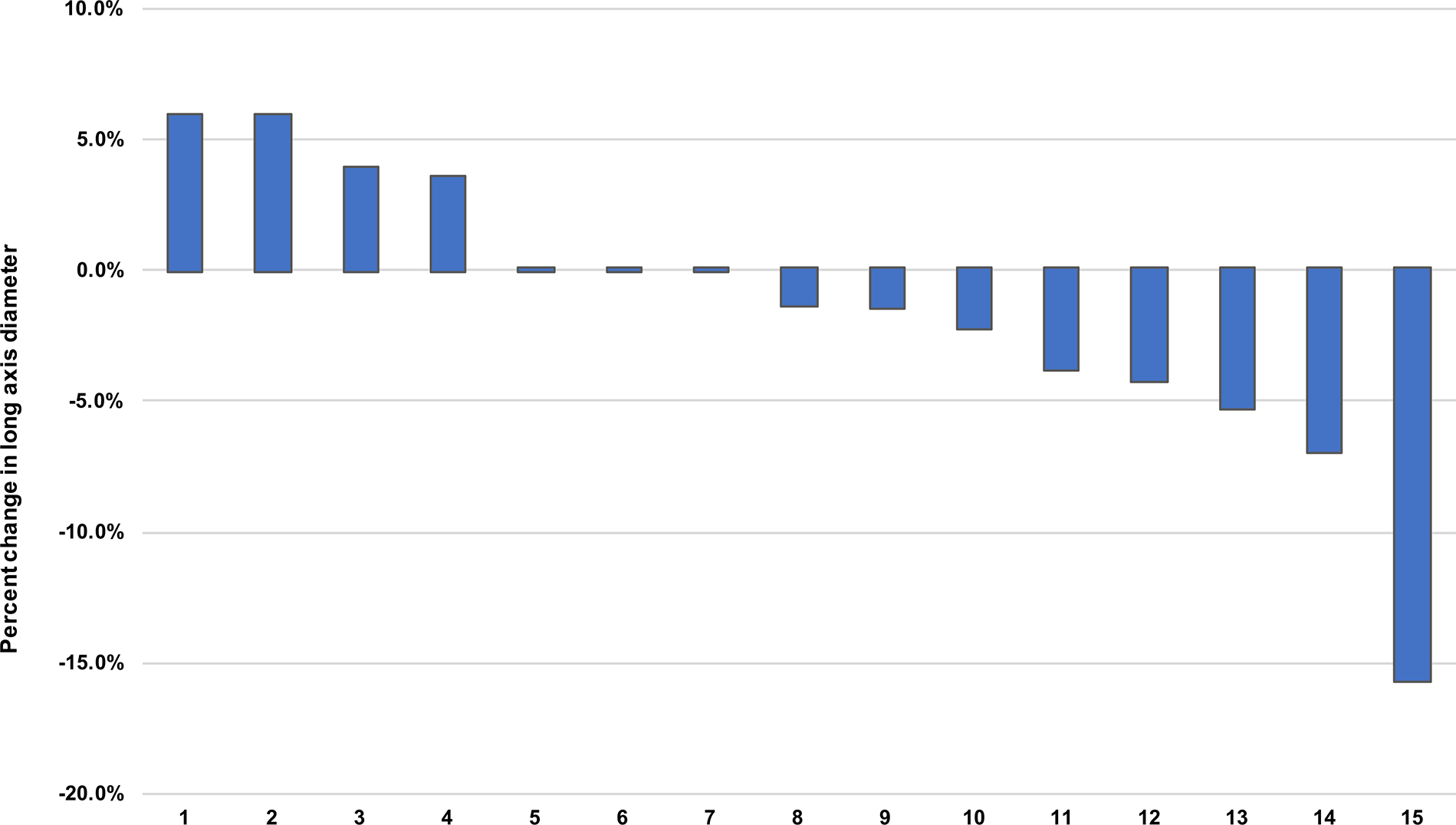
Percentage change in long axis tumor diameter after treatment with neoadjuvant nivolumab.
