Abstract
We review the safety and early oncological outcomes of irreversible electroporation (IRE), a novel non-thermal ablation technique, in small renal masses (SRMs). Following PROSPERO registration (CRD42020197943), a systematic search of MEDLINE, EMBASE and SCOPUS databases according to PRISMA guidelines was performed. Critical appraisal of the included studies was performed using the Newcastle-Ottawa Scale. Of 224 articles screened, 10 met the inclusion criteria. In total, 83 patients were identified. Except for one cohort study (n = 41), the remaining studies were case series of n < 10. Follow up was <12 months in 7/10 articles (range 3–34 months). About 10/10 articles reported safety outcomes. There were no 30-day mortalities. The most frequently reported adverse events were transient haematuria (11/83) and asymptomatic perirenal haematomas (7/83). About 62/63 patients with reported length of stay were discharged within 24 h. No significant long-term changes in renal function were reported. About 7/10 articles reported oncological outcomes. Only one article assessed histopathological outcomes, whilst the remaining studies used cross-sectional imaging modalities to assess efficacy, recurrence or disease progression. About 4/7 patients with histopathology outcomes, showed complete response (CR). About 43/55 patients with radiological outcomes showed CR. No mortalities were reported due to SRMs. These initial findings support IRE as safe and feasible in managing SRMs. However, results from larger studies with longer follow-up are needed to evaluate oncological outcomes and compare these with other ablation methods.
Keywords: Irreversible electroporation, feasibility studies, kidney neoplasms, nanoknife, renal cancer
Introduction
Renal Cell Carcinoma (RCC) is a common cancer, with many patients remaining asymptomatic until late-stage disease. 1 Small renal masses (SRMs) are consistently defined as tumours less than 4 cm in diameter in both the Tumour, Node, Metastases (TNM) staging system (T1a tumours) and the R.E.N.A.L Nephrotomy score (Radius, Exophytic/endophytic, Nearness to the renal collecting system, Anterior or posterior location, Location to the renal poles).1–3 Partial nephrectomy (PN) is the current gold standard for SRMs, as the preservation of renal tissue reduces the risk of associated cardiovascular or metabolic disease. 1
Thermal ablative therapies such as percutaneous radiofrequency ablation (RFA) or cryoablation (CA) provide an alternative management option for SRMs. Indications for ablative therapies currently include frail patients unsuitable for surgical options, those with solitary kidneys or those with bilateral tumours; and are associated with lower complication rates and similar rates of local recurrence.1,4,5 However, RFA and CA therapies are not recommended for central renal tumours, due to increased complication rates, the risk of renal pelvis and/or ureter damage and reduced efficacy due to the heat sink effect (whereby proximity to vessels can limit RFA and CA efficacy, due to heat transfer).1,6–8
Irreversible Electroporation (IRE) or Nanoknife™ is a novel non-thermal ablation method first reported by Rubinsky et al. 9 They demonstrated that by applying a series of high voltage pulses between electrodes placed around the tumour under radiological guidance, irreversible cell permeabilisation can be established leading to tumour cell death. Crucially this leaves the collagen and elastin rich extra-cellular matrix unaffected, which aids in the regeneration of the treated/ablated tissues.9,10 This unique property of IRE leads to its ability to spare vital structures such as blood vessels and the renal collecting system, after which these structures are able to regenerate and re-gain function. 11 Furthermore, IRE is a non-thermal method and so does not suffer from the heat sink effect unlike RFA and CA, further giving it a unique area of clinical application.10,11
This article aims to determine whether a consensus view of the safety and the oncological efficacy of the technique can be determined. To investigate this, we reviewed the current literature on the use of IRE in patients with RCC.
Material and methods
Literature search strategy
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance. 12 The study’s search strategy and design was prospectively registered with PROSPERO (registration number: CRD42020197943).
A systematic review of the literature published up until 29th November 2020 was carried out, using searches of the databases MEDLINE, EMBASE and SCOPUS (Full search strategies available in Supplemental File). Inclusion criteria included articles reporting on safety and oncological outcomes in adult patients (age >18 years) with SRMs treated by IRE. Exclusion criteria included any interim data published prior to the release of full data and animal studies. There were no exclusions based on study design.
