Abstract
Background:
Magnetic resonance imaging (MRI) graft signal intensity is associated with graft damage after anterior cruciate ligament reconstruction (ACLR). However, little is known about the relationship between graft signal intensity and residual laxity of the reconstructed knee based on patient age.
Purpose/Hypothesis:
To evaluate the relationship between graft signal intensity and residual laxity in younger and older patients who underwent ACLR. We hypothesized that higher graft signal intensity would be associated with reduced postoperative knee stability.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A total of 192 patients who underwent double-bundle ACLR were recruited. Proton density–weighted and T2-weighted MRI was performed at 3, 6, and 12 months after surgery, and the signal intensity ratio (SIR) of the anteromedial and posterolateral bundles was measured as the graft signal intensity reference values. At 12 months after surgery, if the KT-1000 arthrometer measurement exhibited a side-to-side difference of ≥2 mm, the patient was determined as having anterior knee laxity. Rotatory knee laxity was defined as a positive pivot shift with International Knee Documentation Committee grade ≥1. The Mann-Whitney U test was used to compare the SIR in patients with and without residual laxity. The Spearman correlation coefficient was used to evaluate the relationship between demographic parameters and the SIR. Based on receiver operating characteristic curves, the optimal SIR cutoff values to predict residual laxity were calculated, and logistic regression analysis was conducted.
Results:
Of 192 patients, 26 (13.5%) had anterior knee laxity, and 20 (10.4%) had rotatory knee laxity. The SIR was negatively correlated with age. In younger patients (<30 years; n = 135), those with residual laxity had a significantly higher SIR than those without laxity; this relationship was not significant in older patients (≥30 years; n = 57). Based on receiver operating characteristic curves and logistic regression analysis, the cutoff values that were determined for the SIR were significantly associated with a higher odds ratio of residual laxity.
Conclusion:
Graft signal intensity decreased with patient age. Patients with higher graft signal intensity in the early postoperative phase after ACLR exhibited a higher prevalence of residual laxity, particularly in younger patients.
Keywords: anterior cruciate ligament, reconstruction surgery, graft signal intensity, age, residual knee laxity
Anatomic anterior cruciate ligament (ACL) reconstruction (ACLR) is an important surgical strategy to restore the kinematics of ACL-injured knees close to that of intact knees. During the past 2 decades, the advantages of anatomic ACLR have focused on improved clinical and kinematic outcomes compared with nonanatomic procedures. 7,12,42,45 Recent studies have highlighted the clinical relevance of anatomic ACLR in terms of preventing the development of knee osteoarthritis. 35,41 Despite several advantages of anatomic ACLR, some patients still have the potential to develop residual knee laxity and experience unsuccessful clinical outcomes. 3,6,28,31,40
The early identification and adequate evaluation of those at risk of ACL graft failure could improve outcomes and eventually reduce the failure risk if surgeons can decide on suitable rehabilitation protocols and the appropriate timing of return to sports based on the condition of the reconstructed graft over time. Therefore, potential biomarkers such as blood samples and imaging studies would be useful to determine the risk of postoperative graft failure or residual knee laxity after anatomic ACLR as early as possible.
Graft signal intensity on magnetic resonance imaging (MRI) has been demonstrated to correspond to the progressive remodeling period of the ACL graft. 29,30,46 Histologically, high graft signal intensity indicates hypervascularity or hypercellular tissue. This histologically higher graft signal can be regularly observed during the early postoperative phase (≤6 months after surgery) and gradually decreases between the first and second years after ACLR. Accordingly, this transition of graft signal intensity suggests a progressive graft remodeling period. 29,30,46 In addition to the concept of the graft remodeling process, previous studies reported that the ACL graft signal increased as a result of damage to the reconstructed graft, caused by an acute graft-bending angle, 1,8,44 tunnel malpositioning, 25 and femoral notch impingement. 18,32 Therefore, persistently high graft signal intensity might indicate graft deterioration, which could lead to worse clinical outcomes after ACLR. However, there is insufficient evidence to explain how higher graft signal intensity affects the clinical outcomes of patients who undergo anatomic ACLR. In particular, limited MRI graft signal data within the first year after ACLR are available regarding whether the graft signal affects future clinical outcomes.
The purpose of the current study was to evaluate the association between graft signal intensity during the first year after anatomic ACLR and the prevalence of residual knee laxity. Our hypothesis was that higher graft signal intensity would be associated with a greater prevalence of residual knee laxity at 12 months after ACLR.
Methods
A total of 459 patients underwent anatomic double-bundle ACLR using a hamstring tendon autograft at our hospital between 2010 and 2018. We excluded patients with incomplete data, open physes, infections, multiple ligament injuries that required repair or reconstruction, and knee osteoarthritis defined as Kellgren-Lawrence grade ≥2 as well as those who underwent meniscal resection. In addition, patients who reinjured their ACL graft were also excluded. Ultimately, 192 patients were enrolled in the current retrospective cohort study. The main reason for exclusion was incomplete MRI data as the result of missing examinations. The study protocol was approved by the ethics committee of our institution, and all participants provided informed consent.
Surgical Procedure of Anatomic Double-Bundle ACLR
Anatomic double-bundle ACLR for the study participants was performed according to a previous study. 37 The harvested semitendinosus tendon was split, and the distal and proximal halves of the tendon were looped and used as the anteromedial bundle (AMB) and posterolateral bundle (PLB), respectively. Each graft was 4.5 to 7.0 mm in diameter. Femoral tunnels for both the AMB and PLB were aimed posterior to the resident’s ridge onto the direct insertion of the ACL using the transportal technique. 20,37,42 A tibial tunnel was created as anteriorly as possible and positioned close to the bony ridge, which corresponded to the anterior boundary of the tibial ACL attachment (Parsons knob). 38,42
The proximal end of each graft was fixed by a fixed-loop cortical suspensory system (Position Suture Plate; B. Braun Aesculap) until August 2014; thereafter, an adjustable-loop cortical suspensory system (TightRope; Arthrex) was applied to retain the graft and provide a longer portion of the graft within the femoral tunnel to maximize the graft-bone interface area. 4,17 The distal end of each graft was fixed by a suture mini-disc (B. Braun Aesculap) with maximum manual force applied as initial tension at 15° to 20° of flexion. 37 Menisci were treated before double-bundle ACLR. An unstable meniscal injury in the red-red or red-white zone by probing was repaired as much as possible.
