Abstract
Objective:
To determine if a 2-day protocol measuring pharmacokinetic and pharmacodynamic characteristics can demonstrate drug-drug interactions when smoked cannabis is added to orally administered hydrocodone/acetaminophen combination products.
Case Summary:
A 51-year-old non-Hispanic white male with chronic pain diagnoses participated in a 2-day pilot protocol. The participant attended two 7-hour in-lab days where he received 10 blood draws each day and completed self-administered pain and anxiety surveys. For both days, the participant took his prescribed dose of hydrocodone/acetaminophen (1/2 tablet of 7.5 mg/325 mg combination product) with the addition of 1 smoked pre-rolled marijuana cigarette (labeled as 0.5 g; 22.17% Δ9-tetrahydrocannabinol; 0.12% cannabidiol) on Day 2. Blood specimens were analyzed using mass spectrometry to quantify the difference of plasma hydrocodone levels between Day 1 and Day 2.
Results:
Compared to Day 1, lower levels of pain and anxiety were reported during Day 2 with the addition of cannabis to oral hydrocodone/acetaminophen. Day 2 pharmacokinetic analysis also revealed more rapid absorption and overall lower levels of hydrocodone in plasma.
Discussion:
Lower hydrocodone plasma levels in Day 2 may indicate cannabis’s effect on metabolism and reduce the risk of opioid toxicity. The quicker absorption rate of hydrocodone could explain lower pain and anxiety scores reported on the second day.
Conclusion and Relevance:
A 2-day protocol was able to capture differences across time in pharmacokinetic and pharmacodynamic measurements. Larger studies can be designed to better characterize the potential drug-drug interaction of cannabis and opioids.
Keywords: pain management, pharmacokinetics, drug interactions, medication safety, analgesics
Introduction and Background
The rising use of cannabis and cannabis-derived products in the United States and other countries increases the need for a more comprehensive understanding of the potential for cannabis-related drug-drug interactions (DDIs). To date, 36 states and 4 U.S. territories have legalized medicinal cannabis for treatment of conditions including multiple sclerosis, epilepsy, neuropathic pain, and mood disorders.1,2 Additionally, 18 states and 3 territories have approved measures to regulate adult-use cannabis. 2 Within the context of historically high rates of opioid overdoses in the U.S., the potential interaction of cannabinoids and opioids is of great interest. Emerging evidence suggests cannabis is being used as a substitute drug for opioids 3 and has been offered as a potential mitigation strategy to reduce overdose risk. 4
Cannabis sativa contains at least 60 cannabinoids that have been well-studied since the early 1960s. 5 The 2 of most interest to clinicians are, (1) (-)-trans Δ9- tetrahydrocannabinol (THC), the main psychoactive component that binds to the CB1 receptor and produces the euphoric sensation and (2) cannabidiol (CBD), the nonpsychoactive component which does not interact with CB1 and has been suggested to attenuate the behavioral effects of THC. 6 CB1 receptors in the brain are involved in both the rewarding effects and tolerance of opioids, as well as for a synergistic effect in the reduction of pain when both components (cannabis and opioids) are administered at sub-analgesic doses. 7 Yet, variations in drug metabolism due to interactions involving metabolizing enzymes may lead to an increased risk of adverse effects. 8 The benefits and harms of cannabis and opioid co-use remain unclear and is the focus of this case report.
