Abstract
Despite numerous studies on bacterial motility, little is known about the regulation of this process by environmental factors in natural isolates. In this study we investigated the control of bacterial motility in response to environmental parameters in two strains isolated from the natural habitat of Lake Baikal. Morphological characterization, carbon source utilization, fermentation analysis, and sequence comparison of 16S rRNA genes showed that these strains belong to two distinct genera, i.e., Enterobacter and Pseudomonas; they were named strains 22 and Y1000, respectively. Both strains swarmed at 25°C and remained motile at low temperatures (4°C), especially the Pseudomonas strain, which further supports the psychrotrophic characteristics of this strain. In contrast, a strong inhibition of motility was observed at above 30°C and with a high NaCl concentration. The existence of flagellar regulatory proteins FlhDC and FleQ was demonstrated in Enterobacter strain 22 and Pseudomonas strain Y1000, respectively, and environmental conditions reduced the expression of the structural genes potentially located at the first level in the flagellar cascade in both organisms. Finally, as in Enterobacter strain 22, a strong reduction in the transcription of the master regulatory gene fleQ was observed in Pseudomonas strain Y1000 in the presence of novobiocin, a DNA gyrase inhibitor, suggesting a link between DNA supercoiling and motility control by environmental factors. Thus, striking similarities observed in the two organisms suggest that these processes have evolved toward a similar regulatory mechanism in polarly flagellated and laterally flagellated (peritrichous) bacteria.
Microorganisms are able to survive under a wide range of environmental conditions (e.g., osmolarity, temperature, and nutrient availability) by rapidly adapting their structure and physiology. These mechanisms are based on the existence of multiple regulatory systems in which gene expression is controlled in a coordinate manner in response to environmental stimuli. One example of such a complex process is the regulation of motility and chemotaxis in bacteria (16).
More than 80% of the known bacterial species are motile by means of flagella (18). The structure and arrangement of flagella differ from species to species and seem to be related to the specific environments in which the cells live (29). Flagella can be arranged on the cell body in a variety of configurations, including single polar, multiple polar, and many peritrichous (or lateral) configurations. Motility by means of flagella is thought to provide a specific advantage for a bacterium (18), because it helps the bacterium to reach the most favorable environment and to successfully compete with other microorganisms. However, the cost of maintenance of a flagellar motility system is high for bacteria (about 2% of biosynthetic energy expenditure in Escherichia coli) due to the number of genes and the energy required for flagellum synthesis and functioning. As a result, the flagellar system is highly regulated (16). Given its importance for bacterial survival under specific conditions, the efficiency of control of this complex system seems to be under strong selective pressure in the environment.
In the present study, we analyzed motility regulation by environmental factors in bacterial strains isolated from a specific natural habitat. Lake Baikal, located in eastern Siberia, is one of the oldest (25 million years) and the deepest (maximum depth, 1,637 m) lakes in the world (11). It represents a particular ecosystem with unique characteristics. Significant seasonal changes in temperature take place only in the top layers of water up to depths of 200 to 250 m. In summer, the surface layers of open deepwater regions of Lake Baikal reach a maximum of 12 to 16°C. The waters of Lake Baikal are poorly mineralized soft waters of the hydrocarbonate class, calcium group. In this oligotrophic lake the sum of the concentrations of major ions is about 100 mg/liter, and the content of biogenic elements and organic matter is insignificant (11). Thus, the aquatic bacteria grow under conditions of low temperature and low contents of mineral and other nutrient compounds. Our results demonstrated that the control of motility in response to changes in these environmental parameters is largely conserved in bacteria with different optimal growth temperatures and with different type of flagellation, despite different organizations of their flagellum regulatory cascades.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. Lake Baikal strains were isolated on 10-fold-diluted RPA solid medium, containing 1.79 g of pancreatic fish hydrolysate per liter, 0.59 g of NaCl per liter, and 11.2 g of Bacto-agar per liter (6). Strains were then grown at 25°C in Luria-Bertani medium (17), tryptone medium (17), or M9 medium (17) supplemented with 0.1% (wt/vol) Casamino Acids. Tryptone swarm plates containing 1% Bacto-tryptone, 0.5% NaCl, and 0.3% Bacto-agar or 10-fold diluted RPA medium (6) with 0.3% agar were used to test bacterial motility as previously described (1). When required, tetracycline was added at a concentration of 15 μg/ml. All experiments were performed in accordance with the European regulation requirements concerning the contained use of genetically modified organisms of group I and group II (agreement no. 2735 and 2736 CAII).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype | Reference or source |
|---|---|---|
| Strains | ||
| Enterobacter strain 22 | Lake Baikal wild type | This study |
| Pseudomonas strain Y1000 | Lake Baikal wild type | L. Denissova (unpublished data) |
| E. coli SM10 | (RP4-2 [Tc::Mu]) Km Tra+ | 24 |
| Plasmids | ||
| pBBR1MCS-3 | Tcr derivative of the broad-host-range cloning vector pBBR1MCS | 10 |
| pDIA575 | pBBR1MCS-3 derivative carrying the flhDC operon of strain 22 | This study |
| pDIA576 | pBBR1MCS-3 derivative carrying the fleQ gene of strain Y1000 | This study |
Phylogenetic analysis.