Postoperative rehabilitation was also consistent with a previous study. 37 Briefly, crutch-assisted weightbearing ambulation and range of motion were permitted the day after ACLR, and if the patients underwent concomitant meniscal repair, their knee joint was immobilized in a brace for 1 week. Running, open kinetic chain exercises, and jump-landing training were allowed at 3 months; the patients started to return to sports activities at 6 to 9 months after surgery. 31
Clinical Assessment
At the 12-month follow-up, several surgeons at our sports medicine clinic examined all participants. All examiners regularly perform ACLR and thus have experience with this procedure, and the postoperative examination was conducted at a single institute. Sagittal knee stability was evaluated using a KT-1000 arthrometer with the Lachman test; if the KT-1000 arthrometer side-to-side difference exhibited ≥2-mm anterior tibial translation relative to the femur, the knee was defined as having residual anterior knee laxity (AKL). For rotatory knee laxity (RKL), the pivot-shift test was administered, and it was assessed by International Knee Documentation Committee (IKDC) grading 15,21 ; if the patients exhibited an IKDC grade ≥1, they were defined as having residual RKL.
Measurement of Graft Signal Intensity on MRI
All patients underwent 1.5-T MRI of their operated knee joint at a mean of 3, 6, and 12 months after ACLR (Signa HDxt; GE Healthcare). The knee joint was kept immobilized, flexed at 10°, and externally rotated at 10° to 15° with a customized frame during the examination. Sagittal fast spin echo proton density–weighted (PDW) (repetition time [TR]/echo time [TE], 3000/16 milliseconds; slice thickness/intersection gap, 4.0/1.0 mm; field of view [FOV], 16 cm; number of excitations [NEX], 2; echo train length [ETL], 14; band width, ±31.25 kHz/FOV) and fast spin echo T2-weighted (T2W) (TR/TE, 3000/98 milliseconds; slice thickness/intersection gap, 4.0/1.0 mm; FOV, 16 cm; NEX, 2; ETL, 14; band width, ±31.25 kHz/FOV) sequences were obtained. The oblique sagittal image was reconstructed by defining the plane that passed through the centers of both the femoral and tibial tunnel outlets.
Based on the oblique sagittal image, signal intensity was measured by calculating the mean region of interest in the traced area of each AMB or PLB. The boundary of the graft was traced along with the intra-articular portion 14 (Appendix Figure A1). An orthopaedic surgeon with 13 years of experience (D.C.), who was blinded to clinical information, measured signal intensity using ImageJ (Version 1.37c; National Institutes of Health). To standardize signal intensity, the value for each region of interest of the graft was divided by that of the posterior cruciate ligament (PCL) because the intra-articular condition of the knee joint, such as inflammation or effusion, affects signal intensity. 14,30 The signal intensity ratio (SIR) was calculated as (AMB intensity or PLB intensity)/PCL intensity. The intrarater reliability to determine and calculate the SIR ranged from 0.742 to 0.870, 9 indicating relatively strong agreement.
Statistical Analysis
Statistical analyses were performed using SPSS (Version 24.0; IBM). Demographic data with and without AKL or RKL were compared using the Mann-Whitney U test because the continuous values in the current data were non-normally distributed. The association between demographic data and the SIR was evaluated using the Spearman correlation coefficient (r).
A receiver operating characteristic (ROC) curve was created to determine the ability of the SIR to discriminate between patients with and without residual knee laxity, and the optimal cutoff value for the SIR was determined. Logistic regression analysis was performed, with the prevalence of residual knee laxity at 12 months postoperatively as the dependent variable and the SIR cutoff value as the independent variable. Statistical significance was set at P < .05.
Results
Of the 192 participants, 26 (13.5%) were defined as having AKL, and 20 (10.4%) were defined as having RKL. Demographic parameters were not significantly different between patients with and without knee laxity (Table 1). Compared to those without knee laxity, patients with knee laxity exhibited a significantly higher SIR at 3 and 6 months after ACLR. At 12 months postoperatively, the differences between patients with and without RKL were no longer significant (Table 1).
Table 1.
Characteristics of Study Participants a
| Anterior Knee Laxity | Rotatory Knee Laxity | |||
|---|---|---|---|---|
| Without (n = 166) | With (n = 26) | Without (n = 172) | With (n = 20) | |
| Age, y | 24.4 ± 11.4 | 23.3 ± 10.9 | 24.6 ± 11.6 | 21.5 ± 8.9 |
| Sex, male/female, n | 55/111 | 9/17 | 58/114 | 6/14 |
| Body mass index, kg/m2 | 23.0 ± 3.5 | 23.0 ± 4.0 | 23.1 ± 3.5 | 22.6 ± 3.8 |
| Tegner score | 6.4 ± 1.6 | 6.5 ± 2.1 | 6.4 ± 1.7 | 6.5 ± 1.9 |
| Time from injury to surgery, mo | 5.5 ± 23.1 | 3.5 ± 4.7 | 5.5 ± 22.7 | 2.9 ± 2.9 |
| KT-1000 arthrometer side-to-side difference, mm | 0.5 ± 0.5 | 2.2 ± 0.4 b | 0.6 ± 0.6 | 1.8 ± 0.9 b |
| Signal intensity ratio | ||||
| AMB on PDW | ||||
| 3 mo | 0.99 ± 0.25 | 1.25 ± 0.45 b | 1.00 ± 0.27 | 1.24 ± 0.42 b |
| 6 mo | 1.22 ± 0.32 | 1.54 ± 0.50 b | 1.23 ± 0.33 | 1.53 ± 0.54 b |
| 12 mo | 1.21 ± 0.40 | 1.36 ± 0.32 b | 1.22 ± 0.40 | 1.31 ± 0.35 |
| AMB on T2W | ||||
| 3 mo | 1.05 ± 0.18 | 1.22 ± 0.28 b | 1.06 ± 0.19 | 1.19 ± 0.30 |
| 6 mo | 1.14 ± 0.22 | 1.33 ± 0.25 b | 1.15 ± 0.23 | 1.32 ± 0.25 b |
| 12 mo | 1.08 ± 0.24 | 1.14 ± 0.22 | 1.08 ± 0.24 | 1.11 ± 0.22 |
| PLB on PDW | ||||
| 3 mo | 1.07 ± 0.27 | 1.38 ± 0.52 b | 1.08 ± 0.30 | 1.38 ± 0.47 b |
| 6 mo | 1.31 ± 0.32 | 1.70 ± 0.48 b | 1.33 ± 0.33 | 1.65 ± 0.53 b |
| 12 mo | 1.31 ± 0.40 | 1.56 ± 0.38 b | 1.32 ± 0.40 | 1.50 ± 0.43 |
| PLB on T2W | ||||
| 3 mo | 1.10 ± 0.23 | 1.32 ± 0.36 b | 1.11 ± 0.25 | 1.27 ± 0.33 b |
| 6 mo | 1.22 ± 0.27 | 1.53 ± 0.44 b | 1.23 ± 0.29 | 1.46 ± 0.46 b |
| 12 mo | 1.15 ± 0.29 | 1.31 ± 0.33 b | 1.16 ± 0.30 | 1.26 ± 0.36 |
a Data are reported as mean ± SD unless otherwise indicated. AMB, anteromedial bundle; PDW, proton density weighted; PLB, posterolateral bundle; T2W, T2 weighted.
