Abstract
Background:
Depression and Binge Eating Disorder (BED) are prevalent among bariatric surgery candidates. Depression subtypes may be differentially related to obesity, such that the atypical subtype predicts poorer outcomes. However, no research has examined depression subtypes, BED, and weight loss in bariatric candidates.
Objective:
To examine whether pre-surgical atypical depressive symptoms, compared to no depressive and melancholic depressive symptoms, were associated with higher rates of pre-surgical BED, binge eating severity, and poorer post-surgical weight loss trajectories among bariatric candidates.
Setting:
An outpatient Midwest bariatric clinic.
Method:
Participants were 345 adults (aged 46.27±12.78 years, 76% female; BMI=49.84±8.51kg/m2) who received a pre-surgical evaluation. Depression subtypes (melancholic, atypical, and no depressive symptoms) were categorized using the Beck Depression Inventory-II. BED diagnosis and severity were evaluated using the Eating Disorder Diagnostic Scale and Binge Eating Scale, respectively. Weight loss trajectories were calculated as percent total body weight loss (%TBWL) post-surgery.
Results:
Using no depression as the referent, participants reporting melancholic symptoms (OR=7.60, p<.001 CI95 [2.59–22.28]) and atypical symptoms (OR=10.11, p<.01 CI95 [2.69–37.94]) were more likely to meet criteria for BED. Patients with atypical depressive symptoms exhibited the highest binge eating severity scores (M=23.03). Depression subtypes did not predict %TBWL trajectories within 18-months post-bariatric surgery.
Conclusions:
Patients reporting pre-operative atypical depressive symptoms were more likely to meet criteria for comorbid BED diagnosis and have greater binge eating severity but did not have poorer weight loss within 18-months post-surgery. Future studies with longer-term follow-up and corresponding measures of post-surgical depression and binge eating pathology are warranted.
Keywords: bariatric surgery, depressive subtypes, binge eating disorder, binge eating
Introduction
Depression and obesity are public health crises and their bidirectional relationship is well-documented, such that obesity predicts depression onset and vice versa.1–2 This relationship may differ by depression subtype, namely atypical versus melancholic-type feature profiles.3–7 Melancholic depression involves more traditional symptoms such as sad mood or thoughts of worthlessness (i.e., cognitive/affective symptoms). Atypical depression features are characterized by significant mood reactivity, interpersonal rejection sensitivity, and at least two associated symptoms of leaden paralysis (fatigue that produces heavy feelings in the arms or legs), hyperphagia, or hypersomnia (i.e., somatic symptoms).8 When considering weight status and cardiometabolic disease, the atypical subtype has been linked to obesity in longitudinal2,6 and cross-sectional studies.3,9–11 The symptoms of hyperphagia (i.e., increased appetite/weight gain) and hypersomnia (i.e., increased sleep), in particular, have been associated with higher body mass index (BMI) when compared to individuals with non-atypical depressive symptoms or those with no history of depression.3–5,7 Given that atypical depressive symptoms have been prospectively associated with increased BMI and waist circumference as well as higher incidence of metabolic disease and fasting glucose over a 5.5-year period in the general population,6,12 patients in this subgroup may be at particularly high risk for maladaptive eating, activity, and/or sleep behaviors that result in weight gain.
Among bariatric surgery candidates, depression is the most commonly reported mental illness, with a recent meta-analysis indicating 19% of patients meeting clinical criteria for depression, a rate over double that of the general population.13 Despite this high prevalence, depression subtypes have yet to be examined in bariatric surgery populations. This is surprising not only due the high rates of depression but also given evidence that 1) depressive symptoms overall have been linked to poorer post-surgical weight loss outcomes – though the literature is mixed14,15 – and 2) atypical depression features, in particular, predict the greatest weight gain over time in non-bariatric samples.6 Failure to examine depressive subtypes may explain some of the inconsistencies in the literature and suggest these subtypes may be important to examine among those seeking surgical weight loss. Given its unique symptom presentation, the atypical depression subtype may also be most strongly related to disordered eating behaviors and Binge Eating Disorder (BED), another psychosocial risk factor in surgical candidates.