After cannabis enters the body, it is rapidly metabolized via hydroxylation or oxidation by hepatic cytochrome P450 (CYP450) enzymes, followed by glucuronidation via the uridine 5′-diphospho-glucuronosyltransferase (UGT) enzymes, and finally excretion in the urine, bile, or feces.9-11 In the case of THC, the primary metabolic pathway is through hydroxylation to the active metabolite, 11-hydroxy (OH)-THC. Of note, 11-OH-THC can pass more readily through the blood-brain barrier and is a 3 to 7 times more potent activator of the CB1 receptor than THC. 12 Further, hydroxylation of 11-OH-THC leads to an inactive metabolite, 11-carboxy-THC (THC-COOH), which becomes glucuronidated to form THC-COOH-glucuronide (THC-Gluc) and is ultimately excreted. Although not as well studied, CBD metabolism proceeds through a similar pathway: hydroxylation followed by excretion either as an intact molecule or as glucuronide conjugates. 13 Overall, cannabinoids are highly lipophilic, eventually concentrating in adipose tissue, liver, lung and spleen, with slow release back into the bloodstream. 14 These properties lead to varying plasma concentrations of active and inactive metabolites that persist in the bloodstream for a wide timeframe after the initial consumption of cannabis. 15
Preliminary in vitro data indicate that several enzymes important in opioid metabolism are inhibited by THC and its metabolites, including CYP2D6 and UGT2B7, 2 of the major enzymes involved in the metabolism of hydrocodone and morphine.16,17 These interactions could, potentially, influence the pharmacokinetic effects of either or both of these drugs. Based upon these data, we hypothesized that DDI may occur in patients who concurrently use cannabis and hydrocodone. The degree to which THC influences the metabolism of opioids is poorly characterized and is of particular interest in the evaluation of patients who smoke cannabis and consume hydrocodone for pain relief. Through 2016, based on milligram of morphine equivalents, hydrocodone has been the most commonly sold, legally prescribed, opioid since 2002. 18
We describe in this case report the pharmacokinetics and dynamics, as expressed in blood plasma levels, and self-reported symptoms in an adult male who used both an oral hydrocodone/acetaminophen combination product and inhaled a cannabis product for pain relief. The primary objective in this paper is to determine whether a metabolic effect on opioid metabolism is observed in a subject who smoked cannabis. A secondary objective is to determine whether changes in self-reported symptoms of pain intensity and anxiety can be detected when smoked cannabis is added to hydrocodone/acetaminophen administration. The outcome can be used to inform future DDI investigations.
Case Report
A 51-year-old non-Hispanic white male with chronic pain diagnoses was recruited to test a protocol designed to study pharmacokinetics and dynamics of hydrocodone/acetaminophen alone and when co-administered with cannabis. The participant reported to an on-campus clinic 2 consecutive mornings to receive blood draws and complete questionnaires. On Day 1, the participant self-administered hydrocodone/acetaminophen (one-half tablet of the 7.5 mg/325 mg combination product). On Day 2, the participant smoked cannabis then self-administered the same dose of hydrocodone/acetaminophen.
Participant History
The participant described in this DDI case study reported daily moderate to severe chronic non-cancer pain from his back, neck, and feet and regular migraine headaches. He also reported a history of depression, anxiety, and an adjustment disorder related to post-traumatic stress disorder and heartburn. A review of his medical history also indicated multiple bouts of kidney stones. The participant’s medications included:
Wellbutrin XL (bupropion) 300 mg by mouth once daily for depression
Desyrel (trazodone) 25 mg orally by mouth for sleep and pain
Prilosec (omeprazole) 20 mg by mouth every morning for heartburn
Ativan (lorazepam) 0.5 mg by mouth as needed for anxiety and sleep
Norco (hydrocodone/acetaminophen) 7.5 mg/325 mg by mouth as needed for pain
The participant reported regular but not daily cannabis use.
Case Study Protocol
The protocol was approved by the Institutional Review Board at Washington State University. The primary aim was to examine whether observable differences in plasma levels of hydrocodone and its metabolites could be detected between two 7-hour days. Secondarily, we studied the pharmacodynamics of cannabis and hydrocodone by examining whether any differences between Day 1 and Day 2 could be captured in measurements of pain intensity and anxiety using a self-reported 0 to 10 Numeric Pain Scale and 0 to 10 Numeric Anxiety Scale.
On the morning of Day 1, the participant arrived at 8:00 am and was escorted to the clinical lab where a study nurse placed a 20-gage intravenous cannula. The participant confirmed no cannabis use 48 hours prior to the visit as required by the study protocol. Blood samples were drawn via 10 cc syringe and collected in a BD 367863 EDTA vacutainer tube. Blood was collected at baseline, 15 minutes, 30 minutes, and then hourly for a total of 10 specimens scheduled through the day over 7 hours (Figure 1). The participant self- administered his prescribed daily dose of oral hydrocodone 7.5 mg/acetaminophen 325 mg, one-half tablet, immediately following the baseline sample collection. Samples were carried to the on-campus lab in the College of Pharmacy and Pharmaceutical Sciences where they were immediately centrifuged at 1000 × g at room temperature for 10 minutes.
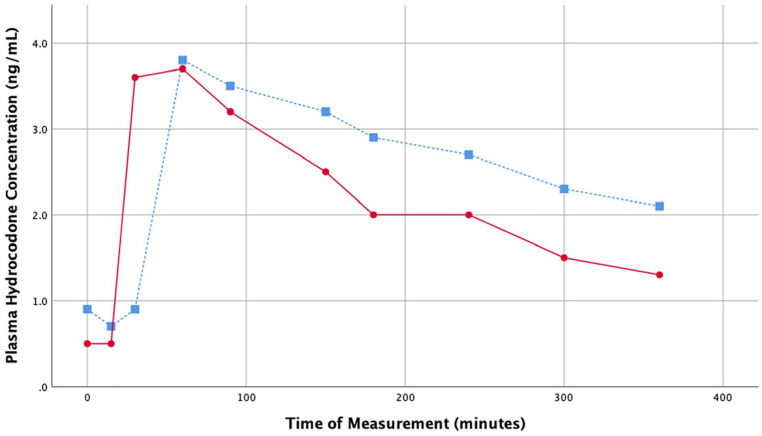
Figure 1.
