Abstract
Rationale & Objective
Information on safety issues of newer glucose-lowering medications from a large population perspective in chronic kidney disease (CKD) patients with type 2 diabetes is limited. Our study aimed to examine hypoglycemia risk associated with sodium-glucose cotransporter 2 inhibitors (SGLT2is) and glucagon-like peptide 1 receptor agonists (GLP-1RAs) versus second-generation sulfonylureas in a general population of older patients with CKD and type 2 diabetes, across race, age, sex, and socioeconomic subgroups.
Study Design
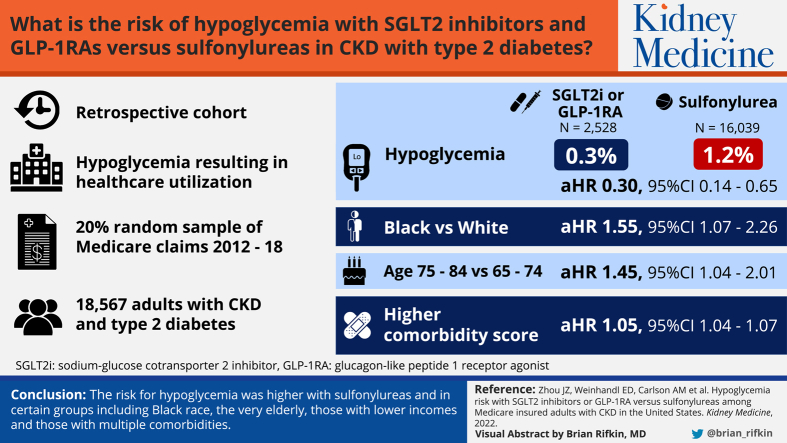
Retrospective cohort.
Setting & Participants
The 20% random sample of Medicare fee-for-service claims, 2012-2018.
Exposures
Use of SGLT2is, GLP-1RAs, or sulfonylureas.
Outcomes
Hypoglycemic events resulting in health care utilization.
Analytical Approach
Cox proportional hazard model evaluated the 90-day risk of hypoglycemia associated with SGLT2is or GLP-1RAs versus sulfonylureas.
Results
A total of 18,567 adults (mean age: 73 years) with CKD and type 2 diabetes was included; 14.0% (n = 2,528) had a prescription for a SGLT2i or GLP-1RA, and 86.0% (n = 16,039) with a sulfonylurea. Compared with sulfonylureas, use of SGLT2is or GLP-1RAs was associated with a significantly lower risk of hypoglycemia (adjusted HR, 0.30; 95% CI, 0.14-0.65). Black individuals had higher risk of developing hypoglycemia than White individuals (adjusted HR, 1.55; 95% CI, 1.07-2.26). Low-income subsidy compared to no low-income subsidy status was associated with higher risk of hypoglycemic events. The risk of hypoglycemia also increased with higher comorbid condition score.
Limitations
CKD and type 2 diabetes diagnosis, CKD stage, and patient clinical status were identified with diagnosis or procedure codes. There is potential for residual confounding with use of retrospective data.
Conclusions
Use of SGLT2is or GLP-1RAs compared with sulfonylureas was associated with a lower risk of hypoglycemia among patients with CKD and type 2 diabetes. Black race was not only associated with lower use of newer agents with demonstrated cardiovascular and kidney benefits and lower hypoglycemia risk, but also with a higher rate of hypoglycemic events as compared with White individuals.
Index Words: Chronic kidney disease (CKD), glucose-lowering medications, hypoglycemia, Medicare claims data, type 2 diabetes
Visual Abstract
Plain-Language Summary.
There is limited information on safety issues of newer glucose-lowering medications from a large population perspective in chronic kidney disease (CKD) patients with type 2 diabetes. We used Medicare 20% claims data to examine hypoglycemia risk associated with second-generation sulfonylureas versus sodium/glucose cotransporter 2 inhibitors or glucagon-like peptide 1 receptor agonists in a general population of older patients with CKD and type 2 diabetes, across race, age, sex, and socioeconomic subgroups. We found that hypoglycemic risk was higher in Black individuals and in patients aged 75-84 years, with low-income subsidy status and higher comorbid condition score. This is important because our work in patients with CKD as well as others in non-CKD patients shows that Black individuals compared with White individuals are less likely to receive newer agents that are less likely to cause hypoglycemia.
An estimated 15% of US adults (aged 18 years or above) (37 million people) have chronic kidney disease (CKD),1 of which the leading cause is diabetes.2 Type 2 diabetes management includes lifestyle modifications, psychosocial care, and pharmacologic approaches for glycemic control. However, glucose-lowering medications can lead to hypoglycemia, the most common adverse effect of diabetes treatment. When severe, it can cause coma and seizures.3,4 A study based on a continuous glucose monitoring system found that hypoglycemia (glucose <70 mg/dL) is associated with cardiac ischemia and symptoms.5
The kidneys play an important role in glucose hemostasis through kidney tubular glucose absorption and gluconeogenesis.6 Hypoglycemia is increased in reduced kidney function. In the Action to Control Cardiovascular Risk in Diabetes trial, higher serum creatinine or higher urinary albumin-creatinine ratio was associated with hypoglycemia requiring medical assistance.7 A prospective observational study found that hypoglycemia is common among patients with CKD and type 2 diabetes; continuous glucose monitoring detected glucose ≤70 mg/dL in 61 of 80 (76%) and glucose ≤60 mg/dL in 49 of 80 (61%); 31 of 80 (39%) experienced a prolonged hypoglycemic events (glucose ≤54 mg/dL for 120 consecutive minutes).8
Large clinical trials have shown benefits of newer glucose-lowering medications on cardiovascular and kidney outcomes in patients with CKD and type 2 diabetes.9, 10, 11, 12, 13, 14, 15, 16 The risk of hypoglycemia was generally low with sodium/glucose cotransporter 2 inhibitors (SGLT2is) or glucagon-like peptide 1 receptor agonists (GLP-1RAs) in these clinical trials. However, data from these clinical trials were based on selected patient populations. It is important to assess whether the results of these clinical trials are applicable to patients with CKD in routine clinical practice. There is limited information on safety issues of newer glucose-lowering medications from a large population perspective in patients with CKD and type 2 diabetes. Additionally, there is no information on comparative hypoglycemia risk in different race, age, sex, or socioeconomic groups. Our study aimed to examine hypoglycemia risk associated with second-generation sulfonylureas versus SGLT2is or GLP-1RAs in a general population of older patients with CKD and type 2 diabetes, across race, age, sex, and socioeconomic subgroups.