A considerable proportion of bariatric surgery candidates report a history of disordered eating prior to surgery16, with data that approximately 43% of patients engage in some type of binge eating behavior.17 Further, a significant proportion of patients meet criteria for BED, which is characterized in the DSM-5 as recurrent binge eating episodes and subjective feelings of loss of control resulting in marked distress.8 Recent prevalence estimates of lifetime and twelve-month BED in the US population are 0.85% and 0.44%, respectively.18 The prevalence of BED among bariatric patients is estimated to be 17%,13 a rate almost twenty times higher than the general population. As with the depression literature, data are mixed regarding the impact of pre-surgical BED on post-surgical weight loss outcomes.19–21 However, data linking pre-surgical BED to the occurrence of post-surgical eating disorder pathology is more consistent.20,22–25 Given the robust body of literature supporting post-surgical eating disorder pathology as a predictor of poor weight-loss outcomes, both pre- and post-surgical eating disorder pathology remain important factors for examination among bariatric surgery patients.22,23
Taken together, these above findings highlight the complex relationships between pre- and post-surgical mood and eating disorders on weight-loss outcomes as well as the potential relationship between depressive subtypes and BED symptoms.26 Evidence in non-bariatric samples suggests that atypical symptoms may contribute relatively more to emotional eating,27 cardiometabolic risk factors,12 and even mortality28 than do melancholic symptoms. Thus, although depressive symptoms in general may be problematic, the potential risk for problematic eating behavior, poor weight loss, and/or weight regain following surgery may be even greater among those with a history of depression with atypical features. Unfortunately, no research to date has examined the association between depression subtypes and BED among individuals with severe obesity presenting for surgical weight loss treatment. Therefore, the objectives of this study were to: 1) examine whether specific depression subtypes were associated with higher rates of self-reported BED and binge eating severity among bariatric surgery candidates, and 2) evaluate the impact of each depression subtype on trajectories of percent total body weight loss (%TBWL). We hypothesized that patients reporting atypical depressive symptoms, indexed primarily by hyperphagia, hypersomnia, and leaden paralysis (e.g., fatigue), would be more likely to meet criteria for DSM-5 BED, exhibit higher binge eating severity in comparison to patients reporting no depressive or melancholic depressive symptoms prior to surgery, and exhibit the poorest weight loss outcomes.
Materials and Methods
Participants
Participants were 345 adults who received a pre-surgical evaluation for laparoscopic Roux-en-Y gastric bypass (LRYGB) or sleeve gastrectomy (SG) bariatric surgery at a bariatric surgical center in the Midwest. Given the data were collected via retrospective chart review, only data from the pre-surgical evaluation and post-surgical follow-up visits were extracted from available charts. The reason for lack of follow-up data was not extracted or coded in this archival study; thus, we do not have detailed information about why participants did not have follow-up data (e.g., whether the reason was elective or not). Additional demographic information, including a breakdown by depressive subtype, can be found in Table 1.
Table 1.
Participant Characteristics by Depressive Symptom Groups
| Depressive Symptoms | ||||
|---|---|---|---|---|
|
| ||||
| Total Sample (N = 341) | No Depressive Symptomsa (n = 120) | Melancholic Depressive Symptomsb (n = 186) | Atypical Depressive Symptomsc (n = 35) | |
| Demographic Factors | ||||
| Age | 46.27 ± 12.78 | 46.52 ± 13.54a | 46.86 ± 12.09a | 41.94 ± 13.33a |
| Sex (% female) | 262 (75.9) | 85 (70.8)a | 147 (79.0)a | 26 (74.3)a |
| Race/Ethnicity (% Caucasian) | 283 (82.0) | 97 (80.8)a | 149 (80.1)a | 33 (94.3)a |
| Marital Status (% married) | 175 (50.7) | 64 (53.3)a | 94 (50.5)a | 17 (48.6)a |
| Education (% high school diploma) | 125 (36.2) | 43 (35.8)a | 64 (34.4)a | 16 (45.7)b |
| Preoperative BMI (kg/m2) | 49.84 ± 8.52 | 49.76 ± 9.50a | 50.24 ± 8.18a | 48.37 ± 6.50a |
Note. Means and standard deviations are presented for continuous variables. Frequencies and percentages are presented for categorical variables. Profiles with the same superscript (a,b) do not statistically differ from one another across each factor.
The group with no depressive symptoms had BDI-II scores < 10.
The group with melancholic depressive symptoms had BDI-II scores ≥ 10 without hyperphagia and hypersomnia.
The group with atypical depressive symptoms had BDI-II scores ≥ 10 with hyperphagia, hypersomnia, and leaden paralysis (fatigue used as proxy).
Measures
Depression subtypes.
Depression subtypes were assessed before surgery using the 21-item Beck Depression Inventory-II (BDI-II),29 a well-validated measure for assessing depressive symptomatology with high internal consistency in the present sample (α=.92). To determine depression subtypes, BDI-II scores were first categorized dichotomously based on the presence or absence of clinically elevated depressive symptoms. Those reporting total scores <10 were identified as having no clinically significant depressive symptoms, while those with scores ≥ 10 were noted as meeting the threshold for clinically significant depressive symptoms in order to capture the widest range of clinically relevant thresholds of mild, moderate, or severe.29 Next, those participants meeting the criteria for clinically significant depression were further categorized according to melancholic versus atypical features based on their scores on individual items. In accordance with the core clinical features of atypical depression as indicated in the DSM-5,8 participants who endorsed hypersomnia (Item 16 ≥ 1 on the increased sleep response option), hyperphagia (Item 18 ≥ 1 on the increased appetite response option), and leaden paralysis/fatigue (Item 20 ≥ 1) were identified as exhibiting atypical depressive symptoms. Given there was no direct measure of leaden paralysis, the fatigue item on the BDI-II was used as a proxy. Subsequently, those participants who did not endorse hypersomnia or hyperphagia symptoms were identified as having melancholic depressive symptoms. Of note, interpersonal rejection sensitivity was not included, as the BDI-II does not assess this symptom and no related data was collected at the time of the pre-surgical evaluation. Further, mood reactivity was not included, which is consistent with methodology used by Lojko and colleagues.11
Binge Eating Disorder Diagnosis and Severity.