Plasma hydrocodone concentrations over study period.
Note. Study Day 1 (hydrocodone/acetaminophen only) = blue square markers with dashed line. Study Day 2 (hydrocodone/acetaminophen and cannabis) = red circle markers with solid line. Patient plasma hydrocodone was quantified at 10 time points over 360 minutes; first assessment (0 minutes), 15, 30, 60, 90, 150, 180, 240, 300, and 360 minutes.
Questionnaires were administered hourly using a 0 to 10 Numeric Pain Scale and 0 to 10 Numeric Anxiety Scale. Additional health questionnaires were administered at baseline to capture participant characteristics: the Personal Health Questionnaire Depression Scale (PHQ-8), and the Generalized Anxiety Disorder 7-item scale (GAD-7), 19 the Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Interference 8a Scale, 20 and the PROMIS Sleep Disturbance 8a Scale. 21 These scales were chosen for their ease of use along with well-established reliability and validity that has been tested previously in populations prescribed opioids.22,23 In addition, the participant was also asked to keep a sleep/wake diary and wear an accelerometer (Actiwatch-2; Philips Respironics, Bend, OR) to track sleep/wake cycles for 3 days prior to and 3 days after the in-lab study days. This is a reliable procedure for determining total sleep duration. 24
On Day 2, the participant followed the identical in-lab blood sample and questionnaire protocol as Day 1, while also requiring the use of 1 pre-rolled marijuana cigarette to be smoked 30 minutes prior to arriving at the on-campus clinic. The participant was asked to use the product he most often purchased and reported consuming 1 pre-rolled 0.5 g (22.17% Δ9-tetrahydrocannabinol; 0.12% cannabidiol) sativa strain marijuana flower cigarette purchased at a legal recreational cannabis dispensary. The label was presented to researchers and indicated the product was named “Jack the Ripper” and produced by “The High Road.” The participant was paid to use a ride-share service to and from the lab. The participant was compensated at a total of $290: $10 for each completed blood draw, $15 for each day of study-mandated transport, and $15 bonus for finishing both study days.
Lab Protocols
Chemical standards and internal standards were obtained from Millipore-Sigma (St. Louis, MO). LCMS grade (Optima) methanol, acetonitrile, and formic acid were obtained from Fisher Scientific (Chicago, IL).
The plasma, buffy coat and red blood cell layers were each extracted and stored separately at −80°C. Samples were prepared by mixing 20 µL plasma with 70 µL LCMS grade methanol and 10 µL internal standard mix containing 5 ppm hydrocodone-D6. Samples were then centrifuged at 17 000 × g at 4°C for 15 minutes and the supernatant was transferred to an LCMS vial. Plasma hydrocodone levels were quantified using a ACQUITY XEVO TQD (Waters Corporation, Milford, MA), using a Waters HSS T3 column, 1.8 µm, 2.1 mm × 100 mm at 30°C. UPLC MS/MS was performed with a gradient elution using mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in methanol) at a flow rate of 0.4 mL/minute under the following conditions: 5% B for 1.0 minute, increasing to 95% B from 1.0 to 4.0 minutes, 95% B held for 2 minutes, followed by a return to initial conditions for 1.5 minutes, for a total time of 7.5 minutes. MS/MS detection of hydrocodone was performed in MRM ES+ mode at the mass transition m/z 300.20→199.20, with a cone voltage of 30 V and a collision energy of 32 eV. The internal standard (hydrocodone-D6) was monitored under the same conditions with a transition of m/z 300.20→199.20. Quantification of hydrocodone in plasma samples was performed against a standard curve, with a limit of quantification of 0.7 ng/mL. Plasma cannabinoid levels were quantified using the same extraction method, with the addition of an internal standard mixture containing 1 ppm of the following: THC-D3, 11-OH-THC-D3, THC-COOH-D3, and THC-Gluc-D3. Separation of cannabinoid compounds was achieved using the same chromatographic system as above, using a Waters BEH C18 column 1.7 µm, 2.1 mm × 50 mm, at 40°C. Additionally, the same gradient elution profile as above was used, however the composition of mobile phase B was changed to 0.1% formic acid in acetonitrile. Detection was cannabinoid compounds was performed in MRM ES+ mode using the following mass transitions: m/z 315.1→193.2 for THC, m/z 331.3→313.1 for 11-OH-THC, m/z 345.2→327.3 for THC-COOH, m/z 521.2→345.0 for THC-Gluc. Cone voltages (V) of 40, 40, 50, and 30, respectively, and collision energies (eV) of 25, 18, 20, and 27, respectively, were used for THC, 11-OH-THC, THC-COOH, THC-Gluc. Internal standards were detected using the same cone voltages and collision energies as their respective standards, with the following mass transitions: m/z 318.1→196.2 for THC-D3, m/z 334.3→316.1 for 11-OH-THC-D3, m/z 348.2→330.3 for THC-COOH-D3, m/z 523.2→348.0 for THC-Gluc-D3. Quantification of cannabis compounds in plasma samples was performed against a standard curve, with a limit of quantification of 1.2 ng/mL for THC, 1.1 ng/mL for 11-OH-THC, 16 ng/mL for THC-COOH, and 190 ng/mL for THC-Gluc.
