Abstract
Background:
Rheumatoid arthritis (RA) is an inflammatory autoimmune condition associated with an increased risk of developing depression and anxiety. Depression and anxiety are associated with worse outcomes in RA, but the magnitude of the effect of each condition on RA outcomes is unclear. It is also unknown how pharmacological treatment of depression affects RA outcomes.
Objective:
The primary aim of this study was to investigate the association of comorbid depression and anxiety with remission in patients with RA. Secondary aims were to determine the association between comorbid depression and anxiety on patient-reported outcomes and the relationship between concomitant use of antidepressants and remission in patients with depression.
Design:
Data from patients with moderate to severe RA were pooled from five randomised controlled trials investigating tocilizumab and conventional synthetic disease-modifying agents.
Methods:
Remission was defined as a clinical disease activity index (CDAI) of ⩽2.8 and simple disease activity index (SDAI) of ⩽3.3. The association between the time to reach remission and depression and anxiety was analysed using Cox proportional hazard analysis.
Results:
Individual patient data were available from 5502 subjects, of whom 511 had depression, 236 had anxiety and 387 were using antidepressants. Depression was significantly associated with reduced remission [adjusted HR (95% CI): 0.62 (0.48–0.80), p < 0.001 and adjusted HR (95% CI): 0.59 (0.44–0.79), p < 0.001] using CDAI and SDAI, respectively. Depression was associated with a lower likelihood of achieving more subjective outcomes (⩽1 physician global assessment, ⩽1 patient global assessment) and ⩽1 28-swollen joint count, but not ⩽1 28-tender joint count or C-reactive protein measurement. Treatment with antidepressants did not improve outcomes for patients with depression. Anxiety was not significantly associated with RA remission.
Conclusion:
Comorbid depression, but not anxiety, was associated with less frequent remission. Concomitant antidepressant use was not associated with improvements in RA outcomes in patients with depression.
Keywords: anxiety, depression, Rheumatoid arthritis, remission, tocilizumab
Significance and Innovation
Depression, but not anxiety, was associated with a lower likelihood of remission.
Rates of remission in rheumatoid arthritis patients with depression were not higher with concurrent treatment of antidepressant medication.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory autoimmune disease, characterised by inflammation in the synovium of diarthrodial joints that can lead to permanent disability and deformity. It is associated with an increased risk of developing additional comorbidities, both physical and psychological, that can complicate treatment paradigms and increase morbidity and mortality.1,2 Depression and anxiety are two similar, yet distinct conditions that, respectively, affect approximately 17% and 7–15%3–5 of RA patients, which represents an increased prevalence in RA compared to the general population.6,7 RA patients with depression or anxiety are more likely to be hospitalised and have longer admission times, more physician visits and, in the case of depression, increased mortality and heightened medication, prognostic and economic burden.8–10
Depression and anxiety are associated with increased sensitivity to pain, disability and fatigue.7,11 In patients with RA, comorbid depression and anxiety are associated with higher disease activity and pain, and lower quality of life.12,13 Previous studies have identified depression and anxiety as negative predictors of remission in RA.14–16 In each of these studies, depression/anxiety symptom severity was determined through a combined symptomatic assessment at baseline rather than being identified from a formal diagnosis, leading to a lack of clarity regarding the magnitude of the effect of each condition on RA outcomes and whether treatment of either condition influences these effects.14,15
First-line treatment of depression and anxiety typically involves the use of antidepressant medication, such as selective-serotonin reuptake inhibitors (SSRIs) and serotonin-noradrenaline reuptake inhibitors (SNRIs).17,18 The effect of antidepressants on RA outcomes in patients with comorbid depression or anxiety has not been elucidated. In RA, antidepressants may reduce pain independently of effects on depression 19 and may modulate plasma inflammatory cytokine profiles. 20 Furthermore, depression is known to have a chronic inflammatory component, with treatment-resistant depression associated with increased peripheral inflammation. 21 Inflammatory mediators, such as those common in RA pathophysiology, can inhibit dopaminergic and hypothalamus–pituitary–adrenal axis functions leading to depressive symptoms.21,22
There are many tools available to measure disease activity in RA. The clinical disease activity index (CDAI) and simplified disease activity index (SDAI) are relatively simple methods of calculating disease activity. Cut-off values provide stricter measures of remission compared to threshold values of the 28-joint Disease Activity Score (DAS28).23–27 Although not directly included in any of these scales, pain is a significant issue in RA 28 and a major concern for patients, and changes in functional ability, measured by the health assessment questionnaire (HAQ), are integral to the disease course of RA. Both the pain and function are patient-reported outcomes and can be influenced by psychological factors.28,29
The primary aim of this study was to investigate the association of comorbid depression and anxiety with remission in patients with RA. Secondary aims were to determine the association between depression and anxiety on patient-reported outcomes (pain, function) and the relationship between concomitant use of antidepressants and remission in RA patients with depression.