DNA fragments of about 1,380 nucleotides encompassing the 16S rRNA gene were PCR amplified and sequenced on both strands by Genome Express (Montreuil, France). The 16S rRNA gene sequences were screened against sequences deposited in databases using the Blast program (2). DNA sequences were aligned, and a phylogenetic tree was constructed as previously described (21).
PCR amplification with degenerate primers.
The degenerate primers fleQ5′ and fleQ3′ (Table 2) used for the PCR amplification of Pseudomonas fleQ-homologous genes were designed on the basis of specifically conserved regions in the nucleotide alignment of the Pseudomonas aeruginosa fleQ (GenBank accession no. L49378) and Vibrio cholerae flrA (GenBank accession no. AF014113) genes.
TABLE 2.
Oligonucleotides used in this study
| Name | Sequencea | Expt |
|---|---|---|
| fleQ5′ | 5′-AAACTCTTGCTGATTGASGACRAT-3′ | PCR amplification |
| fleQ3′ | 5′-CATGYYGTAYTTGCGCATCTTCTC-3′ | PCR amplification |
| flhD3′ | 5′-TTYTGYTCCCADGTCATAAACCA-3′ | Direct sequencing |
| flhC3′ | 5′-SNGAAAGTTTAMGYYTTTTTA-3′ | Direct sequencing |
| Ent1 | 5′-TCGCGTGCTTCCTGAACGATGCTTT-3′ | Direct sequencing |
| Ent2 | 5′-AATTGAGTTTCGCTTTCCAGCAT-3′ | Direct sequencing |
| Ent3 | 5′-AGATCGTCAACGCGAGAATCTTG-3′ | Direct sequencing |
| Ent4 | 5′-TTCCCATCCACAATAACCAACT-3′ | Direct sequencing |
| Ent5 | 5′-AATCAAACGTTGTGTCAAGTA-3′ | Direct sequencing |
| Ent6 | 5′-CAAATAACTAAGATTTTTCCT-3′ | Direct sequencing |
| Ent7 | 5′-ATACAATCCAGCCCTACCAAG-3′ | Direct sequencing |
| Ent8 | 5′-TTTATTACCACACGCTCATCAGCCTGT-3′ | Direct sequencing |
| Y3 | 5′-CGCGGCGGCGAACGCTATCGTC-3′ | Direct sequencing |
| Y4 | 5′-GGCGCGCGCGGCAGAGCGCTTG-3′ | Direct sequencing |
| Y5 | 5′-AAACAGCCACTAGTTAAGTCAAA-3′ | Direct sequencing |
| Y6 | 5′-TTTAGGCACGGGTATTGCTA-3′ | Direct sequencing |
| EntXba5 | 5′-GCTCTAGAGAAATGAGCGTGAGGCACTTTATGG-3′ | PCR amplification |
| EntXho3 | 5′-CCGCTCGAGGAATGATTGACCGGAAAATGCGGGG-3′ | PCR amplification |
| YfleXba | 5′-GCTCTAGATGCGTTGCGCGATAACCTTGAGG-3′ | PCR amplification |
| YfleXho | 5′-CCGCTCGAGGTGCTCTCTCGCTTGGCTGACCG-3′ | PCR amplification |
| E1 | 5′-TTCCCATCCACAATAACCAACT-3′ | Primer extension |
| Y1 | 5′-CGCGGCGGCGAACGCTATCGTC-3′ | Primer extension |
| Y2 | 5′-AAACAGCCACTAGTTAAGTCAAA-3′ | Primer extension |
| Entprom | 5′-GAAATGAGCGTGAGGCACTTTATGG-3′ | PCR amplification |
| Entend | 5′-GAATGATTGACCGGAAAATGCGGGG-3′ | PCR amplification |
S = G + C, R = A + G, Y = C + T, D = G + A + T, M = A + C, N = A + C + G + T.
Direct sequencing of chromosomal DNA.
The sequencing of the flhDC operon of Enterobacter strain 22 was performed as previously described (13) with two degenerate oligonucleotides, i.e., flhD3′ and flhC3′ (Table 2), hybridizing with the more conserved region in homologous sequences available in databases. This was followed by several direct sequencing steps allowing the determination of the entire flhDC operon sequence with oligonucleotides Ent1 to Ent8 (Table 2). To complete the fleQ gene sequence of Pseudomonas strain Y1000, several direct sequencing steps were performed on chromosomal DNA with oligonucleotides Y3 to Y6 (Table 2).
Plasmid construction.
Plasmid pDIA575 was constructed by PCR amplification of the flhDC fragment with primers EntXba5 and EntXho3 (Table 2) from chromosomal DNA of Enterobacter strain 22. The 1,481-nucleotide fragment was cloned into the XbaI and XhoI sites of the broad-host-range vector pBBR1MCS-3 (10).
Plasmid pDIA576 was constructed by PCR amplification of the fleQ fragment with primers YfleXba and YfleXho (Table 2) from Pseudomonas strain Y1000 chromosomal DNA. The 1,922-nucleotide fragment was cloned into the XbaI and XhoI sites of plasmid pBBR1MCS-3. To overexpress fleQ, plasmid pDIA576 was conjugally mobilized from E. coli strain SM10 into strain Y1000 (Table 1).