Presence of BED among participants before surgery was determined from the Eating Disorder Diagnostic Scale (EDDS).30 An algorithm based on DSM-5 is used to detect individuals meeting criteria for an eating disorder, including BED. The EDDS is a robust, self-report instrument and demonstrates high reliability (α=.91) and validity (K=.74) in non-bariatric samples.30 Importantly, the EDDS was previously evaluated as a screening tool for binge eating among the current sample, and shows promise as an adequate and effective instrument for bariatric surgery patients.31 The EDDS demonstrated high internal consistency in the present sample (α=.81).
Severity of binge eating was assessed using the Binge Eating Scale (BES).32 Higher scores on the BES indicate more severe binge eating behavior, with scores greater than 17 indicating clinically significant binge eating symptoms.33 The BES is an effective screening instrument for BED among bariatric surgery patients34 and demonstrated high internal consistency in the present sample (α=.89).
Weight Loss Trajectories.
Trajectories of percent total body weight loss (%TBWL) were calculated as (weight loss in lbs) / (presurgical weight in lbs) x 100. Percent excess body weight loss (%EBWL) was also computed using the formula (weight loss in lbs) / (presurgical weight in lbs – ideal weight in lbs.) x 100, with ideal weight defined as the weight corresponding to a BMI of 25 kg/m2. Both %TBWL and %EBWL trajectories were calculated at 6, 12, and 18 months post-surgery.
Procedure
All data were collected via retrospective chart review within the hospital’s bariatric surgery clinic. Participants aged 18 and older who presented for a pre-surgical evaluation between January 1, 2012 and January 1, 2016 were included the study. This research was approved by the hospital’s Institutional Review Board.
Data Analyses
We examined the depression and binge eating variables both categorically and continuously for the following reasons: analyses of continuous variables (e.g., depressive symptoms from the BDI-II, binge eating severity using the BES) typically yield greater variability, yet analyses of categorical variables (e.g., groups of depressive symptoms, BED diagnosis) often have more clinical utility for providers. For continuous analyses, a series of binary logistic regressions, multinomial logistic regressions, and analysis of variance (ANOVA) with Tukey’s Honest Significant Difference (HSD) post-hoc tests were calculated. For categorical analyses, we used a series of chi-square tests of 140 independence. Education was included as a covariate in the analyses due significant group differences (see Table 1).
A series of mixed linear models with growth curve analyses were calculated in SPSS 25.0 to examine the impact of each pre-surgical depression subtype on trajectories of %TBWL after surgery. Mixed linear models were conducted using all available data, therefore minimizing the impact of missing data in analyses. Growth parameters, including post-surgical time in months (linear slope) and time quadratic (quadratic slope), were entered as main effects into each model. Interaction terms between the two growth parameters and the depressive subtype predictor (with “no depression” as the reference group) were also entered into each model.
Results
Descriptive Statistics
Four participants failed to provide valid data on the BDI-II and were subsequently dropped from analyses. Of the remaining sample (n=341 participants), 221 (64.1%) reported at least mild depressive symptoms (BDI ≥ 10). Of those 221 participants with depressive symptoms, 35 (15.8%) reported atypical depressive features while 186 (84.2%) exhibited a melancholic symptom profile. The average BES score for the sample was 16.92 (SD=9.14), which approaches the cut-off of indicating clinically significant binge eating symptoms.33
Depression and DSM-5 Binge Eating Disorder Diagnosis
Consistent with previous research from this sample31, approximately 16% (n=55) of the total sample met criteria for BED diagnosis utilizing the EDDS DSM-5 algorithm. Results of a chi-square test of independence revealed that participants categorized as meeting the threshold for any depressive symptoms were more likely to meet criteria for BED diagnosis when controlling for education, χ2(1)=18.78, p<.001. A binary logistic regression evaluating the relationship between continuous BDI-II total scores and BED diagnosis was also significant when controlling for education, χ2(7)=20.61, p<.01, such that participants reporting higher scores on the BDI-II were slightly more likely to meet criteria for BED (OR=1.06, p<.001 95% CI [1.03, 1.09]).