Patients and methods
Data were pooled from five randomised phase-III clinical trials: LITHE (NCT00106535), TOWARD (NCT00106574), AMBITION (NCT001109408), SUMMACTA (NCT01194414) and FUNCTION (NCT01007435).30–34 Data were accessed according to Hoffmann-La Roche policy and has been made available through Vivli, Inc. (www.vivli.org). All trials involved the use of either tocilizumab (TCZ) monotherapy, TCZ plus conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or csDMARD monotherapy. All patients were adults (i.e. ⩾18 years of age) and diagnosed with moderate or severe RA as per 2010 American College of Rheumatology (ACR) criteria. 35 LITHE included 1196 patients who had an inadequate response to methotrexate (MTX) despite treatment for >12 weeks at therapeutic doses. Patients were randomised 1:1:1 to receive TCZ 4, 8 mg/kg or MTX monotherapy. TOWARD randomised 1220 patients with moderate to severe RA, despite receiving stable doses of csDMARDs for at least 8 weeks, 2:1 to receive TCZ 8 mg/kg or placebo, with background csDMARD combination therapy in both groups. AMBITION included 673 patients randomised 3:3:1 to receive TCZ 8 mg/kg, MTX or placebo for 8 weeks followed by TCZ 8 mg/kg. Patients were excluded if they had been treated with MTX in the last 6 months, discontinued MTX due to a lack of efficacy or had been unsuccessfully treated with an anti-TNF biologic. SUMMACTA randomised 1262 patients who had previously failed treatment with at least one DMARD or did not receive at least a 20% response with an anti-TNF biologic on a 1:1 ratio to receive TCZ 162 mg weekly or TCZ 8 mg/kg every 4 weeks, followed by an open-label portion whereby the former group was re-randomised 11:1 to continue the current treatment or receive TCZ 8 mg/kg every 4 weeks – the latter group was re-randomised 2:1 to continue current treatment or receive TCZ 162 mg weekly. All patients continued their initial csDMARD therapy regardless of treatment randomisation. FUNCTION randomised 1157 MTX-naïve patients 1:1:1:1 to receive either TCZ 4 mg/kg and MTX, TCZ 8 mg/kg and MTX, placebo and MTX or TCZ 8 mg/kg and placebo.
Data were available regarding each patient’s concomitant medications, comorbidities, disease duration, number of previous DMARDs used, ethnicity, age, weight and sex. Measurements pertaining to disease activity and its constituent measurements, pain scores, the HAQ, laboratory tests and adverse event measures were available from all studies. Patients were excluded from analysis if they did not have CDAI or SDAI scores available. Patient assessment varied depending on clinical trial, but follow-up was typically every fortnight.
Predictors and outcomes
The primary outcome was time to first RA remission according to CDAI ⩽ 2.8 and SDAI ⩽ 3.3.23–26 CDAI- and SDAI-defined remissions were used in separate analysis. Patients were censored at the last known date of follow-up or at the recorded date of death if they had not achieved remission.
Analysis of the 28-swollen joint count (SJC), the 28-tender joint count (TJC), patient global assessment (PtGA) score, physician global assessment (PhGA) score and C-reactive protein (CRP) was conducted to determine if drivers of remission in patients with comorbid depression and anxiety were potentially based on the more patient-orientated subjective-weighted measures (PtGA, TJC) as previously described.14,15 Constituents of the Boolean-based remission criteria (i.e. ⩽ 1 for TJC, SJC, PtGA and CRP) were used to define a terminal event in Kaplan–Meier and Cox proportional hazard models. 36 Although not defined in Boolean-based remission, a PhGA score of ⩽ 1 was classified as a terminal event. 23
Given the association between comorbid depression and anxiety in pain and functional impairment in RA,16,37 individual patient scores for a 100-point visual analogue scale for pain and the HAQ were analysed. Given their role in ACR50 response criteria, a ⩾ 50% improvement from baseline in pain or HAQ scores were used to define a terminal event in Kaplan–Meier and Cox proportional hazard models. 38
Comorbid depression and anxiety were assessed as the primary predictors of remission. Medical history data from each clinical trial were used to determine whether a patient had comorbid depression or anxiety. Patients were categorised as having either comorbid depression or anxiety if a diagnosis was present at baseline and if the disease was considered active/ongoing by the clinical trial investigator. Psychometric scales were not used to determine depression or anxiety. In concert with this limitation is the lack of data clarifying how the severity of mental illnesses changed throughout the trials relative to different DMARD treatments.
Antidepressants were grouped into one variable. Patients were classified as taking an antidepressant if they were recorded as being prescribed either an SSRI (tianeptine inclusive) or SNRI at baseline. Tricyclic antidepressants (comprised primarily of amitriptyline and nortriptyline) were not considered as antidepressants due to their decreasing role as antidepressants comparative to their contemporary use in neuropathic and adjuvant analgesic regimens. 39 Antidepressants were investigated as a covariate in patients with both concomitant depression and anxiety, given the propensity to use SSRIs and SNRIs as antidepressants and anxiolytics. 40
Statistical analysis
To identify potential differences in patient characteristics between and within trials, data were stratified according to individual clinical trial and diagnoses of either depression or anxiety. Data from each trial were pooled regardless of treatment arm after confirming that depression and anxiety had a similar effect on outcome data for patients receiving TCZ or csDMARDs. Cox proportional hazard models were used to assess the association between assessed outcomes (remission according to CDAI and SDAI, remission for single metrics of Boolean criteria and improvements in pain and HAQ) and predictors of interest, including depression, anxiety and antidepressant use. The association between baseline patient characteristics and remission was presented as hazard ratios (HR) with 95% confidence intervals (95% CI). A p-value of less than 0.05 was used to denote statistical significance using the likelihood ratio test. Kaplan–Meier plots were used to estimate the remission probabilities over time and to explore predictor variables in the pooled studies.
Analysis for adjusted models included age, weight, sex, race, baseline disease activity, corticosteroid use, disease duration and the number of DMARDs taken prior to study enrolment. Given the potential for reverse causality whereby more severe disease may contribute to, or cause, depression or anxiety, further analysis was conducted by grouping trials in which TCZ was used as either first-line (AMBITION and FUNCTION) or later-line (LITHE, TOWARD and SUMMACTA). Trials categorised as ‘first-line’ included those in which patients were either treatment-naïve or had not previously failed treatment with DMARDs. Trials were categorised as consisting of ‘later-line’ therapy if it included patients who previously had an inadequate response to one or more DMARDs. Cox proportional hazard models were used to analyse and compare the outcomes between first-line and later-line therapy groups relative to depression or anxiety. Cox proportional hazard models with interaction terms were further used to describe if any effect-modifier relationships existed between variables.