Primer extension.
Total RNA was extracted from 20 ml of culture grown in M9 minimal medium (17) supplemented with 0.1% Casamino Acids or in tryptone medium (17) to an optical density at 600 nm (OD600) of 0.3 using FastPrep system (Bio 101 Savant) and Trizol solution (Gibco BRL) (I. Guillouard, unpublished data). RNA concentration and purity were determined by OD260 and OD280 measurements. Transcriptional start sites were determined as previously described (26). The reactions were performed with 10 and 20 μg of total RNA of Enterobacter and Pseudomonas, respectively, with γ-32P-end-labeled oligonucleotides E1 and Y1 or Y2 (Table 2). As a reference, sequencing reactions were performed on plasmid pDIA575 or pDIA576, using a Thermosequenase radiolabeled terminator cycle sequencing kit from Amersham with the same primer as used in primer extension experiments. Bands from Hyperfilm-MP X-ray film (Amersham) were scanned with a JX-330 SHARP scanner and quantified with the PDI software PDQuest on a SUN computer system.
Nucleotide sequence accession numbers.
The 1,379-nucleotide sequence of the 16S rRNA gene of Pseudomonas strain Y1000 was in accordance with the partial sequence in databases under accession number X99676. The 1,330-nucleotide 16S rRNA sequence of Enterobacter strain 22 has been assigned EMBL nucleotide sequence database accession no. AJ308467. The 2,026- and 1,481-nucleotide sequences containing the complete sequences of the fleQ gene and the flhDC operon have been assigned EMBL nucleotide sequence database accession no. AJ308470 and AJ308469, respectively.
RESULTS
Characterization of natural isolates from Lake Baikal.
Two gram-negative bacteria were isolated from Lake Baikal water samples collected from the South Baikal (strain Y1000) in summer 1995 and from the Central Basin (strain 22) in September 1996 during water sampling expeditions of the Limnological Institute of the Siberian Division of the Russian Academy of Sciences. Water samples were taken at 1,000 and 1,200 m below the surface of the lake for strains Y1000 and 22, respectively. The cultures were enriched by plating on diluted medium (6) and incubation at 5°C or at room temperature for 3 days. All strains were able to grow at a wide range of temperatures, extending from 4 to 37°C, with an optimum growth temperature of around 25°C. Morphological, biochemical, and phenotypic characterization (Table 3) demonstrated that strains 22 and Y1000 belong to the Enterobacter and Pseudomonas genera, respectively, of the Proteobacteria gamma subdivision (12). The taxonomic positions of these strains were further investigated by determination of 16S rRNA gene sequences and their comparative analysis with different DNA sequences present in databases. The construction of a phylogenetic tree (data not shown) further supports the phylogenetic positions of these strains, largely in accordance with morphological characterizations, and suggests that strain 22 was closely related to Enterobacter amnigenus JCM1237 and Enterobacter intermedius JCM1238 (99 and 98% sequence identity, respectively), while the nearest relatives of strain Y1000 were Pseudomonas putida ATCC 17522 and Pseudomonas graminis DSM 11363 (98 and 97% sequence identity, respectively).
TABLE 3.
Morphological, biochemical, and phenotypic characterization of Lake Baikal strainsa
| Characteristic | Strain 22 | Strain Y1000 |
|---|---|---|
| Morphology | ||
| Cell shape | Straight rod | Rod |
| Cell size (μm) | 0.6–1 | 0.5–0.7 to 1–2 |
| Gram stain | − | − |
| Motility | + | + |
| Flagellation | Peritrichous | Polar flagellum |
| Motility on semisolid medium | + | + |
| Temp range for growth (°C) | 4–37 | 4–37 |
| Growth at 41–45°C | ND | − |
| Sporulation | − | − |
| Fermentation analysis | ||
| Oxidase | − | + |
| Catalase | + | + |
| Protease | − | + |
| Amylase | − | − |
| Urease | + | − |
| Carbon sources utilization | ||
| Glucose | − | + |
| Glucose fermentation | − | − |
| Arabinose | + | − |
| Lactose | − | − |
| Mannitol | − | + |
| Xylose | ND | − |
| Maltose | + | + |
| Sucrose | + | − |
| Additional properties | ||
| Nitrate reduction | − | − |
| Indole production | − | + |
| H2S production | − | − |
| Phenylalanine | ND | − |
| Growth with 7% NaCl | +/− | ND |
Morphological, biochemical, and phenotypic characterization was carried out by standard procedures (12). −, negative test or reaction; +, positive test or reaction; +/−, intermediate result, low activity, or reduced growth; ND, not determined.
Effect of environmental factors on bacterial motility.
