Abstract
Background: Vancomycin requires therapeutic drug monitoring (TDM) based on its pharmacokinetic properties, and guidelines have shifted to analyzing area under the curve over 24 hours (AUC24) rather than trough concentrations due to nephrotoxicity concerns and correlation to efficacy. Obesity is an established risk factor for vancomycin-induced nephrotoxicity due to increased drug exposure based on dosing calculations and volume of distribution estimation. The aim of this study is to assess the relationship between AUC-based versus trough-based dosing and nephrotoxicity among obese patients receiving vancomycin. Methods: This research project was conducted as a retrospective, observational, single-centered study which included obese adults who received at least 48 hours of vancomycin. The electronic medical record provided data for patients with vancomycin pharmacokinetic consults either evaluated with trough-only or AUC-based dosing. The primary objective was to compare the development of nephrotoxicity after vancomycin initiation, while secondary objectives included vancomycin loading dose exposure, total daily dose of vancomycin, and whether target TDM was attained. Nominal data were evaluated utilizing the chi-square test and continuous data using the independent samples t-test or Mann-Whitney test. The a priori level of significance was .05. Data analysis was performed using Microsoft Excel and SAS statistical software. Results: Two hundred fifty-four patients were included in the primary analysis. Four patients in the AUC cohort (6.3%) developed nephrotoxicity compared to 32 (17.4%) in the trough cohort (P = .035). Both cohorts received a median of 4 days of therapy; however, the median loading dose per actual body weight in the AUC cohort was 20 mg/kg as compared to 16 mg/kg in the trough cohort. Of the 130 patients with available TDM in the trough cohort, 97 (74.6%) did not meet target attainment as compared to 15 of the 57 in the AUC cohort (26.3%) (P < .001). Conclusions: AUC dosing was associated with a statistically significant reduction in AKI occurrence despite overall higher loading dose exposure as compared to the trough cohort. Though maintenance dose exposure was similar between both cohorts, patients in the AUC cohort maintained therapeutic concentrations at a higher percentage than the trough cohort.
Keywords: anti-infectives, clinical services, drug/medical use evaluation, infectious diseases, monitoring drug therapy, pharmacokinetics
Background
Vancomycin, a glycopeptide antibiotic that prevents cell wall synthesis through binding the cell wall precursor D-alanyl-D-alanine, remains one of the primary antibiotic utilized for empiric methicillin-resistant Staphylococcus aureus (MRSA) coverage worldwide. 1 Early manufacturing processes resulted in vancomycin formulations containing impurities, leading to its representation as “Mississippi mud.” Despite modern purified formulations, nephrotoxicity remains a major concern with vancomycin utilization. 2 Therapeutic drug monitoring (TDM) targets utilized with vancomycin based on its pharmacokinetic properties for maximal efficacy and minimal toxicity have recently been updated in the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society of America and the Society of Infectious Diseases Pharmacists’ guidelines published in June 2020. Previously, guidelines focused on targeting trough concentrations (10-20 mg/L) as a surrogate for a ratio of area under the curve over 24 hours to minimum inhibitory concentration (AUC24/MIC) of ≥400 assuming an MIC of ≤1 mg/L. Target trough concentrations for severe infection ranged from 15 to 20 mg/L; however, such concentrations have been associated with increased propensity for nephrotoxicity. 3 The most recent consensus guidelines have shifted to analyzing area under the curve over 24 hours (AUC24) for TDM as trough concentrations alone demonstrate poor correlation with AUC values. Suzuki et al 4 found that AUC24 values from 600 to 800 mg h/L were associated with acute kidney injury (AKI) as compared to 400-600 mg h/L, while Finch et al 5 found an independent association with individualized AUC dosing and a decrease in AKI (OR 0.52 [95% CI 0.34-0.80], P = .003]).
