Abstract
Background
Serotonin toxicity (also referred to as serotonin syndrome) results from medications that affect the neurotransmitter serotonin. The antibiotic linezolid and the opioids methadone and buprenorphine are all reported to cause serotonin toxicity, but the degree of risk with use of linezolid in combination with methadone or buprenorphine is unknown.
Methods
We conducted a retrospective cross-sectional analysis of adult patients hospitalized from November 2015 to October 2019 who were administered linezolid in combination with methadone and/or buprenorphine within 24 hours and a subgroup that received the combination for ≥3 days. Cases of serotonin toxicity were identified from the clinical notes in the electronic medical record and were classified as possible or definite based on the clinical record. The Hunter diagnostic criteria were retrospectively applied.
Results
There were 494 encounters in which linezolid was administered concurrently with methadone and buprenorphine. The mean patient age was 42.5 years, and 52.4% of encounters were of female patients. The mean duration of concurrent administration was 1.9 days. There were 106 encounters with a duration of concurrent administration ≥3 days (mean, 5.4 days). Two cases of possible serotonin toxicity and 0 cases of definite serotonin toxicity occurred; neither possible case met the Hunter criteria from the available information. Possible cases occurred in 0.40% of all encounters and 1.89% of encounters with ≥3 days of overlap (upper 1-sided 95% CI, 0.87% and 4.06%).
Conclusions
Serotonin toxicity occurring during the administration of linezolid in combination with methadone and/or buprenorphine occurred rarely among 494 hospital encounters, including 106 encounters with ≥3 days of overlap. Limitations include potential missed diagnoses of serotonin toxicity and short durations of overlap. Further study evaluating the short-term risk of this combination is needed.
Keywords: linezolid, methadone, buprenorphine, serotonin, opioid
Serotonin toxicity (also known as “serotonin syndrome”) is a rare but potentially fatal adverse effect of medications that increase the effect of the neurotransmitter serotonin. It is characterized by neuromuscular excitation (tremor, hyperreflexia, or clonus), autonomic stimulation (including pyrexia), and encephalopathy. A variety of medications can directly or indirectly affect serotonin levels and receptors, including certain antidepressants, opioids, antibiotics, and inhibitors of cytochrome p450 enzymes (hereafter all referred to as serotonergic medications). Diagnosis relies on clinical assessment; multiple proposed clinical criteria exist, but there are no confirmatory tests. The Hunter diagnostic criteria require multiple typical signs and symptoms in the presence of serotonergic medications [1]. The risk of serotonin toxicity may be increased when multiple serotonergic medications are used concurrently. Prior studies have correlated serotonergic symptoms with concentrations of causative medications [1].
People with opioid use disorder (OUD) may be at particular risk for serotonin toxicity due to treatment with multiple serotonergic medications, including the opioids methadone (MET) and buprenorphine (BUP), which are medications for OUD (MOUD). People who inject opioids frequently develop skin and soft tissue infections (SSTIs), bacteremia, endocarditis, and osteomyelitis due to nonsterile injection techniques [2]. The antibiotic linezolid (LZD) is a useful option for the treatment of infections in people who use drugs due to excellent coverage of methicillin-resistant Staphylococcus aureus and Streptococcus species and high oral bioavailability. Oral linezolid can be used for outpatient treatment of uncomplicated SSTIs or as step-down therapy for treatment of serious infectious without parenteral antibiotics [3–5]. In recent years, however, the US Food and Drug Administration (FDA) has warned of serotonin toxicity due to LZD and opioids, which may lead to avoidance of a clinically useful antibiotic [6, 7].
It is unknown how commonly serotonin toxicity occurs when LZD is used in combination with MET or BUP (hereafter referred to as LZD + MET or LZD + BUP). We conducted a cross-sectional analysis of the incidence of and risk factors for serotonin toxicity among hospitalized adults treated with LZD + MET or LZD + BUP.