Results
Pharmacokinetics
Plasma hydrocodone levels reached a maximum serum concentration (Cmax) at approximately 1 hour (Tmax) and was cleared faster (half-life; t½) on the second day of the study: 346 minutes on Day 1 versus 137 minutes on Day 2 (Figure 1, Table 1). The area under the curve was similar for both days: 0.95 on Day 1 and 0.80 on Day 2. One notable difference between the 2 days was the absorption rate, which appeared to be quicker on Day 2 than on Day 1, based on hydrocodone plasma level at the 30-minute time point (Day 1 = 0.9 ng/mL; Day 2 = 3.6 ng/mL). Low but detectable levels of THC and 11-OH-THC were observed at time points up to the 2-hour blood draw on Day 2 of the study. The absence of cannabis use on Day 1 was confirmed by observing no THC in the participant’s blood plasma at any of the time points on Day 1. THC-COOH and THC-Gluc were not detected in quantifiable levels in the serum at any of the time points examined in either day (data not shown).
Table 1.
Hydrocodone Pharmacogenetic Parameters.
| Time period | Cmax (ng/mL) | Tmax (min) | t1/2 (min) | AUC0−t* |
|---|---|---|---|---|
| Study Day 1 | 3.8 | 60 | 346 | 0.9465 |
| Study Day 2 | 3.7 | 60 | 137 | 0.7988 |
Note. Cmax = maximum concentration; Tmax = time of maximum concentration ; t1/2 = half-life; AUC0−t* = area under the curve from time point 0 to infinity.
Pharmacodynamics
The baseline questionnaires indicated a PHQ-8 score of 16, corresponding with major depressive symptoms, and a GAD-7 score of 13, corresponding with moderate anxiety symptoms. The participant’s PROMIS Pain Interference and Sleep Disturbance T-scores were 63.5 and 66.1 at baseline, respectively, indicating a higher pain interference and sleep disturbance rating than most healthy adults.
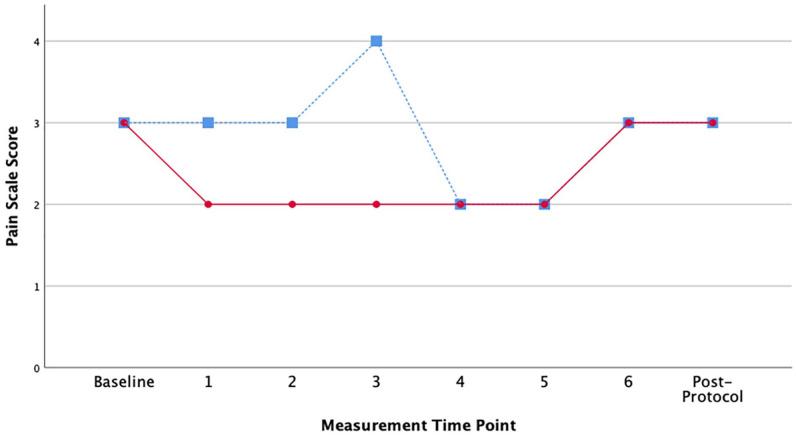
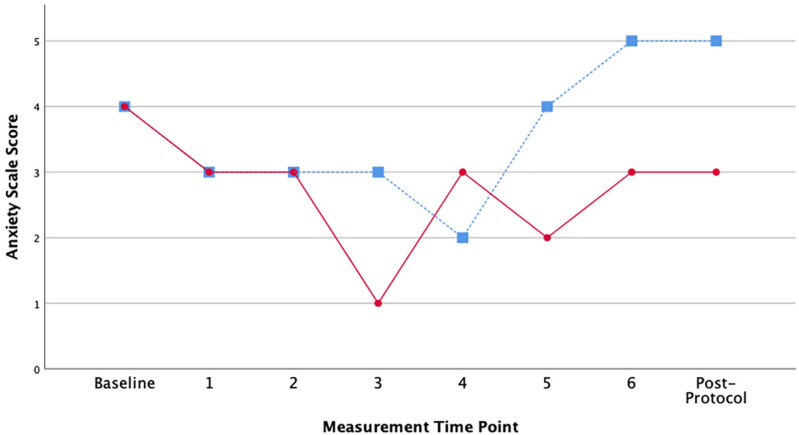
While reported pain intensity scores were low on average across all time points, Day 1 (without cannabis) scores were higher than the Day 2 values (with cannabis) earlier in the day when hydrocodone plasma levels were higher (Mean = 2.875, SD = 0.64 vs Mean = 2.375, SD = 0.52) (Figure 2). The anxiety scores followed a similar pattern, with Day 1 having higher anxiety overall, although the differences were more pronounced later in the day when hydrocodone plasma levels were dropping (Mean = 3.625, SD = 1.06 vs Mean = 2.75, SD = 0.886) (Figure 3). The participant’s actigraphy indicated average sleep duration the 3 nights before Day 1 in the lab (cannabis free) was 6.19 hours. After Day 2 (with cannabis) average sleep duration was 3.93 hours for 2 nights of recorded sleep. Of note, the night of Day 2 (the first night with cannabis) the participant did not sleep at all.
Figure 2.
Patient reported pain scores (0-10 scale) over study period.