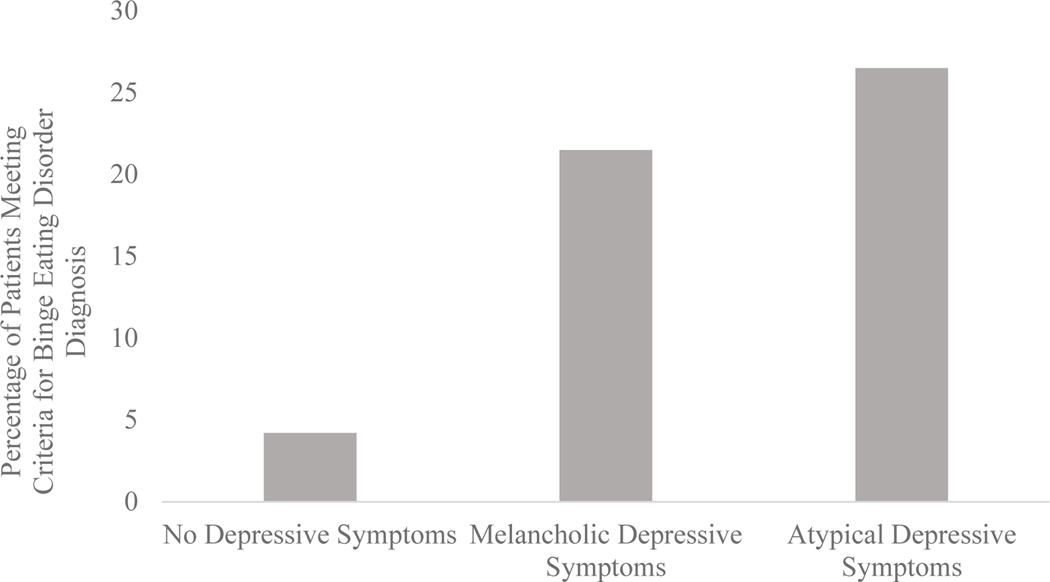
A multinomial logistic regression analysis evaluating the relationships between the depressive symptom groups and BED diagnosis was also significant when controlling for education, χ2(8)=28.43, p<.001. Using the no depressive symptoms group as the reference group, participants reporting melancholic depressive symptoms (OR=7.60, p<.001 95% CI [2.59, 22.28]) and atypical depressive symptoms (OR=10.11, p<.01 95% CI [2.69, 37.94]) were 7.6 and 10.1 times more likely to meet criteria for BED, respectively. See Figure 1 for BED diagnosis prevalence by depressive symptom group.
Figure 1.
Pre-surgical DSM-5 Binge Eating Disorder Diagnosis by Depressive Symptom Groups
Patients were categorized as meeting criteria for DSM-5 Binge Eating Disorder (BED) based on self-reported symptoms using the Eating Disorder Diagnostic Scale (EDDS) algorithm. Depressive symptom groups were categorized using self-reported scores on the Beck Depression Inventory- II (BDI-II). No depressive symptoms were categorized according to BDI-II scores < 10. Melancholic depressive symptoms were categorized according to BDI-II scores ≥ 10 without hyperphagia and hypersomnia. Atypical depressive symptoms were categorized according to BDI-II scores ≥ 10 with hyperphagia, hypersomnia, and leaden paralysis (fatigue used as proxy). In comparison to no depressive symptoms, patients with melancholic and atypical symptoms were 7.6 and 10.1 times as likely to meet criteria for BED when controlling for education, respectively.
Depression and Self-Reported Binge Eating Severity
A binary logistic regression evaluating the relationship between depressive symptoms (dichotomous yes/no) and self-reported binge eating severity was significant when controlling for education, χ2(7)=103.57, p<.001. These findings suggest that participants reporting more binge eating symptoms are 1.2 times more likely to report depressive symptoms, OR=1.20, p<.001 95% CI [1.15, 1.26]). Additionally, a linear regression examining the association between continuous BDI-II scores and binge eating severity revealed that higher depression scores were also associated with more binge eating symptoms when controlling for depression, β=.62, p<.001.
Results from an ANOVA test examining mean differences in self-reported binge eating scores by depressive symptom groups demonstrated a statistically-significant large effect when controlling for education, F (2,254)=56.30, p<.001, ηp2=.31. Post hoc results using Tukey’s HSD test suggested that those reporting no depressive symptoms (M=9.77, SD=6.84) significantly differed from both the melancholic (M=19.87, SD=7.76) and atypical (M=22.97, SD=9.45) depressive symptom groups at the p<.001 level. The melancholic and atypical groups also significantly differed from one another (p<.05).
Weight Loss and Impact of Depression Subtypes on Weight Loss Trajectories
Average %TBWL at each timepoint post-surgery included: 28.61% at 6 months (n = 195), 34.36% at 12 months (n = 171), and 35.30% at 18 months (n = 132). Post-surgical weight was captured for 57.2% of participants in our sample at six months, 50.1% at 12 months, and 38.7% at 18 months. Results of the series of mixed linear models revealed that neither the melancholic nor the atypical depressive subtypes had a significant impact on %TBWL trajectories after surgery (see Table S1 in supplementary materials). Parallel mixed linear models were also conducted using percent excess body weight loss (%EBWL) as the dependent variable. Results did not significantly differ from those of %TBWL.
