Abstract
Objective:
Pharmacological management of heart failure and comorbidities may result in polypharmacy, but there are few population-based studies that portray the use of medications over time. We aimed to describe the trends in polypharmacy and medication use in older adults with heart failure.
Methods:
We performed a study including all adults >65 years with heart failure between 2000 and 2017 using health administrative databases in Quebec, Canada. Medication use was ascertained by the presence of at least one claim in each year. We defined three levels of polypharmacy: ⩾10, ⩾15 and ⩾20 different medications/year, and evaluated the use of guideline-recommended and potentially inappropriate medications. We calculated age- and sex-standardized proportions of users each year.
Results:
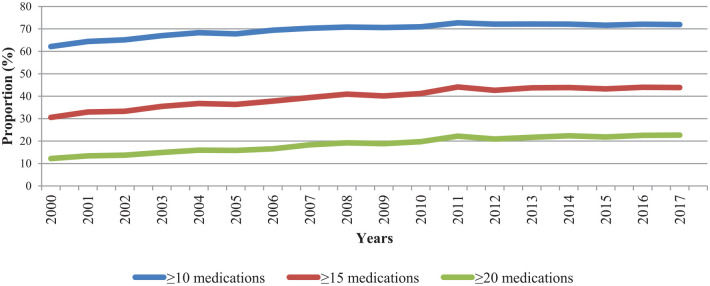
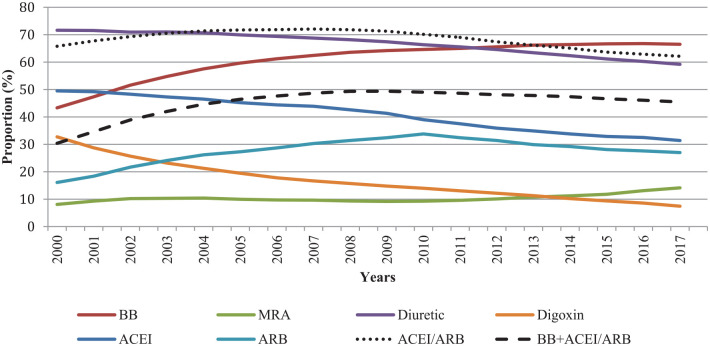
The use of ⩾10, ⩾15 and ⩾20 medications increased from 62.2%, 30.6% and 12.2% in 2000 to 71.9%, 43.9% and 22.7%, respectively, in 2017. The combination of β-blocker and angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) was used by 30.4% of individuals in 2000 and 45.5% in 2017. ACEI/ARB users decreased from 65.8% in 2000 to 62.1% in 2017. Potentially inappropriate medication use decreased over time.
Conclusion:
Polypharmacy is significant among older adults with heart failure. Implications of such medication burden should be investigated.
Keywords: epidemiology, heart failure, treatment, trends
Introduction
In recent years, the combination of an ageing population, an increase in cardiovascular risk factors and a decrease in mortality in patients with coronary heart disease 1 has made heart failure a genuine public health problem. In the province of Quebec, Canada, the prevalence of heart failure was 17.3% among those aged ⩾80 years in 2015–2016. 2 Heart failure imposes a significant burden on healthcare, with increased risks of emergency room visits and hospitalizations 3 and mortality rates that reach almost 45% at 5 years. 4
Pharmacological treatments can reduce complications and improve survival in heart failure patients. 5 Over the last two decades, the cornerstone treatment has remained a combination of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) and β-blocker. 6 In 2017, the Canadian guidelines update recommended the use of valsartan/sacubitril, a new combination proven superior to ACEI/ARB to reduce mortality for symptomatic patients with optimized treatment. 6 Mineralocorticoid receptor antagonists (MRAs) have also been recommended since 2014 for most patients to reduce morbidity and mortality. 7 As for the use of diuretics and digoxin, recommendations have remained consistent over time, with diuretics being indicated to reduce fluid overload and digoxin helping to manage persistent symptoms when optimal treatments are used.7,8 However, clinical context may limit the use of pharmacologic treatments, and gaps between recommendations and clinical practice have been reported. 9 Besides, some medications, such as thiazolidinediones (TZDs) and nonsteroidal anti-inflammatory drugs (NSAIDs), should be avoided in heart failure because their risks often outweigh their benefits.10,11 However, few studies evaluated trends in the use of potentially inappropriate medications in real-world data.