Studies were critically appraised using the Newcastle-Ottawa Quality Assessment Tool (NOQAT) (Full marking criteria are outlined in the Supplemental File). Based on the applicable criteria, articles were designated as either good, fair or poor quality. If all criteria were deemed to be of suitable quality, the article was reported as ‘good quality’. If one criterion was deemed to be of low quality, then the study was labelled ‘fair quality’. If two or more criteria were deemed to be of low quality, then the article was labelled as ‘poor quality’.
Data synthesis and extraction
Two of the authors (AH and GK) independently screened titles and abstracts identified by the literature search. The systematic review web tool Rayyan was used to optimise the screening process. 13 The full relevant articles were reviewed to determine eligibility for inclusion. Any disagreement was discussed and solved by consensus in agreement with the third author (FG).
Variables for which data was collated for included: patient and tumour characteristics, procedural data, safety outcomes including adverse effects and early oncological outcomes included complete response rate. Safety outcomes were determined by assessment of overall mortality, adverse events, and of changes in renal function, whilst early oncological outcomes were determined based on reported complete response (CR) rates and any recurrence rates within the follow-up period reported by the included studies.
Results
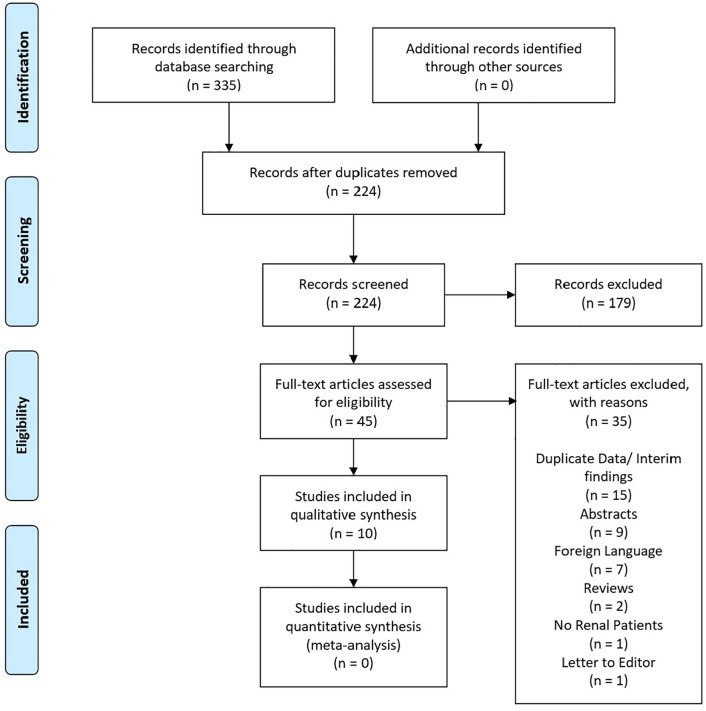
A total of 10 articles met the inclusion criteria and have been included as part of the review (Figure 1). Within these publications a total of 83 patients were identified. Except for one cohort study that included 41 patients the remaining studies were case series of 10 patients or less. Follow up was <12 months in 7/10 articles (range 3–34 months).
Figure 1.
PRISMA flow diagram showing the articles included and excluded at each stage of the assessment process.
The prevailing procedure performed was percutaneous IRE under General Anaesthetic (GA) with muscle paralysis and cardiac synchronisation. Of the articles, six were prospective studies, the remaining four retrospective studies; all were single centre studies. The full study designs of the included studies and the patient characteristics within the studies can be found in Tables 1 and 2.14–23
Table 1.
Study design and details of included studies.
