Abstract
The role of the dormant-like viable but nonculturable (VBNC) condition in the etiology of bacterial infection was examined using a plant system. The plant-pathogenic bacterium Ralstonia solanacearum was first shown to enter into the VBNC state both in response to cupric sulfate when in a saline solution and when placed in autoclaved soil. To determine if the VBNC condition is related to pathogenesis, the physiological status of bacteria recovered from different regions of inoculated tomato plants was determined at different stages of infection. The fraction of in planta bacteria that were VBNC increased during infection and became greater than 99% by the late stage of disease. The possibility that soil-dwelling VBNC bacteria may resuscitate and infect plants was also examined. When tomato seeds were germinated in sterile soil that contained VBNC but no detectable culturable forms of R. solanacearum cells, resuscitation was observed to occur in soil adjacent to plant roots; these resuscitated bacteria were able to infect plants. This is the first report of R. solanacearum entering the VBNC state and of resuscitation of any VBNC plant-pathogenic bacteria and provides evidence that the VBNC state may be involved in explaining the persistent nature of some infections.
Ralstonia solanacearum is a gram-negative plant-pathogenic bacterium that causes bacterial wilt in a variety of plants (12). During infection, the bacteria become motile and travel throughout the vascular system of the plant. As the cell concentration increases, virulence genes are expressed and the cells become nonmotile and secrete exopolysaccharide and pectin-degrading enzymes, leading to the death of the plant (6, 25).
Soil can be considered an oligotrophic environment (34); therefore, the ability of a microbe to survive in soil depends on its ability to resist starvation, dehydration, and exposure to heavy metals (1, 31). In contrast to bulk soil, the rhizosphere is a nutrient-rich environment that is often colonized by bacteria (3). Because infection by R. solanacearum results in plant death, colonization of the host plant rhizosphere by this pathogenic bacterium is likely a temporary event. This suggests that R. solanacearum has the ability to survive for long periods of time in a nutrient-depleted bulk soil environment.
Current strategies aim to prevent recurrent R. solanacearum infections by addition to the soil of biological or chemical control agents (11). The success of these strategies is often based on determining if the target bacterial pathogen is no longer present, as indicated by an absence of growth on the appropriate medium. However, these assays would not detect cells that are in the viable but nonculturable (VBNC) state.
The loss of bacterial culturability, while maintaining viability, was first observed by Xu et al. in 1982 (35). The VBNC state is one in which bacteria are viable but unable to divide sufficiently on nonselective growth medium to yield visible growth (18). VBNC can be considered a long-term dormant-like survival mechanism for non-spore-forming bacteria. The conditions that have been found to induce the VBNC state vary depending on the species of bacteria; they include osmotic stress, temperature shifts, desiccation, starvation, and exposure to the heavy metal copper (9). This condition differs from sublethal cell injury and cell stress in that restoration of culturability is not the result of simply removing the VBNC-inducing signal. Determining that nongrowing cells are VBNC requires the use of a growth-independent viability assay. Common viability assays used to enumerate viable cells measure metabolic activity or the presence of RNA, ATP, or an intact cell membrane (10, 16). The most definitive assay for the presence of VBNC cells is observing restoration of culturability (i.e., resuscitation). Although resuscitation conditions have been reported for several species, it can be difficult to distinguish resuscitation from regrowth of a few remaining culturable cells. Resuscitation does not always occur by a simple reversal of the stress that induced the cells to become VBNC, and there appears to be no universal resuscitation condition (16).
Multiple soil microbes have been reported to become VBNC, including Pseudomonas fluorescens, Salmonella enterica serovar Typhimurium, Agrobacterium tumefaciens, Sinorhizobium meliloti, Xanthomonas campestris pv. vesicatoria, and X. campestris pv. campestris (2, 4, 10, 20, 28, 29, 30, 33). That soil microbes can become VBNC in soil may at least partly explain the observation that the percentage of cells present in soil samples that can be recovered in a culturable form is usually very low (0.01 to 10%) (5, 24). The biological relevance of the VBNC condition is not clear. Certain animal-pathogenic VBNC bacteria including Escherichia coli, Shigella dysenteriae, Campylobacter jejuni, Vibrio vulnificus, and Vibrio cholerae retain virulence or certain virulent characteristics (7, 19, 21, 22, 26). However the virulence of VBNC plant-pathogenic bacteria has not been examined. And no study has monitored the VBNC status of pathogenic bacteria as they progress from a nonhost environment through infection.
This study was initiated based on our hypothesis that the reason some plant diseases such as bacterial wilt are characterized as being recurrent is because pathogenic VBNC bacteria can escape detection by standard assays and can then resuscitate to cause a subsequent infection. To test this hypothesis, we first examined whether R. solanacearum has the ability to enter the VBNC state. We then monitored the physiological status of R. solanacearum during infection of tomato plants to determine if the percentage of cells that are VBNC increases as the infected plant undergoes necrosis. In addition, we determined whether R. solanacearum that becomes VBNC when added to soil could resuscitate and infect plants.