b Statistically significant difference between patients with and without laxity (P < .05; Mann-Whitney U test).
According to the Spearman correlation coefficient, the 3- and 6-month SIRs were significantly negatively correlated with age and significantly positively correlated with the Tegner score; regarding the 12-month SIR, only the PDW sequence had a significant correlation with age and the Tegner score (Table 2).
Table 2.
Spearman Correlation Coefficients for Association Between Patient Characteristics and Signal Intensity Ratio a
| r Value | ||||
|---|---|---|---|---|
| Age | Body Mass Index | Tegner Score | Time From Injury to Surgery | |
| AMB on PDW | ||||
| 3 mo | –0.296 b | –0.010 | 0.217 b | 0.079 |
| 6 mo | –0.287 b | 0.051 | 0.220 b | 0.007 |
| 12 mo | –0.142 b | 0.132 | 0.138 | <0.001 |
| AMB on T2W | ||||
| 3 mo | –0.249 b | –0.038 | 0.121 | 0.151 b |
| 6 mo | –0.211 b | 0.067 | 0.222 b | –0.043 |
| 12 mo | –0.033 | 0.102 | 0.051 | 0.069 |
| PLB on PDW | ||||
| 3 mo | –0.329 b | –0.044 | 0.209 b | 0.060 |
| 6 mo | –0.357 b | –0.024 | 0.240 b | 0.023 |
| 12 mo | –0.168 b | 0.065 | 0.150 b | –0.001 |
| PLB on T2W | ||||
| 3 mo | –0.307 b | –0.046 | 0.169 b | 0.134 |
| 6 mo | –0.214 b | –0.018 | 0.194 b | 0.006 |
| 12 mo | –0.051 | 0.025 | 0.022 | 0.050 |
a Based on the r value, the correlations were interpreted as poor to fair. AMB, anteromedial bundle; PDW, proton density weighted; PLB, posterolateral bundle; T2W, T2 weighted.
b Statistically significant (P < .05).
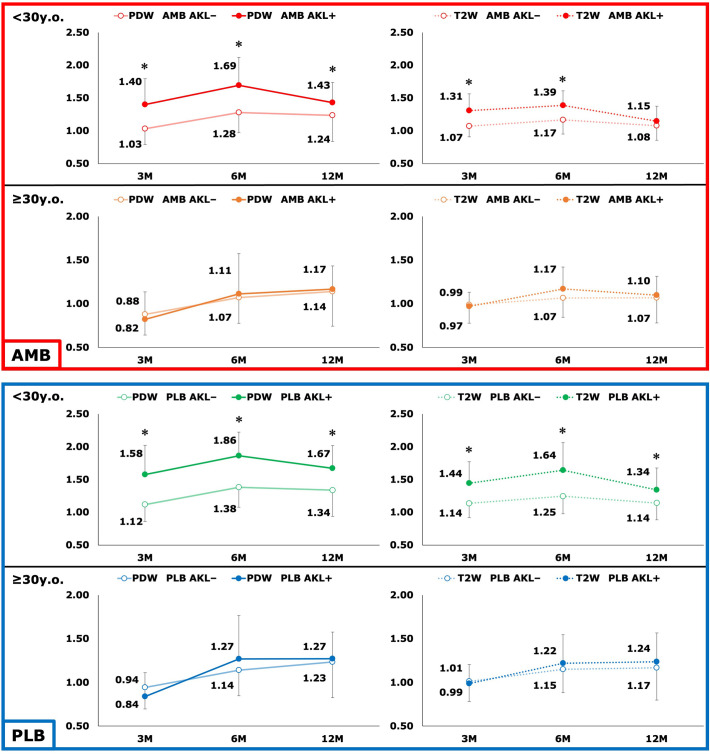
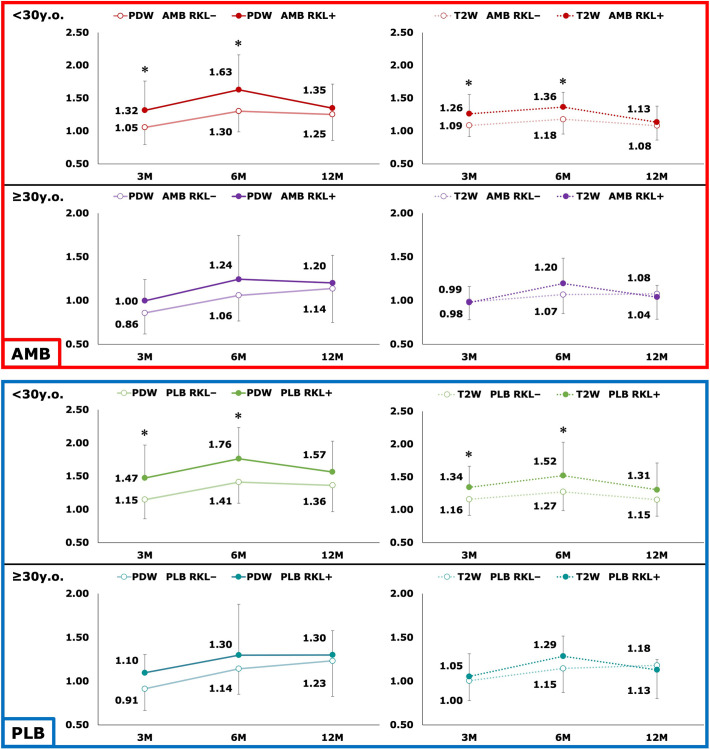
To further study the effect of age on the SIR, we divided the patients into younger (<30 years; n = 135) and older (≥30 years; n = 57) groups; 30 years was chosen because the histogram of participant age showed 2 peaks divided by this age (Appendix Figure A2). In the younger group, the SIR of patients with AKL (n = 19) was significantly higher than those without AKL (n = 116) at 3, 6, and 12 months after ACLR (Figure 1); the SIR of patients with RKL (n = 15) was significantly higher than those without RKL (n = 120) at 3 and 6 months after ACLR, whereas this relationship lost significance at 12 months after surgery (Figure 2). In the older group, the SIR of those without AKL (n = 50) and RKL (n = 52) was not associated with those having AKL (n = 7) and RKL (n = 5), respectively (Figures 1 and 2).