| Authors | Country | Study design | Study duration | Safety/ efficacy | n = | Follow up | Renal cell carcinoma diagnosis modality | Procedure | Other |
|---|---|---|---|---|---|---|---|---|---|
| Thomson et al. 14 | Australia | Prospective Single Centre Cohort | 12 months | Safety Efficacy |
7 (38 including liver and lung malignancies) | 3 months | NR | Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation | – |
| Pech et al. 15 | Germany | Prospective Single Centre Cohort | NR | Safety | 6 | 3 months | Pathology following nephrectomy | Open IRE under GA with muscle paralysis and cardiac synchronisation immediately followed by partial (n = 4) or complete (n = 2) nephrectomy | – |
| Diehl et al. 16 | Germany | Retrospective Single Centre Cohort | 12 months | Safety Efficacy |
5 | Mean 6.4 months (range 3–11) |
RCC recurrence, histopathology from prior nephrectomy | Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation | 5/5 solitary kidneys |
| Vroomen et al. 17 | The Netherlands | Retrospective Single Centre Cohort | 40 months | Safety Efficacy |
1 (8 including other pelvic malignancies) | 4 months | RCC recurrence, histopathology from prior nephrectomy | Percutaneous IRE under GA with muscle paralysis Cardiac synchronisation NR |
Post nephrectomy site of chromophobe RCC recurrence |
| Canvasser et al. 18 | USA | Prospective Single Centre Cohort | 44 months | Safety Efficacy |
41 | Mean 22 months (SD 12.4) |
31/41 patients had histopathological diagnosis pre-IRE or at time of IRE 10/41 on imaging |
Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation | – |
| Liu, et al. 19 | Canada | Retrospective Single Centre Cohort | NR | Safety Efficacy |
5 | Mean 22 months (range 14–31) |
3/5 on CT imaging 2/5 on MRI imaging |
Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation | 2/5 solitary kidneys |
| Wendler et al. 20 | Germany | Prospective Single Centre Cohort | NR | Safety Efficacy |
7 | 27 days to nephrectomy Overall Mean 25 months (range 15–36) |
Histopathology confirmed (pT1acN0cM0) | Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation Open partial (n = 5) or complete (n = 2) nephrectomy 28 days post IRE |
– |
| Wendler et al. 21 | Germany | As above | As above | Safety | As above | As above | As above | As above | Reports on different outcome measures from same patient sample as Wendler et al. 20 |
| Buijs et al. 22 | The Netherlands | Prospective Single Centre Cohort | 16 months | Safety | 10 | Median 6 months (range 3–12) |
Histopathology | Percutaneous IRE under GA with muscle paralysis and cardiac synchronisation | 3/10 solitary kidneys |
| Gul et al. 23 | USA | Retrospective Single Centre Cohort | 84 months | Safety Efficacy |
1 (6 including other ablation modalities) | 34 months | CT diagnosis | NS | Solitary transplanted kidney |
CT: computerised tomography; GA: general anaesthesia; IQR: interquartile range; IRE: irreversible electroporation; MRI: magnetic resonance imaging; NR: not reported; RCC: renal cell carcinoma.
Table 2.
Patient and tumour characteristics in the included studies.
| Authors | n = | Age | Female/male | Tumour characteristics | Nephrometry score | ||||
|---|---|---|---|---|---|---|---|---|---|
| Size (mm) | Type | Fuhrman grade | Location | RENAL | PADUA | ||||
| Thomson et al. 14 | 7 | NR | NR | Median 25 (IQR 19–34) | 4 RCC 2 KMCR 1 KTCM |
NR | NR | NR | NR |
| Pech et al. 15 | 6 | Median 57 (IQR 51–68) | 3 F 3 M |
Median 27 (IQR 24–34) | NR | NR | 4 Central 2 Upper |
NR | NR |
| Diehl, et al. 16 | 5 | Median 66 (IQR 61–71) | 2 F 3 M |
Median 23 (IQR 19–29) | NR | NR | NR | Median 7 (IQR 6–8) | NR |
| Vroomen, et al. 17 | 1 | 52 | 1 M | 30 | 1 cRCC | NR | NR | NR | NR |
| Canvasser et al. 18 | 41 | Mean 63.7 (SD 10.8) | 19 F 23 M |
Mean 20 (SD 6) | 13 ccRCC 4 pRCC 2 cRCC 1 Unclassified RCC 3 Non-diagnostic 2 Oncocytoma 17 No biopsy performed |
NR | 8 Upper pole 24 Interpolar 10 Lower pole |
Median 5 (IQR 4–6) | NR |
| Liu et al. 19 | 5 | Median 34 (IQR 34–68) | 3 F 2 M |
Median 28 (IQR 25–30) | NR | NR | NR | Median 8 (IQR 8–9) | NR |
| Wendler et al.20,21 | 7 | Median 73 (IQR 66–74) | 1 F 6 M |
Median 18 (IQR 17–23) | 5 ccRCC 2 pRCC |
4 Grade 1 3 Grade 2 |
2 Upper pole 4 Interpolar 1 Lower pole |
NR | Median 7 (IQR 6–8) |
| Buijs et al. 22 | 10 | Median 69 (IQR 62–73) | 3 F 7 M |
Median 19 (IQR 17–26) | 7 ccRCC 1 pRCC 2 Non-diagnostic |
2 Grade 1 5 Grade 2 1 Grade 3 2 Non-diagnostic |
4 Upper lobe 2 Middle lobe 2 Lower lober |
Median 6 (IQR 5–7) | Median 8 (IQR 7–9) |
| Gul et al. 23 | 1 | 57 | NR | 16 | 1 ccRCC | Grade 3 | NR | NR | NR |
ccRCC: clear cell renal cell carcinoma; cRCC: chromophobe renal cell carcinoma; IQR: interquartile range, KMCR: kidney metastasis from colorectal carcinoma; KTCM: kidney transitional cell carcinoma; NR: not reported; PADUA: preoperative aspects and dimensions used for anatomic; pRCC: papillary renal cell carcinoma; RENAL: radius exophytic/endophytic nearness anterior/posterior location.