MATERIALS AND METHODS
Strains and chemicals.
R. solanacearum strain AW1 was provided by David Ritchie (Department of Plant Pathology, North Carolina State University). A spontaneously rifampin-resistant strain was isolated and named AS108. AS108 was grown at 28°C on R. solanacearum agar (RSA) (Difco Laboratories, Detroit, Mich.). Rifampin resistance was selected on media containing 50 μg of rifampin/ml.
Unless noted otherwise, all chemicals were from Sigma Chemical Company (St. Louis, Mo.).
Preparation of liquid microcosms.
Cells were grown at 28°C in RSA broth (Difco Laboratories) containing 50 μg of rifampin ml−1 in an orbital air shaker at 250 rpm. When at an optical density at 600 nm of 0.6 to 0.8, cells were harvested by centrifugation, washed three times in 0.9% NaCl, and then suspended in 0.9% NaCl to a concentration of 108 cells ml−1. Liquid microcosms contained 40 ml of cells in 250-ml Erlenmeyer flasks. Initial CFU concentration and direct viable counts were determined as described below prior to addition of copper sulfate to a microcosm.
Preparation of soil microcosms.
Soil was obtained from a site in the Van Landingham Glen on the campus of the University of North Carolina at Charlotte. The clay content was estimated to be 8% using a pipette sedimentation method (8). The total copper content in the soil was found to be 114 μg/g of soil by the Chemical Analysis Laboratory (University of Georgia) via intercoupled plasma mass spectrophotometry analysis. The Geotech Laboratories at The University of North Carolina at Charlotte determined that the soil had a specific gravity of 2.50, a carbon content of 2.5%, and a pH of 6.14. The soil was sifted to a diameter of 64 μm, and 100 g was placed in 250-ml beakers and autoclaved for 90 min. After 2 days, the soil was autoclaved again for 90 min. The moisture content of the soil was determined to be 10% by weighing a sample before and after drying at 85°C overnight. Water loss by autoclaving was replaced to the original hydration level. Experimental soil samples were weighed twice a week, and any lost water was replaced.
When appropriate, bacteria were added to the soil to a final concentration of 1011 cells/kg of dry soil. Bacteria to be added to soil were grown in RSA broth containing rifampin to an optical density at 600 nm of 0.6 to 0.8. Cells were then collected by centrifuging for 6 min at 8,000 rpm, washed three times in 0.9% NaCl, and suspended in 0.9% NaCl to an appropriate concentration.
Bacteria were isolated from soil microcosms by adding approximately 1 g of soil to 3 ml of buffered soil dispersion solution (0.1 M NaCl, 0.01% sodium dodecyl sulfate, and 0.1% sodium pyrophosphate [pH 7.2]) (32). The solution was vortexed, allowed to settle for at least 20 min, and then vortexed again. The bacterial fraction was collected from the supernatant following centrifugation for 5 s at 820 × g. Culturability and viability were then determined as described below. In control experiments, isolation of bacteria from the soil within 1 day resulted in a recovery rate of approximately 60%.
In planta study.
Tomato seeds (Early Girl hybrid) were sterilized by being washed in 10% bleach–0.01% Tween 20 for 30 min and rinsed three times in distilled water. Seeds were placed in a petri dish containing Murashige and Skoog basal medium with 30 g of sucrose and 12 g of agar (M&S agar) and allowed to germinate in growth chambers at 26°C (14 h of light per day). Germinating seeds that showed no signs of contamination after 7 days were then transferred to sterile magenta boxes containing M&S agar and grown in growth chambers at 26°C (14 h of light per day) until the six-leaf stage (∼2 weeks). Tomato plants were inoculated with bacteria prepared as described above in “Preparation of liquid microcosms” by injecting 10 μl containing 107 cells into the root crown with a 26-gauge needle. Control plants were punctured but not inoculated. Some plants were sacrificed 30 min following inoculation and then periodically as the disease progressed. Sections of the plants were cut with a sterile scalpel longitudinally in 5-mm sections to expose the vascular tissue and placed in 2 ml of 0.9% NaCl. The samples were vortexed and allowed to sit for at least 20 min. Following a second vortexing, aliquots were assessed for culturable and viable bacteria. The amount of plant material used for each time point ranged from 0.05 to 0.45 g. Four sections of the plants were sampled: the root tips, the stem between the first and second nodes, the first set of true leaves, and the terminal leaves.
Resuscitation of R. solanacearum.