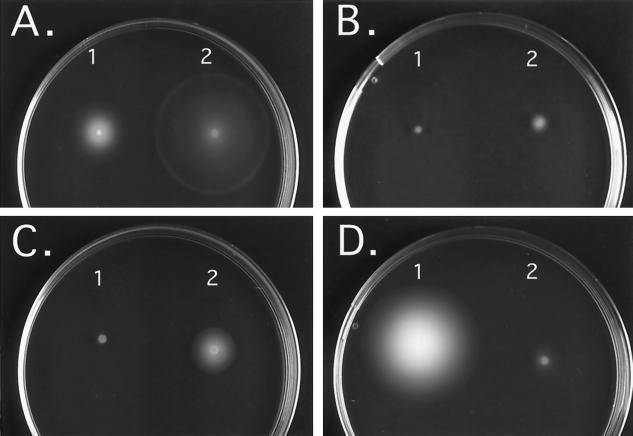
We examined the effects of environmental conditions of Lake Baikal on the motility of isolated strains on semisolid plates at several temperatures ranging from 4 to 37°C and with different concentrations of NaCl. The Enterobacter and Pseudomonas strains were motile at 25°C and remained motile at low temperature (4°C), especially Pseudomonas strain Y1000 (Table 3 and Fig. 1). In contrast, a strong inhibition of motility was observed for both strains at 30 and 37°C or in the presence of NaCl (Fig. 1). Similar effects of these growth conditions on motility were observed by examination of the strains under a light microscope (data not shown).
FIG. 1.
Motility assay on semisolid medium plates. 1, Pseudomonas strain Y1000; 2, Enterobacter strain 22. Assays were performed at 25°C (A), at 25°C in the presence of 500 mM NaCl (B), at 37°C (C), or at 4°C (D). Plates were incubated for 13 to 15 h at 25 and 37°C and for 132 h at 4°C. The results are representative of those from three independent experiments.
Identification of flagellar regulatory proteins.
The taxonomic positions of Lake Baikal motile strains, i.e., within the laterally flagellated (peritrichous) Enterobacteriaceae family or the polarly flagellated Pseudomonadaceae family (12), suggest the existence of FlhDC regulators (16) in Enterobacter strain 22 and of the FleQ regulator (3) in Pseudomonas strain Y1000. This prompted us to identify the genes encoding these putative master regulatory proteins of flagellar biosynthesis.
Attempts to amplify the flhDC operon by PCR with degenerate primers designed on the basis of conserved regions in the coding parts of homologous sequences available in databases failed. Therefore, the sequence of the putative flhDC operon in Enterobacter strain 22 was determined by direct sequencing of chromosomal DNA as described in Materials and Methods. The sequence data we obtained showed a high degree of identity with the corresponding parts of the flhDC operon of E. coli. After several runs of direct sequencing (see Materials and Methods), the whole region was PCR amplified with primers Entprom and Entend (Table 2) and then sequenced on both strands. The amino acid sequence deduced from the 1,481-nucleotide sequence suggests that this fragment encodes both a 116-amino-acid protein and a 192-amino-acid protein. These proteins showed significant homology (i.e., up to 86 and 92%, respectively), with FlhD and FlhC regulatory proteins in various enterobacteria (e.g., E. coli [GenBank accession no. AE005411], Xenorhabdus nematophilus [GenBank accession no. AJ012828], Yersinia enterocolitica [GenBank accession no. AF081587], and Erwinia carotovora [GenBank accession no. AF130387]).
The master regulator gene of Pseudomonas strain Y1000 was identified by PCR amplification with degenerate primers designed based on the coding parts of the corresponding flagellar regulatory genes fleQ of P. aeruginosa (GenBank accession no. L49378) and flrA of V. cholerae (GenBank accession no. AF014113). The resulting 1,424-nucleotide DNA fragment showed 81.3% sequence identity with the fleQ gene of P. aeruginosa, further supporting the existence of a homologous regulator in Pseudomonas strain Y1000. The sequences upstream and downstream of this fragment were obtained by direct sequencing of Pseudomonas strain Y1000 chromosomal DNA (see Materials and Methods) and were subsequently checked by sequencing on both strands the 2,026-bp PCR-amplified fragment containing the complete fleQ gene and its regulatory region (accession no. AJ308470). The deduced FleQ 491-amino-acid sequence showed significant homology with the protein sequences of several master flagellar regulators, i.e., 83% identity with the protein sequence of the FleQ transcriptional activator of P. aeruginosa (GenBank accession no. L49378) and 52% identity with the FlaK and FlrA flagellar regulatory proteins of Vibrio parahaemolyticus (GenBank accession no. AF069392) and V. cholerae (GenBank accession no. AF014113), respectively. The FleQ protein of Pseudomonas strain Y1000 shared common structural and functional domains conserved in master regulators of polar flagellum system belonging to the NtrC family of transcriptional activators of RpoN (ς54)-dependent promoters (3). These included a relatively low homology in the N-terminal region, except for the conservation of residues believed to be involved in the phosphorylation of these proteins (3) and a strong conservation in the ATP-binding site and in the helix-turn-helix DNA-binding element (data not shown).
Effect of environmental conditions on transcription initiation.