Figure 1.
Comparison of graft signal intensity between participants with anterior knee laxity (AKL+) and without anterior knee laxity (AKL–) based on ligament bundle, age group, and imaging sequence. *Statistically significant difference between patients with and without laxity (P < .05; Mann-Whitney U test). AMB, anteromedial bundle; PDW, proton density–weighted; PLB, posterolateral bundle; T2W, T2-weighted.
Figure 2.
Comparison of graft signal intensity between participants with rotatory knee laxity (RKL+) and without rotatory knee laxity (RKL–) based on ligament bundle, age group, and imaging sequence. *Statistically significant difference between patients with and without laxity (P < .05; Mann-Whitney U test). AMB, anteromedial bundle; PDW, proton density–weighted; PLB, posterolateral bundle; T2W, T2-weighted.
In the younger group, the ROC curve analysis indicated that the SIR within the first postoperative year was able to discriminate between patients with and without AKL at 12 months postoperatively (area under the curve, 0.676-0.848). In addition, the SIR at 3 and 6 months postoperatively was able to discriminate between patients with and without RKL (area under the curve, 0.672-0.763) (Table 3).
Table 3.
Results of Receiver Operating Characteristic Curve Analysis of Signal Intensity Ratio and Residual Knee Laxity at 1 Year Postoperatively a
| Anterior Knee Laxity | Rotatory Knee Laxity | |||
|---|---|---|---|---|
| Age <30 y (n = 19) | Age ≥30 y (n = 7) | Age <30 y (n = 15) | Age ≥30 y (n = 5) | |
| AMB on PDW | ||||
| 3 mo | 0.811 (0.691-0.932) b |
0.411 (0.177-0.645) |
0.718 (0.542-0.893) b |
0.638 (0.403-0.874) |
| 6 mo | 0.785 (0.680-0.891) b |
0.420 (0.194-0.646) |
0.688 (0.527-0.850) b |
0.558 (0.317-0.799) |
| 12 mo | 0.676 (0.561-0.791) b |
0.529 (0.327-0.730) |
0.611 (0.465-0.756) |
0.558 (0.315-0.800) |
| AMB on T2W | ||||
| 3 mo | 0.791 (0.679-0.904) b |
0.497 (0.296-0.699) |
0.695 (0.522-0.868) b |
0.477 (0.232-0.722) |
| 6 mo | 0.779 (0.671-0.888) b |
0.597 (0.397-0.798) |
0.763 (0.633-0.893) b |
0.592 (0.353-0.832) |
| 12 mo | 0.613 (0.479-0.748) | 0.563 (0.324-0.801) |
0.581 (0.415-0.746) |
0.535 (0.323-0.746) |
| PLB on PDW | ||||
| 3 mo | 0.833 (0.711-0.955) b |
0.374 (0.144-0.605) |
0.718 (0.548-0.889) b |
0.723 (0.523-0.923) |
| 6 mo | 0.848 (0.758-0.939) b |
0.509 (0.276-0.741) |
0.734 (0.587-0.881) b |
0.485 (0.220-0.750) |
| 12 mo | 0.747 (0.642-0.853) b |
0.526 (0.287-0.764) |
0.654 (0.498-0.810) |
0.588 (0.371-0.806) |
| PLB on T2W | ||||
| 3 mo | 0.804 (0.688-0.919) b |
0.460 (0.244-0.676) |
0.672 (0.495-0.849) b |
0.554 (0.288-0.820) |
| 6 mo | 0.847 (0.771-0.922) b |
0.534 (0.292-0.777) |
0.711 (0.577-0.845) b |
0.681 (0.506-0.856) |
| 12 mo | 0.686 (0.557-0.814) b |
0.577 (0.343-0.812) |
0.619 (0.448-0.791) |
0.581 (0.422-0.739) |
a Data are reported as area under the curve (95% CI). AMB, anteromedial bundle; PDW, proton density weighted; PLB, posterolateral bundle; T2W, T2 weighted.
b Statistically significant for discriminating between patients with and without residual knee laxity at 12 months postoperatively (P < .05).
The optimal SIR cutoff values were determined based on the ROC curve analysis. Logistic regression analysis demonstrated that these optimal cutoff values were significantly associated with higher odds ratios of residual knee laxity only in the younger group (Table 4). For instance, in patients aged <30 years, the cutoff values of the SIR on PDW MRI at 3 months after ACLR demonstrated the highest odds (odds ratio, 17.5 [determined by AMB] or 23.1 [determined by PLB]) of a patient’s having AKL. According to the 3-month SIR of the AMB on PDW MRI, AKL was observed in 14 of 30 patients (46.7%) with an SIR of ≥1.25; on the other hand, AKL was observed in only 5 of 105 patients (4.8%) with an SIR of <1.25 (Table 4). Consistently, according to the 3-month SIR of the PLB on PDW MRI, AKL was observed in 12 of 20 patients (60.0%) with an SIR of ≥1.50; on the other hand, AKL was observed in 7 of 115 patients (6.1%) with an SIR of <1.50 (Table 4).