Soil was sterilized as described above and added to 10- by 20- by 3-in. plastic incubation trays with clear tops that were cleaned and surface sterilized with 95% ethanol followed by UV irradiation. One enclosure was not inoculated with bacteria. In the remaining three enclosures, the sterile soil was inoculated with culturable AS108 and mixed to obtain uniform distribution of the inocula. The enclosures were monitored for culturable cells by the same method as that described above. When no culturable cells were detected over a 5-day period, approximately 150 sterile germinating tomato seeds, prepared as described above in “In planta study,” were added to three-fourths of the surface area of each of the enclosures (on days 7 to 9 following soil inoculation) and incubated in growth chambers at 26°C (14 h of light per day). The enclosures were monitored for culturable cells and wilting of the tomato plants. Plant wilting was observed to occur approximately 8 days following addition of germinating seeds to the soil. At this time samples of soil (between 0.5 and 1 g) were taken from control areas at least 5 cm from the nearest plant; 1, 2, and 3 cm away from the nearest plant crown; loose soil surrounding the roots of the plant; and the soil of the rhizosphere. The lengths of the plant roots varied from 25 to 50 mm, with the average root being 40 mm in length. Rhizosphere soil is soil that remains bound to the extracted roots following shaking; loose soil is soil that is released from the root of the extracted plant following shaking. Bacteria were recovered from the soil as described above and assayed for culturability and viability as described below. Five healthy plants and five plants that exhibited wilting were sampled per enclosure.
Culturability.
Cells were collected from various sources as described below onto a 0.22-μm-pore-size nitrocellulose filter (Whatman, Maidstone, United Kingdom), washed with 0.9% NaCl, and resuspended by repipetting them in 1 ml of 0.9% NaCl. Cells were diluted, and 50 μl of cells was plated in triplicate on RSA plates. Colonies were counted after incubation at 28°C for 2 days and again after 7 days. A lack of growth on all triplicate plates is plotted on the log scale as 1 CFU ml−1.
Viability.
Two assays were used to determine viability. The BacLight LIVE/DEAD bacterial viability kit (Molecular Probes Inc., Eugene, Oreg.) was used as the primary assay. The Kogure assay (17) was performed as an independent viability assay once per trial per treatment.
Assays with the BacLight LIVE/DEAD bacterial viability kit were performed by staining 1 ml of washed cells (collected as described above from each source material) with 1 μl of reagent A and 2 μl of reagent B for 30 min and collecting the cells onto a 0.22-μm-pore-size black polycarbonate filter (Poretics, Livermore, Calif.). Cells were viewed with an Olympus BX60 epifluorescence microscope. The dyes differ in their abilities to penetrate the cell membrane. Reagent A can enter cells with and without an intact cell membrane. Reagent B can only enter cells with a compromised membrane. As a result, red-fluorescing cells are considered to be dead and green-fluorescing cells are considered to be viable. At least 100 cells per sample were scored.
The Kogure assay (17) was performed by incubating 0.1 ml of cells in 1 ml of 0.01% yeast extract and 0.1% nalidixic acid overnight at 25°C. Cells were then stained for 30 min with 0.1 ml of 0.5% acridine orange and collected onto a 0.22-μm-pore-size black polycarbonate filter and viewed via epifluorescence microscopy as described above. Cells that were elongated compared to the control lacking yeast extract and nalidixic acid were scored as live cells. Cells were scored in the same manner described above.
The concentration of VBNC cells is calculated by subtracting the concentration of culturable cells from the concentration of viable cells.
Statistical analysis.
Statistical significance was measured by using a two-way analysis of variance (ANOVA), and the significance of each time point was measured using a paired, two-tailed, Student t test. P values were then measured for confidence using sequential Bonferroni analysis. Bars on graphs represent standard errors (23).
RESULTS
Cupric sulfate induces R. solanacearum to enter the VBNC state in liquid microcosms.
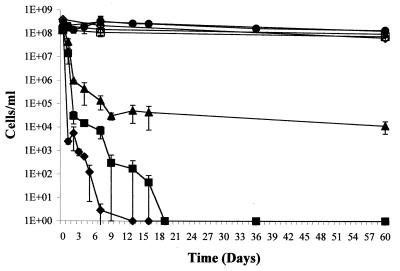
Because other plant-associated bacteria have been shown to become VBNC in response to copper sulfate (2, 10), R. solanacearum was initially examined for the ability to become VBNC by inoculating AS108 into liquid microcosms containing 0.9% NaCl and various concentrations of CuSO4. The average of data obtained in three independent trials is presented in Fig. 1. In the control microcosms over the 60-day period culturability and viability decreased by about the same amount (culturability decreased by approximately 45%, and viability decreased by approximately 40%). For all copper-containing microcosms, however, viability decreased to a significantly lesser degree than did culturability, indicating that many cells were induced to enter the VBNC state. The microcosms containing 5 μM CuSO4 gradually decreased in culturability over 2 weeks from approximately 108 cells ml−1 at day 0 to approximately 104 cells ml−1; culturability remained at this level for the duration of the experiment. In contrast, viability, as determined by the BacLight LIVE/DEAD bacterial viability assay, decreased to only approximately 9 × 107 cells ml−1. Hence the percentage of viable cells that were VBNC increased from less than 5% at the beginning of the experiment to over 99.9% by the end of the experiment. Because viability assays used to study the VBNC condition are indirect (i.e., are growth independent), a Kogure viability assay was used to confirm the results obtained from the LIVE/DEAD assay for the day 60 time point. No significant difference in viability values between these two independent viability assays was observed; the Kogure results are as follows: 0 μM CuSO4, 108 cells/ml; 5 μM CuSO4, 6.8 × 107 cells/ml; 50 μM CuSO4, 4.8 × 107 cells/ml; 500 μM CuSO4, 2.7 × 107 cells/ml. For the microcosms containing the higher concentrations of cupric sulfate, the culturability decreased to nondetectable levels and the rate of decrease was affected by the copper concentration. With 50 μM cupric sulfate, no culturable cells were detected by days 9 to 36; with 500 μM cupric sulfate, no culturable cells were detected by days 6 to 14. For both of these microcosms, 100% of the viable cells present in the microcosms by the end of the experiment were VBNC.