The management of heart failure is often further complicated by comorbidities. In the United States, 58% of patients aged >40 years with heart failure have more than five comorbidities, such as diabetes or chronic obstructive pulmonary disease (COPD). 9 Still in the United States, patients aged ⩾50 years with heart failure use a mean of 7.2 medications annually, and 74% of them meet the common definition of polypharmacy, 12 which is the use of five different medications or more simultaneously. 13 This situation is particularly worrisome because polypharmacy is associated with an increased risk of side effects, drug interactions, inappropriate prescribing, underuse of optimal treatments, hospitalizations and costs, adding on to the burden of heart failure.12,13
With the increase in multimorbidity and the ageing of the population, it is likely that the medication burden among older adults with heart failure is on the rise. However, the evolution of polypharmacy and heart failure medication use over time is poorly described in literature. We therefore aimed at observing the trends in polypharmacy and medication use among older individuals with prevalent heart failure in the province of Quebec, Canada, between 2000 and 2017. Observing those trends will help inform practitioners and provide reliable information to decision-makers and public health.
Methods
Data source and study design
We conducted a retrospective population-based study using the Quebec Integrated Chronic Disease Surveillance System (QICDSS), managed by the Institut national de santé publique du Québec (INSPQ). The QICDSS combines information from five health administrative databases from the public healthcare system of the province of Quebec, Canada. 14 The public health plan covers about 99% of the entire population of the province (8.3 million in 2017). 14 With data available since 1996, the QICDSS includes the following: (1) the health insurance registry, which contains demographic, geographic and public health coverage admissibility data; (2) the MED-ÉCHO database, which contains hospitalization data such as principal and secondary diagnostic codes, length of stay and type of interventions; (3) the vital statistics death database; (4) the physician claims database that contains data on all fee-for-service billings including diagnostic codes and health professional information; and (5) the pharmaceutical services database that contains data on prescription medication claims under the public drug plan, such as medication name, duration of treatment and prescriber. About 90% of individuals aged over 65 years are covered by the public drug plan. A unique, denominated identification number allows the information from all five databases to be linked at the individual level. 14
Study population and case definition
We identified all individuals aged above 65 years with a diagnosis of heart failure 15 in the QICDSS between 2000 and 2017. The case definition requires either the presence of one International Classification of Disease (ICD) 9th revision diagnostic code 428 or ICD-10-CA (Canadian version) code I50 from the MED-ÉCHO database, or the presence of two or more corresponding ICD codes from the physician claims database within 1 year. The sensitivity of this case definition is 84.8% and the specificity is 97.0%. 15 Once an individual is defined as having heart failure, they are considered as such for all following years until their death.
We created unique cohorts of individuals with heart failure for each fiscal year (1 April–31 March) of the study period, resulting in 18 different cohorts. To be included in a specific yearly cohort, the individuals had to be aged 66 years or older on 1 April, be covered by the public drug plan and be alive for the duration of the studied year. Each cohort thus included both incident and prevalent cases of heart failure.
The characteristics of included individuals were retrieved for each cohort; they included age, sex, the region of residence (rural or urban), material and social deprivation index, comorbidities and use of healthcare. Deprivation index are validated proxies of socio-economic status based on postal code. 16 The quality of social networks can be expressed by the social deprivation index, while the quality of material goods owned by the population studied can be described by the material deprivation index. The first quintile of both indexes includes the least deprived individuals, while the fifth quintile contains the most deprived individuals. 16 Comorbidities were assessed using validated QICDSS algorithms for diabetes, hypertension, stroke, COPD, asthma, osteoporosis, mood disorders, schizophrenia and dementia. 14 We used MED-ÉCHO and the physician claims databases to assess the number of hospitalizations and the number of annual visits to general practitioners (GPs), internists and cardiologists.
Medication use and polypharmacy
The common denomination of medications, which is equivalent to the 5th Anatomical Therapeutic Chemical (ATC) classification, was used to ascertain medication. Individuals were considered exposed to the medication if at least one claim has been made in the study year period. The total number of different medications claimed was measured each year. Since the yearly use of five medications is common in heart failure, and that 73% of older adults in Quebec used at least five medications in 2016, 17 we defined polypharmacy as the presence of 10 and more different medication claims for an individual in a given year, regardless of whether the medications were used simultaneously or not. The thresholds of 15 or more and 20 or more different medications were also studied.