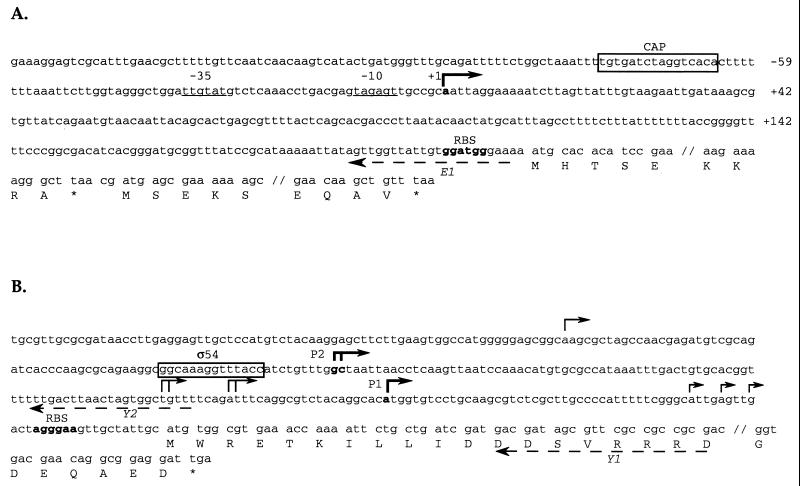
To further characterize the master regulator operon of Enterobacter strain 22, we determined the flhDC transcription start site by primer extension experiments with total RNA and primer E1, located upstream from the translational start site of flhDC (Fig. 2A). A single major band for transcription initiation was detected (data not shown), which indicates that transcription of flhDC arises from the A residue located 211 nucleotides upstream from the putative ATG start codon (Fig. 2A). −35 and −10 hexamers showing 50 and 67% similarity, respectively, with the canonic ς70 consensus sequence were identified upstream from the transcriptional start site. These two boxes are separated by a 17-bp spacer. Moreover, a catabolite gene activator protein (CAP)-like protein-binding site was identified in the regulatory region of the Enterobacter flhDC operon centered at position −71.5 with respect to the transcription start site (Fig. 2A). The presence of a long untranslated region was observed between the transcriptional start site and the ATG initiation codon. Taken together, these observations strongly suggest a mechanism of flhDC regulation similar to the one that we recently characterized for E. coli (26).
FIG. 2.
(A) Regulatory region of the flhDC master operon in Enterobacter strain 22. Nucleotides are numbered relative to the transcriptional start site (+1), indicated by a broken arrow. The unique CAP-binding site consensus sequence is indicated by a box. The positions of the −10 and −35 sequences are underlined. A putative ribosome-binding site (RBS) is indicated in boldface. Only the residues corresponding to the N- and C-terminal parts of FlhD and FlhC are indicated. The dashed arrow labeled E1 represents the oligonucleotide used in +1 mapping. (B) Regulatory region of the fleQ gene in Pseudomonas strain Y1000. Transcriptional start sites are indicated by broken arrows; the two major transcriptional start sites P1 and P2 are indicated in boldface. The position of a putative ς54-binding site is indicated by a box. A putative ribosome-binding site with similarities with corresponding regions in the P. aeruginosa fleQ gene (accession no. L49378) and V. cholerae flrA gene (accession no. AF014113) and close to the consensus sequence of P. aeruginosa 16S rRNA (23) is indicated in boldface. Only the residues corresponding to the N- and C-terminal parts of FleQ are indicated. Dashed arrows labeled Y1 and Y2 represent oligonucleotides used in +1 mapping.
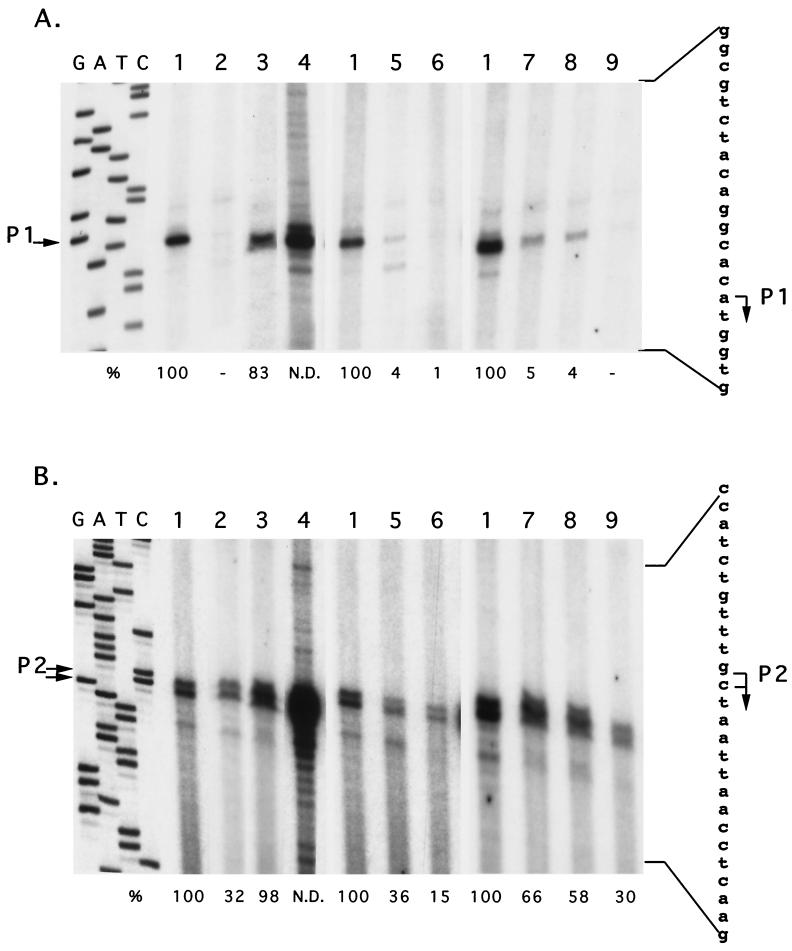
Little is known about the control of the polar flagellum master gene in Pseudomonas. To investigate fleQ expression in Pseudomonas strain Y1000, RNA was isolated from cells grown to early exponential phase or to the entrance into stationary phase. With two primers located 32 bp downstream and 95 bp upstream from the putative ATG initiation codon, we identified multiple transcriptional start sites in primer extension experiments (Fig. 2B). Nevertheless, transcription was initiated mainly at an A residue located 70 nucleotides upstream from the ATG start codon (Fig. 3A) and at GC residues located 177 and 176 nucleotides upstream from the ATG start codon (Fig. 3B). A strong decrease in the levels of these major transcripts was observed when RNA was extracted from cells in late logarithmic phase in comparison with the expression level measured in cells grown to exponential phase (Fig. 3), in accordance with microscopic observations of cell motility (data not shown). Moreover, primer extension experiments performed with RNA isolated from Pseudomonas strain Y1000 overexpressing the fleQ gene from plasmid pDIA576 (Table 1) or from Pseudomonas strain Y1000 grown at 4°C further support the existence of two major transcriptional start sites (Fig. 3). A potential RpoN-binding site was identified between positions −24 and −11 with respect to the C residue located 176 nucleotides upstream from the ATG start codon (Fig. 2B). This site showed 64% identity with the ς54 consensus sequence (14). Finally, it is worth mentioning that 70- and 177-bp untranslated regions were observed between the ATG initiation codon and the major transcriptional start sites P1 and P2, respectively (Fig. 2B).