Methods
Data Source
We used data from a 20% random sample of Medicare fee-for-service claims. Claims data included patient demographic characteristics, health insurance enrollment, institutional (inpatient, outpatient, home health, skilled nursing facility), physician visits, and Part D characteristics files (including prescription events) from January 1, 2012 to December 31, 2018.
Study Design and Cohort Selection
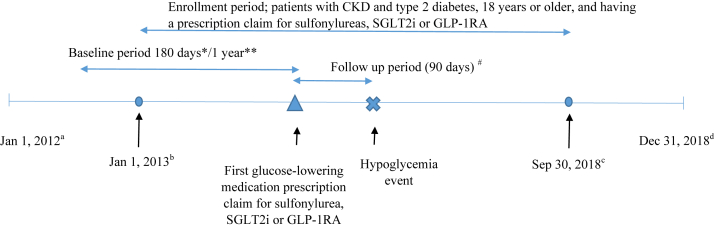
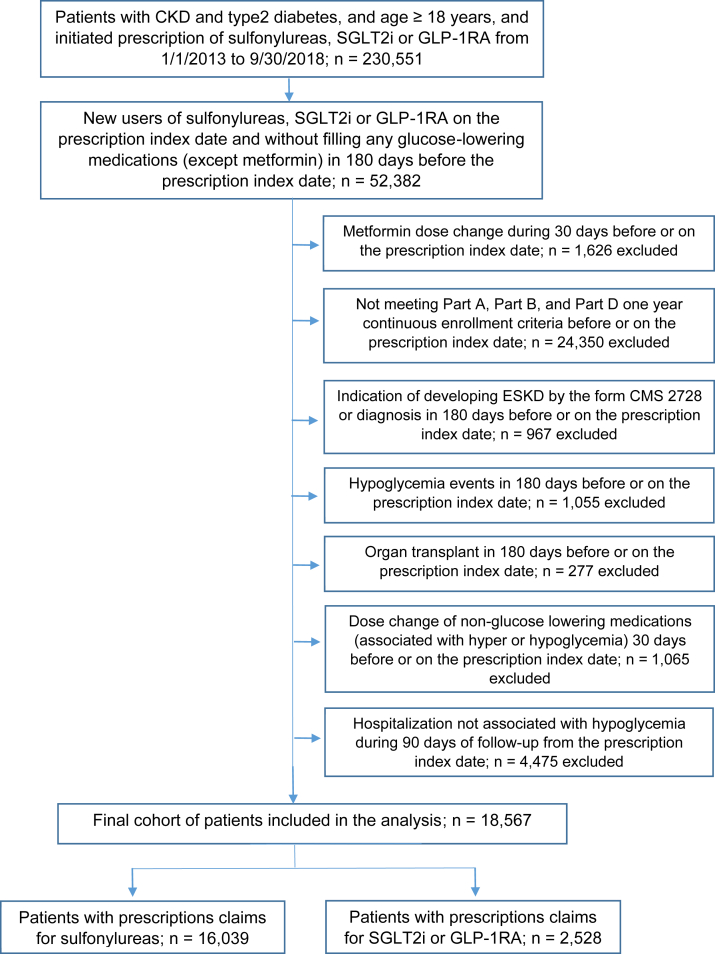
We conducted a retrospective cohort study (Fig 1) of patients with CKD and type 2 diabetes. First, we identified patients with CKD and type 2 diabetes from 2013-2018, and used International Classification of Disease, Ninth/Tenth Revision, Clinical Modification (ICD-9/10-CM) diagnosis codes provided by the US Renal Data System.17 We excluded diagnoses related to type 1 diabetes (ICD-9-CM: 250.X1/250.X3, X=0-9; ICD-10-CM: E10) to select patients more likely to have type 2 diabetes. Patients were considered as having type 2 diabetes if they had 1 or more diagnosis code from inpatient services, home health, or skilled nursing facilities, or 2 or more diagnosis codes from physician claims or outpatient services on different dates within 365 days. The same method was used to identify patients with CKD. This method has been shown to increase sensitivity and specificity compared with using only one claim in patients with diabetes.18 The first claim date was chosen for confirmed diagnosis. To establish CKD and type 2 diabetes diagnoses, the index date was defined by taking the claims date for the later of the 2 diagnoses. For example, if the diabetes diagnosis date was June 15, 2013 and the CKD date was July 12, 2014, then the CKD-type 2 diabetes diagnosis index date was July 12, 2014. Patients younger than 18 years old at the diagnosis index date were excluded.