We characterized the use of recommended medications for heart failure and those that are potentially inappropriate. We included guideline-recommended treatments: 6 ACEI/ARB, β-blockers, MRA, diuretics, digoxin and ivabradine. Valsartan/sacubitril was excluded because there are only data regarding its use in the last year of our study period. Because health administrative databases have limited clinical information, we restricted the list of potentially inappropriate medications to those listed by the 2016 American Heart Association statement 11 that do not require a thorough history to assess appropriateness. In the list, we included medications that have ‘major’ impact on heart failure, with level of evidence ‘A’ or ‘B’. Medications that are not on the regular public drug plan list, such as dipeptidyl peptidase-4 inhibitors, were not included. Therefore, TZDs and nondihydropyridine calcium channel blockers (non-DHP CCBs) were selected as potentially inappropriate medications.
Statistical analyses
Statistical analyses were performed for each cohort (i.e. the 18 years of the study period). Descriptive statistics were used to summarize the population’s characteristics. Using 2001 Quebec standard population weights, we estimated age- and sex-standardized yearly proportions of individuals exposed to polypharmacy and to each recommended and potentially inappropriate medication. The 95% confidence intervals (CIs) were computed using an inverse gamma distribution. We tested age- and sex-standardized relative changes in annual polypharmacy trends using Poisson regression models. Statistical tests were two sided with significance at p < 0.05. SAS 9.4 was used for all analyses.
Ethics
The provincial Public Health Research Ethics Board and the Quebec Commission protecting access to information have approved the use of the QICDSS for surveillance purposes. No written informed consent was required.
Results
The number of individuals with heart failure increased from 45,640 in 2000 to 87,905 in 2017 (Table 1 and Supplemental Table S1). The proportion of individuals aged >85 years increased over the years (from 19.3% to 26.3%). Similarly, the mean number of comorbidities increased from 2.0 in 2000 to 2.6 in 2017. The yearly number of GP visits decreased over the period (from 6.0 to 3.6). Conversely, the yearly number of cardiologist and internist visits, as well as the number of hospitalizations, remained stable. Those results are presented in Supplemental Table S1.
Table 1.
Characteristics of adults aged >65 years with heart failure in Quebec, Canada, from 2000 to 2017.
| Characteristics | 2000 | 2005 | 2010 | 2015 | 2017 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N = 45,640 | N = 61,632 | N = 71,137 | N = 81,900 | N = 87,905 | ||||||
| n | % | n | % | n | % | n | % | n | % | |
| Age (years) (mean ± SD) | 78.9 ± 7.1 | 79.4 ± 7.2 | 79.7 ± 7.5 | 79.8 ± 7.8 | 79.8 ± 7.9 | |||||
| 66–70 | 6184 | 13.5 | 7562 | 12.3 | 9432 | 13.3 | 11,679 | 14.3 | 12,189 | 13.9 |
| 71–75 | 10,091 | 22.1 | 12,520 | 20.3 | 13,176 | 18.5 | 16,143 | 19.7 | 18,121 | 20.6 |
| 76–80 | 11,263 | 24.7 | 14,935 | 24.2 | 15,752 | 22.1 | 16,034 | 19.6 | 17,609 | 20.0 |
| 81–85 | 9312 | 20.4 | 13,869 | 22.5 | 15,797 | 22.2 | 16,822 | 20.5 | 16,853 | 19.2 |
| ⩾86 | 8790 | 19.3 | 12,746 | 20.7 | 16,980 | 23.9 | 21,222 | 25.9 | 23,133 | 26.3 |
| Sex | ||||||||||
| Female | 25,153 | 55.1 | 32,996 | 53.5 | 36,470 | 51.3 | 40,432 | 49.4 | 42,747 | 48.6 |
| Male | 20,487 | 44.9 | 28,636 | 46.5 | 34,667 | 48.7 | 41,468 | 50.6 | 45,158 | 51.4 |
| Number of different medications per year | 11.9 ± 6.0 | 13.0 ± 6.3 | 13.9 ± 6.7 | 14.3 ± 7.1 | 14.4 ± 7.2 | |||||
| 0 | 546 | 1.2 | 749 | 1.2 | 837 | 1.2 | 1130 | 1.4 | 1165 | 1.3 |
| 1–4 | 2875 | 6.3 | 2681 | 4.4 | 2590 | 3.6 | 3038 | 3.7 | 3290 | 3.7 |
| 5–9 | 14,208 | 31.1 | 16,1707 | 26.2 | 16,199 | 22.8 | 17,590 | 21.5 | 18,659 | 21.2 |
| 10–14 | 14,808 | 32.4 | 20,158 | 32.7 | 22,257 | 31.3 | 24,531 | 30.0 | 26,007 | 29.6 |
| 15–19 | 8237 | 18.0 | 12,824 | 20.8 | 16,004 | 22.5 | 18,436 | 22.5 | 19,758 | 22.5 |
| ⩾20 | 4966 | 10.9 | 9043 | 14.7 | 13,250 | 18.6 | 17,175 | 21.0 | 19,026 | 21.6 |
SD, standard deviation.