Table 4.
Results of Logistic Regression Analysis for Signal Intensity Ratio Cutoff Values and ORs for Residual Knee Laxity in Patients Aged <30 Years a
| Anterior Knee Laxity | Rotatory Knee Laxity | |||||
|---|---|---|---|---|---|---|
| Cutoff | B | OR (95% CI) | Cutoff | B | OR (95% CI) | |
| AMB on PDW | ||||||
| 3 mo | 1.25 | 2.862 | 17.5 (5.5-55.2) | 1.25 | 2.303 | 10.0 (3.1-32.4) |
| 6 mo | 1.65 | 2.012 | 7.5 (2.6-21.4) | 1.65 | 1.601 | 5.0 (1.6-15.4) |
| 12 mo | 1.30 | 1.302 | 3.7 (1.3-10.4) | NS | ||
| AMB on T2W | ||||||
| 3 mo | 1.10 | 2.453 | 11.6 (2.6-52.7) | 1.13 | 1.888 | 6.6 (2.0-22.0) |
| 6 mo | 1.25 | 2.120 | 8.3 (2.6-26.9) | 1.20 | 2.277 | 9.8 (2.1-45.2) |
| 12 mo | NS | NS | ||||
| PLB on PDW | ||||||
| 3 mo | 1.50 | 3.142 | 23.1 (7.1-75.1) | 1.50 | 2.331 | 10.3 (3.2-33.4) |
| 6 mo | 1.85 | 2.921 | 18.6 (5.8-59.2) | 1.55 | 1.820 | 6.2 (1.8-20.7) |
| 12 mo | 1.35 | 2.488 | 12.0 (2.7-54.6) | 1.55 | 1.333 | 3.8 (1.3-11.5) |
| PLB on T2W | ||||||
| 3 mo | 1.35 | 2.724 | 15.2 (4.9-47.6) | 1.35 | 2.187 | 8.9 (2.8-28.7) |
| 6 mo | 1.50 | 2.446 | 11.5 (3.9-33.9) | 1.25 | 1.905 | 6.7 (1.5-31.1) |
| 12 mo | 1.35 | 1.292 | 3.6 (1.3-10.0) | 1.35 | 1.201 | 3.3 (1.1-10.1) |
a AMB, anteromedial bundle; NS, not significant; OR, odds ratio; PDW, proton density weighted; PLB, posterolateral bundle; T2W, T2 weighted. Cutoff value indicates the signal intensity ratio.
Discussion
The primary finding of this study was that higher ACL graft signal intensity on MRI in the early postoperative phase (ie, within the first year) was significantly associated with a higher prevalence of residual knee laxity at 1 year after anatomic double-bundle ACLR. Another important finding was that graft signal intensity was negatively correlated with the patient’s age. For the patients younger than 30 years, those with higher graft signal intensity demonstrated a significantly higher prevalence of residual knee laxity. Conversely, in older patients (≥30 years), higher graft signal intensity was not significantly associated with residual knee laxity.
Of the patients who underwent anatomic double-bundle ACLR using a hamstring tendon autograft, those with higher graft signal intensity in the early postoperative phase, such as at 3 or 6 months, showed a significantly higher prevalence of residual knee laxity at 1 year after surgery. With regard to the previous data, Hakozaki et al 14 reported that, using a similar outcome of residual knee laxity as that of the current study, higher graft signals on PDW and T2W MRI at 1 year after anatomic double-bundle ACLR were associated with residual AKL defined by a ≥4-mm side-to-side difference using the KT-2000 arthrometer. Additionally, a more recent clinical study reported by Putnis et al 33 consistently demonstrated that patients with higher graft signal intensity around the femoral tunnel aperture exhibited a higher risk of graft reruptures after single-bundle ACLR. The current study significantly supports these previous data in which higher graft signal intensity is associated with greater laxity derived from intrinsic damage to the reconstructed ACL graft after reconstruction. 1,8,18,25,32,33 Therefore, knee surgeons should pay specific attention to the risk of not only graft reruptures but also residual knee laxity in patients who exhibit higher graft signal intensity on MRI during the first postoperative year.
Moreover, regarding the clinical relevance of ACL graft signal intensity, the current data provide insight on 2 important aspects that should be considered. First, the ability of 3- or 6-month postoperative SIRs to detect residual knee laxity was superior to that of 12-month SIRs (Tables 3 and 4). At this point, few previously published studies support the current finding that earlier postoperative graft signal intensity (ie, within 6 months after ACLR) is superior in detecting future residual knee laxity. It should be noted that several MRI-based studies have reported that the mean graft signal intensity at 3 months after ACLR was lower than that at 6 months because the tissue reparative and revascularization processes are in progress. Thereafter, the mean graft signal intensity is likely to peak around 6 months after reconstruction and then gradually decline and return to near normal levels at 2 years after surgery, corresponding to the healing process of the reconstructed graft. 29,30,46 Based on the initial process of MRI graft signal intensity, an earlier postoperative period such as 3 months is less likely to be affected by the healing process of the graft. Therefore, a higher graft signal in the early postoperative period can be a more specific and appropriate finding to indicate graft deterioration, which may predict residual knee laxity.
Another important finding is that patient age was associated with graft signal intensity; graft signal intensity of older patients was lower than that of younger patients. Notably, a recent study by Putnis et al 33 demonstrated that patient age was negatively correlated with graft signal intensity on MRI. Considering the current and previous data, older patients are less likely to experience similar revascularity bioactivity or tissue reparative reactions compared with younger patients. 29,30,33,46 Moreover, older patients are generally less active and less likely to stress their reconstructed ACL graft compared with younger patients. Knee surgeons should therefore consider patient age when evaluating graft signal intensity after ACLR; older patients attenuate the pathological significance of graft signal intensity with respect to predicting residual knee laxity.