Figure 1.
Retrospective cohort study design. CKD, chronic kidney disease; GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium/glucose cotransporter 2 inhibitor. Note: no prescription claims for any glucose-lowering medications (except metformin) were allowed during the baseline period. Nonglucose-lowering medications associated with hyper- or hypoglycemia were identified in the 90-day period and other covariates were identified in the 180-day period before the prescription index date (∗);Comorbid conditions were identified during the 1-year baseline period (∗∗); The earliest date in database (a); The earliest possible starting time (b); The latest possible starting time (c); The latest date in database (d); Until a hypoglycemia or censoring event (#). Abbreviations: CKD, chronic kidney disease; GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Next, we identified patients who filled a first prescription of a sulfonylurea, SGLT2i, or GLP-1RA from January 1, 2013 to September 30, 2018. The first prescription date of the sulfonylurea, SGLT2i, or GLP-1RA (Table 1) after the CKD-type 2 diabetes diagnosis index date was the prescription index date. We used a new user approach design. New users were patients without any glucose-lowering medication except use of metformin in the 180 days before the prescription index date. Additional inclusion criteria included continuous enrollment in Medicare Part A, Part B, and Part D in the year before or on the prescription index date. Exclusion criteria included the following: (1) hypoglycemic events (Table S1) within the 180 days before or on the prescription index date; (2) organ transplant (Table S2) within 180 days before or on the prescription index date; (3) indication of kidney failure on Centers for Medicare & Medicaid Services form 2728 or by diagnosis codes (ICD9, 5856; ICD10, N186) in the 180-day period before or on the prescription index date; (4) dose change in metformin during the 30 days before or on the prescription index date; (5) dose change of nonglucose-lowering medications associated with hyper- or hypoglycemia (Table S3) during the 30 days before or on the prescription index date; and (6) hospitalization not associated with hypoglycemic event during the 90 days after or on the prescription index date.
Table 1.
Description of Glucose-Lowering Medication Prescriptions Evaluated in the Study
| Class | Medication | FDA Approval Date |
|---|---|---|
| Sulfonylureas (2nd generation) | ||
| Glipizide | 2002 | |
| Glyburide | 2002 | |
| Glimepiride | 1999 | |
| SGLT2is | ||
| canagliflozin | 2013 | |
| dapagliflozin | 2014 | |
| empagliflozin | 2014 | |
| ertugliflozin | 2017 | |
| GLP-1RAs | ||
| albiglutide | 2014 | |
| dulaglutide | 2014 | |
| exenatide | 2005 | |
| exenatide extended-release | 2012 | |
| liraglutide | 2010 | |
| lixisenatide | 2016 | |
| semaglutide | 2017 |
Abbreviations: FDA, US Food and Drug Administration; GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Study Covariates
Baseline covariates included patient demographics (age, sex, and race), low-income subsidy status, CKD stage status, comorbid condition index score, and prescription medication use. The Medicare Part D program offers low-income subsidy benefits to enrollees with limited assets and income. The low-income subsidy provides full or partial waivers for out-of-pocket cost-sharing requirements including premiums, deductibles, and copayments. The low-income subsidy was used as a surrogate for lower socioeconomic status. To define comorbid conditions, we used a 1-year baseline period before the prescription index date. We identified nonglucose-lowering medications associated with hyper- or hypoglycemia in the 90-day period and other covariates in the 180-day period before the prescription index date. Comorbid conditions were identified based on the Elixhauser measure19 and confirmed if at least 1 inpatient or 2 physician/outpatient services claims on different days were identified during the 1-year baseline period. A comorbid condition index score was calculated using van Walraven’s method.20 CKD stage was defined by stage-specific ICD-9/10-CM diagnosis codes (Table S4). The code for the most recent CKD stage (1 to 5) from outpatient or physician visit claims in the 180-day baseline period was used.
Study Outcomes
Our outcome of interest was the first hypoglycemic event resulting in health care utilization within 90 days after the prescription index date. The event was identified by ICD-9/10-CM diagnosis codes (Table S1) from hospital, observation stay, emergency department, urgent care, or clinic visits using Medicare inpatient, outpatient, or physician visit claim files.
Statistical Analysis
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). We described baseline characteristics across individuals initiating sulfonylureas, SGLT2is, or GLP-1RAs as count or percentage for categorical variables and mean for continuous variables. We used a Cox proportional hazard regression model to evaluate the 90-day risk of hypoglycemia associated with sulfonylureas versus SGLT2is or GLP-1RAs. We selected a 90-day follow-up period consistent with published studies on hypoglycemic events.21,22 Patients were followed from the prescription index date until the first hypoglycemic event, death, or censoring events. Censoring events included the following: (1) completion of a 90-day of follow-up; (2) study end, December 31, 2018; (3) end of health insurance coverage (Medicare Part A, Part B, or Part D); (4) development of kidney failure; (5) medication refill gap of sulfonylureas, SGLT2is, or GLP-1RAs, metformin, or nonglucose-lowering medications that may be associated with hyper- or hypoglycemia during the 90-day follow-up period from the index date. More than 15 days gap between 2 prescription fill dates was considered a refill gap; (6) dosing change of metformin or nonglucose-lowering medications (associated with hyper- or hypoglycemia) during the 90-day follow-up period from the prescription index date; and (7) having claims for new glucose-lowering medications or new nonglucose-lowering medications (associated with hyper- or hypoglycemia) during the 90-day follow-up period from the prescription index date.