FIG. 3.
Identification of the fleQ transcriptional start site and effects of growth conditions on fleQ expression with primer Y1 (A) and with primer Y2 (B) (see Materials and Methods). Primer extension analysis was performed with RNA extracted from Pseudomonas strain Y1000 grown in exponential phase at room temperature (lanes 1); to late logarithmic phase (lanes 2); at 4°C (lanes 3); in the presence of 500 mM NaCl (lanes 5); at 30°C (lanes 6); or in the presence of 100 μM (lanes 7), 200 μM (lanes 8), and 400 μM (lanes 9) novobiocin and from Pseudomonas strain Y1000 carrying plasmid pDIA576 grown in exponential phase at 25°C (lanes 4). As a reference, a DNA sequencing ladder is shown (lanes G, A, T, and C). The sequence is complementary to the strand shown on the right and was obtained with the same primer as that used for primer extension. Major transcription start sites P1 and P2 are indicated by arrows. The mRNA level was quantified with the PDI software PDQuest on a SUN computer system. Quantitative data are indicated at the bottom of each lane and were expressed relative to the standard condition level (lanes 1), which was assigned a value of 100%. -, background level undetectable by quantification procedure; N.D., not determined.
To determine whether the growth conditions could play a role in fleQ gene expression, primer extension experiments were performed with RNA extracted from Pseudomonas strain Y1000 grown at 30°C or in the presence of 500 mM NaCl. As seen in Fig. 3, growth under both conditions resulted in a strong decrease in the levels of both fleQ major transcripts, i.e., more than 90 and 50% for the P1 and P2 transcripts, respectively.
Role of DNA supercoiling level in control of motility.
A link between DNA topology and regulation of gene expression in response to environmental cues has been proposed (28). In particular, a reduction in motility has been observed in E. coli in the presence of DNA gyrase inhibitors, suggesting the involvement of DNA supercoiling in the regulation of bacterial motility (22). To investigate the role of DNA topology in the control of flagellar gene expression in Pseudomonas strain Y1000, we performed a motility assay in the presence of novobiocin, a DNA gyrase inhibitor. A strong reduction in motility was observed in the presence of 200 μM novobiocin, as in Enterobacter strain 22 (data not shown). To further characterize the effect of these conditions on the master regulator gene expression, we carried out primer extension experiments with RNA isolated from Pseudomonas strain Y1000 grown in the presence of 100, 200, and 400 μM novobiocin. No significant effect on growth was observed in the presence of 100 μM novobiocin, and only a small decrease in growth rate was observed in the presence of 200 and 400 μM novobiocin (data not shown). In contrast, a progressive and important decrease in the fleQ major transcript level was measured with the increase in novobiocin concentration. In particular, low expression of fleQ was measured at a 400 μM concentration of DNA gyrase inhibitor (30% and undetectable for P2 and P1 transcripts, respectively), which is quite similar to that observed under various environmental conditions (Fig. 3). Thus, these results suggest a role for DNA supercoiling in the regulation of polar flagellum master gene fleQ expression in Pseudomonas strain Y1000.