METHODS
To find eligible hospital encounters, pharmacy records were queried for all medication administrations of LZD, MET, and BUP at the University of Maryland Medical Center in Baltimore, Maryland, from November 2015 to October 2019. Encounters were included if an order for LZD was administered within 24 hours of an order for BUP or MET. The electronic medical record (Epic, Verona, WI, USA) of each encounter was manually searched (by E.C.T.) using the free-text search function for the term “serotonin.” This function returns matching results in medications, problem lists, and clinical notes. It does not return visit diagnoses. Encounters were selected for further review if the clinical notes or hospital problem lists mentioned any level of concern for serotonin toxicity. The medical record of these encounters was closely reviewed, and each encounter was further classified by 2 physician authors (E.C.T. and S.A.S.). When there was no concern in the clinical record for serotonin toxicity or when the suspected episode of serotonin toxicity occurred before the coadministration of LZD + MET or LZD + BUP, the encounter was classified as a “noncase.” When serotonin toxicity was included as part of the differential diagnosis for a patient’s signs or symptoms but was not confirmed or definitively diagnosed by the clinical team, the encounter was classified as a “possible case.” “Definite cases” were defined as confirmed or final diagnosis of serotonin toxicity in the clinical record. The same 2 authors also applied the Hunter diagnostic criteria for serotonin toxicity to the possible and definite cases as permitted by the available information. The Hunter criteria were not met if the information in the clinical record did not allow definitive classification as serotonin toxicity. Disagreements were settled by consensus.
Data, including age, sex, duration of hospitalization, and number of unique encounters per patient, were collected or calculated from the pharmacy order records. The average doses of MET and BUP orders administered during or within 24 hours of LZD were calculated per encounter. The dose interval was not standardized, and thus the dose does not reflect a particular interval (such as every 24 hours). The longest duration of concurrent administration of LZD + MET or LZD + BUP during each encounter was calculated in days; if the orders did not overlap but occurred within 24 hours, the overlap duration was reported as 0 days. The pharmacy records of each hospital encounter were queried for a list of other serotonergic medications (Appendix A) [8]. The number of serotonergic medications administered during each encounter was reported, regardless of the timing in relation to LZD, BUP, or MET.
We hypothesized that the risk of serotonin toxicity would be increased by a higher number of other serotonergic medications, higher doses of MET and BUP, and a longer period of overlap of LZD + MET or LZD + BUP. We measured these risk factors for noncases, possible cases, and definite cases. We used the interquartile range (IQR) method of outlier detection to determine if the cases were outliers compared with noncases. The upper bound for the noncases was calculated as [3rd quartile + IQR * 1.5].
Data processing and graphing were conducted in Access (Microsoft 365), Excel (Microsoft 365), and GraphPad (Prism, version 9.3.1). This study was approved by the institutional review board of the University of Maryland, Baltimore, School of Medicine, and the requirement for informed consent was waived.
RESULTS
Three-hundred eighty-three patients in 494 unique hospital encounters were administered LZD + MET and/or LZD + BUP within 24 hours (Table 1). In 52% of the encounters, the patient’s sex was recorded as female, and the mean patient age was 42.5 years. Most encounters involved MET (83%); a minority involved BUP (16%) or both MET and BUP (1%). The mean number of encounters per patient was 1.3, and the encounters averaged 15.7 days in duration. In a subgroup of the 106 encounters with prolonged (≥3 days) overlap of LZD + MET or LZD + BUP, the demographics were similar, except for longer hospital duration.
Table 1.
Demographics
| All Cases | Cases With ≥3 Days of Overlap | |
|---|---|---|
| No. | 494 | 106 |
| Female, % | 52.4 | 49.1 |
| Age, mean, y | 42.5 | 43.8 |
| Methadone only, No. (%) | 411 (83) | 90 (85) |
| Buprenorphine only, No. (%) | 78 (16) | 15 (14) |
| Both, No. (%) | 5 (1%) | 1 (1%) |
| Encounter duration, d | 15.7 | 33.6 |
Two encounters met criteria for possible cases; both received LZD + MET for ≥3 days (Table 2). No patients met criteria for a definite case, and no cases were found in the combination of LZD + BUP. Possible cases of serotonin toxicity occurred in 0.40% of all encounters (upper 1-sided 95% CI, 0.87%) and 1.89% of encounters with ≥3 days of overlap (upper 1-sided 95% CI, 4.06%).
Table 2.