This study was approved by the Hennepin Healthcare Human Subjects Research Committee (IRB-FY2021-35). A waiver of consent was issued due to data anonymity, use of secondary data, and large population.
Results
The study cohort comprised 18,567 adults (aged 18 years or above) with CKD and type 2 diabetes after applying study inclusion and exclusion criteria; 14.0% (n = 2,528) had a prescription for a SGLT2i or GLP-1RA, and 86.0% (n = 16,039) for a sulfonylurea. SGLT2i or GLP-1RA use was 3.4% in 2013, 12.9% in 2016, and 21.5% in 2018. A Consolidated Standards of Reporting Trials diagram for patient selection is provided in Fig 2. The mean age (standard deviation) of all users was 72.9 (±10.0) years, 50.1% were women, 12.9% were Black, and 33.3% had the low-income subsidy. Sulfonylurea users had a higher mean Elixhauser comorbidity score than SGLT2i or GLP-1RA users. The proportion of patients with CKD stage 4-5 also was higher in sulfonylurea compared with SGLT2i or GLP-1RA users. Baseline characteristics in the overall cohort and by each treatment group are summarized in Table 2. The proportion of patients with new use of a SGLT2i or GLP-1RA was lower among Black patients than White patients or other patients (Table 3).
Figure 2.
Consolidated Standards of Reporting Trials diagram for patient selection. Abbreviations: CKD, chronic kidney disease; CMS, Centers for Medicare & Medicaid Services; GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Table 2.
Baseline Characteristics of CKD patients Aged 18 Years or Above With Type 2 Diabetes, Medicare 20% CKD claims, 2013-2018
| Baseline characteristics | Overall cohort | SGLT2i/GLP-1RA | Sulfonylurea |
|---|---|---|---|
| Total (n) | 18,567 | 2,528 | 16,039 |
| Age, y | |||
| mean (SD) | 72.9 (10.0) | 68.8 (9.9) | 73.5 (9.9) |
| Age category, y | |||
| 18-64 | 2,594 (14.0%) | 551 (21.8%) | 2,043 (12.7%) |
| 65-74 | 7,929 (42.7%) | 1,334 (52.8%) | 6,595 (41.1%) |
| 75-84 | 5,865 (31.6%) | 549 (21.7%) | 5,316 (33.1%) |
| ≥85 | 2,179 (11.7%) | 94 (3.7%) | 2,085 (13.0%) |
| Sex | |||
| Male | 9,262 (49.9%) | 1,223 (48.4%) | 8,039 (50.1%) |
| Female | 9,305 (50.1%) | 1,305 (51.6%) | 8,000 (49.9%) |
| Race/Ethnicity | |||
| White | 14,598 (78.6%) | 2,069 (81.8%) | 12,529 (78.1%) |
| Black | 2,396 (12.9%) | 244 (9.7%) | 2,152 (13.4%) |
| Other/unknown | 1,573 (8.5%) | 215 (8.5%) | 1,358 (8.5%) |
| Low-income subsidy (LIS) status | |||
| Non-LIS | 12,384 (66.7%) | 1,721 (68.1%) | 10,663 (66.5%) |
| LIS | 6,183 (33.3%) | 807 (31.9%) | 5,376 (33.5%) |
| CKD stage | |||
| 1/2 | 1,832 (9.9%) | 312 (12.3%) | 1,520 (9.5%) |
| 3 | 6,474 (34.9%) | 697 (27.6%) | 5,777 (36.0%) |
| 4/5 | 738 (4.0%) | 37 (1.5%) | 701 (4.4%) |
| Unk/Unspc | 9,523 (51.3%) | 1,482 (58.6%) | 8,041 (50.1%) |
| Metformin | 11,241 (60.5%) | 1,691 (66.9%) | 9,550 (59.5%) |
| Nonglucose-lowering medications associated with hyperglycemia | 11,164 (60.1%) | 1,599 (63.3%) | 9,565 (59.6%) |
| Statins | 9,862 (53.1%) | 1,424 (56.3%) | 8,438 (52.6%) |
| Tricyclic antidepressants | 659 (3.5%) | 137 (5.4%) | 522 (3.3%) |
| Corticosteroids | 2,246 (12.1%) | 287 (11.4%) | 1,959 (12.2%) |
| Nonglucose-lowering medications associated with hypoglycemia | 4,884 (26.3%) | 743 (29.4%) | 4,141 (25.8%) |
| Antibiotics | 1,890 (10.2%) | 248 (9.8%) | 1,642 (10.2%) |
| SSRIs | 3,094 (16.7%) | 514 (20.3%) | 2,580 (16.1%) |
| MAOIs | ∗ | ∗ | ∗ |
| Antihypertensives (noncardioselective) | 431 (2.3%) | 56 (2.2%) | 375 (2.3%) |
| Elixhauser comorbidity index score | |||
| mean (SD) | 8.5 (8.9) | 6.1 (8.0) | 8.8 (9.0) |
Note: ∗refers to counts of 10 or fewer patients.
Abbreviations: CKD, chronic kidney disease; SD, standard deviation; GLP-1RA, glucagon-like peptide 1 receptor agonist; LIS, low-income subsidy; MAOI, monoamine oxidase inhibitor; SGLT2i, sodium/glucose cotransporter 2 inhibitor; SSRI, selective serotonin reuptake inhibitor; Unk/unspc, CKD stage unknown or unspecified.
Table 3.
