Abstract
COVID-19 is caused by coronavirus (SARS-CoV-2) is a worldwide health crisis. This severe acute respiratory syndrome was declared an outbreak after the first case was reported in Wuhan, the capital city of Hubei Province in China. On March 11th, 2020, the World Health Organization (WHO) declared it as a pandemic. The pharmaceutical treatment of COVID-19 has garnered significant critical attention due to the unavailability of medications to treat COVID-19. Recently, researchers have shown an increased interest in the monoclonal antibody drugs to treat COVID-19 especially REGEN-COV (casirivimab and imde-vimab). This review aims to highlight the relation between the drug and COVID-19 and the recently updated information on the monoclonal antibody REGEN-COV from the U.S. Food and Drug Administration and other authorities. It is also designed to focus on the bibliometric data of REGEN-COV for the last three years (2020, 2021, and 2021) in PubMed and Google Scholar.
Keywords: REGEN-COV, casirivimab and imdevimab, COVID-19, SARS-CoV-2, Ronapreve
Significance for public health
REGEN-COV is one of the drugs that have been prescribed for the treatment of the novel SARS-CoV-2. More attention was paid to the monoclonal antibody drugs since the SARS-CoV-2 started. The drug has been used to treat SARS-CoV-2 patients since November 21st, 2020. However, on January 24th, 2022, and due to its weak activity against the new SARS-CoV-2 variants, the FDA limited the use of REGEN-COV drug. As a result, drug prescribers should be cautious of prescribing the medicine to the public for the reasons stated above, and prescribing this drug, particularly for the Omicron variant, will not result in the desired therapeutic outcomes and may result in unwanted and unnecessary side effects.
Bibliometric analysis of REGEN-COV
A growing body of literature recognises the importance of REGEN-COV since the SARS-CoV-2 started. The availability of this literature could be translated and analysed as bibliometric data to give a clear picture of the REGEN-COV importance in the treatment of the novel SARS-CoV-2 chronologically. The bibliometrics data were obtained from PubMed and Google Scholar. The key phrases used in this article were picked based on the authors’ previous experience. The main phrases used in PubMed and Google Scholar were “REGEN-COV” and “Ronapreve”. The data was taken from the platforms indicated above on January 27th, 2022. According to PubMed, there were 152 published studies between 2020 and 2022. (7 in 2020, 118 in 2021, and 27 in 2022) when the key phrase “REGEN-COV” is used in the search. At the same time, four published studies were noted when the key phrase “Ronapreve” was used in the same platform (Figure 1). The importance of this medicine in treating SARS-CoV-2 and the attractiveness of the REGEN-COV drug name compared to Ronapreve may be inferred from the findings. When the key phrase “REGEN-COV” was searched on Google Scholar, there were 471 published data (5 in 2020, 402 in 2021, and 64 in 2022; Figure 2). About 60 articles were found on Google Scholar when the key phrase “Ronapreve” was used in the search. This result indicated that the researchers often use the keyword “ REGEN-COV “ more than “Ronapreve”.
Figure 1.
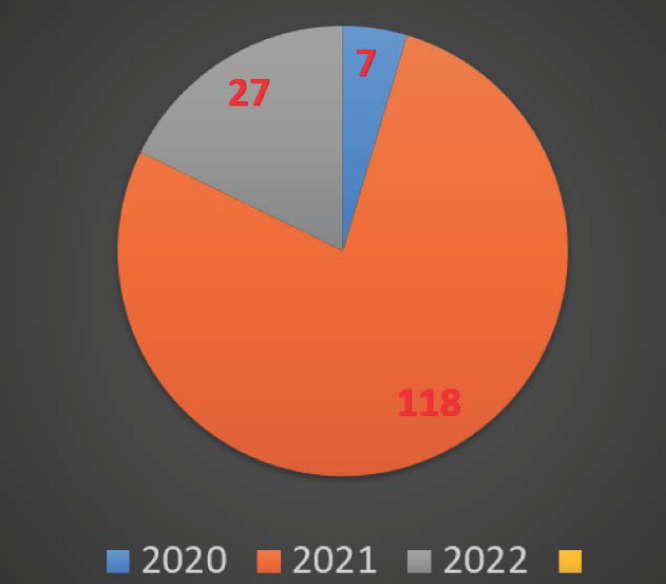
Bibliometric data from PubMed.