Overall NOQAT Evaluation Scores were assigned to each article (Table 3). Generally, articles performed better at reporting safety and efficacy, as 6/10 were scored as good, however only 1/7 studies reporting on oncological outcomes was deemed to be of good quality.
Table 3.
Critical appraisal via overall NOQAT Evaluation for both safety and efficacy, and oncological outcomes for included studies.
| Author | n = | Overall NOQAT evaluation across both investigated outcomes | |
|---|---|---|---|
| Safety and efficacy | Oncological outcomes | ||
| Thomson et al. 14 | 7 | Poor | Poor |
| Pech et al. 15 | 6 | Good | – |
| Diehl et al. 16 | 5 | Good | Fair |
| Vroomen et al. 17 | 1 | Fair | Poor |
| Canvasser et al. 18 | 41 | Good | Fair |
| Liu et al. 19 | 5 | Fair | Fair |
| Wendler et al. 20 | 7 | Good | Good |
| Wendler et al. 21 | As above | Good | – |
| Buijs et al. 22 | 10 | Good | – |
| Gul et al. 23 | 1 | Fair | Fair |
Safety Outcomes
All 10 articles reported safety outcomes (Table 4). There were no 30-day mortalities reported in any of the studies.14–23
Table 4.
Safety and efficacy outcomes of included studies.
| Authors | n = | Follow up | 30 day mortality | Procedural time (min) | Length of stay | Renal function | Complications | Other |
|---|---|---|---|---|---|---|---|---|
| Thomson, et al. (2011)(14) | 7 | 3 months | 0 | Range 90–120 (excluding anaesthesia) | 7/7 1 day | No significant change | 1/7 Obstruction of upper ureter (previously damaged by RFA) 1/7 Unintentional adrenal gland electroporation 2/7 Transient frank haematurias (<24 h duration) |
In all 38 patients, there were also 6 transient ventricular arrhythmias and 2 upper limb neurapraxias due to prolonged arm extension. |
| Pech et al. 15 | 6 | 3 months | 0 | Median 201 (IQR 176–204) | NR | Decrease in function due to nephrectomy | 1/6 Intraoperative supraventricular extrasystole | No changes on 5-min pre- and post-IRE Arterial Blood Gas analysis. |
| Diehl et al. 16 | 5 | Mean 6.4 months (range 3–11) |
0 | NR | NR | No significant change ΔGFR −3 mL/min |
1/5 Transient frank haematuria 1/5 Stage 1 AKI |
|
| Vroomen, et al. 17 | 1 | 4 months | 0 | NR | NR | NR | 1/1 Upper limb motor loss with sensory involvement with partial recovery at 4 months (due to arm position) | |
| Canvasser et al. 18 | 41 | Mean 22 months (SD 12.4) |
0 | Median 94 (IQR 72–131) | 29/41 0 days 12/41 1 day |
No significant change ΔGFR −6 mL/min |
4/41 Asymptomatic perirenal haematomas 2/41 Postoperative urinary retentions 1/41 Postoperative pain requiring overnight admission 2/41 Patients with morbid obesity needing postoperative NIV and 24-h admission |
|
| Liu et al. 19 | 5 | Mean 22 months (range 14–31) |
0 | Range 120–270 | 5/5 1 day | No significant change ΔGFR −6 mL/min |
0 Adverse events reported | |
| Wendler et al. 20 | 7 | 27 days to nephrectomy Overall Mean 25 months (range 15–36) |
0 | Mean 129 (range 53–203) | NR | No significant change | 7/7 Transient frank haematurias 7/7 Post-puncture pain needing medication 2/7 Self-limiting perirenal haematomas |
|
| Wendler et al. 21 | As above | As above | As above | As above | As above | As above | As above | Normal post-IRE morphological appearances on MRI urogram. Post-nephrectomy histopathology found regeneration of urothelium with permanent tissue necrosis of tumour and parenchyma below. Urine cytology showed transient cell vacuolisation in first 7 days post-IRE. |
| Buijs et al. 22 | 10 | Median 6 months (range 3–12) |
0 | Mean 126 (range 105–150) | 9/10 1 day 1/10 7 days |
No significant change | 1/10 Blocked ureter due to blood clot 1/10 Transient frank haematuria 1/10 Self-limiting perirenal haematoma 1/10 Painful micturition 1/10 Pyelonephritis (17 days post-IRE) |
|
| Gul, et al. 23 | 1 | 34 months | 0 | NR | NR | No significant change | 0 Adverse events reported | IRE in renal graft, function intact at 34 months follow up. |
ΔGFR: change in GFR; IRE: irreversible electroporation; NIV: non-invasive ventilation; NR: not reported; RFA: radiofrequency ablation.