Distribution of Cases by Methadone or Buprenorphine and Duration Overlap With Linezolid
| Overlap Duration <3 Days | Overlap Duration ≥3 Days | |||||
|---|---|---|---|---|---|---|
| Case | No | Possible | Definite | No | Possible | Definite |
| Methadone | 321 | 0 | 0 | 88 | 2 | 0 |
| Buprenorphine | 63 | 0 | 0 | 15 | 0 | 0 |
| Both | 4 | 0 | 0 | 1 | 0 | 0 |
In the first possible case, a 28-year-old man developed tachycardia, hyperreflexia, and ankle clonus while taking LZD and trazodone. He was receiving MET 10 mg by mouth 3 times a day, which overlapped with LZD for 48.5 days and was discontinued 7 days before the episode of possible serotonin toxicity. His symptoms resolved after cessation of trazodone. It was not noted if the clonus was spontaneous or inducible, so the Hunter criteria were not met. In the second possible case, a 56-year-old man developed encephalopathy and tremor while taking LZD, MET 30 mg by mouth 3 times a day, and bupropion. The patient’s symptoms resolved after discontinuation of the LZD and MET. In the episode, hyperreflexia was not described in addition to tremor, so the Hunter criteria were not met.
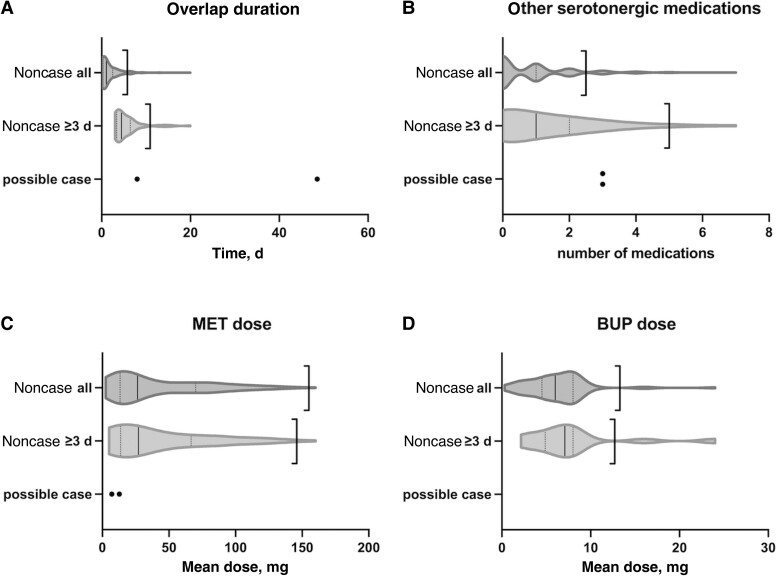
The 2 possible cases had long durations of LZD + MET overlap and frequent use of other serotonergic medications compared with noncases (Figure 1). Possible case #1 featured 48.5 days of overlap, above both the upper bounds and the longest duration of overlap in all 494 encounters. Possible case #2 featured 8.0 days of overlap, above the mean (1.9 days) and upper bound (5.8 days) for all noncases but below the upper bound for noncases with ≥3 days of overlap (10.9 days). The mean overlap of noncases with ≥3 days of overlap was 5.4 days. Both possible cases were administered 3 other serotonergic medications during the hospital encounter, which is above the upper bound for all noncases (2.5 medications) but below the upper bound for noncases with ≥3 days of overlap (5.0 medications). The noncases were administered a mean of 0.9 other serotonergic medications overall, and those with ≥3 days of overlap were administered a mean of 1.4 other serotonergic medications. The average doses of MET orders in the 2 possible cases (12.7 mg and 7.0 mg) were below the means for all noncases (41.0 mg) and noncases with ≥3 days of overlap (42.4 mg) (Figure 1C). The mean and upper bounds of doses of BUP orders were 7.9 mg and 13.3 mg for all noncases and 7.9 mg and 12.7 mg for noncases with ≥3 days of overlap.
Figure 1.
Distributions of noncase encounters of any overlap duration (noncase all) and noncase encounters with ≥3 days of overlap (noncase ≥3 d) as violin plots; solid vertical lines show the median; dotted vertical lines show the first (Q1) and third quartiles (Q3). The left-facing bracket represents the upper bound [Q3 + (Q3–Q1) * 1.5]. Abbreviations: BUP, buprenorphine; MET, methadone.
DISCUSSION
In our analysis of hospitalized patients administered LZD + MET or LZD + BUP, no definitive cases of serotonin toxicity occurred among 494 encounters, and 2 possible cases of serotonin toxicity were identified. Among our subgroup of prolonged coadministration of LZD + MET and/or LZD + BUP, the upper 95% CI for the incidence of possible serotonin toxicity was just above 4%. Both possible cases received prolonged courses of LZD + MET and received 3 other serotonergic medications during the hospitalization. The 2 possible cases featured high numbers of other serotonergic medications and longer periods of co-administration of LZD + MET or LZD + BUP compared with the noncases.