The current data indicated the clinical relevance of postoperative MRI graft signal intensity that could be useful to validate the prognosis of patients who undergo anatomic ACLR. In particular, younger patients with higher physical activity levels would benefit from undergoing follow-up MRI between 3 and 6 months after ACLR to determine the risk of developing residual knee laxity. If the graft signal is far higher than its mean value, surgeons should pay attention to the risk of residual knee laxity and alter postoperative treatment to preserve the graft, for instance, by delaying the progression of rehabilitation. Future MRI studies are required to further explain the mechanisms underlying graft signal intensity after anatomic ACLR.
As for the concept of double-bundle ACLR, previous biomechanical studies have put an emphasis on the different functions of the AMB and PLB in restraining anterior laxity and controlling tibial axial rotation laxity. 2,13,34,36 The AMB has been reported to have a more specific biomechanical behavior to stabilize anterior tibial translation with the knee flexed past 30°. 2,36 On the other hand, the PLB has been reported to stabilize anterior tibial translation when the knee is at full extension or 15° of flexion, 2,36 and the oblique orientation of the PLB in the coronal plane allows greater ability to stabilize tibial rotatory laxity than a more vertical AMB. 2,13,34 Notably, the current data conflict with these previous studies. In other words, the SIRs of the AMB could predict the development of both AKL and RKL; consistently, that of the PLB could predict the development of both AKL and RKL. However, previous studies have reported that both the AMB and PLB are important for stabilizing anterior and rotatory tibial laxity relative to the femur after ACLR. In fact, 2 clinical studies using navigation systems clarified that isolated fixation of either the AMB or PLB reduced anterior tibial translation against both anterior tibial loading and tibial internal rotation at the same time. 22,24 The findings of the current clinical study support the latter 2 navigation system studies 22,24 ; for patients who undergo ACLR, both the AMB and the PLB have an important role to stabilize AKL and RKL.
Limitations
The current study had several limitations. First, the graft that was harvested in the current study was a hamstring tendon autograft. Therefore, the current findings would not be applicable to ACL-injured patients reconstructed with an allograft or bone–patellar tendon–bone graft. Second, the follow-up period was relatively short, up to 12 months after surgery, and further relationships remain unclear. In addition, we did not include laxity data at 3 and 6 months after ACLR. Third, RKL was evaluated using IKDC grading. Quantitative methods 16,26 would clarify the detailed mechanism between RKL and graft signal intensity. Fourth, the current study did not obtain an enhanced MRI sequence, which would be more effective for evaluating the revascularization or tissue reparative process. 10,29,30 Fifth, we switched from a fixed-loop cortical suspension device to an adjustable-loop device to fix the proximal end of the hamstring tendon graft. This may have affected the process of graft healing and its following alteration of the graft signal on MRI. Finally, multiple factors are associated with the development of residual laxity after ACLR, the most important being nonanatomic tunnel placement. 5,19,48 Other factors include a concomitant meniscal tear with the ACL injury 11,23,27,39,43 as well as intrinsic joint laxity before the ACL injury. 40,47 Future large-sample cohort studies that include graft signal intensity data would reveal the interrelationship among these multiple factors in the development of residual laxity after ACLR.
Conclusion
The current study elucidated the relationship between graft signal intensity on MRI and residual knee laxity after anatomic double-bundle ACLR. The graft signal was negatively correlated with patient age. In patients younger than 30 years, higher graft signal intensity in the early postoperative period (between 3 and 6 months after ACLR) was associated with a higher prevalence of residual knee laxity at 1 year postoperatively.
Acknowledgment
The authors thank Editage (https://www.editage.jp) for its English-language editing.
APPENDIX
Appendix Figure A1.
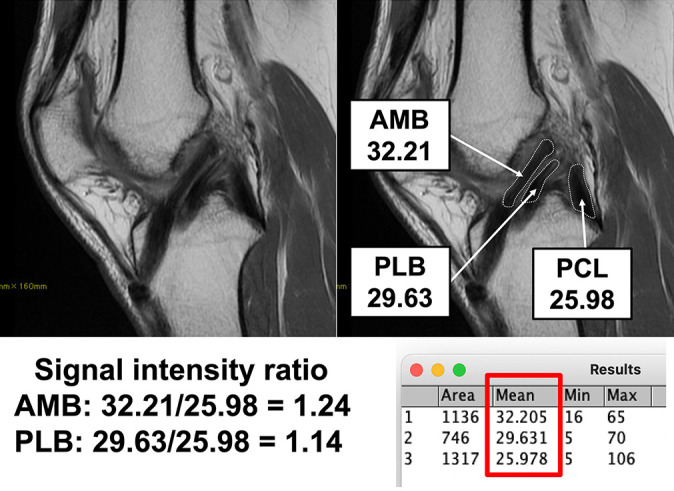
Example of evaluating graft signal intensity on magnetic resonance imaging. The signal intensity ratio was calculated as (AMB intensity or PLB intensity)/PCL intensity. AMB, anteromedial bundle; PCL, posterior cruciate ligament; PLB, posterolateral bundle.
Appendix Figure A2.
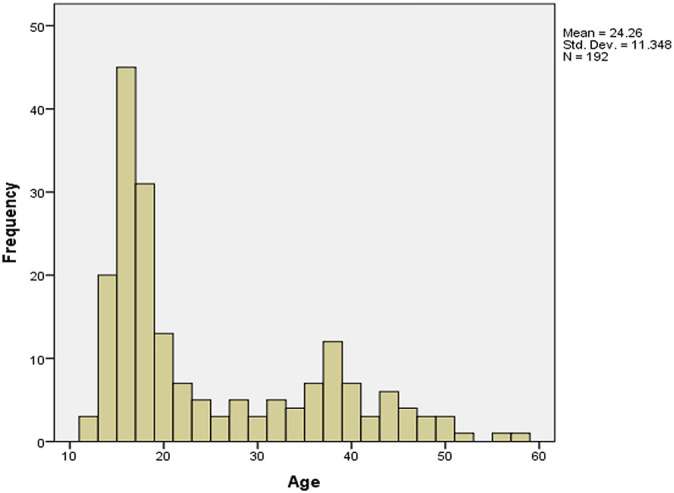
Histogram of patient age.
Footnotes
Final revision submitted March 20, 2022; accepted March 31, 2022.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Hirosaki University Graduate School of Medicine and Hirosaki University Hospital.