The association of vancomycin and AKI development was demonstrated in one meta-analysis with a relative risk of 2.45 [95% CI 1.69-3.55]. AKI usually developed within 4 to 17 days after vancomycin was initiated. 6 Various risk factors have been established for vancomycin-associated AKI which include concurrent nephrotoxic medications (ie, loop diuretics, aminoglycosides, amphotericin B, intravenous contrast dye, as well as piperacillin/tazobactam when used concurrently with vancomycin), pre-existing renal dysfunction, critical illness, and obesity. 7
Vancomycin-associated nephrotoxicity in obese patients has been attributed to increased drug exposure as dosing calculations are based on actual body weight (ABW). A key component to vancomycin pharmacokinetic dosing is volume of distribution (Vd). Although vancomycin Vd has been demonstrated to increase with body fat, studies have shown it does not necessarily increase in a proportional fashion. 8 The new consensus guidelines note various analyses demonstrating this information and have recommended estimating Vd as 0.5 L/kg in obese adults compared to Vd of 0.7 L/kg in patients with average weight. The guidelines recommend utilizing lower milligram/kilogram (mg/kg) dosing for obese patients to accommodate for the nonlinear relationship between Vd and body weight. Compared to typical loading doses of 25 to 30 mg/kg, lower doses of 20 to 25 mg/kg are recommended with a maximum dose of 3000 mg. Guidelines recommend generally not exceeding 4500 mg/day for empiric maintenance. 3
Our study focused specifically on the obese population as data on AUC-based approaches is limited within this subgroup. Obesity is defined as a BMI ≥ 30 kg/m2 and is stratified in 3 classes (class I obesity = 30-34.9 kg/m2, class II obesity = 35-39.9 kg/m2, and class III obesity, or morbid obesity = ≥40 kg/m2). According to the Centers for Disease Control and Prevention (CDC), the prevalence of obesity in the United States has increased from 30.8% to 42.4% from 1999 to 2018. 9 As of 2019, Kentucky ranks as the fifth highest state in the country for obesity rates with 36.6% obesity reported. 10
At our 217-bed community hospital, a trough-only TDM approach was utilized for vancomycin dosing until 2019. For trough based dosing, typically a loading dose of 25 to 30 mg/kg ABW capped at 2000 mg (an institutional decision) was delivered followed by a regimen of 15 to 20 mg/kg ABW at an interval determined by calculation of population based pharmacokinetic parameters. A trough level would be obtained at presumed steady state, typically with the fourth or fifth maintenance dose. In 2019 an AUC-based dosing scheme was implemented in an attempt to optimize vancomycin outcomes and mitigate adverse events including nephrotoxicity. During this conversion, the previous institutional imposed maximum loading dose of 2000 mg was increased but still limited to 2500 mg based on prescriber consensus. For AUC based dosing 2 vancomycin serum concentrations are obtained after the distribution phase of the first dose (the first 1-2 hours after the end of dose infusion and the second at the time of a single estimated half-life). Volume of distribution (Vd), drug elimination (k) and calculated half-life (t1/2) are then calculated and a patient specific dose and dosing interval are determined. Follow up levels are obtained at presumed steady state, typically with the fourth or fifth maintenance dose.