Trends in polypharmacy
The age- and sex-standardized prevalence of polypharmacy increased steadily from 2000 to 2017, for the three definitions used (Table 2, Figure 1 and Supplemental Table S2). The age- and sex-standardized proportion of individuals using 10 or more different medications showed an absolute increase of 9.7% (from 62.2% to 71.9%) in the period, corresponding to a relative increase of 0.8% (95% CI = −0.7 to 2.3) per year. Similarly, the proportion of individuals using 15 or more different medications increased by 13.3% (from 30.6% to 43.9%) with a relative increase of 2.0% (95% CI = −0.2 to 4.2) per year, while the proportion of individuals using 20 or more different medications showed an increase of 10.5% (from 12.2% to 22.7%) with a relative increase of 3.5% (95% CI = 1.8–5.2) per year. Accordingly, the mean number of different medication use increased, from 11.9 [standard deviation (SD) = 6.0] in 2000 to 14.4 (SD = 7.2) in 2017 (Table 1).
Table 2.
Age- and sex-standardized proportions of polypharmacy and medication users in adults aged >65 years with heart failure in Quebec, Canada, from 2000 to 2017.
| Proportion of users (95% confidence interval) | |||||
|---|---|---|---|---|---|
| 2000 | 2005 | 2010 | 2015 | 2017 | |
| Polypharmacy | |||||
| ⩾10 medications/year | 62.2% | 67.8% | 70.9% | 71.6% | 71.9% |
| (60.2–63.7) | (65.9–69.7) | (69.2–72.7) | (70.0–73.3) | (70.3–73.5) | |
| ⩾15 medications/year | 30.6% | 36.3% | 41.2% | 43.3% | 43.9% |
| (29.4–31.3) | (35.2–37.5) | (40.1–42.3) | (42.2–44.3) | (42.8–44.9) | |
| ⩾20 medications/year | 12.2% | 15.8% | 19.7% | 21.9% | 22.7% |
| (11.7–12.8) | (15.3–16.4) | (19.2–20.3) | (21.3–22.4) | (22.1–23.2) | |
| Guideline-recommended medication | |||||
| ACEIs/ARBs | 65.8% | 71.7% | 70.1% | 63.6% | 62.1% |
| (64.6–67.0) | (70.6–72.8) | (69.1–71.2) | (62.8–64.5) | (61.3–63.0) | |
| β-blockers | 43.3% | 59.7% | 64.6% | 66.7% | 66.5% |
| (42.4–44.3) | (58.7–60.7) | ( 63.6–65.6) | (65.8–67.6) | (65.6–67.4) | |
| β-blockers + ACEIs/ARBs | 30.4% | 46.4% | 49.0% | 46.6% | 45.5% |
| (29.6–31.2) | (45.6–47.3) | (48.2–49.9) | (45.9–47.4) | (44.7–46.2) | |
| Add-on medications | |||||
| MRA | 8.1% | 10.0% | 9.3% | 11.8% | 14.1% |
| (7.7–8.5) | (9.6–10.4) | (8.9–9.7) | (11.4–12.2) | (13.7–14.6) | |
| Diuretics | 71.6% | 69.9% | 66.3% | 61.2% | 59.2% |
| (70.4–72.8) | (68.9–71.0) | (65.4–67.3) | (60.3–62.0) | (58.4–60.0) | |
| Digoxin | 32.7% | 19.5% | 14.0% | 9.4% | 7.4% |
| (31.9–33.6) | (18.9–20.0) | (13.6–14.5) | (9.0–9.7) | (7.2–7.7) | |
| Ivabradine | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Potentially inappropriate medications | |||||
| Non-DHP CCBs | 16.2% | 14.3% | 12.1% | 9.6% | 8.8% |
| (15.6–16.8) | (13.8–14.8) | (11.7–12.6) | (9.2–9.9) | (8.5–9.2) | |
| Thiazolidinediones | 1.1% | 3.2% | 1.6% | 0.2% | 0.1% |
| (0.9–1.2) | (3.0–3.5) | (1.4–1.7) | (0.2–0.3) | (0.1–0.2) | |
Age- and sex-standardized proportions using 2001 Quebec standard population weights.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonist; non-DHP CCBs, nondihydropyridine calcium channel blockers.