FIG. 1.
Culturability and viability of R. solanacearum in liquid microcosms. Cells of strain AS108 were inoculated into microcosms containing various concentrations of cupric sulfate. At various times, aliquots were removed and the concentrations of viable (open symbols) and culturable (solid symbols) forms of bacteria were determined as described in Materials and Methods. Microcosms containing 0 (circles), 5 (triangles), 50 (squares), and 500 μM (diamonds) cupric sulfate are shown. The results are the average values of three trials. Bars, standard errors.
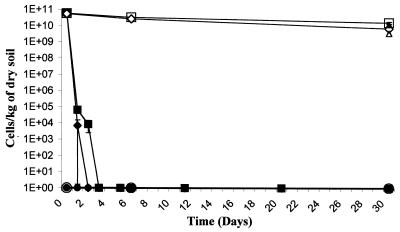
R. solanacearum enters the VBNC state in sterile soil.
To determine if AS108 would enter the VBNC state in a more complex environment, exponentially growing cells were added to sterile soil to an approximate concentration of 1011 cells kg of dry soil−1. Three soil microcosms were examined; one was not inoculated with bacteria and served as a control, a second was inoculated with AS108, and in a third the soil was supplemented to 500 mg of cupric sulfate/kg prior to bacterial inoculation. The averages of data obtained in three independent trials are presented in Fig. 2. In both inoculated microcosms, culturability dropped to nondetectable levels within 2 days for the copper-supplemented soil and within 3 days for the nonsupplemented soil. However, at the end of the trials, the microcosms contained at least 2 × 1010 viable (and VBNC) cells kg of dry soil−1 for the soil lacking supplemented copper and at least 1 × 1010 viable (and VBNC) cells kg of dry soil−1 for the copper-supplemented soil. Viability results obtained with the Kogure assay for samples taken at the end of the trial were similar to those obtained with the LIVE/DEAD assay (Fig. 2).
FIG. 2.
Culturability and viability of R. solanacearum in sterile soil. Cells of AS108 were inoculated into sterile soil as described in Materials and Methods. At various times, soil samples were removed, bacteria were isolated, and the concentrations of viable (open symbols) and culturable (solid symbols) forms of bacteria were determined as described in Materials and Methods. Circles, uninoculated soil; squares, inoculated soil; diamonds, inoculated soil supplemented with cupric sulfate. Also shown are viabilities determined at the terminal time point by the Kogure assay with inoculated soil (▴) and inoculated soil supplemented with cupric sulfate (▵). The results are the averages of three trials. Bars, standard errors.
R. solanacearum becomes VBNC in planta.
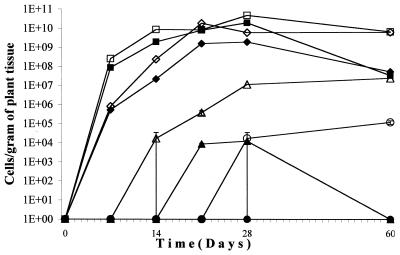
If VBNC is a long-term survival mechanism induced by an oligotrophic environment, it is possible that, as an infected plant undergoes necrosis, the pathogenic bacteria become VBNC in response to a signal such as decreased nutrient availability prior to the return of the bacteria to the soil. To test this idea, sterile tomato plants were inoculated at the root crown with 107 cells of AS108, an amount that consistently resulted in infected plants (unpublished results). For each of three trials, one plant was sacrificed per time point and the root tips, stem between the first and second leaf node, the first true leaves, and the terminal leaves were removed and assayed for viable and culturable forms of the bacteria. The time points were 30 min and days 7, 14, 21, 28, and 39 for all trials and additionally day 60 for trials 2 and 3. The time points for visible signs of disease progression were as follows: blackening of the primary root, between 4 and 5 days; browning of the peripheral roots, between 8 and 13 days; brown streaks in the stems, between 12 and 18 days; leaf wilting, between 15 and 22 days; signs of necrosis for the entire plant, between 22 and 29 days. The results of one of the three experiments are given in Fig. 3.
FIG. 3.
Culturability and viability of R. solanacearum isolated from infected plants. Tomato plants were inoculated with culturable forms of R. solanacearum. At various times after inoculation, bacteria were isolated from four different tissue samples and the concentrations of viable (open symbols) and culturable (solid symbols) forms of the bacteria were determined as described in Materials and Methods. The four tissues assayed were root tips (squares), stem from the first to second node (diamonds), first true leaf (triangles), and terminal leaf (circles). Bars, standard errors.