References
- 1. Ahn JH, Jeong HJ, Lee YS, Park JH, Lee JH, Ko TS. Graft bending angle is correlated with femoral intraosseous graft signal intensity in anterior cruciate ligament reconstruction using the outside-in technique. Knee. 2016;23(4):666–673. [DOI] [PubMed] [Google Scholar]
- 2. Amis AA. The functions of the fibre bundles of the anterior cruciate ligament in anterior drawer, rotational laxity and the pivot shift. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):613–620. [DOI] [PubMed] [Google Scholar]
- 3. Baba R, Kondo E, Iwasaki K, et al. Impact of surgical timing on clinical outcomes in anatomic double-bundle anterior cruciate ligament reconstruction using hamstring tendon autografts. Orthop J Sports Med. 2019;7(11):2325967119880553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrow AE, Pilia M, Guda T, Kadrmas WR, Burns TC. Femoral suspension devices for anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(2):343–349. [DOI] [PubMed] [Google Scholar]
- 5. Bedi A, Maak T, Musahl V, et al. Effect of tibial tunnel position on stability of the knee after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(2):366–373. [DOI] [PubMed] [Google Scholar]
- 6. Büyükdoğan K, Laidlaw MS, Fox MA, Kew ME, Miller MD. Effect of tibial tunnel placement using the lateral meniscus as a landmark on clinical outcomes of anatomic single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2021;49(6):1451–1459. [DOI] [PubMed] [Google Scholar]
- 7. Byrne KJ, Hughes JD, Gibbs C, et al. Non-anatomic tunnel position increases the risk of revision anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2022;30(4):1388–1395. doi: 10.1007/s00167-021-06607-7 [DOI] [PubMed] [Google Scholar]
- 8. Chen L, Wu Y, Lin G, et al. Graft bending angle affects allograft tendon maturity early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3048–3054. [DOI] [PubMed] [Google Scholar]
- 9. Chiba D, Yamamoto Y, Kimura Y, Sasaki S, Tsuda E, Ishibashi Y. Combination of anterior tibial and femoral tunnels makes the signal intensity of antero-medial graft higher in double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2021;29(3):783–792. [DOI] [PubMed] [Google Scholar]
- 10. Covey DC, Sandoval KE, Riffenburgh RH. Contrast-enhanced MRI evaluation of bone–patellar tendon–bone and hamstring ACL autograft healing in humans: a prospective randomized study. Orthop J Sports Med. 2018;6(10):2325967118800298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cristiani R, Rönnblad E, Engström B, Forssblad M, Stålman A. Medial meniscus resection increases and medial meniscus repair preserves anterior knee laxity: a cohort study of 4497 patients with primary anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(2):357–362. [DOI] [PubMed] [Google Scholar]
- 12. Diermeier T, Meredith SJ, Irrgang JJ, et al. Patient-reported and quantitative outcomes of anatomic anterior cruciate ligament reconstruction with hamstring tendon autografts. Orthop J Sports Med. 2020;8(7):2325967120926159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22(1):85–89. [DOI] [PubMed] [Google Scholar]
- 14. Hakozaki A, Niki Y, Enomoto H, Toyama Y, Suda Y. Clinical significance of T2*-weighted gradient-echo MRI to monitor graft maturation over one year after anatomic double-bundle anterior cruciate ligament reconstruction: a comparative study with proton density-weighted MRI. Knee. 2015;22(1):4–10. [DOI] [PubMed] [Google Scholar]
- 15. Hefti E, Müller W, Jakob RP, Stäubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226–234. [DOI] [PubMed] [Google Scholar]
- 16. Hoshino Y, Araujo P, Ahldén M, et al. Quantitative evaluation of the pivot shift by image analysis using the iPad. Knee Surg Sports Traumatol Arthrosc. 2013;21(4):975–980. [DOI] [PubMed] [Google Scholar]
- 17. Houck DA, Kraeutler MJ, McCarty EC, Bravman JT. Fixed- versus adjustable-loop femoral cortical suspension devices for anterior cruciate ligament reconstruction: a systematic review and meta-analysis of biomechanical studies. Orthop J Sports Med. 2018;6(10):2325967118801762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Howell SM, Berns GS, Farley TE.Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiology. 1991;179(3):639–643. [DOI] [PubMed] [Google Scholar]
- 19. Hussein M, van Eck CF, Cretnik A, Dinevski D, Fu FH. Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(3):512–520. [DOI] [PubMed] [Google Scholar]
- 20. Iriuchishima T, Suruga M, Yahagi Y, Iwama G, Aizawa S. Morphology of the resident’s ridge, and the cortical thickness in the lateral wall of the femoral intercondylar notch correlate with the morphological variations of the Blumensaat’s line. Knee Surg Sports Traumatol Arthrosc. 2020;28(8):2668–2674. [DOI] [PubMed] [Google Scholar]
- 21. Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6(2):107–114. [DOI] [PubMed] [Google Scholar]
- 22. Ishibashi Y, Tsuda E, Yamamoto Y, Tsukada H, Toh S. Navigation evaluation of the pivot-shift phenomenon during double-bundle anterior cruciate ligament reconstruction: is the posterolateral bundle more important? Arthroscopy. 2009;25(5):488–495. [DOI] [PubMed] [Google Scholar]
- 23. Katakura M, Horie M, Watanabe T, et al. Effect of meniscus repair on pivot-shift during anterior cruciate ligament reconstruction: objective evaluation using triaxial accelerometer. Knee. 2019;26(1):124–131. [DOI] [PubMed] [Google Scholar]
- 24. Koga H, Muneta T, Yagishita K, Ju YJ, Sekiya I. The effect of graft fixation angles on anteroposterior and rotational knee laxity in double-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(3):615–623. [DOI] [PubMed] [Google Scholar]
- 25. Lee SM, Yoon KH, Lee SH, Hur D. The relationship between ACL femoral tunnel position and postoperative MRI signal intensity. J Bone Joint Surg Am. 2017;99(5):379–387. [DOI] [PubMed] [Google Scholar]