Figure 2.
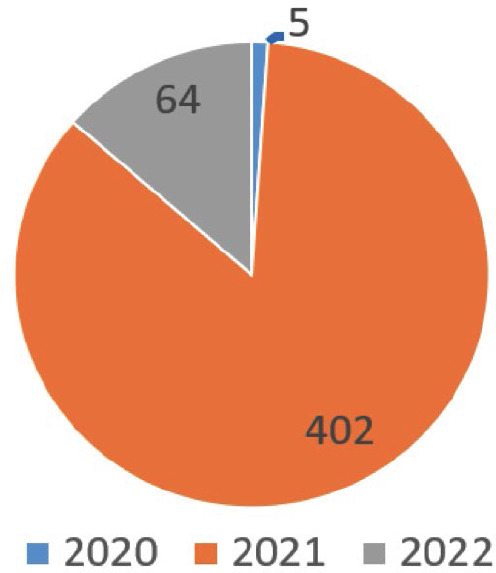
Bibliometric data from Google Scholar.
Nonetheless, Google Scholar's findings differ from PuMed's prior findings; Google Scholar produced more results, but no indication of this disparity was found. This inconsistency could be due to the results being repeated while searching in Google Scholar, a more general platform for the published work than PubMed (Figure 2). Both platforms, however, demonstrated frequent use of “REGEN-COV” in SARS-CoV-2 research.
REGEN-COV (casirivimab and imdevimab)
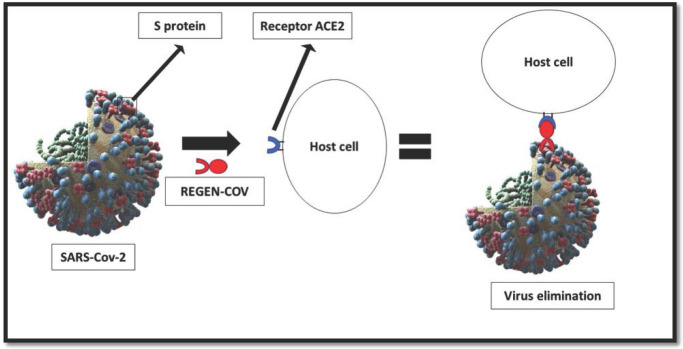
“REGEN-COV”, also known as “Ronapreve,” is a drug suggested to treat the novel SARS-CoV-2 that combines casirivimab and imdevimab; two monoclonal antibodies.1,2 This combination works by targeting the SARS-CoV-2 spike protein, inhibiting its interaction with the human angiotensin-converting enzyme 2 (ACE2) receptors, and eliminating the virus (Figure 3).2,3 Despite the importance of SARS-CoV-2, there remains a paucity of evidence on the treatment of the novel pandemic SARS-CoV-2. However, in a preliminary analysis of an ongoing double-blind, randomised trial of 275 SARS-CoV-2 outpatients, the REGN-COV2 antibody (1200mg, 8000mg casirivimab, and 1200mg and 400mg imdevimab) have been found to reduce viral load better than placebo. 4 In a separate study, the combination of casirivimab and imdevimab reduced hospital admission or mortality in high-risk non-admitted patients by 70% in a phase III trial. 5 For the novel Omicron type of SARS-CoV-2, a combination of monoclonal antibodies has been reported as ineffective. 6 The main adverse effects reported for REGEN-COV were dizziness, nausea, rash, chills, and lymphadenopathy. 7 Whereas flushing, urticaria, anaphylaxis, and pruritus were rarely reported. 7
Figure 3.
Mechanism of action of REGEN-CoV for the treatment of SARS-CoV-2.