Differences in New Use of SGLT2is/GLP-1RAs Versus Sulfonylureas Across Age, Sex, Race/Ethnicity, and Low-Income Subsidy Groups in Patients With CKD and Type 2 Diabetes
| Baseline characteristics | Overall cohort | SGLT2i/GLP-1RA | Sulfonylurea |
|---|---|---|---|
| Total (n) | 18,567 | 2,528 | 16,039 |
| Age category, y | |||
| 18-64 | 2,594 | 551 (21.2%) | 2,043 (78.8%) |
| 65-74 | 7,929 | 1,334 (16.8%) | 6,595 (83.2%) |
| 75-84 | 5,865 | 549 (9.4%) | 5,316 (90.6%) |
| ≥85 | 2,179 | 94 (4.3%) | 2,085 (95.7%) |
| Sex | |||
| Male | 9,262 | 1,223 (13.2%) | 8,039 (86.8%) |
| Female | 9,305 | 1,305 (14.0%) | 8,000 (86.0%) |
| Race/ethnicity | |||
| White | 14,598 | 2,069 (14.2%) | 12,529 (85.8%) |
| Black | 2,396 | 244 (10.2%) | 2,152 (89.8%) |
| Other/unknown | 1,573 | 215 (13.7%) | 1,358 (86.3%) |
| Low-income subsidy (LIS) status | |||
| Non-LIS | 12,384 | 1,721 (13.9%) | 10,663 (86.1%) |
| LIS | 6,183 | 807 (13.1%) | 5,376 (86.9%) |
Abbreviations: CKD, chronic kidney disease; GLP-1RA, glucagon-like peptide 1 receptor agonist; LIS, low-income subsidy; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
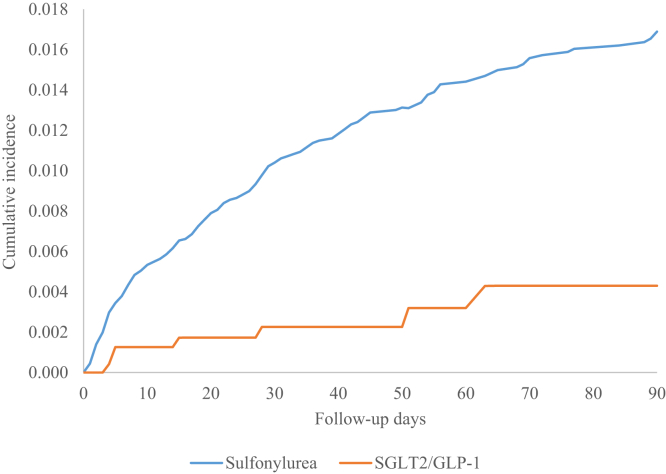
Hypoglycemic events occurring during treatment using SGLT2is or GLP-1RAs versus sulfonylureas were 0.3% and 1.2%, and the hypoglycemic events related to acute care were 43% and 76%, respectively (data not shown). The cumulative probability of hypoglycemic events is shown in Fig 3. Adjusted risk for hypoglycemic events during the follow-up period is provided in Table 4. Compared with sulfonylureas, use of SGLT2is or GLP-1RAs was significantly associated with a reduced risk of hypoglycemic events (adjusted hazard ratio [aHR], 0.30; 95% confidence interval [CI], 0.14-0.65) after adjustment for age, sex, race/ethnicity, CKD stage, comorbid condition score, and baseline use of nonglucose-lowering medications associated with hyper- or hypoglycemia. Black individuals had a higher risk of developing hypoglycemia than White individuals (aHR, 1.55; 95% CI, 1.07-2.26). Patients aged 75-84 years versus 65-74 years had higher risk of hypoglycemic events (aHR, 1.45; 95% CI, 1.04-2.01). Low-income subsidy compared with no low-income subsidy status was associated with a higher risk of hypoglycemic events. The risk of hypoglycemic events also increased with increased comorbid condition score (aHR, 1.05; 95% CI, 1.04-1.07).
Figure 3.
The Nelson-Aalen estimated 90-day cumulative incidence of hypoglycemic events. Abbreviations: GLP-1RA, glucagon-like peptide 1 receptor agonist; SGLT2i, sodium/glucose cotransporter 2 inhibitor.
Table 4.
Hazard Ratios for Risk of Hypoglycemia in CKD Patients Aged 18 Years or Above With Type 2 Diabetes
| Analysis | Hazard Ratio | 95% CI | P value | |
|---|---|---|---|---|
| Glucose-lowering medication | ||||
| Sulfonylurea | Ref. | |||
| SGLT2i/GLP-1RA | 0.30 | 0.14 | 0.65 | 0.002 |
| Age category, y | ||||
| 18-64 | 0.93 | 0.57 | 1.52 | 0.78 |
| 65-74 | Ref. | |||
| 75-84 | 1.45 | 1.04 | 2.01 | 0.02 |
| ≥85 | 1.02 | 0.64 | 1.61 | 0.95 |
| Sex | ||||
| Male | 0.80 | 0.60 | 1.06 | 0.12 |
| Female | Ref. | |||
| Race/ethnicity | ||||
| White | Ref. | |||
| Black | 1.55 | 1.07 | 2.26 | 0.02 |
| Other/unknown | 1.17 | 0.70 | 1.95 | 0.54 |
| Low-income subsidy (LIS) | ||||
| Non-LIS | Ref. | |||
| LIS | 1.56 | 1.14 | 2.14 | 0.005 |
| CKD stage | ||||
| 1/2 | 1.02 | 0.60 | 1.73 | 0.96 |
| 3 | Ref. | |||
| 4/5 | 1.68 | 0.97 | 2.89 | 0.06 |
| Unk/Unspc | 1.15 | 0.84 | 1.57 | 0.38 |
| Elixhauser comorbidity index score (per 1-unit change) | 1.05 | 1.04 | 1.07 | <.0001 |
| Metformin | ||||
| No | Ref. | |||
| Yes | 0.91 | 0.68 | 1.21 | 0.51 |
| Nonglucose-lowering medications associated with hyperglycemia | ||||
| No | Ref. | |||
| Yes | 0.94 | 0.71 | 1.25 | 0.69 |
| Nonglucose-lowering medications associated with hypoglycemia | ||||
| No | Ref. | |||
| Yes | 1.12 | 0.81 | 1.55 | 0.49 |
Abbreviations: CKD, chronic kidney disease; CI, confidence interval; GLP-1RA, glucagon-like peptide 1 receptor agonist; LIS, low-income subsidy; Ref., reference; SGLT2i, sodium/glucose cotransporter 2 inhibitor; Unk/Unspc, CKD stage unknown or unspecified.