All data analysis was performed using R software. 41
Results
Patient population
The pooled dataset included 5502 patients, of whom 1376 (25%) were treated with csDMARDs and 4126 (75%) with TCZ ± csDMARDs. Data were missing for 10 and 2 patients for SDAI and CDAI, respectively, leaving 5492 and 5500 patients available for the analysis of primary outcomes. Median follow-up time varied for each clinical trial, ranging from 24 weeks in AMBITION and TOWARD to 260 weeks in LITHE.
A total of 511 (9.3%) patients had comorbid depression and 236 (4.3%) had comorbid anxiety. Of these, 98 had concomitant depression and anxiety. A total of 387 (7.1%) patients were prescribed antidepressant medication at baseline (Supplementary Table 1). There were 360 (6.5%) patients taking an SSRI, 18 (0.33%) an SNRI and 9 (0.16%) an SSRI and SNRI. Of the 413 patients with depression alone, 240 (58.1%) were prescribed an antidepressant. Of the 138 with anxiety alone, 33 (23.9%) were prescribed an antidepressant. Of the 98 with concomitant depression and anxiety, 58 (59.2%) were prescribed an antidepressant. There were 56 patients prescribed antidepressant medication who did not have either depression or anxiety recorded at baseline.
There was some heterogeneity between individual clinical trials, primarily around the duration of RA, and the balancing of treatment arms. However, there were no significant differences between the proportion of patients with depression or anxiety across each trial (Supplementary Table 14) and patients with depression or anxiety did not have different baseline disease activity, as measured by SDAI, than those without. Across studies, the characteristics of patients with depression or anxiety within trials were largely similar. Patients with depression or anxiety were more likely to be female, older and weigh more than other patients (Supplementary Tables 14–16). Further demographic data stratifying patients by clinical trial, depression and anxiety are described in Supplementary Tables 11–16.
Association between depression and remission
Depression was significantly associated with a decreased probability of remission defined by both CDAI and SDAI on univariable analysis (Table 1, CDAI: p < 0.001, SDAI: p < 0.001). On adjusted analysis, depression was similarly associated with a decreased probability of CDAI [HR (95% CI): 0.62 (0.48–0.80), p < 0.001] and SDAI [HR (95% CI): 0.59 (0.44–0.79), p < 0.001] defined remission (Table 1).
Table 1.
Univariable and adjusted analysis of the association of depression with CDAI and SDAI remission.
| Univariable analysis | CDAI-remission | SDAI-remission | ||||
|---|---|---|---|---|---|---|
| Events/ patients | HR [95% CI] | p-value | Events/ patients | HR [95% CI] | p-value | |
| Comorbid depression | ||||||
| No | 1677/4989 | 1 | 1396/4982 | 1 | ||
| Yes | 103/512 | 0.55 [0.45–0.67] | <0.001 | 78/510 | 0.50 [0.40–0.63] | <0.001 |
| Adjusted analysis | CDAI-remission | SDAI-remission | ||||
| Events/ patients | HR [95% CI] | p-value | Events/ patients | HR [95% CI] | p-value | |
| Comorbid depression | ||||||
| No | 1677/4989 | 1 | 1396/4982 | 1 | ||
| Yes | 103/512 | 0.62 [0.48–0.80] | <0.001 | 78/510 | 0.59 [0.44–0.79] | <0.001 |
CDAI, clinical disease activity index; CI, confidence interval; HR, hazard ratio; SDAI, simple disease activity index.
Adjustment variables: age, weight, sex, race, baseline disease activity, antidepressant use, corticosteroids, disease duration and number of previous DMARDs.
Concomitant use of antidepressants was not associated with a change in time to remission in RA patients with depression (Table 2). Patients with depression who were taking concomitant antidepressant medications had a similar association with remission defined by CDAI [HR: 0.67 (0.52–0.87), p = 0.002] to those who had depression but were not taking concomitant antidepressant medication [HR: 0.58 (0.41–0.81), p = 0.001]. A similar observation was made when remission was defined by SDAI in those who were on concomitant antidepressant medication [HR: 0.56 (0.42–0.75), p < 0.001] and were not [HR: 0.55 (0.38–0.81), p = 0.002]. No heterogeneity was observed in the association between depression [CDAI: p(interaction) = 0.139, SDAI: p(interaction) = 0.261] and time to remission according to antidepressant use. Use of antidepressants was significantly associated with CDAI- and SDAI-defined remission on univariable analysis, but when analysis was adjusted using depression as a covariate, the association was not significant for either CDAI- or SDAI-defined remission (data not shown).
Table 2.
Adjusted analysis of the association of depression with CDAI- and SDAI-defined remission stratified by antidepressant treatment (pharmacotherapy).
| CDAI-remission | SDAI-remission | |||||
|---|---|---|---|---|---|---|
| HR [95% CI] | p-value | p-interaction | HR [95% CI] | p-value | p-interaction | |
| Depression with pharmacotherapy | 0.672 [0.52–0.87] | 0.002 | 0.139 | 0.563 [0.42–0.75] | <0.001 | 0.261 |
| Depression without pharmacotherapy | 0.578 [0.41–0.81] | 0.001 | 0.553 [0.38–0.81] | 0.002 | ||
CDAI, clinical disease activity index; CI, confidence interval; HR, hazard ratio; SDAI, simple disease activity index.
Adjustment variables: age, weight, sex, race, baseline disease activity, corticosteroids, disease duration and number of previous DMARDs.