REGEN-COV FDA and other authorities approval
REGEN-COV acquired its first emergency use license in the United States to treat COVID-19 on November 21st, 2020. 7 Moreover, The FDA issued a Letter of Authorization (LOA) to Regeneron (the pharmaceutical company that applied for the authorization) on February 3, 2021, for the emergency use of casirivimab and imdevimab in combination to prevent sickness in patients who have been exposed to someone with laboratory-confirmed SARS-CoV-2. 8 Moreover, it was reported that Japan became the first country to license this combination to treat SARS-CoV-2 in July 2021. 5 The LOA includes a stipulation that Regeneron provides instructional and educational materials to the FDA for review and approval prior to their initial distribution. The REGEN-COV LOA was issued for the treatment of mild to moderate coronavirus disease 2019 (COVID-19) in adults and paediatric patients (12 years of age and older, weighing at least 40 kg) who have positive results from direct SARS-CoV-2 viral testing and are at high risk of developing severe COVID-19 and/or hospitalisation. 8 Later on, the medicine acquired conditional marketing authorisation in the United Kingdom to prevent and treat SARS-CoV-2 on August 20th, 2021. 5
January 24th, 2022, FDA update for REGEN-COV
On January 24th, 2022, due to the data that showed that REGEN-COV is ineffective against the Omicron variant, the FDA changed the authorizations for the monoclonal antibody treatments REGEN-COV (casirivimab and imdevimab) to restrict their use to patients who are likely to have been infected with or exposed to a variation susceptible to these treatments. 9 Monoclonal antibodies are lab-made proteins that imitate the immune system's capacity to combat diseases like SARS-CoV-2 viruses; as a result, specific variants, such as Omicron, are ineffective against specific variations. 9 The FDA reauthorization of this drug is to prevent patients from being exposed to potentially dangerous side effects such as injection site responses or allergic reactions from certain medications that are not likely to help individuals who have been infected with or exposed to the Omicron form. 9
Conclusions
The purpose of this review was to highlight the link between the drug and COVID-19, as well as the FDA's and other authorities' recently updated information on the monoclonal antibody REGEN-COV (casirivimab and imdevimab). It's also concentrated on REGEN-COV bibliometric data in PubMed and Google Scholar over the last three years (2020, 2021, and 2021). One of the more significant findings to emerge from this study is that the drug was showed no activity against the Omicron variant of the SARS-CoV-2 virus. Therefore, the FDA has limited drug use based on the drug's action on the SARS-CoV-2 infection.
Footnotes
Contributions: Conceptualization, SMM; Methodology, SMM, and NO; Data Formal Analysis, SMM, YMA, SSAT, and SAH; Investigation, SMM, JK; Data Curation, SMM, and SAO Writing— Original Draft SMM; Writing—Review & Editing, SMM, NO, SOA, YMA, SSAT SAH, RRM.
Conflict of interest: The authors declare no conflict of interest.
Availability of data and material: All data generated or analysed during this study are included in this published article.
Ethical approval: Not applicable.
Patient consent for publication: Not applicable.
Informed consent: Not applicable.
References
- 1.Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 2020;109:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidebottom DB, Gill D. Ronapreve for prophylaxis and treatment of COVID-19. BMJ 2021;374:n2136. [DOI] [PubMed] [Google Scholar]
- 3.Hussain M, Jabeen N, Raza F, et al. Structural variations in human ACE2 may influence its binding with SARS-CoV-2 spike protein. J Med Virol 2020;92:1580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with COVID-19. N Engl J Med 2021;384:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahase E. COVID-19: UK approves first monoclonal antibody treatment. BMJ 2021;374:n2083. [DOI] [PubMed] [Google Scholar]
- 6.Ikemura N, Hoshino A, Hihuchi Y, et al. SARS-CoV-2 Omicron variant escapes neutralization by vaccinated and convalescent sera and therapeutic monoclonal antibodies. medRxiv 2021. 10.1101/2021.12.13.21267761 [DOI]
- 7.Deeks ED. Casirivimab/Imdevimab: First Approval. Drugs 2021;81:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA. Emergency Use Authorization (EUA) for REGEN-COV Center for Drug Evaluation and Research (CDER) Memorandum. 2022. Available from: https://www.fda.gov/media/146273/download
- 9.FDA, U. Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant. 2022. Available from: fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-limits-use-certain-monoclonal-antibodies-treat-COVID-19-due-omicron