We conducted sensitivity analysis to compare SGLT2i versus sulfonylurea use and GLP-1RA versus sulfonylurea use, separately. Compared with sulfonylureas, SGLT2i agents were significantly associated with reduced risk of hypoglycemic events (aHR, 0.19; 95% CI, 0.05-0.75) after covariate adjustment. GLP-1RA use was marginally but not statistically significantly associated with reduced risk of hypoglycemic events (aHR, 0.47; 95% CI, 0.21-1.07, P = 0.07).
Discussion
Among patients with CKD and type 2 diabetes, use of newer glucose-lowering medications (SGLT2is or GLP-1RAs) compared with sulfonylureas was associated with decreased risk of hypoglycemia. These results add to limited observational evidence for the association of newer glucose-lowering medications compared with sulfonylureas with safety issues among patients with reduced kidney function. The association was independent of age, sex, race/ethnicity, baseline medication use, CKD stage condition, and comorbid conditions. The sensitivity analysis comparing SGLT2is versus sulfonylureas and GLP-1RAs versus sulfonylureas showed that the risk of hypoglycemia was lower with SGLT2is compared with GLP-1RAs, relative to sulfonylureas. We also observed that Black race, older age (75-84 years), higher Elixhauser comorbidity index score, and low-income subsidy status were associated with a higher rate of developing hypoglycemic events.
Sulfonylureas are widely used as a diabetes treatment because they effectively lower blood glucose and hemoglobin A1c and are available as generics. The second-generation agents (glyburide, glipizide, glimepiride) have largely replaced first generation drugs (chlorpropamide, tolazamide, tolbutamide) used by the general population because of lower risk of hypoglycemia. Recently, a meta-analysis of randomized controlled trials examined efficacy and safety of newer glucose-lowering medications. The study compared SGLT2is with sulfonylureas as a second-line therapy in patients with type 2 diabetes inadequately controlled on metformin. The study included 5 trials involving 4,300 participants and found that SGLT2is were associated with less hypoglycemia as an add-on therapy to metformin (odds ratio, 0.12; 95% CI, 0.07-0.21) compared with sulfonylureas.23
Although the benefits of SGLT2is or GLP-1RAs among patients with type 2 diabetes and CKD have been demonstrated, there is no evidence that sulfonylureas reduce either cardiovascular or kidney progression risk. The Kidney Disease: Improving Global Outcomes (KDIGO) provides more specific clinical guidelines for patients with CKD and type 2 diabetes. In the 2021 KDIGO guideline, metformin and a SGLT2i are recommended as the first-line treatment choice for patients with CKD stage 3 or higher (estimated glomerular filtration rate ≥30 mL/min/1.73 m2). An SGLT2i is also recommended as a second-line treatment to these patients. In patients with type 2 diabetes and CKD who have not achieved individualized glycemic targets despite use of metformin and a SGLT2i, or who are unable to use those medications, a GLP-1RA is recommended.24 Our data showed that SGLT2i or GLP-1RA versus sulfonylurea use across the study time period was lower, but these agents showed a rapid increase in use from 2013-2018. We expect the trend will continue given the proven cardiovascular and kidney benefits of these agents.
Cost is an important factor that influences selection of a newer medication such as a SGLT2i or GLP-1RA. Luo et al25 recently assessed annual out-of-pocket costs associated with commonly used SGLT2is or GLP-1RAs across Part D plans. Median estimated annual out-of-pocket costs ranged from $1,211 (interquartile range, $1,167-$1,221) for ertugliflozin to $2,447 (interquartile range, $2,441-$2,464) for liraglutide with the standard Part D benefit design. Medicare beneficiaries not eligible for the low-income subsidy face very high out-of-pocket costs annually for SGLT2is or GLP-1RAs. Moreover, canagliflozin, one of 200 drugs with the highest utilization by dual eligible patients having both Medicare and Medicaid benefits, was included on <75% of Part D plan formularies in 2019 and 2020.26 High out-of-pocket costs and a low rate of formulary inclusion in Part D plans likely limit access to these medications by many patients that may receive clinical benefits from these newer medications. A recent retrospective study using Medicare claims data showed that SGLT2is or GLP-1RAs were only used in 3.3% and 6.1% in patients with CKD and type 2 diabetes, respectively, in 2016.27 Another retrospective analysis using 2015-2019 data from Optum Clinformatics Data Mart also suggested that prescription of SGLT2is was low but increasing in commercially insured patients with type 2 diabetes. Furthermore, the study showed that there were racial/ethnic, sex, and socioeconomic disparities in receipt of SGLT2i therapy. SGLT2i use was lower in Black patients.28 We observed that Black and older patients with CKD were less likely to receive these newer agents, were more likely to receive sulfonylureas, which have higher risk for hypoglycemia, and were also at a significantly higher risk of developing hypoglycemia after adjustment for other medications and covariates.