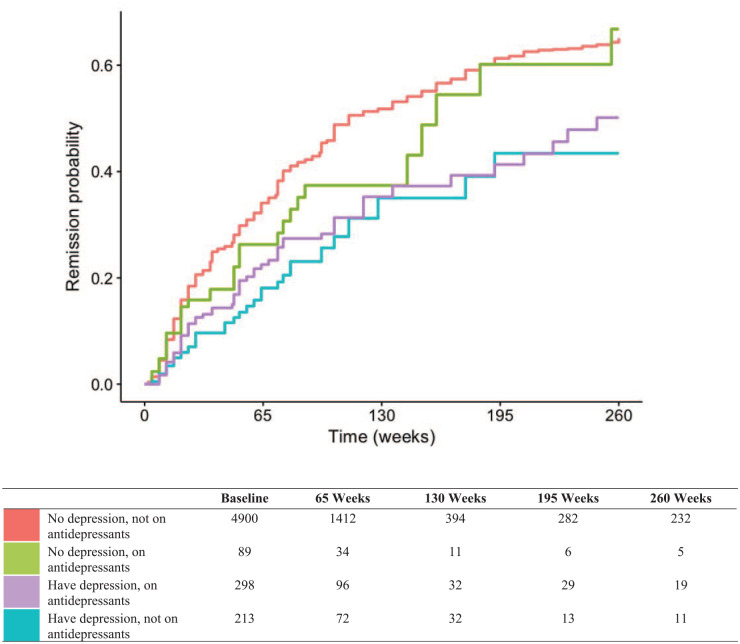
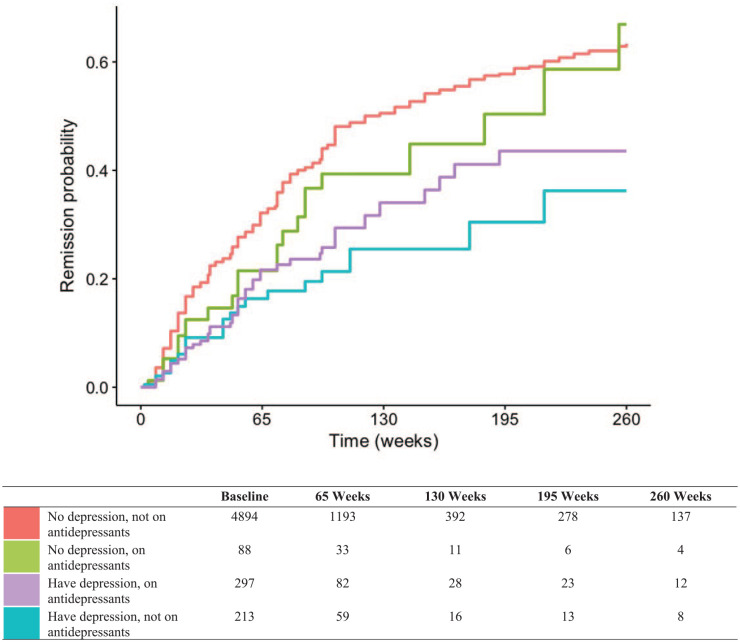
Kaplan–Meier estimates of time to remission (defined by CDAI and SDAI) according to depression and antidepressant use are displayed in Figures 1 and 2, respectively.
Figure 1.
CDAI-remission, no confidence interval.
Figure 2.
SDAI remission, no confidence interval.
No heterogeneity was observed in the association between depression and remission outcomes according to the type of DMARD taken [CDAI: p(interaction) = 0.45, SDAI: p(interaction) = 0.61] or individual clinical trial [CDAI: p(interaction) = 0.15, SDAI: p(interaction) = 0.75] (Supplementary Table 2).
Depression was associated with a decreased likelihood of achieving ⩽ 1 SJC, PtGA and PhGA scores on adjusted analysis (Supplementary Table 5). No association was observed between depression and the likelihood of achieving ⩽ 1 TJC and CRP, although there was a trend towards the significance for TJC (Supplementary Table 5).
On adjusted analysis, concomitant use of antidepressants was not associated with a change in the likelihood of achieving ⩽ 1 SJC, ⩽ 1 PhGA or ⩽ 1 CRP in RA patients with depression (data not shown). Patients with depression on concomitant antidepressants had a lower likelihood of achieving ⩽ 1 TJC, but a higher likelihood of achieving ⩽ 1 PtGA relative to patients with depression but who were not taking antidepressants (data not shown). However, there was no significant treatment interaction effect between depression and antidepressant use for the likelihood of achieving ⩽ 1 TJC, SJC, PtGA, PhGA or CRP.
In the pooled first-line treatment cohort, depression was associated with a decreased probability of remission defined by both CDAI [adjusted HR: 0.51 (0.34–0.76), p = 0.001] and SDAI [adjusted HR: 0.53 (0.35–0.81), p = 0.004] (Supplementary Table 4). Similarly, within the later-line treatment cohort, depression was also associated with a decreased probability of remission defined by both CDAI [adjusted HR: 0.70 (0.55–0.89), p = 0.004] and SDAI [adjusted HR: 0.58 (0.44–0.76), p < 0.001] (Supplementary Table 4).
Association between anxiety and remission
Anxiety was associated with decreased probability of remission defined by CDAI but not SDAI on univariable analysis (Table 3, CDAI: p = 0.005, SDAI: p = 0.084). On adjusted analysis, anxiety was not associated with a decreased probability of remission defined by either CDAI [HR: 0.80 (0.60–1.06), p = 0.114] or SDAI [HR: 0.93 (0.69–1.25), p = 0.647] (Table 3). No heterogeneity was observed in the association between anxiety and remission according to the type of DMARD taken [CDAI: p(interaction) = 0.09, SDAI: p(interaction) = 0.06] or individual clinical trials [CDAI: p(interaction) = 0.40, SDAI: p(interaction) = 0.47] (Supplementary Table 3).
Table 3.