Formal assessment tools for categorising complications were used in 6 of the 10 papers.16–18,20,22,23 The most frequently used (4/6) was the Clavien-Dindo classification. The most reported adverse events were transient haematuria occurring in 11/83 patients, and asymptomatic peri-renal haematomas occurring in 7 patients.
Of the patients with a reported length of stay, 62/63 were discharged in 24 h.
Renal function was reported via changes in estimated glomerular filtration rate (eGFR), or changes in urea or creatinine levels. No significant changes were reported in any of the included articles.
Oncological outcomes
Of the included articles, 7 out of 10 reported on oncological outcomes (Table 5). Regarding the NOQAT critical appraisal, only one study was regarded to be off good quality. The remaining articles were rated as fair or poor, often due to their sub-optimal follow-up periods (as 3/7 had a follow-up of less than 12 months).
Table 5.
Oncological outcomes of included studies.
| Authors | n = | Follow up | Modality of outcome assessment | Tumour response to IRE | Further interventions | Survival |
|---|---|---|---|---|---|---|
| Thomson et al. 14 | 7 | 3 months | CT | 5/7 CR at 3 months 2/7 DP |
2 of 5 CR patients had one more IRE procedure | NR |
| Diehl et al. 16 | 5 | Mean 6.4 months (range 3–11) |
MRI | NR | NR | 100% OS at 3 months |
| Vroomen et al. 17 | 1 | 4 months | PET-CT | 1/1 CR at 3 months | NR | 100% OS at 4 months |
| Canvasser et al. 18 | 41 | Mean 22 months (SD 12.4) |
CT | 92% local recurrence free survival at 2 years (NB: Of 35/41 patients with sufficient follow up data) | 3/41 had RFA salvage with CR 1/41 had robotic assisted partial nephrectomy salvage with CR |
95% OS at 2 years No mortality due to RCC (NB: Of 35/41 patients with sufficient follow up data) |
| Liu, et al. 19 | 5 | Mean 22 months (range 14–31) |
Gadolinium enhanced MRI | 4/5 CR 1/5 Recurrence |
1 recurrence patient had RFA salvage with CR at 3 months | NR |
| Wendler, et al.20,21 | 7 | 27 days to nephrectomy Overall Mean 25 months (range 15–36) |
Histopathology | 4/7 CR (ypT0V0N0Pn0R0) 3/7 incomplete ablation (ypT1aV0N0Pn0R1) |
Group with nephrectomy on day 28 post-IRE for all patients | NR |
| Gul et al. 23 | 1 | 34 months | NR | 1/1 CR | NR | Alive at latest follow up of 34 months |
CR: complete response; CT: computerised tomography; DP: disease progression; IRE: irreversible electroporation; MRI: magnetic resonance imaging; NR: not reported; OS: overall survival; PET: positron emission tomography; RCC: renal cell carcinoma; RFA: radiofrequency ablation.