Methods
This was a retrospective, observational, single center study that compared the development of nephrotoxicity in obese patients receiving vancomycin either based on trough-only or AUC-based TDM. Due to its design, the study was reviewed by the Catholic Health Initiatives Institution for Research and Innovation Institutional Review Board and determined to be exempt according to federal regulations. The initial cohort data were selected from various vancomycin administration reports at Saint Joseph East (a 217-bed community hospital in Lexington, Kentucky) from February 2017 to August 2020. As our institution transitioned to AUC-based dosing in March 2019, this served as an appropriate division of the study’s 2 cohorts. The trough cohort data ranged from February 2017 to February 2019, and the AUC cohort data ranged from March 2019 to August 2020. The electronic medical record was surveyed for patient specific parameters including demographics (age, gender, weight, height, body-mass index, comorbidities and location [general medical ward vs intensive care unit]), serum creatinine (baseline and follow up), vancomycin dosing as documented in the medication administration record (MAR), vancomycin TDM concentrations, and other potential nephrotoxic medications documented in the MAR. Nephrotoxic medications were included if administered for at least 24 prior to or concomitantly with vancomycin. One-time doses of contrast dye were included. This study listed the following medications as nephrotoxic: loop diuretics (furosemide, bumetanide, torsemide, or ethacrynic acid), nonsteroidal anti-inflammatory agents (ibuprofen, naproxen, ketorolac, celecoxib, diclofenac, or indomethacin), iopamidol, antimicrobials (aminoglycosides, amphotericin B, piperacillin/tazobactam, or intravenous acyclovir), and other medications (angiotensin converting enzyme inhibitors or angiotensin receptor blockers). Specific comorbidities assessed included chronic kidney disease; chronic viral illness defined as history of HIV and/or hepatitis C; and cardiovascular disease defined as history of myocardial infarction, angina, heart failure, stroke or intermittent claudication as documented in the EMR. Patients who were considered for inclusion were at least 18 years of age with a calculated BMI of at least 30 kg/m2 and received at least 48 hours of vancomycin. BMI was divided per obesity class. Initially, inclusion criteria was based on 72 hours of vancomycin administration; however, due to the limited sample available, this was adjusted to 48 hours for a more robust evaluation. Patients were excluded if they had documented allergies to glycopeptides, were pregnant or breastfeeding, were on baseline hemodialysis, or had received vancomycin at an outside facility.
The primary objective of this study was to compare the development of nephrotoxicity after vancomycin initiation. As nephrotoxicity has been defined by multiple organizations, the primary outcome was nephrotoxicity as defined by a simplified Kidney Disease: Improving Global Outcomes (KDIGO) score (an increase in serum creatinine (SCr) ≥ 0.3 mg/dL within 48 hours) to provide a more inclusive outcome. The KDIGO parameter of a decrease in urine output was removed as it was not possible to obtain consistently recorded urine output data outside of the intensive care unit. Nephrotoxicity according to RIFLE criteria (risk, injury, failure, loss, end stage kidney disease) was utilized as a secondary outcome (an increase in SCr at least 2 times baseline SCr within 1 to 7 days persisting for at least 24 hours). Other secondary outcomes included vancomycin loading dose calculations, total daily dose of vancomycin, and target TDM attainment. Total daily dose of vancomycin was defined as the total milligrams of vancomycin received within a 24-hour period as displayed on the MAR. Target attainment for trough-based dosing was determined per individual clinical pharmacist recommendations but was limited to either 10 to 20 mg/L for non-severe infections such as cellulitis or 15 to 20 mg/L for more severe infections including (but not limited to) bacteremia, sepsis, moderate to severe skin infections, bone and joint infections, endocarditis and pneumonia. All AUC cohort TDM targets were defined as an AUC of 400 to 600 mg h/L per guideline recommendations.
Statistical Analysis
Nominal data were evaluated utilizing the chi-square test, and continuous data were evaluated with either the independent samples t-test if parametric or Mann-Whitney test if non-parametric. All findings were reported with descriptive statistics. The a priori level of significance was .05. Data analysis was performed using Microsoft Excel and SPSS statistical software.