Health disparities in Black patients with diabetes and CKD have been well demonstrated. A cohort study with 4,251 participants found that the chance of developing diabetes was significantly higher for Black than for White adults (about 66 more cases of diabetes per 1,000 people).29 A large cohort study in multiethnic patients free of cardiovascular disease and with estimated glomerular filtration rate >60 mL/min/1.73 m2 at baseline found that kidney function decline varied significantly by race/ethnicity. Black individuals had a significantly higher rate of kidney function decline than White individuals (0.31 mL/min/1.73 m2/year faster on average, P = 0.001) after adjusting for multiple potential confounders.30 The US Renal Data System 2020 annual data report indicated that the adjusted prevalence of kidney failure was 3.4 times higher in Black individuals than White individuals in 2018.17 The results of our study highlight the importance of developing policies to address these disparities in Black patients at higher risk for development of CKD and kidney failure and to mitigate health disparities due to financial burden.
Our study has several strengths. We focused on health disparities regarding hypoglycemia risk in a large population of older patients with CKD and type 2 diabetes filling prescriptions for newer glucose-lowering medications. Additionally, SGLT2i or GLP-1RA clinical trials have included patients with CKD, but the majority were conducted in patients with estimated glomerular filtration rate ≥30 mL/min/1.73 m2. There is limited data on hypoglycemia risk of these agents among patients with type 2 diabetes and CKD stages 4-5. Also, we used a new user design, which reduces the risk of selection bias that can occur when patients have previously been exposed to these drug classes. To capture more potential confounding effects, we adjusted for the effect of nonglucose-lowering medications associated with hyper- or hypoglycemia and censored follow-up at medication change, refill gap, and dosing change. Compared with small data sources, we used the large Medicare claims database to capture comprehensive longitudinal information on patient demographics, inpatient and outpatient diagnoses and procedures, and prescriptions. We used actual medication claims dispensing records rather than other data sources that measure prescribing patterns not patient use.
The study has several limitations. CKD and type 2 diabetes diagnosis, CKD stage, and patient clinical status were identified with diagnosis or procedure codes because laboratory values were not available from the data sources. We may have underestimated the overall incidence of hypoglycemia by excluding patients with a history of hypoglycemic events before the prescription index date. There is potential for residual confounding with use of retrospective data. We adjusted our analysis by important risk factors (age, kidney function, and other chronic conditions), however we could not adjust for all potential confounders, especially lifestyle factors. Our analysis cohort consisted of patients with CKD enrolled in Medicare Part D coverage; utilization patterns may differ for patients enrolled in non-Part D prescription plans, Medicare Advantage plans, or other types of health insurance. The Medicare data set does not include patients aged younger than 65 years, except for those with disabilities. Generalizability to other populations should be considered carefully. We excluded patients with kidney failure, so our results cannot be extrapolated to this population. The low-income subsidy status was used as surrogate for socioeconomic status; other socioeconomic data was not available. Dose titration was not considered in the analysis. Our study investigated initial dose of the agents, and we used a short 90-day follow-up period to reduce the possibility of dose adjustment. Finally, information provided in Medicare Part D claims is based on dispensed prescriptions, which reflect prescription acquisition patterns and does not reflect patient consumption behavior.
In conclusion, among patients with CKD and type 2 diabetes, use of SGLT2is or GLP-1RAs compared with sulfonylureas was associated with a decreased risk of hypoglycemia. Our results provide real-world evidence on the association of SGLT2i or GLP-1RA use with the risk of hypoglycemia. Importantly, our results demonstrate that Black race was not only associated with lower use of newer agents with demonstrated cardiovascular and kidney benefits and lower hypoglycemia risk, but also with a higher rate of hypoglycemia events as compared with White patients. These results are a call for action for new policies that eliminate disparities in access and use of these newer agents in Black individuals and those with lower socioeconomic status.
Article Information
Authors’ Full Names and Academic Degrees
Julie Z. Zhao, MPH, PhD, Eric D. Weinhandl, PhD, MS, Angeline M. Carlson, PhD, and Wendy L. St. Peter, PharmD
Authors’ Contributions
Research idea and study design: JZZ, AMC, WLSP; data acquisition: JZZ, EDW, WLSP; data analysis/interpretation: JZZ, EDW, WLSP; statistical analysis: JZZ, EDW, WLSP; supervision or mentorship: WLSP. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We would like to acknowledge the support that Jon Schommer, PhD and Weihua Guan, PhD, University of Minnesota, provided as advisors on JZZ’s PhD dissertation committee. The data reported here have been supplied by the Centers for Medicare & Medicaid Services.
Disclaimer
The authors received no specific funding for this work. The data reported here have been supplied by the Centers for Medicare & Medicaid Services (CMS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. Government.
Peer Review
Received January 17, 2022. Evaluated by 2 external peer reviewers, with direct editorial input by a Statistical Editor and the Editor-in-Chief. Accepted in revised form May 11, 2022.