For the root tip and stem sections, the concentration of culturable cells increased during the first 28 days. At later times, the culturable-cell concentration decreased while the viable-cell concentration decreased by a lesser amount. The difference in culturability and viability as the disease progressed was more pronounced for the plant sections located farther away from the site of inoculation. In the first true leaf, the concentrations of both culturable and viable cells increased during infection. However, by 1 month after the entire plant showed signs of necrosis, there were no detected culturable cells and more than 107 viable cells per g of tissue. For the terminal leaf, no culturable cells were detected at any time point although there were more than 104 viable cells per g of tissue detected in the terminal leaf at day 60.
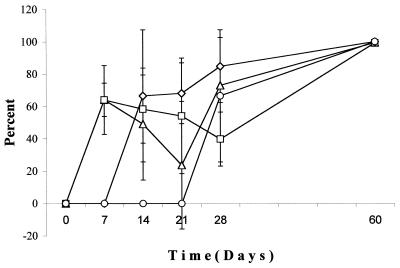
To present the data in a way that directly relates to testing the hypothesis that in planta cells become VBNC as disease progresses, the percentages of viable cells that were VBNC were calculated for the four plant tissue sections and averaged for the three trials (Fig. 4). In the inoculum, less than 5% of viable cells were VBNC. For all plant sections that contained cells, there were between 40 and 80% of the viable cells in the VBNC state up to day 28. At the last time point (day 60), when there was extensive necrosis throughout the plant, greater than 99% of the viable cells were in the VBNC state.
FIG. 4.
Percentages of viable R. solanacearum cells isolated from an infected plant that are VBNC. Tomato plants were inoculated with culturable forms of R. solanacearum. At various times after inoculation, bacteria were isolated from four different tissue samples and the concentrations of viable and culturable cells were determined as described in Materials and Methods. The percentage of viable cells that were VBNC was calculated for each of the four tissue samples: root tip (Δ), stem from the first to second node (□), first true leaf (⋄), and terminal leaf (○). Bars, standard errors.
Resuscitation of R. solanacearum
The above experiments indicate that R. solanacearum becomes VBNC in planta and that the percentage of viable cells that are VBNC increases as does plant tissue necrosis. If VBNC is a part of disease etiology, then soil-dwelling VBNC cells should resuscitate in response to some change to the environment, such as rhizosphere formation at growing plants. To test this idea, culturable R. solanacearum cells were added to sterile soil in each of four 10- by 20- by 3-in. plastic incubation trays to a starting concentration of 1011 cells per kg of soil as described above. After inoculation, each enclosure was monitored for culturability. No culturable cells were detected by day 4 in samples taken from any enclosure. For the next 5 consecutive days, samples from each of the enclosures contained no detectable culturable cells. On days 7 to 9, approximately 100 sterile germinating tomato seeds were then added to each of the enclosures. For each enclosure, one-fourth of the area was not seeded.
On days 15 to 17, when wilting of some plants was observed in the experimental enclosures, samples of soil were taken at various distances from both nonwilted and wilted plants, from soil loosely associated with plant roots (loose soil), and from soil tightly associated with plant roots (rhizosphere). No wilted plants were observed in the control enclosure. For each of the experimental enclosures, five wilted and five nonwilted plants were assayed per time point. All samples were assayed for culturable cells as described in Materials and Methods.
Because resuscitated bacteria will grow when in the presence of nutrients, it cannot be determined whether any observed culturable cells isolated from soil samples were resuscitated or growing bacteria; hence, although the total number of culturable bacteria could be quantitated, the number of resuscitated bacteria could not. None of the 44 samples taken during the course of the experiment from the control area of each of the three enclosures were found to contain detectable levels of culturable AS108. By contrast, the highest levels of culturable AS108 were found in samples isolated from the rhizosphere of infected (i.e., wilted) plants, with there being at least 1,000 colonies per plated sample. The concentration of culturable cells decreased as the distance from the wilting plant increased, with culturable cells being detected 3 cm from the base of the plant. Culturable AS108 was also isolated from the rhizosphere and 1 cm away from the bases of nonwilted plants in all three enclosures, although at smaller numbers than seen from similar samples obtained from wilted plants.
Thus, VBNC bacteria located close to growing plants can be resuscitated. However, the percentage of VBNC cells that resuscitated could not be determined because it is likely that the culturable cells found in these soil samples represent both resuscitated cells and growth of resuscitated cells.
DISCUSSION
In liquid microcosms, copper can induce R. solanacearum to enter the VBNC state. The percentage of viable cells that are VBNC is dependent on the copper concentration. In the control microcosm, approximately 20% of the cells became VBNC, likely induced by an absence of nutrients as has been shown to occur with other microbes (13, 33). In 5 μM cupric sulfate, over 99.9% of the viable cells were VBNC, while for the 50 μM and 500 μM microcosms 100% of viable cells were VBNC. Therefore, although copper sulfate is not required for R. solanacearum to enter the VBNC state, it can induce cells to do so. Similar observations have been made with other plant-associated microbes, including A. tumefaciens, S. meliloti, and X. campestris (2, 10).