Of the seven articles, only one assessed oncological efficacy using histopathological outcomes by carrying out IRE ablation followed by subsequent resection. 21 The remainder used imaging modalities spanning CT, PET, and MRI for assessment. Complete response (CR) rates were reported in 43/55 (78%) via imaging outcomes and only 4/7 (57%) when using histopathological outcomes. There were no mortalities due to SRMs reported by any study
Discussion
This review found IRE to be safe for use in SRMs; as it was not associated with any mortalities, there were few clinically significant adverse events, and negligible effects on renal function. The two most observed adverse effects were transient haematuria and asymptomatic peri-renal haematomas. Both of which are expected and of little clinical concern. When evaluating safety and efficacy as the outcome, most studies were deemed to be of good quality, giving a higher degree of confidence in the safety of IRE.
Ablative therapies are becoming more popular in managing SRMs, especially due to the increasing age of affected individuals and associated co-morbidities including frailty that may potentially deem them high risk or not suitable to receive general anaesthesia (GA).1,24,25 Current data suggest that thermal ablative therapies may have a similar rate of local recurrence compared to PN in T1aN0M0 tumours, however; this may not be accurate for T1b tumours and there is the risk of selection bias when comparing against patients fit for surgery.5,26–30 Thermal ablative therapies are not suitable for use on centrally located renal tumours, due to the potential risk of damaging the collecting system and the risk of heat sink.6,8 IRE may have a unique role in clinical practice as an alternative to radical nephrectomy for this group of patients. This is supported by Wendler et al.20,21 in their ablate-and-resect trial, that shows that the urothelium can recover following the effects of IRE ablation and that the collecting system is spared, whilst IRE causes substantial damage to the tumour.
With regards to oncological outcomes, the most significant limitations identified were in patient sample size, follow-up duration, and variation in assessment modality. The largest study included was carried out by Canvasser et al. 18 (n = 41), however their sub-optimal biopsy rate of 60% (25/42 tumours) led to their oncological data giving a local recurrence free survival (LRFS) of 83%, compared to 92% LRFS rate in their intent to treat (ITT) cohort. When compared to the 5-year LRFS rates of 94.6% for PN and 91.7% for RFA reported by Olweny et al., 28 IRE is potentially clinically inferior. Canvasser et al. 18 also acknowledge their own selection bias of tumours with low complexity, as measured by their R.E.N.A.L scores. The rest of the included studies had 10 or less patients, 2 of which only included 1 patient relevant to this review.17,23 Clearly this makes meaningful clinical implications difficult in the absence of larger studies supporting any oncological findings.
Follow up time presented an additional barrier in determining robust oncological outcomes. Of the seven studies, three had follow up less than a year, which we defined as the minimum duration necessary for any meaningful early oncological assessment to be made.
There was significant heterogeneity in the assessment modality used to carry out the oncological assessment, and this made any meta-analysis of the data impossible to perform. Six of the articles included used imaging-based assessment, though there was variation in the type of imaging (CT, MRI and PET-CT) and of the assessment scales used for each.14–18,23 Wendler et al. 21 used histopathological outcomes instead, by resecting the ablated kidney. They reported lower CR rates than those described by other studies via their imaging-based assessments (57% and 78% respectively). However, their conclusions are based on a small number of participants (n = 7). The decreased CR rates from the histopathological analysis indicates it may have greater sensitivity at detecting residual tumour than the imaging-based assessment tools. However, reviews of positive surgical margins (PSMs) in nephron sparing surgery indicated that a “watch and wait’ approach may be more appropriate than immediate re-intervention as many patients with PSMs do not develop local recurrence.31,32 Studies of longer duration could provide a more definite answer than histopathology as to how the 5-year survival rates from IRE compare against other ablative methods.
One of the limitations preventing widespread use of IRE is the need for muscle paralysis and cardiac synchronisation, as the concurrent need for a GA means some patients unsuitable for surgery due to co-morbidities may also be unsuitable for IRE. 1 Though beyond the scope of this article recent next generation bi-polar high-frequency IRE (H-FIRE) has been shown to reduce the level of muscle contractions and does not require cardiac synchronisation.33–35 As this technique develops it could overcome some of the current limitations in IRE ablation. Comparison studies against other ablative techniques and against current surgical standards are also required before IRE is adopted into standard clinical practice.
Conclusions
IRE appears to be safe for the management of SRMs. The treatment may be efficacious; however, larger studies, with longer follow-up, will help to further clarify the role IRE in future management algorithms for SRMs.