Footnotes
Complete author and article information provided before references.
Table S1: ICD-9/10-CM diagnosis codes for hypoglycemia
Table S2: ICD-9/10-CM diagnosis and CPT codes for solid organ transplants
Table S3: Nonglucose-lowering medications associated with hyperglycemia or hypoglycemia
Table S4: Chronic kidney disease stage-specific ICD-9/10-CM diagnosis codes
Supplementary Material
Tables S1-S4.
References
- 1.Centers for Disease Control and Prevention Chronic kidney disease in the United States, 2021. https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html Published July 8, 2021.
- 2.National Institute of Diabetes and Digestive and Kidney Diseases Diabetic kidney disease. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/diabetic-kidney-disease
- 3.Arieff A.I., Doerner T., Zelig H., Massry S.G. Mechanisms of seizures and coma in hypoglycemia. Evidence for a direct effect of insulin on electrolyte transport in brain. J Clin Invest. 1974;54(3):654–663. doi: 10.1172/JCI107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imad H., Zelano J., Kumlien E. Hypoglycemia and risk of seizures: a retrospective cross-sectional study. Seizure. 2015;25:147–149. doi: 10.1016/j.seizure.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Desouza C., Salazar H., Cheong B., Murgo J., Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26(5):1485–1489. doi: 10.2337/diacare.26.5.1485. [DOI] [PubMed] [Google Scholar]
- 6.Gerich J.E. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller M.E., Bonds D.E., Gerstein H.C., et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong S., Presswala L., Harris Y.T., et al. Hypoglycemia in patients with type 2 diabetes mellitus and chronic kidney disease: A prospective observational study. Kidney360. 2020;1(9):897–903. doi: 10.34067/KID.0001272020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B., Perkovic V., Mahaffey K.W., et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 10.Mahaffey K.W., Jardine M.J., Bompoint S., et al. Canagliflozin and cardiovascular and renal outcomes in type 2 diabetes mellitus and chronic kidney disease in primary and secondary cardiovascular prevention groups. Circulation. 2019;140(9):739–750. doi: 10.1161/CIRCULATIONAHA.119.042007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiviott S.D., Raz I., Bonaca M.P., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 12.Heerspink H.J.L., Stefánsson B.V., Correa-Rotter R., et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–1446. doi: 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 13.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394(10193):121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 14.Gerstein H.C., Colhoun H.M., Dagenais G.R., et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;394(10193):131–138. doi: 10.1016/S0140-6736(19)31150-X. [DOI] [PubMed] [Google Scholar]
- 15.Wanner C., Inzucchi S.E., Lachin J.M., et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi: 10.1056/nejmoa1515920. [DOI] [PubMed] [Google Scholar]
- 16.Zinman B., Wanner C., Lachin J.M., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 17.US Renal Data System. USRDS 2020 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MA: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. https://adr.usrds.org/2020/chronic-kidney-disease/ckd-analytical-methods. Accessed July 12, 2022.
- 18.Hebert P.L., Geiss L.S., Tierney E.F., Engelgau M.M., Yawn B.P., McBean A.M. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270–277. doi: 10.1177/106286069901400607. [DOI] [PubMed] [Google Scholar]
- 19.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.van Walraven C., Austin P.C., Jennings A., Quan H., Forster A.J. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 21.van Dalem J., Brouwers M.C.G.J., Stehouwer C.D.A., et al. Risk of hypoglycaemia in users of sulphonylureas compared with metformin in relation to renal function and sulphonylurea metabolite group: population based cohort study. BMJ. 2016;354:i3625. doi: 10.1136/bmj.i3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens K.K., McArthur E., Dixon S.N., Fleet J.L., Hramiak I., Garg A.X. The hypoglycemic risk of glyburide (glibenclamide) compared with modified-release gliclazide. Can J Diabetes. 2015;39(4):308–316. doi: 10.1016/j.jcjd.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z., Li G. Sodium-glucose co-transporter 2 inhibitors compared with sulfonylureas in patients with Type 2 diabetes inadequately controlled on metformin: a meta-analysis of randomized controlled trials. Clin Drug Investig. 2019;39(6):521–531. doi: 10.1007/s40261-019-00781-w. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes in CKD. https://kdigo.org/guidelines/diabetes-ckd/
- 25.Luo J., Feldman R., Rothenberger S.D., Hernandez I., Gellad W.F. Coverage, formulary restrictions, and out-of-pocket costs for sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists in the Medicare part D program. JAMA Netw Open. 2020;3(10) doi: 10.1001/jamanetworkopen.2020.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Department of Health and Human Services Part D plans generally include drugs commonly used by dual eligibles. https://oig.hhs.gov/oei/reports/oei-05-20-00190.asp
- 27.Zhao J.Z., Weinhandl E.D., Carlson A.M., St. Peter W.L. Glucose-lowering medication use in CKD: analysis of US Medicare beneficiaries between 2007 and 2016. Kidney Med. 2021;3(2):173–182.e1. doi: 10.1016/j.xkme.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eberly L.A., Yang L., Eneanya N.D., et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6139. e216139-e216139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bancks M.P., Kershaw K., Carson A.P., Gordon-Larsen P., Schreiner P.J., Carnethon M.R. Association of modifiable risk factors in young adulthood with racial disparity in incident type 2 diabetes during middle adulthood. JAMA. 2017;318(24):2457–2465. doi: 10.1001/jama.2017.19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peralta C.A., Katz R., DeBoer I., et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1-S4.