Results
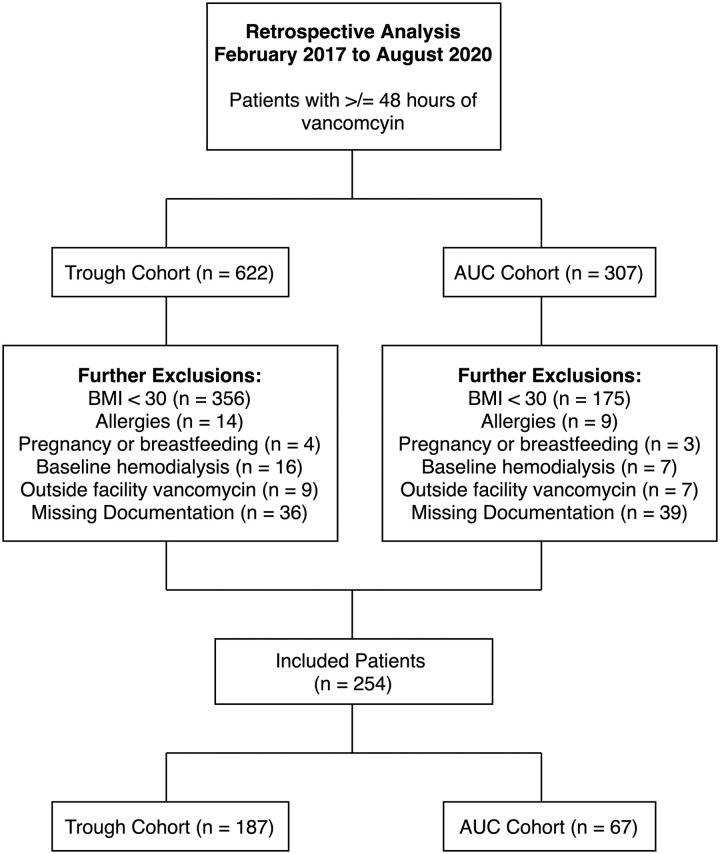
Surveyed vancomycin administration reports at our institution included 929 patients who had received at least 48 hours of vancomycin from February 2017 to August 2020 as shown in Figure 1. After further inclusion and exclusion criteria, the remaining sample size was 254 with 187 patients in the trough cohort and 67 patients in the AUC cohort. As patient charts were reviewed, 531 patients (57.2%) were excluded due to BMI below 30, 23 (2.5%) for documented allergies to vancomycin, 7 (0.8%) for pregnancy or breastfeeding, 23 (2.5%) for baseline hemodialysis requirements, and 16 (1.7%) for receipt of vancomycin at outside facilities. An additional 75 patients (22.8%) were excluded upon data collection due to the patient being unable to receive a complete dose at the specified dosing time or interval, or an inability to obtain clinically evaluable serum drug levels.
Figure 1.
Eligibility criteria.
There were no statistically significant differences among baseline characteristics between the trough and AUC cohorts as seen in Table 1. The majority of patients were female (54.3%) and were located on the general medicine floor (73.6%). The mean age was 59.0 and 55.7 years in the trough and AUC cohorts, respectively. The median ABW was 110 kg in both cohorts, and the majority of patients were classified as class I or class II obesity (63.0%). The most common comorbidities included diabetes (43.7%), cardiovascular disease (24.4%), and chronic kidney disease (7.1%). Although 7.1% of patients had a history of chronic kidney disease, the median serum creatinine prior to vancomycin administration was 0.97 and 0.92 mg/dL in the trough and AUC cohorts respectively without any statistically significant differences noted. The only statistically significant difference among other nephrotoxic medications included contrast dye (11.8% in the trough cohort vs 20.9% in the AUC cohort, P = .041). Overall, 57 patients (22.4%) received loop diuretics, 19 (7.5%) received nonsteroidal anti-inflammatory agents, 36 (14.2%) received contrast dye, 112 (44.1%) received antimicrobials.
Table 1.
Baseline Characteristics.