Fig. 2 –
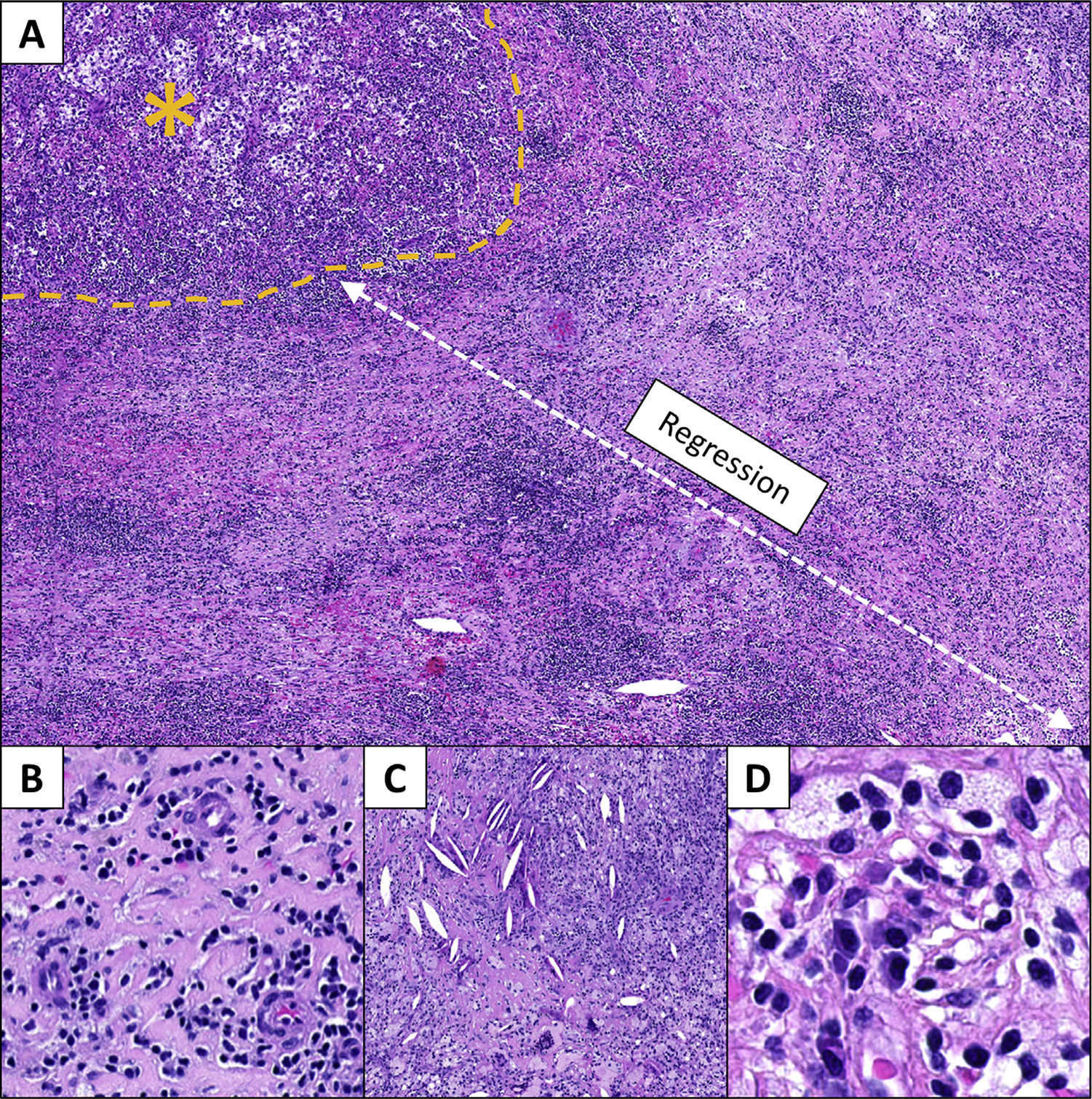
Features of immune-related pathologic response (irPR) are seen in the nephrectomy specimen from a responder to neoadjuvant nivolumab. (A) The irPR is characterized by a regression bed, that is, where the tumor used to be, with remaining residual viable tumor marked by a yellow asterisk (40× original magnification). This regression bed is characterized by histologic features of (B) wound healing, including neovascularization and fibrosis (200×), (C) immune infiltration and cholesterol clefts secondary to tumor cell clearance (100×), and (D) plasma cells and foamy macrophages (400×; H&E staining, all panels). H&E = hematoxylin and eosin.