Note. Study Day 1 (hydrocodone/acetaminophen only) = blue square markers with dashed line. Study Day 2 (hydrocodone/acetaminophen and cannabis) = red circle markers with solid line. Patient self-reported pain scores at 6 time points over 360 minutes (once every hour) as well as once at arrival (baseline) and at once after completing the study protocol.
Figure 3.
Patient reported anxiety scores (0-10 scale) over study period.
Note. Study Day 1 (hydrocodone/acetaminophen only) = blue square markers with dashed line. Study Day 2 (hydrocodone/acetaminophen and cannabis) = red circle markers with solid line. Patient self-reported anxiety scores at 6 time points over 360 minutes (once every hour) as well as once at arrival (baseline) and at once after completing the study protocol.
Discussion
The pharmacokinetic results in our case study demonstrate that changes in hydrocodone plasma levels can be captured after smoked cannabis is added to an oral opioid. Additionally, we were able to capture changes across time using simple self-report instruments measuring pain intensity and anxiety. Our participants’ symptom results were also expected with improvements in perceived pain intensity and anxiety on Day 2 when cannabis was added. Many who use opioid medications for pain management also report using cannabis to treat pain, anxiety, and related symptoms.25,26 The lower plasma level of opioids on Day 2 could indicate some effect on metabolism. It appears that hydrocodone was absorbed faster on Day 2, potentially leading to the observed lower pain and anxiety scores. The lower opioid level on Day 2 could also indicate, possibly, that the risk of opioid toxicity was reduced when cannabis was added. In the context of historically high rates of opioid overdose, the news that cannabis may aid in comfort while not heightening hydrocodone blood plasma levels is encouraging. At a minimum, less hydrocodone levels on Day 2 suggests that smoking a single cannabis cigarette was not likely any more dangerous from an opioid overdose standpoint. In fact, it could be reasonable to ask whether cannabis may even be protective for this individual by lowering opioid levels. It is also possible that adding cannabis actually interfered with metabolism and could even decrease the effectiveness of the opioids. However, given the low levels of THC and 11-OH-THC, and no detectable THC-COOH and THC-Gluc observed in the plasma of the subject on Day 2, it is also possible that the 0.5 g dose of smoked marijuana as performed in this study may not be sufficient to inhibit hydrocodone metabolism in vivo, with the variability in hydrocodone metabolism observed between study Day 1 and study Day 2 caused by the participant’s natural daily variation.