The findings from our cross-sectional analysis are supported by a large cohort of almost 500 hospitalized patients receiving LZD and MET and/or BUP. The study is further strengthened by a methodology to detect suspected but unconfirmed cases of serotonin toxicity. It also has several limitations. The clinical diagnosis of serotonin syndrome was not standardized in the clinical record. We attempted to mitigate this by retrospectively applying the Hunter diagnostic criteria to the possible cases, an approach that has been used previously in studies attempting to retrospectively diagnose serotonin toxicity [9, 10]. As in other studies, missing clinical data often made retrospective application of these criteria inconclusive. The Hunter criteria are more sensitive and specific than the previously proposed Sternbach criteria, although they were validated only in a cohort suffering overdoses of serotonergic medications, rather than toxicity on appropriately dosed medications [1]. Our classification of cases as probable or definite was subjective, although we mitigated this by having 2 physician authors independently adjudicate cases. Our methodology may have also missed patients for whom the clinical team did not suspect or document suspicion of serotonin toxicity.
The duration of concurrent administration in our study was often shorter than a typical course of LZD. Due to the nature of our data set, we do not know if patients were discharged on linezolid, so the overlap duration reported here is likely an underestimate. If patients developed serotonin toxicity after discharge and were readmitted within the University of Maryland Medical System, the case would have been detected by our methods. In our study, only 2 possible cases were identified, which precluded statistical comparison of the risk factors among possible cases with noncases. In particular, the low number of identified cases and the short duration of medication overlap limit the applicability of these findings to clinical care.
Our findings are supported by other data that suggest that serotonin toxicity due to LZD is rare. A reanalysis of the phase III and IV randomized trials of LZD and comparator antibiotics included 2208 patients who received LZD and another serotonergic medication [11]. Serotonergic toxicity was not reported in any patient, and only 0.14% of the patients were diagnosed by retrospective application of the Hunter criteria, which was not significantly increased relative to the comparator antibiotic group. Retrospective cohort studies of hospitalized patients taking LZD and selective serotonin or serotonin-norepinephrine reuptake inhibitors, which would be expected to be more serotonergic than BUP or MET, have reported incidences between 1.1% and 4.2% [9, 12].
Analyses of medication toxicity reports have also shown that serotonin toxicity is rarely caused by either MET or BUP. A review of 1641 cases of serotonin toxicity associated with prescription of opioids in the World Health Organization Global Database of Individual Case Safety Reports found that only 5.6% of those cases involved MET and only 1.2% involved BUP [13]. MET and BUP are also rarely reported to cause serotonin toxicity in combination with LZD. A review of 669 reports of serotonin toxicity in the FDA Adverse Event Reporting System found 27 cases of serotonin toxicity involving LZD + MET and 1 case involving LZD + BUP [14]. In our literature search, we found 2 published case reports of serotonin toxicity resulting from LZD + MET [15, 16]. Outcomes of cases of serotonin toxicity due to these medications have not been systemically reported. We are unaware of any case reports of serotonin toxicity resulting from LZD + BUP. MET may be more likely to cause serotonin toxicity than BUP due to the more frequent use of MET compared with BUP. The difference may also be due to how the opioids interact with neurotransmitter receptors. In vitro pharmacokinetic studies have demonstrated that MET inhibits serotonin uptake and binds the 5-HT receptors, but BUP does not [13, 17].
MOUDs are highly effective for treating OUD, including reducing opioid use, overdose, and death [18]. Initiation during hospitalization increases retention in addiction care and reduces future opioid use [19], reduces early and unplanned discharge, and increases completion of antibiotics [20]. Despite these benefits, MOUD is underutilized in hospitalized patients who may require antibiotics for infectious complications of drug use [21, 22]. This is likely in part due concerns about drug–drug interactions between MOUD and other medications, including LZD.
Despite the rarity of serotonin toxicity resulting from linezolid with other serotonergic medications, the potential for toxicity is a concern among infectious disease specialists. A survey of members of the Infectious Diseases Society of America found that 23% of the 207 respondents who had prescribed linezolid and a selective serotonin reuptake inhibitor reported observing serotonin toxicity [23]. It is unclear if concern about serotonin toxicity with LZD among infectious disease specialists is proportionate to the true risk and if it unnecessarily limits use of LZD.