Addition of copper does not induce all cells to become VBNC, only those that are not killed by the copper treatment. The percentage of cells that were killed by the copper increased as the copper concentration increased (Fig. 1). In the control microcosm, approximately 60% of the cells survived to the end of the trials. In 5 μM and 50 μM copper, approximately 42 and 49%, respectively, of the starting cell population survived, whereas at 500 μM only approximately 14% of the starting cell population survived. Therefore, only a portion of the original cell population, but the majority of surviving cells, became VBNC in response to copper.
AS108 was also able to become VBNC in a more complex environment, sterile soil. Because the goal was to determine if bacteria have the ability to enter the VBNC state in soil, biotic factors such as protozoan predation and competition with other microbes that could complicate analysis were removed by sterilization. All viable cells in soil microcosms containing either no supplemental copper or 500 mg of soil copper kg−1 entered the VBNC condition within 3 days (Fig. 2). This amount of supplemental copper was chosen because it is equal to the 99th percentile of the average amount of copper in soil of the United States (15). However, viability dropped by less than 1 log unit in both soils. These results indicate that unsupplemented soil presents the bacterium with conditions that induce the VBNC state. Similar observations have been made with other soil microbes including Flavobacterium sp. strain P25 (13), P. fluorescens (33), Salmonella (29), and X. campestris pv. campestris (10). One difference between the results presented here and those presented in these other studies is that all viable R. solanacearum cells entered the VBNC condition and did so within a relatively short period of time. A previous study using soil from the same site and X. campestris also found that cells entered the VBNC condition within a few days, though not all of the viable cells did so (10). Because there are few studies in which the VBNC condition has been examined in soil, and none using R. solanacearum, it is not known what soil or bacterial parameters influence entry of cells into the VBNC state. It is possible that conditions known to influence the survival of bacteria in soil (soil moisture, temperature, pH, texture, O2 availability, nutrient availability, and physiological status of the introduced bacteria) can also affect VBNC entry. It is also possible that autoclaving the soil created compounds that influenced the culturability of the bacteria.
The ability of R. solanacearum to enter the VBNC state suggests that detection methods to determine the effectiveness of soil treatment strategies that rely on cell culturing may not be counting all viable forms of this pathogen. These results also suggest that copper-based biocides used to treat soil may in fact induce a small population to enter the dormant-like VBNC condition.
The VBNC condition in bacteria can be considered to be a long-term survival mechanism employed primarily by gram-negative bacteria in response to a variety of environmental stresses. If the VBNC condition is involved in the etiology of bacterial plant-pathogenic disease, either via bacterial survival or in the infection process itself, then it would be expected that the appearance of VBNC cells might occur as a host plant undergoes necrosis and that resuscitation of VBNC cells in soil might occur when the bacteria encounter a host plant. Both of these situations were observed.
During plant infection, a significant percentage of cells entered the VBNC condition (Fig. 4). This percentage increased to greater than 99% after the plant underwent extensive necrosis. Although the environment in the infected plant tissues likely changes in that there is an increase in bacterial concentration and less access to readily metabolized nutrients, it was not determined what was the signal that induced cells to become VBNC during infection. Interestingly, in two of three trials, VBNC cells were found in the first true leaf and terminal leaf tissues before culturable forms of the cells were detected (Fig. 4). This suggests that VBNC cells move through the vascular system of the plant prior to movement of growing, culturable forms of the bacteria.
To assay for the presence of VBNC cells requires using a viability assay that is not dependent on bacterial growth. To consider bacteria that do not grow on medium that normally supports growth to be viable, cells must have the ability to resume growth under the appropriate conditions. When observed, resuscitation serves as a definitive confirmation that nonculturable cells were indeed VBNC. To date, resuscitation of plant-pathogenic bacteria has not been reported.
In this study, R. solanacearum resuscitation was studied in soil to which was added sterile tomato seeds. Culturable forms of R. solanacearum were observed only after plants germinated and were found associated with the rhizosphere of both symptomatic and asymptomatic plants. The concentration of culturable R. solanacearum decreased along with the distance from the plant. And lower concentrations of culturable R. solanacearum were found, in a similar concentration gradient, associated with asymptomatic rather than with symptomatic plants. These results indicate that VBNC R. solanacearum resuscitated when in the presence of a plant rhizosphere. The number of VBNC cells that resuscitated cannot be determined because the culturable cells in the rhizosphere contain both resuscitated cells and those that represent growth of resuscitated cells. Appearance of culturable cells near nonwilted plant roots suggests that these cells had not yet reached a concentration sufficient to infect the host plant or had infected the plant but had not yet induced visible symptoms.