Cannabis has been suggested as a harm reduction strategy for people who are using opioids. In some cases, patients using medical cannabis to treat intractable pain have been found to significantly reduce the quantity of opioids utilized in treatment based on milligram of morphine equivalents. 27 There are a lack of studies evaluating the effects of cannabis when used in combination with opioids to effectively treat pain and the corresponding adverse effects. The drug interaction probability scale (DIPS), an objective scale used to assess the causation of DDI in the clinical setting, 28 was applied to the 2-day evaluation period. Based on the standard set of 10-questions, a probable interaction (total score = 6) was observed between orally administered hydrocodone/acetaminophen and inhalation of combusted cannabis (Table 2). In the clinical setting, as with this case, there are often multiple confounders, such as multiple medical conditions and prescribed medications, which complicate the identification of potential DDIs, but is aided by completing objective review with pharmacokinetic and dynamic information as well as other clinical tools such as the DIPS. Also, not every individual administered 2 interacting medications will experience a clinically relevant event, so it is of importance to document potential DDIs to aid in patient care.
Table 2.
Drug Interaction Probability Scale.
| Question | Score | Comment |
|---|---|---|
| Are there previous credible reports of this interaction in humans? | Yes (+1) | There has been at least one published study indicating that marijuana use alters the measured opioid levels when used for pain management 29 |
| Is the observed interaction consistent with the known interactive properties of precipitant drug (cannabis)? | Yes (+1) | There were no unexpected results observed on Day 2 of the study—day that marijuana was used prior to arrival at the clinic; all observed events were consistent with documented effects of cannabis |
| Is the observed interaction consistent with the known interactive properties of the object drug (hydrocodone/acetaminophen)? | Yes (+1) | There were no unexpected results observed on Day 1 or Day 2 of the study; hydrocodone was ingested prior to arrival at the clinic on both days; all observed events were consistent with documented effects of hydrocodone |
| Is the event consistent with the known or reasonable time course of the interaction (onset and/or offset)? | Yes (+1) | All observed events and laboratory results occurred within a reasonable pharmacokinetic timeline of both orally administered hydrocodone and inhaled marijuana |
| Did the interaction remit upon de-challenge of the precipitant drug (cannabis) with no change in the object drug (hydrocodone/acetaminophen)? | Unknown (0) | Current study did not continue monitoring of the patient after Day 2—future study may warrant examination of hydrocodone/acetaminophen levels upon de-challenge of cannabis |
| Did the interaction reappear when the precipitant drug (cannabis) was re-administered in the presence of continued use of object drug (hydrocodone/acetaminophen)? | Unknown (0) | There was no re-administration of cannabis and continued monitoring of patient after Day 2—future study may warrant re-exposure of cannabis with continual monitoring of patient and hydrocodone/acetaminophen levels |
| Are there reasonable alternative causes for the event? | Unknown (0) | Although all effects are consistent, there is the possibility that the patient was not completely honest with the researchers |
| Was the object drug (hydrocodone/acetaminophen) detected in the blood or other fluids in concentrations consistent with the proposed interaction? | Yes (+1) | See Table 1 and Figure 1 for additional information on pharmacokinetic data |
| Was the drug interaction confirmed by any objective evidence consistent with the effects on the object drug (hydrocodone/acetaminophen) other than drug concentrations? | Yes (+1) | See Table 1 and Figure 1 for additional information on pharmacokinetic data |
| Was the interaction greater when the precipitant drug (cannabis) dose was increased or less when the precipitant drug (cannabis) dose was decreased? | Unknown (0) | There were no dose ranging studies performed |
| Total score | 6 | There is a “probable” drug-drug interaction between marijuana and hydrocodone/acetaminophen |
Although sleep disturbance was not noted in the review of the participant’s medical history, it was noted that multiple prescribed medications were indicated for sleep and the participant scored high on the sleep disturbance scale. Possibly the participant suffered some cannabis withdrawal symptoms that caused disrupted sleep. While it was self-reported that he did not use cannabis daily as a requirement for study eligibility, we could not verify. Our participant’s sleep duration was seriously curtailed after cannabis was added on Day 2, as measured by actigraphy. The participant’s overall sleep habits were irregular; therefore, this variation may not be related to cannabis use. Poor sleep is also a symptom commonly reported by adults with chronic pain and one that is often self-managed with cannabis.25,26 Future investigations on the effect of cannabis on sleep are therefore warranted.