Discussion
Results suggest that bariatric surgery candidates who present with atypical depressive symptoms before surgery are also more likely to meet criteria for self-reported, pre-operative BED than those without depression or those with the melancholic depressive subtype when controlling for education. This pattern was present when examining depressive symptoms both categorically and continuously. In comparison to patients reporting no depressive symptoms before surgery, those reporting melancholic and atypical depressive symptoms pre-operatively were 7.6 and 10.1 times more likely to meet criteria for pre-operative BED diagnosis, respectively. In the sample, 21% of patients with melancholic depressive symptoms and 26% of patients with atypical depressive symptoms met criteria for BED diagnosis. In contrast, less than 1% of patients with no depressive symptoms met criteria for BED, suggesting that the presence of depressive symptoms is associated with greater self-reported BED diagnosis among bariatric surgery candidates, and those patients presenting with atypical depressive symptoms may be at greatest risk for comorbid mood and eating pathology.
Similar results were found with regards to self-reported binge eating severity. Patients reporting clinically significant depressive symptoms were slightly more likely to endorse more severe binge eating pathology before surgery when controlling for education. In exploring differences between the depressive symptom groups, patients reporting melancholic and atypical depressive symptoms exhibited more severe binge eating behaviors. Further, those in the atypical group exhibited the highest binge eating severity scores, which were significantly higher than those in the melancholic group. Therefore, patients presenting with pre-operative atypical depressive symptoms appear to be at the highest risk for comorbid pre-operative problematic binge eating behaviors.
Contrary to our hypotheses, endorsement of pre-operative melancholic nor the atypical depressive subtypes was not associated with %TBWL trajectories within 18-months post-bariatric surgery. Although no prior data exist evaluating the relationships between specific pre-operative depressive subtypes and post-surgical weight-loss outcomes, our finding are consistent with previous studies showing that pre-surgical depressive symptoms overall may not predict post-operative weight loss outcomes up to 24- to 36-months post-surgery.15,22 However, it would be premature to conclude that depressive subtypes do not affect post-surgical weight-loss, especially over longer follow-up periods. Specifically, more recent literature suggests that mood disorders may impact long-term outcomes (i.e., seven years post-surgery).35 In addition, post-surgical depressive and eating disorder symptoms have been associated with poorer post-surgical weight-loss outcomes.15,22,23 Unfortunately, neither depressive subtypes nor BED symptoms were assessed post-surgery using clinical diagnosis in our sample. Thus, the impact of depressive subtypes on post-surgical weight-loss outcomes may depend on the interaction between depressive subtypes and BED symptoms post-bariatric surgery, may manifest at later term follow-ups, and may depend on greater clinical severity. Future studies evaluating the relationships between clinically diagnosed depressive subtypes and BED on long-term post-surgery are still warranted.
Overall, this research is the first to examine the associations between depression symptom groups, self-reported BED diagnosis, binge eating severity, and weight-loss trajectories among adults with severe obesity seeking bariatric surgery. Given that depression is the most common comorbidity with eating disorders36, our findings both support and advance the literature by suggesting that patients presenting pre-operatively with atypical depressive symptoms may be at the highest risk for comorbid BED and problematic binge eating behaviors. Education levels differed across depression groups in our study, such that patients in the atypical depressive symptoms group had significantly lower education levels (i.e., lower % having a high school diploma) compared to patients in the melancholic or no depressive symptoms groups. Higher levels of education are shown to be protective against depressive symptoms, specifically among people from disadvantaged backgrounds.37 Low education and socioeconomic status (SES) are also strong risk factors for negative health outcomes broadly.38 Additional studies are needed to better understand the role of lower education and SES as risk factors for depression among different depression subtypes. Although no relationships were found between pre-surgical depressive subtypes and post-surgical weight-loss trajectories within our relatively short follow-up period, our findings highlight a need for future research to elucidate the potential interactive effects of depressive subtypes and BED symptoms on post-surgical weight-loss outcomes.
Previous research in non-bariatric samples shows higher prevalence of atypical depressive symptoms among patients with obesity when compared to counterparts who have depression but do not have obesity.11 There are several potential reasons why atypical depressive symptoms contribute to the maintenance of disordered eating behaviors or vice versa. First, there are shared biological and pathophysiological pathways that underlie the relationship between depression, eating pathology, and obesity.26 Genetics, homeostatic disruptions, and neurobiological factors are proposed mechanisms by which depression and obesity onset and co-occur.39–41 Evidence also suggests that depressive subtypes may contribute to variability in these pathways. A recent study evaluated the associations between depressive subtypes (e.g., melancholic, atypical) and biological measures, and concluded that patients presenting with atypical depression exhibited greater inflammation and metabolic abnormalities.39 Although a paucity of research exists evaluating inflammation and metabolic disturbance within eating disorders, previous research has shown that binge eating results in metabolic changes in young lean women, and that these effects are likely to be worsened among adults with obesity or insulin resistance.42 Given the distinct pathophysiological mechanisms associated with atypical depression, these pathways explain why atypical depression may predispose adults with severe obesity to develop disordered eating behaviors.