Figure 1.
Age- and sex-standardized proportions of adults aged >65 years with heart failure in Quebec, Canada, exposed to three levels of polypharmacy between 2000 and 2017.
Age- and sex-standardized proportions using 2001 Quebec standard population weights.
Confidence intervals being very narrow, they are not shown in the figure (see Supplemental Table S2).
Trends in recommended medication use
Trends in medication use differed according to the medication studied (Table 2, Figure 2 and Supplemental Table S3). The proportion of β-blocker users showed an absolute increase of 23.2% (from 43.3% to 66.5%) between 2000 and 2017. Similarly, the proportion of individuals using a β-blocker in combination with an ACEI/ARB showed an increase of 15.1% (from 30.4% to 45.5%).
Figure 2.
Age- and sex-standardized proportions of heart failure medication users among adults aged >65 years with heart failure in Quebec, Canada, between 2000 and 2017.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BB, β-blocker; MRA, mineralocorticoid receptor antagonist.
Age- and sex-standardized proportion using 2001 Quebec standard population weights.
Confidence intervals being very narrow, they are not shown in the figure (see Supplemental Table S3).
The proportion of ARB users increased by 17.7% from 2000 to 2010 (from 16.1% to 33.8%) and then decreased by 6.8% from 2010 to 2017 (from 33.8% to 27.0%) (Figure 2). ACEI showed a 18.1% decrease during the study period (from 49.5% to 31.4%). Between 2000 and 2008, the use of all ACEIs diminished, except for ramipril, perindopril and trandolapril, while the use of ARBs increased for all except losartan (Supplemental Figures S1 and S2). From 2008, only perindopril and trandolapril increased within the ACEI class, while it was only telmisartan and olmesartan use that increased within the ARBs. These increases were not sufficient to offset the decreases in other medications in these classes.
The proportions of MRA users were stable, between 8.1% and 11.3%, from 2000 to 2013 then increasing to 14.1% in 2017 (Figure 2 and Table 2). The proportion of diuretic users showed an absolute decrease of 12.4% (from 71.6% to 59.2%). The decrease in the proportion of digoxin users was 25.3% (from 32.7% to 7.4%), which represents the most important absolute reduction in proportion of users among all the medications studied (Figure 2 and Table 2).
Trends in potentially inappropriate medication use
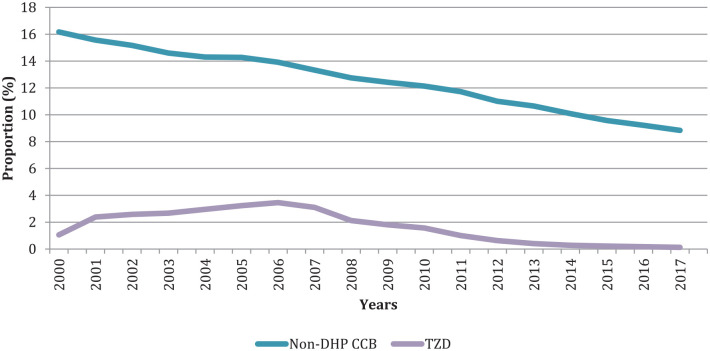
The use of potentially inappropriate medications decreased over time (Table 2, Figure 3 and Supplemental Table S4). The proportion of non-DHP CCB users showed a decrease of 7.4% (from 16.2% to 8.8%). The use of TZDs reached a peak of 3.5% in 2006 before decreasing at 0.1% in 2017.
Figure 3.
Age- and sex-standardized proportions of potentially inappropriate medication users among adults aged >65 years with heart failure in Quebec, Canada, between 2000 and 2017.
Non-DHP CCBs, nondihydropyridine calcium channel blockers; TZD, thiazolidinediones.
Age- and sex-standardized proportions using 2001 Quebec standard population weights.
Confidence intervals being very narrow, they are not shown in the figure (see Supplemental Table S4).
Discussion
Our population-based study shows that the pharmacological burden of older adults with heart failure has significantly increased over the past decades. The use of potentially inappropriate medications is becoming less common. The use of ACEIs/ARBs has also declined in recent years.