Limitations of our case-study report include the single patient format and inability to control the amount or type of cannabis smoked due to federal and state restrictions regarding cannabis. Also, due to the pilot nature of this study, a physical assessment and regular vital sign checks were not completed and could have helped to validate changes in subjective anxiety and pain reporting. Our participant also had multiple comorbidities and other medications that could confound the results. Specifically, lorazepam and acetaminophen have anxiety and pain-relieving properties, and could contribute to the patient’s perceived levels of discomfort throughout the study duration. In addition, several of the other medications, including bupropion, trazadone, omeprazole, and acetaminophen are metabolized in the liver by enzymes that have been shown to be inhibited by cannabis and its metabolites.16,17 These additional DDI effects could contribute to the overall pharmacodynamics and pharmacokinetics of the drugs in this study and may be having an effect on the patient’s level of pain and anxiety. Nonetheless, the combination of acetaminophen and hydrocodone is the most commonly prescribed opioid product, 18 providing a realistic drug profile for a patient with pain. While a hydrocodone-only product was approved by the Food and Drug Administration in 2013, it has not been prescribed with the same frequency as the combination products that include acetaminophen. 18
The decision to ask our participant to smoke cannabis and arrive to the campus lab safely was feasible and the protocol with multiple blood draws and questionnaires was well-tolerated. Numerous variables can affect drug metabolism and a case study of one person cannot be generalized to the larger population. Yet, we were able to show that collecting in-lab hourly plasma levels was feasible and, potentially, sufficient to capture changes in metabolism over a day’s time. Future studies could include quantification of all potential DDI, including medications which have not been prescribed for pain management, as well as the possibility of better control over variability in CBD/THC administration when permissible by federal and local laws.
Conclusions and Relevance
Our previous research found that cannabis and opioids are used together regularly by approximately 50% of adults who are prescribed opioids for pain.26,30 Yet, much of the available research on cannabis use is inconsistent, often of poor quality, and fraught with limitations due to the vast number of cannabis-based products. 31 A critical need exists to improve the quality and breadth of cannabis research for people with pain who use opioids. This case study was intended to test our protocol and collect pharmacokinetic and pharmacodynamic data concurrently, allowing for a synchronous look at opioid plasma levels and reported effects. After adding inhaled cannabis, plasma hydrocodone levels did not increase, in fact they decreased, potentially leading to decreased hydrocodone toxicity. Performing replication studies with multiple patients is necessary for confidence in the effects of cannabis on hydrocodone toxicity. Future studies could add other variables, such as a physical assessment at study entry, regular vital signs such as heart rate and blood pressure, as well as respiratory assessments such as respiratory rate and pulse oximetry, or sedation. This would allow for potential physiological differences between the multiple daily assessment timepoints as well as Day 1 and Day 2 to be detected, while validating self-reported pain and anxiety scales as well as explore interactions further and help address questions about the role of cannabis in opioid overdose risks. Future DDI investigations can adapt this protocol for larger samples with more rigorous designs that control for opioid and cannabis dose and types of cannabis smoked including using single ingredient opioids rather than combination products as was the case for this report. As the use of cannabis grows with loosening state regulations, the need for high quality evidence increases.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The state of Washington and Washington State University had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript or the decision to submit the paper for publication.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This investigation was supported in part by funds provided by the State of Washington Initiative Measure No. 502.
ORCID iD: Ross Jason Bindler  https://orcid.org/0000-0002-7259-2549
https://orcid.org/0000-0002-7259-2549
References
- 1. Freeman TP, Hindocha C, Green SF, Bloomfield MAP. Medicinal use of cannabis based products and cannabinoids. BMJ. 2019;365:l1141. doi: 10.1136/bmj.l1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Conference of State Legislatures. State medical marijuana laws. Accessed October 25, 2021. https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx
- 3. Corroon JM, Jr, Mischley LK, Sexton M. Cannabis as a substitute for prescription drugs—a cross-sectional study. J Pain Res. 2017;10:989-998. doi: 10.2147/JPR.S134330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999-2010. JAMA Intern Med. 2014;174(10):1668-1673. doi: 10.1001/jamainternmed.2014.4005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770-1804. doi: 10.1002/cbdv.200790152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hložek T, Uttl L, Kadeřábek L, et al. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur Neuropsychopharmacol. 2017;27(12):1223-1237. doi: 10.1016/j.euroneuro.2017.10.037 [DOI] [PubMed] [Google Scholar]