DISCUSSION
In the past years, the hierarchical organization of regulatory systems in gram-negative bacteria has been extensively studied. Bacterial flagellum genes form an ordered cascade in which the expression of one gene located at a given level requires the transcription of another gene at a higher level (16). At the top of the hierarchy are located the flhDC master operon in enterobacteria (16) and the fleQ and flrA master genes in P. aeruginosa (3) and V. cholerae (9), respectively. Flagellar regulatory cascades have also been recently identified in other bacteria, such as Caulobacter crescentus (30), Sinorhizobium (Rhizobium) meliloti (25), and Helicobacter pylori (27). In enterobacteria, the ς70-dependent flhDC operon is controlled by numerous environmental signals (15, 22) and global regulatory proteins such as H-NS and the cyclic AMP-CAP complex (4, 26). In contrast, the regulation of master genes governing the synthesis of polar flagella remains largely unknown. In the present study we characterized two gram-negative motile bacteria isolated from Lake Baikal. Morphological and phylogenetic analyses suggested that they belong to the laterally flagellated (peritrichous) Enterobacter and polarly flagellated Pseudomonas genera (Table 3). Both isolated strains are psychrotrophic bacteria, especially strain Y1000, suggesting that this organism is more adapted to deep-layer water conditions of Lake Baikal (7). These psychrotrophic properties were further supported by conservation of the motility of these strains at low temperatures (Fig. 1). The physical and chemical characteristics of Lake Baikal waters (11) prompted us to test whether isolated bacteria are able to respond to modifications in these parameters. We showed here that an increase in temperature and a high salt concentration resulted in inhibition of motility in both laterally and polarly flagellated psychrotrophic bacteria isolated from natural environment (Fig. 1). The stationary phase of growth also suppressed motility (Fig. 3 and data not shown), in accordance with growth phase dependence of flagellar gene expression in E. coli (19). Moreover, our results suggest that the master regulatory protein-encoding genes located at the first level of the cascade, i.e., flhDC in Enterobacter and fleQ in Pseudomonas (Fig. 2 and 3), are the major targets for environmental factors and growth phase. Indeed, increasing the fleQ gene dosage from plasmid pDIA576 in Pseudomonas strain Y1000 reversed the reduced motility caused by environmental conditions (data not shown).
A unique transcriptional start site was identified in the flhDC promoter region of Enterobacter strain 22 (Fig. 2A), consistent with the flhDC single major start site observed in E. coli (26) and in Proteus mirabilis (5). By primer extension experiments we identified multiple transcriptional start sites in Pseudomonas strain Y1000, including two major fleQ transcription initiation sites (Fig. 2B and 3). Such a complex promoter structure suggests possible multiple regulations of this gene. In this respect, it should be emphasized that transcription from the first major transcriptional start site (P1 in Fig. 3A) was more sensitive to all adverse conditions tested, including the presence of a gyrase inhibitor, than the second major transcriptional start site (P2 in Fig. 3B). This could result from different rates of transcription from the promoters and/or different mRNA stabilities of the corresponding transcripts. Finally, we have previously shown that a long untranslated region plays an important role in the transcriptional control of the master flagellar operon in E. coli (26). Whether the presence of such a regulatory region (Fig. 2) is involved in the regulation of motility and flagellum biosynthesis in Pseudomonas strain Y1000 and Enterobacter strain 22 remains to be determined.
Despite several studies devoted to motility control in response to environmental conditions (15, 22), the mechanism by which bacteria sense environmental changes remains unknown. Nevertheless, environmental factors such as high temperature and high osmolarity, which are known to induce changes in DNA topology and regulation of gene expression (8, 28), also affect bacterial motility in various microorganisms, such as H. pylori (27) and Y. enterocolitica (20). The loss of motility in E. coli in the presence of novobiocin, a gyrase inhibitor (22), further supports a link between the DNA supercoiling level and motility regulation. Consistent with these data, we demonstrated here that novobiocin and adverse conditions strongly reduce motility and/or flagellar gene expression in Enterobacter strain 22 (data not shown) and Pseudomonas strain Y1000 (Fig. 3), while they have no significant effect on growth. This suggests that the regulation of flagellar systems in both strains could correlate with specific variations in DNA supercoiling.
In this study we demonstrated the existence of remarkable similarities in responses to environmental factors, not only between the flagellar system of natural isolates and that of mesophilic bacteria but also between bacteria with different types of flagellation and regulatory cascades. These observations suggest that the control of bacterial motility has evolved toward a similar mechanism in different bacterial systems. Even though stable physicochemical conditions, i.e., low temperature and low salt concentration, characterize Lake Baikal, bacteria may encounter different temperature and salt conditions due to multiple currents and seasonal or local changes on surface layers or shallow parts (11). Thus, the ability of Lake Baikal bacteria to react to adverse conditions can be of prime importance in the adaptive process of these strains in response to environmental challenges.
ACKNOWLEDGMENTS
We are grateful to E. Krin, E. Zaychikov, and L. Denissova for helpful advice and discussions and to G. Karimova for critical reading of the manuscript. We thank M. E. Kovach for providing us with plasmid pBBR1MCS-3. We also thank E. Turlin for technical assistance.