The results from this study have several important clinical implications. Our results suggest that BED diagnosis and problematic binge eating behaviors are the greatest among patients reporting atypical depressive symptoms. However, we did not find any direct relationships between pre-surgical depressive subtype (melancholic vs. atypical) and post-surgical weight-loss trajectories, which may be a function of our relatively short follow-up period. We suggest that screening for the co-occurrence of depressive and BED symptoms at both pre- and post-bariatric surgery may allow health care providers to better determine those who may be at an increased risk for poorer eating- and weight-related outcomes post-surgery. Next, tailored interventions aimed at addressing both atypical depression symptoms and binge eating may improve patient outcomes at pre- and post-surgery. For example, cognitive behavioral therapy (CBT) is a well-established treatment for BED43,44 and depression (including depression with melancholic and atypical features).45 Additionally, previous research has shown that four-session CBT treatment for binge eating is effective in reducing these behaviors among bariatric surgery candidates.46 As such, CBT may be a viable adjunctive therapy to administer to patients in combination with bariatric surgery in order to address both atypical depression and binge eating symptoms.
Our results should be considered within the context of their limitations. With the exception of our weight-loss trajectory analyses, data from the present study is cross-sectional in nature which limits our ability to make causal inferences on the depression-binge eating relationship. Patients presenting with atypical depressive symptoms may be at increased risk to meet criteria for BED diagnosis and clinically significant binge eating severity; however, it is also possible that atypical depressive symptoms are a consequence of binge eating, or that the relationship is bidirectional. Future studies should utilize longitudinal designs with repeated measures of depressive and eating disorder symptoms to infer the causality of these relationships. Next, this study utilized retrospective paper chart review, so we do not have detailed information on patients that did not have follow-up data within the time that data was extracted or the subsequent reasons as to why this occurred (e.g., excluded due to psychiatric comorbidity, elected not to proceed with surgery, moved, etc.). Prospective studies should collect more data on patients presenting for pre-bariatric surgery evaluations to determine reasons patients do/do not move forward with surgery. We also do not have data on depressive symptoms or BED symptoms post-bariatric surgery. While our weight-loss trajectory analyses show no relationship between depressive symptom groups and weight-loss outcomes, it is unclear if post-surgical BED symptoms in conjunction with depression would negatively impact weight-loss over a longer follow-up. Further, there was a drop in available chart data across the post-surgical follow-up period (57% at 6 months vs. 38% at 18 months) for our weight-loss trajectory analyses. Patients with poorer weight loss outcomes and elevated mood or eating pathology may have been more likely to be lost to follow-up, which could have contributed to the non-significant findings with regard to weight loss outcomes. Future studies are needed evaluating the co-occurrence of depression and BED both pre- and post-bariatric surgery to determine the impact of these symptoms on long-term weight-loss outcomes. Additionally, our primary symptom measures required patients to self-report their symptoms. Future studies should utilize more robust instruments, such as structured clinical interviews, to assess depression and binge eating disorder diagnoses. Lastly, no direct measurement of leaden paralysis or interpersonal rejection sensitivity was included in the current study. Given that leaden paralysis and interpersonal rejection sensitivity are elements of the atypical specifier in DSM-5, it is unknown whether they contribute to the relationship between atypical depressive symptoms and binge eating among bariatric surgery patients. While we included fatigue as a proxy for leaden paralysis, future studies should include more comprehensive measures of atypical depressive symptoms to determine which symptoms are most salient with regards to binge eating.
Conclusions
The findings from this study are the first to delineate the association between pre-surgical depressive symptom groups and self-reported binge eating diagnosis and severity among bariatric surgery candidates. Our results suggest that individuals reporting atypical depressive symptoms are 10.1 times more likely to meet criteria for comorbid BED diagnosis and report clinically significant binge eating severity. Bariatric surgery candidates reporting atypical depressive symptoms appear to be at most risk for binge eating diagnosis and severity. While our findings do not support a relationship between depressive subgroups and weight-loss trajectories within 18-month post-surgery, future studies are needed evaluating the impact of co-occurring depression and BED symptoms on post-surgery weight related outcomes over longer follow-up periods. Future studies aimed at identifying treatment targets and developing structured interventions to improve atypical depressive symptoms and binge eating behaviors within this population may be warranted.
Supplementary Material
Highlights.