- 7. Roberts JD, Gennings C, Shih M. Synergistic affective analgesic interaction between delta-9-tetrahydrocannabinol and morphine. Eur J Pharmacol. 2006;530(1-2):54-58. doi: 10.1016/j.ejphar.2005.11.036 [DOI] [PubMed] [Google Scholar]
- 8. Wahawisan J, Kolluru S, Nguyen T, Molina C, Speake J. Methadone toxicity due to smoking cessation—a case report on the drug-drug interaction involving cytochrome P450 isoenzyme IA2. Ann Pharmacother. 2011;45(6):e34. doi: 10.1345/aph.1P759 [DOI] [PubMed] [Google Scholar]
- 9. Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I. Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes. Life Sci. 2007;80(15):1415-1419. doi: 10.1016/j.lfs.2006.12.032 [DOI] [PubMed] [Google Scholar]
- 10. Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613-624. doi: 10.1016/S0025-6196(11)60750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89(5-6):165-170. doi: 10.1016/j.lfs.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 12. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327-360. doi: 10.2165/00003088-200342040-00003 [DOI] [PubMed] [Google Scholar]
- 13. Ujváry I, Hanuš L. Human metabolites of cannabidiol: a review on their formation, biological activity, and relevance in therapy. Cannabis Cannabinoid Res. 2016;1(1):90-101. doi: 10.1089/can.2015.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101-106. doi: 10.1192/bjp.178.2.101 [DOI] [PubMed] [Google Scholar]
- 15. Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clin Chem. 2016;62(12):1579-1592. doi: 10.1373/clinchem.2016.263475 [DOI] [PubMed] [Google Scholar]
- 16. Al Saabi A, Allorge D, Sauvage F-L, et al. Involvement of UDP-glucuronosyltransferases UGT1A9 and UGT2B7 in ethanol glucuronidation, and interactions with common drugs of abuse. Drug Metab Dispos. 2013;41(3):568-574. doi: 10.1124/dmd.112.047878 [DOI] [PubMed] [Google Scholar]
- 17. Bansal S, Maharao N, Paine MF, Unadkat JD. Predicting the potential for cannabinoids to precipitate pharmacokinetic drug interactions via reversible inhibition or inactivation of major cytochromes P450. Drug Metab Dispos. 2020;48(10):1008-1017. doi: 10.1124/dmd.120.000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Food and Drug Administration. FDA analysis of long-term trends in prescription analgesic products: quantity, sales, and price trends. FDA. Accessed January 28, 2021. https://www.fda.gov/media/111695/download [Google Scholar]
- 19. Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi: 10.1016/j.genhosppsych.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 20. Cook KF, Schalet BD, Kallen MA, Rutsohn JP, Cella D. Establishing a common metric for self-reported pain: linking BPI Pain Interference and SF-36 Bodily Pain Subscale scores to the PROMIS Pain Interference metric. Qual Life Res. 2015;24(10):2305-2318. doi: 10.1007/s11136-015-0987-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6-24. doi: 10.1080/15402002.2012.63626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781-792. doi: 10.1093/sleep/33.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Askew RL, Cook KF, Revicki DA, Cella D, Amtmann D. Evidence from diverse clinical populations supported clinical validity of PROMIS pain interference and pain behavior. J Clin Epidemiol. 2016;73:103-111. doi: 10.1016/j.jclinepi.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342-392. doi: 10.1093/sleep/26.3.342 [DOI] [PubMed] [Google Scholar]
- 25. Sexton M, Cuttler C, Finnell JS, Mischley LK. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 2016;1(1):131-138. doi: 10.1089/can.2016.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilson M, Finlay M, Orr M, et al. Engagement in online pain self-management improves pain in adults on medication-assisted behavioral treatment for opioid use disorders. Addict Behav. 2018;86:130-137. doi: 10.1016/j.addbeh.2018.04.019 [DOI] [PubMed] [Google Scholar]
- 27. O’Connell M, Sandgren M, Frantzen L, Bower E, Erickson B. Medical cannabis: effects on opioid and benzodiazepine requirements for pain control. Ann Pharmacother. 2019;53(11):1081-1086. doi: 10.1177/1060028019854221 [DOI] [PubMed] [Google Scholar]
- 28. Horn JR, Hansten PD, Chan LN. Proposal for a new tool to evaluate drug interaction cases. Ann Pharmacother. 2007;41(4):674-680. doi: 10.1345/aph.1H423 [DOI] [PubMed] [Google Scholar]
- 29. Goggin MM, Shahriar BJ, Stead A, Jannis GC. Reduced urinary opioid levels from pain management patients associated with marijuana use. Pain Manag. 2019;9(5):441-447. doi:10.2217/pmt-2019-0017 [DOI] [PubMed] [Google Scholar]
- 30. Clem SN, Bigand TL, Wilson M. Cannabis use motivations among adults prescribed opioids for pain versus opioid addiction. Pain Manag Nurs. 2020;21(1):43-47. doi: 10.1016/j.pmn.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 31. Inglet S, Winter B, Yost SE, et al. Clinical data for the use of cannabis-based treatments: a comprehensive review of the literature. Ann Pharmacother. 2020;54(11):1109-1143. doi: 10.1177/1060028020930189 [DOI] [PubMed] [Google Scholar]