Univariable and adjusted analysis of the association of anxiety with CDAI and SDAI remission.
| Univariable analysis | CDAI-remission | SDAI-remission | ||||
|---|---|---|---|---|---|---|
| Events/ patients | HR [95% CI] | p-value | Events/ patients | HR [95% CI] | p-value | |
| Comorbid anxiety | ||||||
| No | 1725/5264 | 1 | 1424/5257 | 1 | ||
| Yes | 55/236 | 0.68 [0.52–0.89] | 0.005 | 50/235 | 0.78 [0.59–1.03] | 0.084 |
| Adjusted analysis | CDAI-remission | SDAI-remission | ||||
| Events/ patients | HR [95% CI] | p-value | Events/ patients | HR [95% CI] | p-value | |
| Comorbid anxiety | ||||||
| No | 1725/5264 | 1 | 1424/5257 | 1 | ||
| Yes | 55/236 | 0.80 [0.60–1.06] | 0.114 | 50/235 | 0.93 [0.69–1.25] | 0.647 |
CDAI, clinical disease activity index; CI, confidence interval; DMARD, disease-modifying antirheumatic drugs; HR, hazard ratio; SDAI, simple disease activity index.
Adjustment variables: age, weight, sex, race, baseline disease activity, antidepressant use, corticosteroids, disease duration and number of previous DMARDs.
On sensitivity analysis, there was no evidence that concomitant depression and anxiety were associated with worse outcomes than depression alone [CDAI: p(interaction) = 0.75, SDAI: p(interaction) = 0.06]. Anxiety was not significantly associated with remission when analysis was restricted to only RA patients with comorbid depression [CDAI – HR: 1.02 (0.61–1.71), p = 0.95, and SDAI – HR: 1.65 (0.96–2.83), p = 0.07] (Supplementary Table 10).
Association of depression, anxiety and antidepressants with patient-reported outcomes
Depression was associated with a decreased probability of achieving a ⩾ 50% improvement in HAQ and pain scores on univariable analysis (Supplementary Table 6). On adjusted analysis, depression was associated with a decreased probability of a ⩾ 50% improvement in HAQ score from baseline [HR: 0.63 (0.52–0.76), p < 0.001, Supplementary Table 7]. Likewise, depression was associated with a decreased probability of a ⩾ 50% improvement in pain score from baseline [adjusted HR: 0.82 (0.71–0.94), p = 0.006, Supplementary Table 7].
Anxiety was associated with a decreased probability of achieving a ⩾ 50% improvement in HAQ and pain scores from baseline on univariable analysis (Supplementary Table 6), but these associations were not apparent on adjusted analysis [⩾ 50% improvement in HAQ, adjusted HR: 0.92 (0.76–1.12), p = 0.42, and ⩾ 50% improvement in pain score, adjusted HR: 0.89 (0.76–1.06), p = 0.18, Supplementary Table 8].
Concomitant antidepressant use in patients with depression had no effect on the proportion of patients who achieved a ⩾ 50% improvement in HAQ or pain scores from baseline [⩾ 50% HAQ: p(interaction) = 0.99, and ⩾ 50% pain: p(interaction) = 0.23, Supplementary Table 9].
Discussion
In a pooled adjusted analysis of five randomised controlled trials (RCTs) where patients with RA were treated with TCZ, csDMARDs or a combination of both, comorbid depression, but not anxiety, was associated with an approximately 40% reduced likelihood of achieving remission at any given time. Concomitant use of antidepressants did not modify the negative association between depression and remission.
This is the largest study analysing the specific association of depression, anxiety and antidepressant use with outcomes in patients with RA. More specifically, the association between antidepressant use in RA patients with comorbid depression and disease activity measures has not previously been reported. While causality cannot be established, there is a strong negative association between depression and the likelihood of remission. This association was consistent across analysis of multiple sub-groups, and RA patients with depression did not appear to have any modifying or mitigating factors that improved their outcomes. Notably, antidepressant therapy did not have a significant association with remission in patients with depression, indicating that pharmacological treatment of depression is not associated with improvement in RA-related outcomes. This is in contrast to psychological interventions in RA, particularly behavioural interventions, which have previously been reported to demonstrate minor improvements in PtGA, pain and fatigue, 42 but were of no benefit in TJC and SJC, indicative of a potential psychological element to some patient-reported outcomes.
The association between depression and remission was treatment agnostic – patient outcomes were similar irrespective of TCZ or csDMARD usage. Furthermore, the association did not appear to be different in patients who were receiving first-line or later-line treatment after adjustment for baseline disease activity and disease duration – indicative of an association that is not constrained to patients with more severe disease. This indicates a pervasive issue that is unlikely to be alleviated through change in RA therapy and provides a challenge for rheumatologists. Although depression was identified as a negative prognostic marker, anxiety appeared to be relatively benign with regard to RA-related outcomes. While depression and anxiety can often occur together, patients with both anxiety and depression did not have worse outcomes than those with depression alone.
When considered as a combined operator, depression and anxiety have previously been associated with decreased likelihood of remission in RA patients, and this association was rationalised by a lack of responsiveness of more subjective measurements used in disease activity indexes, such as TJC and PtGA.14,16 However, our study found that only depression was associated with a decreased likelihood of achieving a ⩽ 1 score for SJC, PtGA and PhGA, with a trend towards significance for a ⩽ 1 TJC. While the PtGA and PhGA are more subjective-weighted measures, it should be noted that SJC is not entirely objective, displaying significant assessor variability and bias. 43 Interestingly, depression was not associated with any difference in time to reach ⩽ 1 CRP – arguably the most objective constituent of disease activity measures. This may indicate that, given the similar results across both patient- and physician-orientated metrics, patients and physicians may have inadvertently conflated symptoms of depression and RA.
It has also been noted that depression may influence adherence to treatment in chronic diseases, 44 leading to sub-optimal treatment outcomes. 45 However, there was no apparent treatment effect modifier in remission between patients with depression undergoing treatment with either csDMARDs or intravenous TCZ. Patients undergoing intravenous therapy are typically more adherent in practice. 46 This diminishes the likelihood of medication adherence being a factor in the observed association. However, this does not discount non-compliance with non-pharmacological RA therapies, as (poor) adherence to medication typically translates to other aspects of therapy as well. 47 These factors indicate that drivers of the negative association in RA patients between outcomes and depression may not be based exclusively on psychological symptoms and perceptions of disease.