Depressive symptoms are associated with Binge Eating Disorder diagnosis
Atypical depressive symptoms associated with highest risk of Binge Eating Disorder
Patients with atypical depressive symptoms exhibit greater binge eating severity
Pre-operative depression subtypes did not predict 18-month post-surgical weight-loss
Funding:
Kent State University Community Research Fellowship Award (awarded to MAWH)
Footnotes
Disclosure: The authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Schweinfurth N, Walter M, Borgwardt S, Lang UE. Depression and Obesity. Obesity: Springer; 2016:235–244. [Google Scholar]
- 2.Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Archives of general psychiatry. 2010;67(3):220–229. [DOI] [PubMed] [Google Scholar]
- 3.Glaus J, Vandeleur C, Gholam-Rezaee M, et al. Atypical depression and alcohol misuse are related to the cardiovascular risk in the general population. Acta Psychiatrica Scandinavica. 2013;128(4):282–293. [DOI] [PubMed] [Google Scholar]
- 4.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765. [DOI] [PubMed] [Google Scholar]
- 5.Lamers F, de Jonge P, Nolen WA, et al. Identifying depressive subtypes in a large cohort study: results from the Netherlands Study of Depression and Anxiety (NESDA). Journal of Clinical Psychiatry. 2010;71(12):1582. [DOI] [PubMed] [Google Scholar]
- 6.Lasserre AM, Glaus J, Vandeleur CL, et al. Depression with atypical features and increase in obesity, body mass index, waist circumference, and fat mass: a prospective, population-based study. JAMA psychiatry. 2014;71(8):880–888. [DOI] [PubMed] [Google Scholar]
- 7.Levitan RD, Davis C, Kaplan AS, Arenovich T, Phillips D, Ravindran AV. Obesity comorbidity in unipolar major depressive disorder: refining the core phenotype. The Journal of clinical psychiatry. 2012;73(8):1119–1124. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: 2013. [Google Scholar]
- 9.Chou KL, Yu KM. Atypical depressive symptoms and obesity in a national sample of older adults with major depressive disorder. Depression and anxiety. 2013;30(6):574–579. [DOI] [PubMed] [Google Scholar]
- 10.De Wit L, Luppino F, van Straten A, Penninx B, Zitman F, Cuijpers P. Depression and obesity: a meta-analysis of community-based studies. Psychiatry research. 2010;178(2):230–235. [DOI] [PubMed] [Google Scholar]
- 11.Łojko D, Buzuk G, Owecki M, Ruchała M, Rybakowski JK. Atypical features in depression: association with obesity and bipolar disorder. Journal of affective disorders. 2015;185:76–80. [DOI] [PubMed] [Google Scholar]
- 12.Lasserre AM, Strippoli MPF, Glaus J, et al. Prospective associations of depression subtypes with cardio-metabolic risk factors in the general population. Molecular Psychiatry. 2017;22(7):1026–1034. [DOI] [PubMed] [Google Scholar]
- 13.Dawes AJ, Maggard-Gibbons M, Maher AR, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: a meta-analysis. Jama. 2016;315(2):150–163. [DOI] [PubMed] [Google Scholar]
- 14.Marek RJ, Ben-Porath YS, van Dulmen MH, Ashton K, Heinberg LJ. Using the presurgical psychological evaluation to predict 5-year weight loss outcomes in bariatric surgery patients. Surgery for Obesity and Related Diseases. 2017;13(3):514–521. [DOI] [PubMed] [Google Scholar]
- 15.White MA, Kalarchian MA, Levine MD, Masheb RM, Marcus MD, Grilo CM. Prognostic significance of depressive symptoms on weight loss and psychosocial outcomes following gastric bypass surgery: a prospective 24-month follow-up study. Obesity surgery. 2015;25(10):1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Opolski M, Chur-Hansen A, Wittert G. The eating-related behaviours, disorders and expectations of candidates for bariatric surgery. Clinical obesity. 2015;5(4):165–197. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell JE, King WC, Courcoulas A, et al. Eating behavior and eating disorders in adults before bariatric surgery. International Journal of Eating Disorders. 2015;48(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Udo T, Grilo CM. Prevalence and correlates of DSM-5–defined eating disorders in a nationally representative sample of US adults. Biological psychiatry. 2018;84(5):345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meany G, Conceição E, Mitchell JE. Binge eating, binge eating disorder and loss of control eating: effects on weight outcomes after bariatric surgery. European Eating Disorders Review. 2014;22(2):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams-Kerver GA, Steffen KJ, Mitchell JE. Eating Pathology After Bariatric Surgery: an Updated Review of the Recent Literature. Current psychiatry reports. 2019;21(9):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wimmelmann CL, Dela F, Mortensen EL. Psychological predictors of mental health and health-related quality of life after bariatric surgery: a review of the recent research. Obesity Research & Clinical Practice. 2014;8(4):e314–e324. [DOI] [PubMed] [Google Scholar]