At a median follow-up of 24.7 mo, metastasis-free survival and overall survival were 100% and 100% at 1 yr, 85.1% and 100% at 2 yr, and 85.1% and 85.7% at 3 yr, respectively (Supplementary Fig. 4). One patient died following a cerebrovascular accident and none died of RCC. Two patients developed metastatic disease, one at 13 mo and the other at 20 mo postoperatively.
This early phase study of neoadjuvant nivolumab in patients with nonmetastatic high-risk RCC demonstrated acceptable rates of AEs with preserved quality of life during treatment. Although patients experienced only minimal decreases in radiographic tumor size, one showed features of an irPR. Consistent with our findings, Forde and coworkers [7] reported a clinical response rate of only 10% but a pathologic response rate of 45% in a trial of patients with resectable non–small-cell lung cancer treated with neoadjuvant nivolumab. Our observation of a patient exhibiting an irPR supports the hypothesis that neoadjuvant PD-1/PD-L1 inhibition may augment an antitumor response in a subset of patients with nonmetastatic RCC. It is the hope that this antitumor response remains in memory for future recurrence of disease or induces the elimination of existing micrometastatic clones. It is reasonable to question, however, whether continued PD-1/PD-L1 inhibition in the postoperative period is required to achieve these outcomes. Indeed, combined treatment with both neoadjuvant and adjuvant nivolumab is the subject of study in an ongoing phase III clinical trial (PROSPER RCC, ClinicalTrials.gov identifier NCT03055013).
Prior studies have suggested a potential role for neoadjuvant tyrosine kinase inhibitors (TKIs) to downstage tumors prior to surgical resection (reviewed by Gleeson et al [2] and Borregales et al [8]). For example, in a phase 2 trial by Karam and coworkers [9], neoadjuvant axitinib demonstrated a median 28.3% reduction in tumor diameter and 11 (45.8%) partial responses by RECIST criteria. Furthermore, in a follow-up study, the authors demonstrated a significant rate of conversion of candidacy from radical to partial nephrectomy following TKI administration [10]. Unfortunately, the significant side-effect profile and decreased quality of life experienced by patients treated with preoperative TKIs weigh against the potential benefits of this treatment strategy [2]. This contrasts with the observations of this study, where we saw relatively fewer AEs and preserved quality of life.
In conclusion, in this study we demonstrate the safety and tolerability of neoadjuvant nivolumab in patients with nonmetastatic high-risk RCC. Our data suggest that although patients are unlikely to experience a radiographic response with only three doses of drug, a small proportion may have evidence of an immunologic response on pathologic evaluation.
Supplementary Material
Funding/Support and role of the sponsor:
This study was supported by funding from Bristol-Myers Squibb. No funder/sponsor had a role in the collection, management, analysis, or interpretation of the data. The preparation, review, approval, and decision to submit the manuscript for publication was made solely by the study’s authors.