Financial support came from the Institut Pasteur, the Centre National de la Recherche Scientifique (URA 2171), and in part from grant N 225 of 6th contest for young scientists from the Russian Academy of Sciences in 1999. O.A.S. was supported by a French Government fellowship.
REFERENCES
- 1.Adler J. Chemotaxis in bacteria. Science. 1966;153:708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment research tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Arora S K, Ritchings B W, Almira E C, Lory S, Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J Bacteriol. 1997;179:5574–5581. doi: 10.1128/jb.179.17.5574-5581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin P, Terao E, Lee E H, Lejeune P, Colson C, Danchin A, Collatz E. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J Bacteriol. 1994;176:5537–5540. doi: 10.1128/jb.176.17.5537-5540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furness R B, Fraser G M, Hay N A, Hughes C. Negative feedback from a Proteus class II flagella export defect to the flhDC master operon controlling cell division and flagellum assembly. J Bacteriol. 1997;179:5585–5588. doi: 10.1128/jb.179.17.5585-5588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbenko Y A. About the most suitable amount of “dry nutritious agar” in medium for cultivation of marine heterotrophic microorganisms. Microbiologia. 1961;30:168–172. [PubMed] [Google Scholar]
- 7.Gounot A-M. Psychrophilic and psychrotrophic microorganisms. Experientia. 1986;42:1192–1197. doi: 10.1007/BF01946390. [DOI] [PubMed] [Google Scholar]
- 8.Higgins C F, Cairney J, Stirling D A, Sutherland L, Booth I R. Osmotic regulation of gene expression: ionic strength as an intracellular signal? Trends Biochem Sci. 1987;12:339–344. [Google Scholar]
- 9.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 10.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 11.Kozhova O M, Izmest'eva L R. Lake Baikal. Evolution and biodiversity. Leiden, Germany: Backhus Publishers; 1998. [Google Scholar]
- 12.Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams and Wilkins; 1984. [Google Scholar]
- 13.Krin E, Hommais F, Soutourina O, Ngo S, Danchin A, Bertin P. Description and application of a rapid method for genomic DNA direct sequencing. FEMS Microbiol Lett. 2001;199:229–233. doi: 10.1111/j.1574-6968.2001.tb10679.x. [DOI] [PubMed] [Google Scholar]
- 14.Kustu S, Santero E, Keener J, Popham D, Weiss D. Expression of sigma54 (ntrA)-dependent genes is probably united by a common mechanism. Microbiol Rev. 1989;53:367–376. doi: 10.1128/mr.53.3.367-376.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Louise C J, Shi W, Adler J. Adverse conditions which cause lack of flagella in Escherichia coli. J Bacteriol. 1993;175:2229–2235. doi: 10.1128/jb.175.8.2229-2235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 17.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 18.Moens S, Vanderleyden J. Function of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 19.Pruss B M, Matsumura P. Cell cycle regulation of flagellar genes. J Bacteriol. 1997;179:5602–5604. doi: 10.1128/jb.179.17.5602-5604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohde J R, Fox J M, Minnich S A. Thermoregulation in Yersinia enterocolitica is coincident with changes in DNA supercoiling. Mol Microbiol. 1994;12:187–199. doi: 10.1111/j.1365-2958.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 21.Semenova E A, Kuznedelov K D, Grachev M A. Nucleotide sequences of fragments of 16S rRNA of the Baikal natural populations and laboratory cultures of cyanobacteria. Mol Biol. 2001;35:405–410. [PubMed] [Google Scholar]
- 22.Shi W, Li C, Louise C, Adler J. Mechanism of adverse conditions causing lack of flagella in Escherichia coli. J Bacteriol. 1993;175:2236–2240. doi: 10.1128/jb.175.8.2236-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shine J, Dalgarno L. Terminal sequence analysis of bacterial ribosomal RNA. Eur J Biochem. 1975;57:221–230. doi: 10.1111/j.1432-1033.1975.tb02294.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology. 1983;1:784–794. [Google Scholar]
- 25.Sourjik V, Muschler P, Scharf B, Schmitt R. VisN and VisR are global regulators of chemotaxis, flagellar, and motility genes in Sinorhizobium (Rhizobium) meliloti. J Bacteriol. 2000;182:782–788. doi: 10.1128/jb.182.3.782-788.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soutourina O, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spohn G, Scarlato V. Motility of Helicobacter pylori is coordinately regulated by the transcriptional activator FlgR, an NtrC homolog. J Bacteriol. 1999;181:593–599. doi: 10.1128/jb.181.2.593-599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tse-Dinh Y C, Qi H, Menzel R. DNA supercoiling and bacterial adaptation: thermotolerance and thermoresistance. Trends Microbiol. 1997;5:323–326. doi: 10.1016/s0966-842x(97)01080-9. [DOI] [PubMed] [Google Scholar]
- 29.Wilson D R, Beveridge T J. Bacterial flagellar filaments and their component flagellins. Can J Microbiol. 1993;39:451–472. doi: 10.1139/m93-066. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Newton A. Regulation of the Caulobacter flagellar gene hierarchy: not just for motility. Mol Microbiol. 1997;24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]