This study supports the safety of LZD + MET or LZD + BUP for short durations (<3 days), such oral LZD if a patient temporarily loses intravenous access or to finish a course after initial intravenous therapy. Future studies should investigate the effects of overlap duration of LZD and MOUD and the number of other serotonergic medications on the incidence of serotonin toxicity. Systematically reported outcomes of serotonin toxicity would help clinicians assess the true risks from these medications. Studies performed in self-contained medical systems with better data on discharge medications and outpatient visits may be useful to measure the effect of outpatient treatment with LZD and MOUD.
CONCLUSIONS
LZD and MOUD may be beneficial in people with OUD and infections. We found only 2 possible cases and no definite cases of serotonin toxicity among 494 encounters where LZD was administered with MET or BUP. Possible serotonin toxicity occurred only in patients administered LZD + MET for more than 8 days, and always in the setting of multiple other serotoninergic medications.
Acknowledgments
Financial support . None reported.
Potential conflicts of interest. The authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. E.C.T.: conceptualization, data curation, writing – original draft, review, and editing; E.L.H.: conceptualization, writing – review and editing; S.A.S.: conceptualization, writing – review and editing.
Patient consent . This study was approved by the University of Maryland Baltimore Institutional Review Board, with a waiver of individual patient consent.
Data . Data not publicly available.
Appendix A. Serotonergic Medications
amitriptyline
amoxapine
bupropion
buspirone
citalopram
clomipramine
cocaine
cyclobenzaprine
desipramine
desvenlafaxine
dexfenfluramine
dexmethylphenidate
dextroamphetamine
dextromethorphan
dihydroergotamine
dolasetron
doxepin
duloxetine
eletriptan
ergotamine
escitalopram
fenfluramine
fentanyl
fluoxetine
fluvoxamine
frovatriptan
granisetron
imipramine
isocarboxazid
lasmiditan
levomilnacipran
lithium
lorcaserin
lysergic acid diethylamide
maprotiline
mdma
meperidine
metaxalone
methamphetamine
methylene blue
methylergonovine
methylphenidate
milnacipran
mirtazapine
moclobemide
naratriptan
nefazodone
nortriptyline
ondansetron
oxitriptan
palonosetron
paroxetine
pentazocine
phenelzine
phentermine
protriptyline
rasagiline
rizatriptan
safinamide
selegiline
sertraline
sibutramine
St. John’s wort (Hypericum perforatum)
sumatriptan
Syrian rue (Peganum harmala, harmine)
tramadol
tranylcypromine
trazodone
trimipramine
tryptophan
venlafaxine
vilazodone
vortioxetine
zolmitriptan
Contributor Information
Edward C Traver, Division of Infectious Disease, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Emily L Heil, Department of Pharmacy Practice and Science, University of Maryland School of Pharmacy, Baltimore, Maryland, USA.
Sarah A Schmalzle, Division of Infectious Disease, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland, USA; Institute of Human Virology, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- 1. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–42. doi: 10.1093/qjmed/hcg109 [DOI] [PubMed] [Google Scholar]
- 2. Murphy EL, DeVita D, Liu H, et al. Risk factors for skin and soft-tissue abscesses among injection drug users: a case-control study. Clin Infect Dis 2001; 33:35–40. doi: 10.1086/320879 [DOI] [PubMed] [Google Scholar]
- 3. Li JZ, Willke RJ, Rittenhouse BE, Rybak MJ. Effect of linezolid versus vancomycin on length of hospital stay in patients with complicated skin and soft tissue infections caused by known or suspected methicillin-resistant staphylococci: results from a randomized clinical trial. Surg Infect 2003; 4:57–70. doi: 10.1089/109629603764655290 [DOI] [PubMed] [Google Scholar]
- 4. Hepburn MJ, Dooley DP, Skidmore PJ, Ellis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med 2004; 164:1669–74. doi: 10.1001/archinte.164.15.1669 [DOI] [PubMed] [Google Scholar]
- 5. Moran GJ, de Anda C, Das AF, Green S, Mehra P, Prokocimer P. Efficacy and safety of tedizolid and linezolid for the treatment of acute bacterial skin and skin structure infections in injection drug users: analysis of two clinical trials. Infect Dis Ther 2018; 7:509–22. doi: 10.1007/s40121-018-0211-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Food and Drug Administration . FDA drug safety communication: FDA warns about several safety issues with opioid pain medicines; requires label changes. 2016. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-several-safety-issues-opioid-pain-medicines-requires. Accessed October 14, 2021.