- 22.Kalarchian MA, King WC, Devlin MJ, et al. Psychiatric disorders and weight change in a prospective study of bariatric surgery patients: a 3-year follow-up. Psychosomatic medicine. 2016;78(3):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao AM, Wadden TA, Faulconbridge LF, et al. Binge-eating disorder and the outcome of bariatric surgery in a prospective, observational study: Two-year results. Obesity. 2016;24(11):2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conceição EM, Utzinger LM, Pisetsky EM. Eating disorders and problematic eating behaviours before and after bariatric surgery: characterization, assessment and association with treatment outcomes. European Eating Disorders Review. 2015;23(6):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marino JM, Ertelt TW, Lancaster K, et al. The emergence of eating pathology after bariatric surgery: a rare outcome with important clinical implications. International Journal of Eating Disorders. 2012;45(2):179–184. [DOI] [PubMed] [Google Scholar]
- 26.Sarwer DB, Allison KC, Wadden TA, et al. Psychopathology, disordered eating, and impulsivity as predictors of outcomes of bariatric surgery. Surgery for Obesity and Related Diseases. 2019;15(4):650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paans NPG, Bot M, van Strien T, Brouwer IA, Visser M, Penninx BWJH. Eating styles in major depressive disorder: Results from a large-scale study. Journal of Psychiatric Research. 2018;97:38–46. [DOI] [PubMed] [Google Scholar]
- 28.Roest AM, Thombs BD, Grace SL, Stewart DE, Abbey SE, de Jonge P. Somatic/affective symptoms, but not cognitive/affective symptoms, of depression after acute coronary syndrome are associated with 12-month all-cause mortality. Journal of Affective Disorders. 2011;131(1-3):158–163. [DOI] [PubMed] [Google Scholar]
- 29.Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio. 1996;78(2):490–498. [Google Scholar]
- 30.Stice E, Telch CF, Rizvi SL. Development and validation of the Eating Disorder Diagnostic Scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychological assessment. 2000;12(2):123. [DOI] [PubMed] [Google Scholar]
- 31.Williams GA, Hawkins MA, Duncan J, Rummell CM, Perkins S, Crowther JH. Maladaptive eating behavior assessment among bariatric surgery candidates: evaluation of the Eating Disorder Diagnostic Scale. Surgery for Obesity and Related Diseases. 2017;13(7):1183–1188. [DOI] [PubMed] [Google Scholar]
- 32.Gormally J, Black S, Daston S, Rardin D. The assessment of binge eating severity among obese persons. Addictive behaviors. 1982;7(1):47–55. [DOI] [PubMed] [Google Scholar]
- 33.Marcus MD, Wing RR, Hopkins J. Obese binge eaters: Affect, cognitions, and response to behavioral weight control. Journal of Consulting and Clinical Psychology. 1988;56(3):433. [DOI] [PubMed] [Google Scholar]
- 34.Grupski AE, Hood MM, Hall BJ, Azarbad L, Fitzpatrick SL, Corsica JA. Examining the Binge Eating Scale in screening for binge eating disorder in bariatric surgery candidates. Obesity surgery. 2013;23(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalarchian MA, King WC, Devlin MJ, et al. Mental disorders and weight change in a prospective study of bariatric surgery patients: 7 years of follow-up. Surgery for Obesity and Related Diseases. 2019;15(5):739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker CB, Plasencia M, Kilpela LS, Briggs M, Stewart T. Changing the course of comorbid eating disorders and depression: what is the role of public health interventions in targeting shared risk factors? Journal of Eating Disorders. 2014;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauldry S. Variation in the protective effect of higher education against depression. Society and mental health. 2015;5(2):145–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marmot M. The health gap: the challenge of an unequal world. The Lancet. 2015;386(10011):2442–2444. [DOI] [PubMed] [Google Scholar]
- 39.Lamers F, Vogelzangs N, Merikangas K, De Jonge P, Beekman A, Penninx B. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Molecular psychiatry. 2013;18(6):692. [DOI] [PubMed] [Google Scholar]
- 40.Milaneschi Y, Simmons WK, Rossum EF, Penninx BW. Depression and obesity: evidence of shared biological mechanisms. Molecular psychiatry. 2018:1. [DOI] [PubMed] [Google Scholar]
- 41.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N . Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC medicine. 2013;11(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor AE, Hubbard J, Anderson EJ. Impact of binge eating on metabolic and leptin dynamics in normal young women. The Journal of Clinical Endocrinology & Metabolism. 1999;84(2):428–434. [DOI] [PubMed] [Google Scholar]
- 43.Wilfley DE, Cohen LR. Psychological treatment of bulimia nervosa and binge eating disorder. Psychopharmacology Bulletin. 1997;33(3):437. [PubMed] [Google Scholar]
- 44.Wilfley DE, Welch RR, Stein RI, et al. A randomized comparison of group cognitive-behavioral therapy and group interpersonal psychotherapy for the treatment of overweight individuals with binge-eating disorder. Archives of general psychiatry. 2002;59(8):713–721. [DOI] [PubMed] [Google Scholar]
- 45.Tolin DF. Is cognitive–behavioral therapy more effective than other therapies?: A meta-analytic review. Clinical psychology review. 2010;30(6):710–720. [DOI] [PubMed] [Google Scholar]
- 46.Ashton K, Drerup M, Windover A, Heinberg L. Brief, four-session group CBT reduces binge eating behaviors among bariatric surgery candidates. Surgery for Obesity and Related Diseases. 2009;5(2):257–262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.