The increased prevalence of polypharmacy in patients with heart failure is consistent with the results of other studies targeting patients with this disease, as well as those targeting the general older population or individuals with other chronic diseases.18–22 The proportion of inpatients with heart failure and polypharmacy in the United States leaped from 25% to 55% between 2003 and 2014. 21 The increase of comorbidities in our population is potentially responsible for the increase in polypharmacy. With an ageing population with multimorbidity, following guidelines for each disease can lead to a complex medication regimen. This can be a concern when managing a disease like heart failure, as the resulting polypharmacy could be the cause of further harms.12,13 Unfortunately, there is yet only few guidelines that address the issue of multimorbidity.23,24
Most of our findings regarding trends in medication use are consistent with others. In a study conducted in the United States analysing medication trends in individuals with heart failure from 1988 to 2008, β-blockers and ACEIs/ARBs use increased by 36% (from 15.4% to 51.8%) and 31% (from 24% to 55%), respectively. 9 In individuals aged ⩾30 years in Denmark, the use of ACEIs/ARBs increased from 10% to 35% between 1997 and 2015, while the use of β-blockers increased from 20% to 70%. 25 Conversely, the use of digoxin and diuretics decreased by an absolute 20% and 15%, 25 a trend that we also observed in our data. The decrease in the use of diuretics and digoxin over time might be explained by the more appropriate control of heart failure in the population.
The global reduction of ACEI use that we observed in our study is hardly explainable. It is conceivable that the decrease in ACEI use could result from changes in practice following the publication of studies exposing benefits of ARBs. For example, the Val-HeFT study was published in 2001 and concluded that the use of valsartan 160 mg twice daily reduced the combined endpoint of mortality and morbidity and improved clinical signs and symptoms of heart failure. 26 Then, the CHARM-Alternative trial, published in 2003, concluded that the use of candesartan 32 mg once daily reduced cardiovascular deaths and hospital admissions for heart failure. 27 Hence, some practitioners might have switched their patients from an ACEI to an ARB after taking notice of those studies. The arrival of valsartan/sacubitril, approved in Canada in 2015 and reimbursed by the public drug plan in 2017, could also have led to a reduction in the use of ACEIs. However, these elements do not explain why the total proportion of ACEI/ARB use decreased in the population, since a transition from one class to another would not affect the total measurement. One hypothesis that could not be evaluated in our study is that the proportions of individuals with different diagnoses of heart failure may have changed over time. Indeed, our data do not differentiate between systolic heart failure and diastolic heart failure, nor between heart failure with preserved and reduced ejection fraction. Since these conditions are managed differently, it can influence medication trends if their proportions vary over time. In fact, a recent study observed a reduction in the incidence of heart failure with reduced ejection fraction. 28 Nevertheless, even considering this possible change over time, it remains difficult to explain the overall decrease in the use of ACEIs/ARBs, as they are also the first choice of treatment for conditions very prevalent in the study population, such as hypertension or diabetes. These prescribing trends that go against guidelines are of massive interest in terms of public health, in regard of the benefits of ACEIs/ARBs in the management of heart failure, and other chronic conditions. Future studies should address this concern.
The use of potentially inappropriate medications decreased over the study period, which may reflect an increased awareness of the harm of potentially inappropriate prescribing. For example, the 2006 Canadian guideline of heart failure diagnosis and management update introduced the concept of polypharmacy and of potentially inappropriate medications in heart failure 6 and may have instigated behavioural change in prescribing practices. In 2014, Bermingham et al. created and evaluated a list of potentially inappropriate medications in heart failure, which included TZDs and non-DHP CCBs, that have been associated with an increased risk of all-cause hospitalizations in newly diagnosed heart failure patients. 10 In 2016, the American Heart Association published a statement regarding drugs that may aggravate heart failure with the purpose of assisting healthcare providers’ decision-making. 11 Observational studies suggesting increased risk of hospitalizations with the use of potentially inappropriate drugs 29 may also have contributed to increased awareness.
Our study has the strength of population-based analysis spanning 18 years. The QICDSS database provided detailed accurate information on individuals’ characteristics and medication use, avoiding the recall bias occurring with questionnaires, and thus providing a comprehensive portrait of medication use over nearly two decades.