Footnotes
Financial disclosures: Michael A. Gorin certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Noah M. Hahn: paid consultant/advisor for Merck & Co., Inc., Genentech, Inc., Pfizer, Inc., Boehringer Ingelheim, and EMD Serono, Inc.; research/grant support from AstraZeneca, plc., Bristol-Myers Squibb, Genentech, Inc., Inovio Pharmaceuticals, Inc., and Pieris Pharmaceuticals, Inc. Hans J. Hammers: paid consultant/advisor for ARMO Biosciences, Inc., Bayer AG, Bristol-Myers Squibb, Corvus Pharmaceuticals, Exelixis, Inc., Eli Lilly and Company, Merck & Co., Inc., Pfizer, Inc., Novartis International AG, and Surface Oncology, Inc.; research/grant support from Bristol-Myers Squibb and Merck & Co., Inc. Janis M. Taube: paid consultant/advisor for Akoya Biosciences, Inc., AstraZeneca, plc., Bristol-Myers Squibb, Compugen, Ltd., and Merck & Co., Inc.; equipment loan, reagent provision, and stock options with Akoya Biosciences, Inc. Charles G. Drake: paid consultant/advisor for Bayer AG, Bristol-Myers Squibb, Compugen, Ltd., F-star Biotechnology Ltd., Genentech, Inc., Genocea Biosciences, Inc., Janssen Pharmaceuticals, Inc., Kleo Pharmaceuticals, Inc., Merck & Co., Inc., EMD Serono, Inc., Pfizer, Inc., Pierre Fabre Dermo-Cosmetique, Shattuck Labs, Inc., Tizona Therapeutics, Inc., and Werewolf Therapeutics, Inc.; patents licensed to Bristol-Myers Squibb and Janssen Pharmaceuticals, Inc.; employment at Janssen Pharmaceuticals, Inc.
Study concept and design: Gorin, Drake, Hammers, Trock, Allaf.
Acquisition of data: Gorin, Patel, Rowe, Hahn, Hammers, Pons, Pierorazio, Nirschl, Salles, Stein, Lotan, Taube, Drake, Allaf.
Analysis and interpretation of data: Gorin, Patel, Hahn, Stein, Taube, Hammers, Drake, Allaf.
Drafting of the manuscript: Gorin, Patel.
Critical revision of the manuscript for important intellectual content: Gorin, Patel, Rowe, Hahn, Hammers, Pons, Pierorazio, Nirschl, Salles, Stein, Lotan, Taube, Drake, Allaf.
Statistical analysis: Patel, Trock.
Obtaining funding: Allaf, Drake.
Administrative, technical, or material support: Pons, Nirschl.
Supervision: Gorin, Allaf.
Other: None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euo.2021.04.002.
References
- [1].Rappold PM, Silagy AW, Kotecha RR, Hakimi AA. Immune checkpoint blockade in renal cell carcinoma. J Surg Oncol 2021;123:739–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gleeson JP, Motzer RJ, Lee CH, et al. The current role for adjuvant and neoadjuvant therapy in renal cell cancer. Curr Opin Urol 2019;29:636–42. [DOI] [PubMed] [Google Scholar]
- [3].Patel HD, Puligandla M, Shuch BM, et al. The future of perioperative therapy in advanced renal cell carcinoma: how can we PROSPER? Future Oncol 2019;15:1683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Labbate C, Hatogai K, Werntz R, et al. Complete response of renal cell carcinoma vena cava tumor thrombus to neoadjuvant immunotherapy. J Immunother Cancer 2019;7:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Singla N, Elias R, Ghandour RA, et al. Pathologic response and surgical outcomes in patients undergoing nephrectomy following receipt of immune checkpoint inhibitors for renal cell carcinoma. Urol Oncol 2019;37:924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pignot G, Thiery-Vuillemin A, Walz J, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: a new surgical challenge? Eur Urol 2020;77:761–3. [DOI] [PubMed] [Google Scholar]
- [7].Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Borregales LD, Adibi M, Thomas AZ, Wood CG, Karam JA. The role of neoadjuvant therapy in the management of locally advanced renal cell carcinoma. Ther Adv Urol 2016;8:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Karam JA, Devine CE, Urbauer DL, et al. Phase 2 trial of neoadjuvant axitinib in patients with locally advanced nonmetastatic clear cell renal cell carcinoma. Eur Urol 2014;66:874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Karam JA, Devine CE, Fellman BM, et al. Variability of inter-observer agreement on feasibility of partial nephrectomy before and after neoadjuvant axitinib for locally advanced renal cell carcinoma (RCC): independent analysis from a phase II trial. BJU Int 2016;117:629–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