There is also the possibility that a lack of difference in RA outcomes between patients with depression undergoing treatment with or without antidepressants can be explained by depressive symptomatology that is attributable to inflammation. Dysfunction of the hypothalamus–pituitary–adrenal axis is associated with depression, and RA patients are more likely to have dysfunction of this axis.22,48,49 Inflammation can also lead to potential reductions in dopaminergic functioning, affecting motor function, reward pathways and leading to symptoms, such as anhedonia. 21 As such, inflammatory effects on neurological function may lead to depressive symptoms that are not responsive to traditional antidepressant treatment.21,22 These factors may explain the apparent lack of difference in outcomes between patients with depression with and without antidepressant treatment, leading to ineffective treatment of depression and a subsequent lack of modulation in patient-orientated subjective constituents of disease activity and pain.
Strengths of this study are the large, multi-trial cohort and thorough clinical trial dataset and protocols, allowing the ability to adjust for multiple variables in the analysis. This allowed for the stratification of patients by disease duration and type of trial, enabling discrepancy between patients with more advanced disease, thereby reducing the potential for identified associations to be attributed to reverse causality. Included in these strengths were the ability to analyse diagnosed depression and anxiety as separate conditions and to determine if pharmacological treatment of either condition was associated with improvements in outcomes and to minimise questions of RA treatment adherence.
The prevalence of anxiety in the pooled cohort was 4.3%, which is lower than that of other studies in RA patients where it is quoted as being 7–15%, but is similar to the proportion of RA and arthritis patients who have received a diagnosis of generalised anxiety disorder.6,48 The presence of comorbid depression in this cohort (9.3%) was relatively low compared to other estimates in RA, where the prevalence is likely 17%, but can vary significantly depending on the diagnostic method. 3 This raises the concern of either potential undiagnosed anxiety or depression in the present cohort, which has been noted in previous studies. 50 However, it could be symptomatic of the nature of clinical trials that exclude participants with comorbid conditions, raising concerns regarding external validity to real-life cohorts, inhibiting generalisability of the study.51,52
Antidepressants were prescribed in approximately 60% of participants who had an underlying diagnosis of depression, which is similar to the rate of prescribing in the general population.53–55 The remaining proportion of patients with depression not treated with antidepressants may be due to management of depression using non-pharmacological techniques or clinician adherence to guidelines that recommend the cessation of antidepressant medication after an initial period.56,57 There were a small number of patients on antidepressant therapy despite not having a history of depression or anxiety. This may be partly due to the propensity for clinicians to use antidepressants, predominately SSRIs, as an established treatment in alternative psychological conditions or for use in off-label conditions where there is a limited evidence base.58–60
A potential weakness of this study is that we were not able to account for patients undergoing psychotherapy for depression or anxiety and to describe how this interacts with outcomes. However, given the similarities between patients with depression taking antidepressants and those who are not, it is likely this effect was not sizable. In addition, the severity of a patient’s depression or anxiety was not assessed, and these comorbidities were categorised as either being present or not at baseline. This leads to the grouping of patients who may have differences in severity of their psychological comorbidities, which could partly explain why anxiety did not appear to be significant in this study and limited our ability to draw robust comparisons between patients with depression who were prescribed antidepressants and those who were not. Given that the categorisation of depression and anxiety was based off medical histories, rather than psychometric analyses at baseline, there may be the possibility that patients with historical depression or anxiety were included among patients actively suffering from the disease. However, ensuring only patients with active depression or anxiety were included in analysis may have minimised this risk.
These findings affirm the significant negative association between depression, but not anxiety and remission in RA. Despite the results, they do not provide evidence to support approaches on how to mitigate the negative association of depression on outcomes in RA, nor provide the underlying reasons for the correlation. Multiple variables, such as the use of pharmacotherapy for depression or DMARD choice failed to moderate the association, providing little guidance for rheumatologists regarding medication selection. Furthermore, the association was present in RA patients who had previously failed DMARD treatment and those who had not. In contrast, anxiety was not associated with RA remission, which may indicate a comparatively more positive RA prognosis for these patients. Further research is warranted and should focus on whether these results are translatable to clinical practice and to determine if psychological support of patients with depression delivers different associations with outcomes in RA compared to antidepressants.
Conclusion
Comorbid depression, but not anxiety, was associated with less frequent remission. The negative association of depression with remission was not moderated by differences in RA treatment or pharmacotherapy for depression and was consistent across patients who had previously failed treatment with DMARDs and patients who had not. Concomitant antidepressant use in patients with depression was not associated with improvements in RA outcomes, including in pain and HAQ scores.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221111613 for The association of depression and anxiety with treatment outcomes in patients with rheumatoid arthritis – a pooled analysis of five randomised controlled trials by Arkady T. Manning-Bennett, Ashley M. Hopkins, Michael J. Sorich, Susanna M. Proudman, David J.R. Foster, Ahmad Y. Abuhelwa and Michael D. Wiese in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
This publication is based on research using data from data contributors Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved, and is not in any way responsible for, the contents of this publication.
Footnotes
ORCID iD: Arkady T. Manning-Bennett  https://orcid.org/0000-0002-6592-5675
https://orcid.org/0000-0002-6592-5675
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Arkady T. Manning-Bennett, UniSA: Clinical & Health Sciences, University of South Australia, North Terrace, Adelaide, SA 5000, Australia.
Ashley M. Hopkins, Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Bedford Park, SA, Australia
Michael J. Sorich, Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Bedford Park, SA, Australia
Susanna M. Proudman, Rheumatology Unit, Royal Adelaide Hospital, Adelaide, SA, Australia Discipline of Medicine, University of Adelaide, Adelaide, SA, Australia.
David J.R. Foster, UniSA: Clinical & Health Sciences, University of South Australia, Adelaide, SA, Australia
Ahmad Y. Abuhelwa, Discipline of Clinical Pharmacology, College of Medicine and Public Health, Flinders University, Bedford Park, SA, Australia College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates.