Some limits must also be mentioned. Because the case definition of heart failure is a life-time definition, some of the included individuals could be false positives that are followed over the years. This could have led to an underestimation of the use of recommended medication, as false positive cases would not require heart failure treatment. We also underestimated polypharmacy and the use of potentially inappropriate medications, such as NSAIDs, because over-the-counter medications are not included in the database. Although our definition of polypharmacy presents the overall burden of prescribed medications in a year for an individual, it does not allow an evaluation of concomitant medication use. The lack of clinical data, such as prognosis, ejection fraction, presence of symptoms, contraindications or side effects, limited us from evaluating proper medication use in accordance with clinical presentation and context. Future research could assess the quality of polypharmacy, for example, by describing the drug–drug and drug–disease interactions. Our results cannot be extrapolated to younger individuals or individuals living in nursing homes, as they were not included in our study. Also, there are a small proportion of older adults who have private drug coverage and therefore were not included in the study. Those individuals are usually healthier and therefore less likely to use polypharmacy. The results may also not apply to other jurisdictions that have different healthcare systems, particularly those that do not offer public drug plans like the one in the province of Quebec. We did not evaluate the effect of the geographical area, including possible differences between urban and rural areas. Differential provision of health services could influence health service use and consequently, medication use. Finally, we did not evaluate the associations between polypharmacy and heart failure medication use with clinical consequences, such as medication interactions, health service use or mortality, which should be explored in future research.
Conclusion
Our study characterized trends in medication use among older Quebecers with heart failure. The prevalence of polypharmacy increased over the years, which may raise questions about the possible consequences of such medication burden. As for specific medications for heart failure management, most of our results are consistent with published guidelines and literature. However, the decrease in ACEI/ARB use is difficult to explain and needs to be further investigated to assess the possible consequences in terms of public health.
Supplemental Material
Supplemental material, sj-docx-1-tak-10.1177_17539447221113946 for Polypharmacy among older individuals with heart failure: trends between 2000 and 2017 in the province of Quebec, Canada by Alexandre Campeau Calfat, Marc Simard, Amina Ouali, Claudia Blais and Caroline Sirois in Therapeutic Advances in Cardiovascular Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Alexandre Campeau Calfat  https://orcid.org/0000-0002-3734-2165
https://orcid.org/0000-0002-3734-2165
Claudia Blais  https://orcid.org/0000-0002-5299-9691
https://orcid.org/0000-0002-5299-9691
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Alexandre Campeau Calfat, Faculty of Medicine, Université Laval, Québec, QC, Canada.
Marc Simard, Faculty of Medicine, Université Laval, Québec, QC, Canada; Institut national de santé publique du Québec, Québec, QC, Canada.
Amina Ouali, Faculty of Medicine, Université Laval, Québec, QC, Canada.
Claudia Blais, Institut national de santé publique du Québec, Québec, QC, Canada; Faculty of Pharmacy, Université Laval, Québec, QC, Canada.
Caroline Sirois, Faculty of Pharmacy, Université Laval, CEVQ, 1050 Chemin Ste-Foy, Québec, QC G1S 4L8, Canada; Institut national de santé publique du Québec, Québec, QC, Canada.
Declarations
Ethics approval and consent to participate: The provincial Public Health Research Ethics Board and the Quebec Commission protecting access to information have approved the use of the QICDSS for surveillance purposes. No written informed consent was required.
Consent for publication: Not applicable.
Author contribution(s): Alexandre Campeau Calfat: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Marc Simard: Conceptualization; Data curation; Formal analysis; Methodology; Resources; Software; Writing – original draft; Writing – review & editing.
Amina Ouali: Writing – original draft; Writing – review & editing.
Claudia Blais: Data curation; Methodology; Writing – original draft; Writing – review & editing.
Caroline Sirois: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Alexandre Campeau Calfat received a scholarship from the ‘Centre de recherche sur les soins et les services de première ligne de l’Université Laval (CERSSPL-UL)’. Caroline Sirois is a recipient of a Junior 2 Research Scholar from the Quebec Research Funds in Health [Fonds de recherche du Québec – Santé (FRQS)].
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The datasets analysed during the current study are not publicly available. Some data may be available from the authors upon reasonable request and with permission of the Institut national de santé publique du Québec.