| Demographic (n = 254) | Trough cohort (n = 187) | AUC cohort (n = 67) | P-Value |
|---|---|---|---|
| Sex | |||
| Male, n (%) | 87 (46.5) | 29 (43.3) | .65 |
| Female, n (%) | 100 (53.5) | 38 (56.7) | |
| Age, years, mean (SD) | 59.0 (44.8-73.2) | 55.7 (41.3-70.1) | .47 |
| ABW, kg, median (IQR) | 109.6 (80.5-138.7) | 110.0 (80.9-139.1) | .70 |
| BMI, n (%) | |||
| Class I | 78 (41.7) | 25 (37.3) | .73 |
| Class II | 41 (21.9) | 16 (23.9) | |
| Class III | 67 (35.8) | 26 (34.3) | |
| Location, n (%) | |||
| General medicine floor | 156 (83.4) | 31 (86.6) | .54 |
| Intensive care unit | 31 (16.6) | 9 (13.4) | |
| Documented comorbidities, n (%) | |||
| CKD | 14 (7.5) | 4 (6.0) | .54 |
| Diabetes mellitus, type 2 | 80 (42.7) | 31 (46.2) | .62 |
| Chronic viral illness | 7 (3.7) | 3 (4.5) | .62 |
| Cardiovascular disease | 48 (25.7) | 14 (20.9) | .79 |
| Nephrotoxic medications, n (%) | |||
| Loop diuretics | 44 (23.5) | 13 (19.4) | .64 |
| NSAID | 16 (8.6) | 3 (4.5) | .33 |
| Contrast dye | 22 (11.8) | 14 (20.9) | .04 |
| Antimicrobials | 84 (44.9) | 28 (41.8) | .95 |
| Others (ACE inhibitor or ARB) | 43 (23.0) | 13 (19.4) | .97 |
| Serum creatinine, mg/dL, median | 0.97 | 0.92 | .91 |
Note. ABW = actual body weight; CKD = chronic kidney disease; NSAID = nonsteroidal anti-inflammatory; ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker.
When observing the incidence of KDIGO nephrotoxicity, 32 patients (17.4%) in the trough cohort developed acute kidney injury as compared to 4 (6.3%) in the AUC cohort (P = .035). Similarly, 9 patients (4.8%) developed acute kidney injury according to RIFLE criteria in the trough cohort compared to 1 (1.6%) in the AUC cohort (P = .256). Overall, the median duration of therapy for both cohorts was 4 days (P = .943). Patients in the trough cohort received a median of 2188 mg of vancomycin per day as compared to 2083 mg of vancomycin per day in the AUC cohort (P = .787). Of the 163 patients who received loading doses, the median loading dose per actual body weight for patients in the trough cohort was 16 mg/kg and 20 mg/kg in the AUC cohort (P < .001). The median loading dose was 2000 mg for the trough cohort and 2500 mg for the AUC cohort (P < .001). Of the 187 patients (130 in the trough cohort and 57 in the AUC cohort) who underwent steady state pharmacokinetic (SSPK) evaluation, 40 (30%) in the trough cohort, and 25 (44%) in the AUC cohort achieved target attainment (P < .001). Sixty-seven patients (35.8%) with steady state pharmacokinetic analysis in both cohorts (50 in the trough cohort and 17 in the AUC cohort) could not be evaluated for target attainment due to documentation inconsistencies. Of the 112 patients with SSPK evaluation who did not reach target attainment, 27 (28%) in the trough cohort and 3 (20%) in the AUC cohort were above goal (P = .524) (Table 2).
Table 2.
Outcomes.
| Outcomes (n = 254) | Trough cohort (n = 187) | AUC cohort (n = 64) | P-Value |
|---|---|---|---|
| Primary outcome | |||
| KDIGO nephrotoxicity, n (%) | 32 (17.4) | 4 (6.3) | .04 |
| Secondary outcomes | |||
| RIFLE nephrotoxicity, n (%) | 9 (4.8) | 1 (1.6) | .26 |
| Duration of therapy, days, median (IQR) | 4.0 (2.0-6.0) | 4.0 (2.0-6.0) | .94 |
| Total daily dose, mg, median (IQR) | 2188 (1049-3327) | 2083 (943-3223) | .79 |
| Total LD, mg, median (IQR) | 2000 (1625-2375) | 2500 (2000-3000) | <.001 |
| LD per ABW, mg/kg, median (IQR) | 16 (11-21) | 20 (17-23) | <.001 |
| Target attainment, n (%) | 40 (30) | 25 (44) | <.001 |
Note. LD = loading dose.
Discussion
The findings of this study suggest that there is a statistically significant correlation between the utilization of AUC-based dosing and the reduced risk of nephrotoxicity in obese patients receiving vancomycin. Moreover, our study demonstrated that despite patients in the AUC cohort receiving guideline-recommended loading doses and similar total daily doses of maintenance therapy as compared to the trough cohort, the AUC cohort had a statistically significant association with therapeutic target attainment as compared to higher rates of subtherapeutic targets in the trough cohort.