- 26. Lopomo N, Signorelli C, Bonanzinga T, Muccioli GMM, Visani A, Zaffagnini S. Quantitative assessment of pivot-shift using inertial sensors. Knee Surg Sports Traumatol Arthrosc. 2012;20(4):713–717. [DOI] [PubMed] [Google Scholar]
- 27. Mouton C, Magosch A, Pape D, Hoffmann A, Nührenbörger C, Seil R. Ramp lesions of the medial meniscus are associated with a higher grade of dynamic rotatory laxity in ACL-injured patients in comparison to patients with an isolated injury. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1023–1028. [DOI] [PubMed] [Google Scholar]
- 28. Nakamae A, Adachi N, Deie M, et al. Risk factors for progression of articular cartilage damage after anatomical anterior cruciate ligament reconstruction. Bone Joint J. 2018;100-B(3):285–293. [DOI] [PubMed] [Google Scholar]
- 29. Ntoulia A, Papadopoulou F, Ristanis S, Argyropoulou M, Georgoulis AD. Revascularization process of the bone–patellar tendon–bone autograft evaluated by contrast-enhanced magnetic resonance imaging 6 and 12 months after anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(7):1478–1486. [DOI] [PubMed] [Google Scholar]
- 30. Ntoulia A, Papadopoulou F, Zampeli F, Ristanis S, Argyropoulou M, Georgoulis A. Evaluation with contrast-enhanced magnetic resonance imaging of the anterior cruciate ligament graft during its healing process: a two-year prospective study. Skeletal Radiol. 2013;42(4):541–552. [DOI] [PubMed] [Google Scholar]
- 31. Nukuto K, Hoshino Y, Yamamoto T, et al. Anatomic double-bundle anterior cruciate ligament reconstruction could not achieve sufficient control of pivot-shift when accompanying tibial tunnel coalition. Knee Surg Sports Traumatol Arthrosc. 2021;29(11):3743–3750. [DOI] [PubMed] [Google Scholar]
- 32. Oshima T, Putnis S, Grasso S, Klasan A, Parker DA. Graft size and orientation within the femoral notch affect graft healing at 1 year after anterior cruciate ligament reconstruction. Am J Sports Med. 2019;48(1):99–108. [DOI] [PubMed] [Google Scholar]
- 33. Putnis SE, Oshima T, Klasan A, et al. Magnetic resonance imaging 1 year after hamstring autograft anterior cruciate ligament reconstruction can identify those at higher risk of graft failure: an analysis of 250 cases. Am J Sports Med. 2021;49(5):1270–1278. [DOI] [PubMed] [Google Scholar]
- 34. Robinson J, Carrat L, Granchi C, Colombet P. Influence of anterior cruciate ligament bundles on knee kinematics. Am J Sports Med. 2007;35(12):2006–2013. [DOI] [PubMed] [Google Scholar]
- 35. Rothrauff BB, Jorge A, de Sa D, Kay J, Fu FH, Musahl V. Anatomic ACL reconstruction reduces risk of post-traumatic osteoarthritis: a systematic review with minimum 10-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2020;28(4):1072–1084. [DOI] [PubMed] [Google Scholar]
- 36. Sakane M, Fox RJ, Glen SLW, Livesay A, Li G, Fu FH. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15(2):285–293. [DOI] [PubMed] [Google Scholar]
- 37. Sasaki S, Tsuda E, Hiraga Y, et al. Prospective randomized study of objective and subjective clinical results between double-bundle and single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(4):855–864. [DOI] [PubMed] [Google Scholar]
- 38. Shimodaira H, Tensho K, Akaoka Y, Takanashi S, Kato H, Saito N. Tibial tunnel positioning technique using bony/anatomical landmarks in anatomical anterior cruciate ligament reconstruction. Arthrosc Tech. 2017;6(1):e49–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shybut TB, Vega CE, Haddad J, et al. Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament–deficient knee. Am J Sports Med. 2015;43(4):905–911. [DOI] [PubMed] [Google Scholar]
- 40. Song GY, Zhang H, Zhang J, Zhang ZJ, Zheng T, Feng H. Excessive preoperative anterior tibial subluxation in extension is associated with inferior knee stability after anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2019;48(3):573–580. [DOI] [PubMed] [Google Scholar]
- 41. Sundemo D, Mårtensson J, Senorski EH, et al. No correlation between femoral tunnel orientation and clinical outcome at long-term follow-up after non-anatomic anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(11):3400–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tachibana Y, Shino K, Mae T, Iuchi R, Take Y, Nakagawa S. Anatomical rectangular tunnels identified with the arthroscopic landmarks result in excellent outcomes in ACL reconstruction with a BTB graft. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2680–2690. [DOI] [PubMed] [Google Scholar]
- 43. Tang X, Marshall B, Wang JH, et al. Lateral meniscal posterior root repair with anterior cruciate ligament reconstruction better restores knee stability. Am J Sports Med. 2019;47(1):59–65. [DOI] [PubMed] [Google Scholar]
- 44. Tashiro Y, Gale T, Sundaram V, et al. The graft bending angle can affect early graft healing after anterior cruciate ligament reconstruction: in vivo analysis with 2 years’ follow-up. Am J Sports Med. 2017;45(8):1829–1836. [DOI] [PubMed] [Google Scholar]
- 45. Uchida R, Shino K, Iuchi R, et al. Anatomical triple bundle ACL reconstructions with hamstring tendon autografts: tunnel locations and two-year clinical outcomes. Arthroscopy. 2021;37(9):2891–2900. [DOI] [PubMed] [Google Scholar]
- 46. Weiler A, Peters G, Mäurer J, Unterhauser FN, Südkamp NP. Biomechanical properties and vascularity of an anterior cruciate ligament graft can be predicted by contrast-enhanced magnetic resonance imaging. Am J Sports Med. 2001;29(6):751–761. [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto Y, Tsuda E, Maeda S, et al. Greater laxity in the anterior cruciate ligament–injured knee carries a higher risk of postreconstruction pivot shift: intraoperative measurements with a navigation system. Am J Sports Med. 2018;46(12):2859–2864. [DOI] [PubMed] [Google Scholar]
- 48. Zantop T, Wellmann M, Fu FH, Petersen W. Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2008;36(1):65–72. [DOI] [PubMed] [Google Scholar]