- 7. Food and Drug Administration . FDA drug safety communication: updated information about the drug interaction between linezolid (Zyvox) and serotonergic psychiatric medications. 2011. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-updated-information-about-drug-interaction-between-linezolid-zyvox-and. Accessed April 16, 2021.
- 8. Boyer EW. Serotonin syndrome (serotonin toxicity). UpToDate. May 6, 2022. Available at: https://www.uptodate.com/contents/serotonin-syndrome-serotonin-toxicity. Accessed May 2, 2022. [Google Scholar]
- 9. Woytowish MR, Maynor LM. Clinical relevance of linezolid-associated serotonin toxicity. Ann Pharmacother 2013; 47:388–97. doi: 10.1345/aph.1R386 [DOI] [PubMed] [Google Scholar]
- 10. Lawrence KR, Adra M, Gillman PK. Serotonin toxicity associated with the use of linezolid: a review of postmarketing data. Clin Infect Dis 2006; 42:1578–83. doi: 10.1086/503839 [DOI] [PubMed] [Google Scholar]
- 11. Butterfield JM, Lawrence KR, Reisman A, Huang DB, Thompson CA, Lodise TP. Comparison of serotonin toxicity with concomitant use of either linezolid or comparators and serotonergic agents: an analysis of phase III and IV randomized clinical trial data. J Antimicrob Chemother 2012; 67:494–502. doi: 10.1093/jac/dkr467 [DOI] [PubMed] [Google Scholar]
- 12. Karkow DC, Kauer JF, Ernst EJ. Incidence of serotonin syndrome with combined use of linezolid and serotonin reuptake inhibitors compared with linezolid monotherapy. J Clin Psychopharmacol 2017; 37:518–23. doi: 10.1097/JCP.0000000000000751 [DOI] [PubMed] [Google Scholar]
- 13. Rickli A, Liakoni E, Hoener MC, Liechti ME. Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol 2018; 175:532–43. doi: 10.1111/bph.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gatti M, Raschi E, de Ponti F. Serotonin syndrome by drug interactions with linezolid: clues from pharmacovigilance-pharmacokinetic/pharmacodynamic analysis. Eur J Clin Pharmacol 2021; 77:233–9. doi: 10.1007/s00228-020-02990-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinberg M, Morin AK. Mild serotonin syndrome associated with concurrent linezolid and fluoxetine. Am J Health Syst Pharm 2007; 64:59–62. doi: 10.2146/ajhp060227 [DOI] [PubMed] [Google Scholar]
- 16. Mastroianni A, Ravaglia G. Serotonin syndrome due to co-administration of linezolid and methadone. Infez Med 2017; 25:263–6. [PubMed] [Google Scholar]
- 17. Codd EE, Shank RP, Schupsky JJ, Raffa RB. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 1995; 274:1263–70. [PubMed] [Google Scholar]
- 18. Babu KM, Brent J, Juurlink DN. Prevention of opioid overdose. N Engl J Med 2019; 380:2246–55. doi: 10.1056/NEJMra1807054 [DOI] [PubMed] [Google Scholar]
- 19. Liebschutz JM, Crooks D, Herman D, et al. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern Med 2014; 174:1369–76. doi: 10.1001/jamainternmed.2014.2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marks LR, Munigala S, Warren DK, et al. A comparison of medication for opioid use disorder treatment strategies for persons who inject drugs with invasive bacterial and fungal infections. J Infect Dis 2020; 222(Suppl 5):S513–20. doi: 10.1093/infdis/jiz516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med 2016; 129:481–5. doi: 10.1016/j.amjmed.2015.09.024 [DOI] [PubMed] [Google Scholar]
- 22. Jicha C, Saxon D, Lofwall MR, Fanucchi LC. Substance use disorder assessment, diagnosis, and management for patients hospitalized with severe infections due to injection drug use. J Addict Med 2019; 13:69–74. doi: 10.1097/ADM.0000000000000454 [DOI] [PubMed] [Google Scholar]
- 23. Beekmann SE, Gilbert DN, Polgreen PM. Toxicity of extended courses of linezolid: results of an Infectious Diseases Society of America Emerging Infections Network survey. Diagn Microbiol Infect Dis 2008; 62:407–10. doi: 10.1016/j.diagmicrobio.2008.08.009 [DOI] [PubMed] [Google Scholar]