References
- 1. Blais C, Rochette L. Trends in prevalence, incidence and mortality of diagnosed and silent coronary heart disease in Quebec. Health Promot Chronic Dis Prev Can 2015; 35: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Massamba VK, Rochette L, Trépanier P-L, et al. Surveillance de l’insuffisance cardiaque au Québec: prévalence, incidence et mortalité de 2005-2006 à 2015-2016. Québec, QC, Canada: Institut national de santé publique du Québec, 2019. [Google Scholar]
- 3. Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep 2014; 11: 212–219. [DOI] [PubMed] [Google Scholar]
- 4. Jones NR, Roalfe AK, Adoki I, et al. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail 2019; 21: 1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah A, Gandhi D, Srivastava S, et al. Heart failure: a class review of pharmacotherapy. P T 2017; 42: 464–472. [PMC free article] [PubMed] [Google Scholar]
- 6. Ezekowitz JA, O’Meara E, McDonald MA, et al. 2017 comprehensive update of the Canadian Cardiovascular Society guidelines for the management of heart failure. Can J Cardiol 2017; 33: 1342–1433. [DOI] [PubMed] [Google Scholar]
- 7. Moe GW, Ezekowitz JA, O’Meara E, et al. The 2014 Canadian Cardiovascular Society heart failure management guidelines focus update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol 2015; 31: 3–16. [DOI] [PubMed] [Google Scholar]
- 8. Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol 2006; 22: 23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong CY, Chaudhry SI, Desai MM, et al. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med 2011; 124: 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bermingham M, Ryder M, Travers B, et al. The St Vincent’s potentially inappropriate medicines study: development of a disease-specific consensus list and its evaluation in ambulatory heart failure care. Eur J Heart Fail 2014; 16: 915–922. [DOI] [PubMed] [Google Scholar]
- 11. Page RL, O’Bryant CL, Cheng D, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation 2016; 134: e32–e69. [DOI] [PubMed] [Google Scholar]
- 12. Goyal P, Bryan J, Kneifati-Hayek J, et al. Association between functional impairment and medication burden in adults with heart failure: medication burden in heart failure. J Am Geriatr Soc 2019; 67: 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol 2012; 65: 989–995. [DOI] [PubMed] [Google Scholar]
- 14. Blais C, Jean S, Sirois C, et al. Quebec Integrated Chronic Disease Surveillance System (QICDSS), an innovative approach. Chronic Dis Inj Can 2014; 34: 226–235. [PubMed] [Google Scholar]
- 15. Schultz SE, Rothwell DM, Chen Z, et al. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis Inj Can 2013; 33: 160–166. [PubMed] [Google Scholar]
- 16. Pampalon R, Hamel D, Gamache P. A comparison of individual and area-based socio-economic data for monitoring social inequalities in health. Health Rep 2009; 20: 85–94. [PubMed] [Google Scholar]
- 17. Gosselin E, Simard M, Dubé M, et al. Portrait de la polypharmacie chez les aînés québécois entre 2000 et 2016. Québec, QC, Canada: Institut national de santé publique du Québec, 2020. [Google Scholar]
- 18. Zhang N, Sundquist J, Sundquist K, et al. An increasing trend in the prevalence of polypharmacy in Sweden: a nationwide register-based study. Front Pharmacol 2020; 11: 326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kantor ED, Rehm CD, Haas JS, et al. Trends in prescription drug use among adults in the United States from 1999-2012. JAMA 2015; 314: 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sirois C, Ouali A, Simard M. Polypharmacy among older individuals with COPD: trends between 2000 and 2015 in Quebec, Canada. COPD 2019; 16: 234–239. [DOI] [PubMed] [Google Scholar]
- 21. Unlu O, Levitan EB, Reshetnyak E, et al. Polypharmacy in older adults hospitalized for heart failure. Circ Heart Fail 2020; 13: e006977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. von Lueder TG, Atar D. Comorbidities and polypharmacy. Heart Fail Clin 2014; 10: 367–372. [DOI] [PubMed] [Google Scholar]
- 23. Guthrie B, Payne K, Alderson P, et al. Adapting clinical guidelines to take account of multimorbidity. BMJ 2012; 345: e6341. [DOI] [PubMed] [Google Scholar]
- 24. Muth C, Blom JW, Smith SM, et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: a systematic guideline review and expert consensus. J Intern Med 2019; 285: 272–288. [DOI] [PubMed] [Google Scholar]
- 25. Vinter N, Jensen M, Skjøth F, et al. Twenty-year time trends in use of evidence-based heart failure drug therapy in Denmark. Basic Clin Pharmacol Toxicol 2020; 127: 30–38. [DOI] [PubMed] [Google Scholar]
- 26. Cohn JN, Tognoni G. A Randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001; 345: 1667–1675. [DOI] [PubMed] [Google Scholar]
- 27. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362: 772–776. [DOI] [PubMed] [Google Scholar]
- 28. Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018; 6: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Girouard C, Grégoire J-P, Poirier P, et al. Effect of contraindicated drugs for heart failure on hospitalization among seniors with heart failure: a nested case-control study. Medicine 2017; 96: e6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tak-10.1177_17539447221113946 for Polypharmacy among older individuals with heart failure: trends between 2000 and 2017 in the province of Quebec, Canada by Alexandre Campeau Calfat, Marc Simard, Amina Ouali, Claudia Blais and Caroline Sirois in Therapeutic Advances in Cardiovascular Disease