Michael D. Wiese, UniSA: Clinical & Health Sciences, University of South Australia, Adelaide, SA, Australia
Declarations
Ethics approval and consent to participate: Advice from the University of South Australia’s Human Research Ethics Committee was that ethical review and approval was not required to undertake this research. These studies were conducted by F. Hoffmann-La Roche Ltd. (Basel, Switzerland) in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from all participants prior to enrolment.
Consent for publication: Not applicable.
Author contributions: Arkady T. Manning-Bennett: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft.
Ashley M. Hopkins: Data curation; Investigation; Methodology; Writing – review & editing.
Michael J. Sorich: Methodology; Writing – review & editing.
Susanna M. Proudman: Methodology; Writing – review & editing.
David J.R. Foster: Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
Ahmad Y. Abuhelwa: Data curation; Formal analysis; Methodology; Writing – review & editing.
Michael D. Wiese: Investigation; Methodology; Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Competing Interests: The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Availability of data and materials: Not applicable.
References
- 1. van Onna M, Boonen A. The challenging interplay between rheumatoid arthritis, ageing and comorbidities. BMC Musculoskelet Disord 2016; 17: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dougados M, Soubrier M, Antunez A, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis 2014; 73: 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matcham F, Rayner L, Steer S, et al. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol 2013; 52: 2136–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lok EY, Mok CC, Cheng CW, et al. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics 2010; 51: 338–338. [DOI] [PubMed] [Google Scholar]
- 5. Isik A, Koca SS, Ozturk A, et al. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol 2007; 26: 872–878. [DOI] [PubMed] [Google Scholar]
- 6. Marrie RA, Hitchon CA, Walld R, et al. Increased burden of psychiatric disorders in rheumatoid arthritis. Arthritis Care Res 2018; 70: 970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Publishing, 2013. [Google Scholar]
- 8. Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ 2019; 22: 372–378. [DOI] [PubMed] [Google Scholar]
- 9. Hitchon CA, Walld R, Peschken CA, et al. The impact of psychiatric comorbidity on health care use in rheumatoid arthritis: a population-based study. Arthritis Care Res 2021; 73: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marrie RA, Walld R, Bolton JM, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry 2018; 53: 65–72. [DOI] [PubMed] [Google Scholar]
- 11. Ebrahimzadeh MH, Moradi A, Bidgoli HF, et al. The relationship between depression or anxiety symptoms and objective and subjective symptoms of patients with frozen shoulder. Int J Prev Med 2019; 10: 38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rathbun AM, Harrold LR, Reed GW. A prospective evaluation of the effects of prevalent depressive symptoms on disease activity in rheumatoid arthritis patients treated with biologic response modifiers. Clin Ther 2016; 38: 1759–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matcham F, Ali S, Irving K, et al. Are depression and anxiety associated with disease activity in rheumatoid arthritis? A prospective study. BMC Musculoskelet Disord 2016; 17: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Michelsen B, Kristianslund EK, Sexton J, et al. Do depression and anxiety reduce the likelihood of remission in rheumatoid arthritis and psoriatic arthritis? Data from the prospective multicentre NOR-DMARD study. Ann Rheum Dis 2017; 76: 1906–1910. [DOI] [PubMed] [Google Scholar]
- 15. Boer AC, Huizinga TWJ, Van Der Helm-Van Mil AHM. Depression and anxiety associate with less remission after 1 year in rheumatoid arthritis. Ann Rheum Dis 2018; 78: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matcham F, Norton S, Scott DL, et al. Symptoms of depression and anxiety predict treatment response and long-term physical health outcomes in rheumatoid arthritis: secondary analysis of a randomized controlled trial. Rheumatology 2015; 55: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Psychological Association. Clinical practice guideline for the treatment of depression across three age cohorts. Washington, DC: American Psychological Association, 2019. [Google Scholar]
- 18. Baldwin DS, Anderson IM, Nutt DJ, et al. Evidence-based pharmacological treatment of anxiety disorders, post-traumatic stress disorder and obsessive-compulsive disorder: a revision of the 2005 guidelines from the British Association for Psychopharmacology. J Psychopharmacol 2014; 28: 403–439. [DOI] [PubMed] [Google Scholar]
- 19. Perrot S, Maheu E, Javier RM, et al. Guidelines for the use of antidepressants in painful rheumatic conditions. Eur J Pain 2006; 10: 185–192. [DOI] [PubMed] [Google Scholar]
- 20. Wie˛dłocha M, Marcinowicz P, Krupa R, et al. Effect of antidepressant treatment on peripheral inflammation markers – a meta-analysis. Progr Neuro-Psychopharmacol Biol Psych 2018; 80: 217–226. [DOI] [PubMed] [Google Scholar]
- 21. Felger JC, Treadway MT. Inflammation effects on motivation and motor activity: role of dopamine. Neuropsychopharmacol 2017; 42: 216–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lwin MN, Serhal L, Holroyd C, et al. Rheumatoid arthritis: the impact of mental health on disease: a narrative review. Rheumatol Ther 2020; 7: 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Arthritis Rheum 2011; 63: 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005; 7: R796–R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smolen JS, Breedveld FC, Schiff MH, et al. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 2003; 42: 244–257. [DOI] [PubMed] [Google Scholar]
- 26. Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23(5, Suppl. 39): S100–S108. [PubMed] [Google Scholar]