Supplemental Material
Supplemental material, sj-docx-1-urj-10.1177_03915603221077590 for Irreversible electroporation in renal tumours: A systematic review of safety and early oncological outcomes by Aidan Hilton, Georgios Kourounis and Fanourios Georgiades in Urologia Journal
Footnotes
Author contributions: Conceptualisation/protocol development: FG; Data collection: AH, GK; Data analysis: AH, GK, FG; Manuscript writing/editing: AH, GK, FG.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: FG is funded by the National Institute of Health Research (NIHR) in partnership with NHS Blood and Transplant (NHSBT) as an Academic Clinical Fellow. No specific funding was received to assist with the preparation of this manuscript.
Ethics approval: This is a systematic review of the literature, registered and approved in PROSPERO (registration number: CRD42020197943).
Consent to participate and for publication: As this is a secondary study of already published studies, no informed consent or ethical approvals are required.
ORCID iD: Fanourios Georgiades  https://orcid.org/0000-0003-0440-2720
https://orcid.org/0000-0003-0440-2720
Data availability statement (data transparency): The raw data that support the findings of this study are available from the corresponding author upon reasonable request. The search strategy is available in the supplementary material accompanying this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol 2019; 75(5): 799–810. [DOI] [PubMed] [Google Scholar]
- 2. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. Urol J 2009; 182(3): 844–853. [DOI] [PubMed] [Google Scholar]
- 3. Finelli A, Ismaila N, Bro B, et al. Management of small renal masses: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2017; 35(6): 668–680. [DOI] [PubMed] [Google Scholar]
- 4. Larcher A, Fossati N, Tian Z, et al. Prediction of complications following partial nephrectomy: implications for ablative techniques candidates. Eur Urol 2016; 69(4): 676–682. [DOI] [PubMed] [Google Scholar]
- 5. Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015; 67(2): 252–259. [DOI] [PubMed] [Google Scholar]
- 6. Atwell TD, Carter RE, Schmit GD, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012; 23(1): 48–54. [DOI] [PubMed] [Google Scholar]
- 7. Littrup PJ, Ahmed A, Aoun HD, et al. CT-guided percutaneous cryotherapy of renal masses. J Vasc Interv Radiol 2007; 18(3): 383–392. [DOI] [PubMed] [Google Scholar]
- 8. Lay AH, Faddegon S, Olweny EO, et al. Oncologic efficacy of radio frequency ablation for small renal masses: clear cell vs papillary subtype. Urol J 2015; 194(3): 653–657. [DOI] [PubMed] [Google Scholar]
- 9. Rubinsky B, Onik G, Mikus P. Irreversible electroporation: a new ablation modality – clinical implications. Technol Cancer Res Treat 2007; 6(1): 37–48. [DOI] [PubMed] [Google Scholar]
- 10. Lee EW, Thai S, Kee ST. Irreversible electroporation: a novel image-guided cancer therapy. Gut Liver 2010; 4(Suppl.1): S99–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kourounis G, Paul Tabet P, Moris D, et al. Irreversible electroporation (Nanoknife® treatment) in the field of hepatobiliary surgery: current status and future perspectives. J BUON 2017; 22(1): 141–149. [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Internet J Surg 2010; 8(5): 336–341. [DOI] [PubMed] [Google Scholar]
- 13. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan: a web and mobile app for systematic reviews. Syst Rev 2016; 5(1): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011; 22(5): 611–621. [DOI] [PubMed] [Google Scholar]
- 15. Pech M, Janitzky A, Wendler JJ, et al. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol 2011; 34(1): 132–138. [DOI] [PubMed] [Google Scholar]
- 16. Diehl SJ, Rathmann N, Kostrzewa M, et al. Irreversible electroporation for surgical renal masses in solitary kidneys: short-term interventional and functional outcome. J Vasc Interv Radiol 2016; 27(9): 1407–1413. [DOI] [PubMed] [Google Scholar]
- 17. Vroomen LGPH, Scheffer HJ, Melenhorst MCAM, et al. Irreversible electroporation to treat malignant tumor recurrences within the Pelvic cavity: a case series. Cardiovasc Intervent Radiol 2017; 40(10): 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Canvasser NE, Sorokin I, Lay AH, et al. Irreversible electroporation of small renal masses: suboptimal oncologic efficacy in an early series. World J Urol 2017; 35(10): 1549–1555. [DOI] [PubMed] [Google Scholar]