An alternative explanation for these data is that the soil thought to contain only VBNC cells also contained culturable bacteria at a concentration low enough to escape detection and that these bacteria grew when in a rhizosphere. If culturable cells were present in the soil, they would be expected to be evenly distributed. That is, the likelihood of a control soil sample containing a culturable cell would equal the likelihood that a rhizosphere sample would contain a culturable cell. Therefore, for the rhizosphere-associated culturable cells to represent regrowth of undetected culturable cells and not resuscitation of VBNC cells, it would be required that, by chance, all of the soil samples collected from the 30 plants, and none of the 132 control soil samples, contained at least one culturable cell. Even if all culturable cells found in a sample associated with a given plant are considered to have originated from a single culturable bacterium, for the alternative explanation to be true, among 162 total samples all 30 random locations where a seed happened to germinate would have to have contained at least one culturable cell while none of the remaining 132 nonseeded locations contained a culturable cell. Using a chi-square analysis with a two-way contingency table, and assuming that the presence of a culturable bacterium is independent of the presence of a plant, there is less than a 10−19 probability of obtaining these data. The low probability of this occurrence argues that the results presented in this study indicate that R. solanacearum was VBNC in the soil and underwent resuscitation when in the presence of a host plant rhizosphere.
This is the first report of resuscitation of VBNC plant-pathogenic bacteria and provides evidence that the VBNC state may be involved in explaining the persistent nature of some infections. These data also give insight into the life cycle of this and perhaps other pathogenic bacteria. Upon entering bulk soil, R. solanacearum likely becomes VBNC and remains so until encountering a rhizosphere. Unidentified factors in the rhizosphere allow the bacteria to resuscitate and infect a host plant. As infection progresses, a greater proportion of the viable bacteria become VBNC. Upon plant death, the bacteria enter the soil and repeat the process.
We suggest that the VBNC state should be considered to be a physiological condition that most gram-negative bacteria can undergo in response to exposure to varying environmental conditions. Because VBNC bacteria are not detected using standard culturing assays, their presence could help explain a number of observations such as the low efficiency of culturing bacteria found in soil samples (5, 24), the difficulty in maintaining genetically modified bacteria introduced into fields that contain indigenous bacteria (14, 27), and the persistent nature of bacterial infections in fields to which biocides have been added to remove pathogenic bacteria. Interestingly, copper, a biocide used in agriculture, has been shown to induce multiple plant-pathogenic bacteria that are not killed by the agent to become VBNC (20, 29).
ACKNOWLEDGMENTS
We thank Nicholas Parker and the Geotech Laboratories at UNC-Charlotte for soil analysis and Albert Flavier for strain AW1.
This work was supported by the North Carolina Biotechnology Center (grant 9705-ARG-0019) and The University of North Carolina at Charlotte.
REFERENCES
- 1.Acea M, Moore C, Alexander M. Survival and growth of bacteria introduced into soil. Soil Biol Biochem. 1988;20:509–515. [Google Scholar]
- 2.Alexander E, Salazar C, Steck T R. The viable but nonculturable condition is induced by copper in Agrobacterium tumefaciens and Rhizobium meliloti. Appl Environ Microbiol. 1999;65:3754–3756. doi: 10.1128/aem.65.8.3754-3756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bazin M J, Markham P, Scott E M, Lynch J M. Population dynamics and rhizosphere interactions. In: Lynch J M, editor. The rhizosphere. Chichester, England: John Wiley & Sons; 1990. pp. 99–128. [Google Scholar]
- 4.Binnerup S J, Jensen D F, Thordal-Christensen H, Sorensen J. Detection of viable, but non-culturable Pseudomonas fluorescens DF57 in soil using a microcolony epifluorescence technique. FEMS Microbiol Ecol. 1993;12:97–105. [Google Scholar]
- 5.Campbell R, Greaves M. Anatomy and community structure of the rhizosphere, 11–34. In: Lynch J M, editor. The rhizosphere. Chichester, England: John Wiley & Sons; 1990. [Google Scholar]
- 6.Clough S, Flavier A, Schell M, Denny T. Differential expression of virulence genes and motility in Ralstonia (Pseudomonas) solanacearum during exponential growth. Appl Environ Microbiol. 1997;63:844–850. doi: 10.1128/aem.63.3.844-850.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colwell R R, Brayton P, Harrington D, Tall B, Huq A, Levine M M. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- 8.Folk R L. Petrology of Rocks. Austin, Tex: Hemphill Publishing Co.; 1974. [Google Scholar]
- 9.Gauthier M J. Environmental parameters associated with the viable but nonculturable state. In: Colwell R R, Grimes D J, editors. Nonculturable microorganisms in the environment. Washington D.C.: ASM Press; 2000. pp. 87–112. [Google Scholar]