- 27. Gaujoux-Viala C, Mouterde G, Baillet A, et al. Evaluating disease activity in rheumatoid arthritis: which composite index is best? A systematic literature analysis of studies comparing the psychometric properties of the DAS, DAS28, SDAI and CDAI. Joint Bone Spine 2012; 79: 149–155. [DOI] [PubMed] [Google Scholar]
- 28. Walsh DA, McWilliams DF. Pain in rheumatoid arthritis. Curr Pain Headache Rep 2012; 16: 509–517. [DOI] [PubMed] [Google Scholar]
- 29. Imran MY, Saira Khan EA, Ahmad NM, et al. Depression in rheumatoid arthritis and its relation to disease activity. Pak J Med Sci 2015; 31: 393–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011; 63: 609–621. [DOI] [PubMed] [Google Scholar]
- 31. Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008; 58: 2968–2980. [DOI] [PubMed] [Google Scholar]
- 32. Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010; 69: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Burmester GR, Rubbert-Roth A, Cantagrel A, et al. Efficacy and safety of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional DMARDs in patients with RA at week 97 (SUMMACTA). Ann Rheum Dis 2016; 75: 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann Rheum Dis 2017; 76: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324 [DOI] [PubMed] [Google Scholar]
- 36. Felson D. Defining remission in rheumatoid arthritis. Ann Rheum Dis 2012; 71(Suppl. 2): i86–i88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kojima M, Kojima T, Suzuki S, et al. Depression, inflammation, and pain in patients with rheumatoid arthritis. Arthritis Rheum 2009; 61: 1018–1024. [DOI] [PubMed] [Google Scholar]
- 38. Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38: 727–735. [DOI] [PubMed] [Google Scholar]
- 39. Murnion BP. Neuropathic pain: current definition and review of drug treatment. Aust Prescr 2018; 41: 60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jakubovski E, Johnson JA, Nasir M, et al. Systematic review and meta-analysis: dose–response curve of SSRIs and SNRIs in anxiety disorders. Depress Anxiety 2019; 36: 198–212. [DOI] [PubMed] [Google Scholar]
- 41. Team RC. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2018. [Google Scholar]
- 42. Prothero L, Barley E, Galloway J, et al. The evidence base for psychological interventions for rheumatoid arthritis: a systematic review of reviews. Int J Nurs Stud 2018; 82: 20–29. [DOI] [PubMed] [Google Scholar]
- 43. Sokka T, Pincus T. Quantitative joint assessment in rheumatoid arthritis. Clin Exp Rheumatol 2005; 23: S58–S62. [PubMed] [Google Scholar]
- 44. Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med 2011; 26: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wabe NT, Sorich MJ, Wechalekar MD, et al. Effect of adherence to protocolized targeted intensifications of disease-modifying antirheumatic drugs on treatment outcomes in rheumatoid arthritis: results from an Australian Early Arthritis Cohort. J Rheumatol 2016; 43: 1643–1649. [DOI] [PubMed] [Google Scholar]
- 46. Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care 2003; 9: S136–S143. [PubMed] [Google Scholar]
- 47. Wabe N, Lee A, Wechalekar M, et al. Factors associated with medication adherence in a longitudinal study of rheumatoid arthritis patients. Int J Clin Pract 2019; 73: e13375. [DOI] [PubMed] [Google Scholar]
- 48. Sturgeon JA, Finan PH, Zautra AJ. Affective disturbance in rheumatoid arthritis: psychological and disease-related pathways. Nat Rev Rheumatol 2016; 12: 532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eijsbouts AM, van den Hoogen FH, Laan RF, et al. Hypothalamic-pituitary-adrenal axis activity in patients with rheumatoid arthritis. Clin Exp Rheumatol 2005; 23: 658–664. [PubMed] [Google Scholar]
- 50. Englbrecht M, Alten R, Aringer M, et al. New insights into the prevalence of depressive symptoms and depression in rheumatoid arthritis – implications from the prospective multicenter VADERA II study. PLoS ONE 2019; 14: e0217412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vashisht P, Sayles H, Cannella AC, et al. Generalizability of patients with rheumatoid arthritis in biologic agent clinical trials. Arthritis Care Res (Hoboken) 2016; 68: 1478–1488. [DOI] [PubMed] [Google Scholar]
- 52. Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply?’ Lancet (London, England) 2005; 365: 82–93. [DOI] [PubMed] [Google Scholar]
- 53. Davidson SK, Romaniuk H, Chondros P, et al. Antidepressant treatment for primary care patients with depressive symptoms: data from the diamond longitudinal cohort study. Aust N Z J Psychiatry 2020; 54: 367–381. [DOI] [PubMed] [Google Scholar]
- 54. González HM, Croghan T, West B, et al. Antidepressant use in Black and White populations in the United States. Psychiatr Serv 2008; 59: 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hämäläinen J, Isometsä E, Sihvo S, et al. Treatment of major depressive disorder in the Finnish general population. Depress Anxiety 2009; 26: 1049–1059. [DOI] [PubMed] [Google Scholar]
- 56. Cleare A, Pariante CM, Young AH, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 2015; 29: 459–525. [DOI] [PubMed] [Google Scholar]
- 57. Clinical practice guideline for the treatment of depression across three age cohorts . Washington, DC: American Psychological Association, 2019. [Google Scholar]
- 58. Abbing-Karahagopian V, Huerta C, Souverein PC, et al. Antidepressant prescribing in five European countries: application of common definitions to assess the prevalence, clinical observations, and methodological implications. Eur J Clin Pharmacol 2014; 70: 849–857. [DOI] [PubMed] [Google Scholar]
- 59. Ables AZ, Baughman OL, 3rd. Antidepressants: update on new agents and indications. Am Fam Phys 2003; 67: 547–554. [PubMed] [Google Scholar]
- 60. Jannini TB, Lorenzo GD, Bianciardi E, et al. Off-label uses of selective serotonin reuptake inhibitors (SSRIs). Curr Neuropharmacol 2022; 20: 693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221111613 for The association of depression and anxiety with treatment outcomes in patients with rheumatoid arthritis – a pooled analysis of five randomised controlled trials by Arkady T. Manning-Bennett, Ashley M. Hopkins, Michael J. Sorich, Susanna M. Proudman, David J.R. Foster, Ahmad Y. Abuhelwa and Michael D. Wiese in Therapeutic Advances in Musculoskeletal Disease