- 19. Liu B, Clark J, Domes T, et al. Percutaneous irreversible electroporation for the treatment of small renal masses: the first Canadian case series. Can Urol Assoc J 2019; 13(9): E263–E267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wendler JJ, Pech M, Fischbach F, et al. Initial assessment of the efficacy of irreversible electroporation in the focal treatment of localized renal cell carcinoma with delayed-interval kidney tumor resection (Irreversible Electroporation of Kidney Tumors Before Partial Nephrectomy [IRENE] Trial: an Ablate-and-Resect Pilot Study). Urology 2018; 114: 224–232. [DOI] [PubMed] [Google Scholar]
- 21. Wendler JJ, Pech M, Köllermann J, et al. Upper-urinary-tract effects after irreversible electroporation (IRE) of human localised renal-cell carcinoma (RCC) in the IRENE pilot phase 2a ablate-and-resect study. Cardiovasc Intervent Radiol 2018; 41(3): 466–476. [DOI] [PubMed] [Google Scholar]
- 22. Buijs M, Zondervan PJ, de Bruin DM, et al. Feasibility and safety of irreversible electroporation (IRE) in patients with small renal masses: results of a prospective study. Urol Oncol Semin Original Investig 2019; 37(3): 183.e1–183.e8. [DOI] [PubMed] [Google Scholar]
- 23. Gul ZG, Griffith JJ, Welch C, et al. Focal ablative therapy for renal cell carcinoma in transplant allograft kidneys. Urology 2019; 125: 118–122. [DOI] [PubMed] [Google Scholar]
- 24. Marchioni M, Amparore D, Ingels A, et al. Renal tumors ablation. Minerva Urol Nephrol 2021; 73(4): 549–551. [DOI] [PubMed] [Google Scholar]
- 25. Campi R, Berni A, Amparore D, et al. Impact of frailty on perioperative and oncologic outcomes in patients undergoing surgery or ablation for renal cancer: a systematic review. Minerva Urol Nephrol. Epub ahead of print 29 October 2021. DOI: 10.23736/S2724-6051.21.04583-3. [DOI] [PubMed] [Google Scholar]
- 26. Prins FM, Kerkmeijer LGW, Pronk AA, et al. Renal cell carcinoma: alternative nephron-sparing treatment options for small renal masses, a systematic review. J Endourol 2017; 31(10): 963–975. [DOI] [PubMed] [Google Scholar]
- 27. Rivero JR, De La Cerda J, Wang H, et al. Partial nephrectomy versus thermal ablation for clinical stage T1 renal masses: systematic review and meta-analysis of more than 3900 patients. J Vasc Interv Radiol 2018; 29(1): 18–29. [DOI] [PubMed] [Google Scholar]
- 28. Olweny EO, Park SK, Tan YK, et al. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 2012; 61(6): 1156–1161. [DOI] [PubMed] [Google Scholar]
- 29. Andrews JR, Atwell T, Schmit G, et al. Oncologic outcomes following partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2019; 76(2): 244–251. [DOI] [PubMed] [Google Scholar]
- 30. Shi L, He Y, Liu C, et al. Local ablation vs partial nephrectomy in T1N0M0 renal cell carcinoma: an inverse probability of treatment weighting analysis. Cancer Med 2020; 9(21): 7988–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marszalek M, Carini M, Chlosta P, et al. Positive surgical margins after nephron-sparing surgery. Eur Urol 2012; 61(4): 757–763. [DOI] [PubMed] [Google Scholar]
- 32. Steinestel J, Steffens S, Steinestel K, et al. Positive surgical margins in nephron-sparing surgery: risk factors and therapeutic consequences. World J Surg Oncol 2014; 12(1): 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Arena CB, Sano MB, Rossmeisl JH, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomed Eng Online 2011; 10(1): 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sano MB, Fan RE, Cheng K, et al. Reduction of muscle contractions during irreversible electroporation therapy using high-frequency bursts of alternating polarity pulses: a laboratory investigation in an ex vivo swine model. J Vasc Interv Radiol 2018; 29(6): 893–898.e4. [DOI] [PubMed] [Google Scholar]
- 35. Partridge BR, O’Brien TJ, Lorenzo MF, et al. High-frequency irreversible electroporation for treatment of primary liver cancer: a proof-of-principle study in canine hepatocellular carcinoma. J Vasc Interv Radiol 2020; 31(3): 482–491.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-urj-10.1177_03915603221077590 for Irreversible electroporation in renal tumours: A systematic review of safety and early oncological outcomes by Aidan Hilton, Georgios Kourounis and Fanourios Georgiades in Urologia Journal