- 10.Ghezzi J, Steck T R. Induction of the viable but non-culturable condition in Xanthomonas campestris pv. campestris in liquid microcosms and sterile soil. FEMS Microbiol Ecol. 1999;30:203–208. doi: 10.1111/j.1574-6941.1999.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 11.Hartman G L, Elphinstone J G. Advances in the control of Pseudomonas solanacearum. In: Hayward A C, Hartman G L, editors. Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. Wallingford, United Kingdom: CAB International Press; 1994. pp. 157–177. [Google Scholar]
- 12.Hayward A C. The hosts of Pseudomonas solanacearum. In: Hayward A C, Hartman G L, editors. Bacterial wilt: the disease and its causative agent, Pseudomonas solanacearum. Wallingford, United Kingdom: CAB International Press; 1994. pp. 9–24. [Google Scholar]
- 13.Heijnen C, Page S, van Elsas J. Metabolic activity of Flavobacterium strain P25 during starvation and after introduction into bulk soil and the rhizosphere of wheat. FEMS Microbiol Ecol. 1995;18:129–138. [Google Scholar]
- 14.Hirsch P R. Population dynamics of indigenous and genetically modified rhizobia in the field. New Phytol. 1996;133:159–171. [Google Scholar]
- 15.Holmgren G G S, Meyer M, Chaney R, Daniels R. Cadmium, lead, zinc, copper, and nickel in agricultural soils of the United States of America. J Environ Qual. 1993;22:335–348. [Google Scholar]
- 16.Kell D, Kaprelyants A, Weichart D, Harwood C, Barer M. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 17.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 18.McDougald D, Rice S, Weichart D, Kjelleberg S. Nonculturability: adaptation or debilitation? FEMS Microbiol Ecol. 1998;25:1–9. [Google Scholar]
- 19.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pernezny K, Collins J. Epiphytic populations of Xanthomonas campestris pv. vesicatoria on pepper: relationships to host-plant resistance and exposure to copper sprays. Plant Dis. 1997;81:791–794. doi: 10.1094/PDIS.1997.81.7.791. [DOI] [PubMed] [Google Scholar]
- 21.Pommepuy M, Butin M, Derrien A, Fourmelon M, Colwell R R. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/aem.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman I, Shahamat M, Chowdhury M A R, Colwell R R. Potential virulence of viable but nonculturable Shigella dysenteriae type I. Appl Environ Microbiol. 1996;62:115–220. doi: 10.1128/aem.62.1.115-120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice W R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 24.Richaume A, Steinberg D, Jocteur-Monrozier L, Faurie G. Differences between direct and indirect enumeration of soil bacteria: the influence of soil structure and cell location. Soil Biol Biochem. 1993;25:641–643. [Google Scholar]
- 25.Saile E, McGarvey J, Schell M, Denny T. Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology. 1997;87:1264–1271. doi: 10.1094/PHYTO.1997.87.12.1264. [DOI] [PubMed] [Google Scholar]
- 26.Shaha S K, Shaha S N, Sangal S C. Recovery of injured Campylobacter jejuni cells after animal passage. Appl Environ Microbiol. 1991;57:3388–3389. doi: 10.1128/aem.57.11.3388-3389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Streeter J G. Failure of inoculant rhizobia to overcome the dominance of indigenous strains for nodule formation. Can J Microbiol. 1994;40:513–522. [Google Scholar]
- 28.Troxler J, Zala M, Moenne-Loccoz Y J, Keel C, Defago G. Predominance of nonculturable cells of the biocontrol strain Pseudomonas fluorescens CHA0 in the surface horizon of large outdoor lysimeters. Appl Environ Microbiol. 1997;63:3776–3782. doi: 10.1128/aem.63.10.3776-3782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turpin P, Maycroft K, Rowland C, Wellington E. Viable but non-culturable salmonellas in soil. J Appl Bacteriol. 1993;74:421–427. doi: 10.1111/j.1365-2672.1993.tb05149.x. [DOI] [PubMed] [Google Scholar]
- 30.Van Dyke M, Prosser J. Effect of cell density and attachment on resuscitation in soil of starved Pseudomonas fluorescens MON787. FEMS Microbiol Ecol. 1998;26:63–70. [Google Scholar]
- 31.van Elsas J D, van Overbeek L S. Bacterial responses to soil stimuli. In: Kjelleberg A, editor. Starvation in bacteria. New York, N.Y: Plenum; 1993. pp. 55–79. [Google Scholar]
- 32.van Elsas J D, Smalla K. Methods for sampling soil microbes. In: Hurst C H, et al., editors. Manual of environmental microbiology. Washington, D.C.: ASM Press; 1997. pp. 383–390. [Google Scholar]
- 33.van Overbeek L, Eberl L, Givskov M, Molin S, van Elsas J. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl Environ Microbiol. 1995;61:4202–4208. doi: 10.1128/aem.61.12.4202-4208.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams S T. Oligotrophy in soil: fact or fiction? In: Fletcher M, Floodgate G, editors. Bacteria in the natural environment: the effect of nutrient conditions. New York, N.Y: Academic Press; 1985. pp. 81–110. [Google Scholar]
- 35.Xu H, Roberts N, Singleton F, Atwell R, Grimes D, Colwell R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. FEMS Microbiol Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]