Previous studies have demonstrated that trough-only TDM for the general patient population receiving vancomycin may lead to increased risk of nephrotoxicity. Bosso et al 11 colleagues found upwards of a 3-fold risk in nephrotoxicity with trough-based dosing after multivariate analysis including consideration of additional nephrotoxins and comorbidities among other factors (OR 3.643, 95% CI 1.749-7.587). In one meta-analysis that compared 14 observational trials in patients with Staphylococcus aureus bacteremia, trough concentrations >15 mg/dL were not associated with reduction in key outcomes such as mortality or persistent bacteremia as compared to statistically significant reduction of such outcomes with AUC-based dosing. 12 However, the role of AUC-based TDM reducing nephrotoxicity in obese patients remains understudied. Generally data available for obese patients receiving vancomycin via AUC-based dosing stems from subgroup analyses. One simulation reported by Langton et al 13 demonstrated that targeting AUC values rather than trough values led to an overall lower exposure to vancomycin in obese patients. As the major risk for nephrotoxicity in obese patients hinges on increased drug exposure, this provides insight into the potential benefits of AUC-based dosing.
Defining terms remains an important component of study design, and ensuring an appropriate definition for acute kidney injury was key to the applicability of this study. AKI is defined by various methods which may lead to inconsistencies in individual patient evaluation for vancomycin-associated nephrotoxicity. Both the KDIGO and Acute Kidney Injury Network (AKIN) have defined AKI as an increase in serum creatinine of ≥0.3 mg/dL within 48 hours.14,15 RIFLE—or risk, injury, failure, loss, end stage renal disease (ESRD)—categorizes kidney injury as an increase in serum creatinine ≥2 times above baseline within 1 to 7 days that persists over 24 hours. 16 As compared to KDIGO or AKIN definitions, RIFLE criteria limits the number of patients who would be classified as developing acute kidney injury.
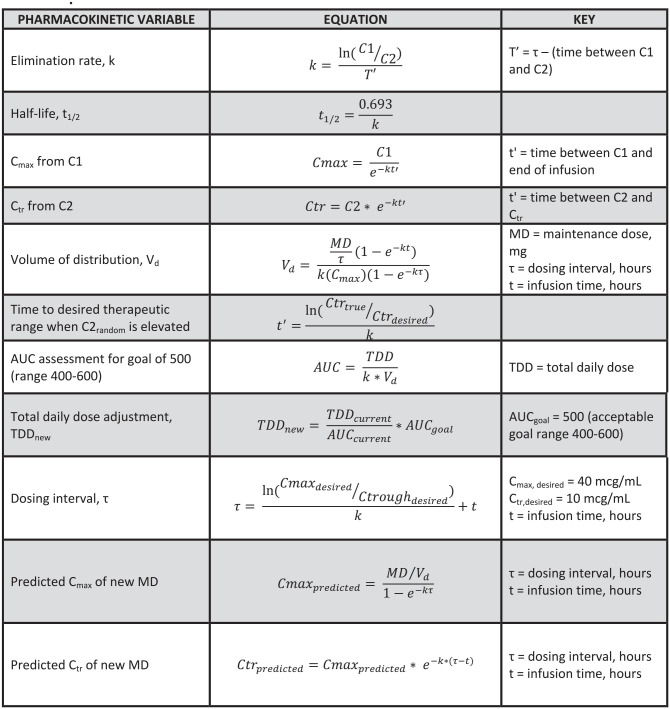
There were several limitations to this study. Firstly, guideline recommendations for vancomycin TDM are focused on serious infections caused by MRSA. Due to sample size limitations within our smaller community hospital, any patient who received vancomycin with appropriate inclusion and exclusion criteria was evaluated regardless of indication or pathogen. Although this is extrapolating recommendations beyond the updated guidelines, vancomycin TDM approaches that are recommended specifically for MRSA infections continue to be utilized beyond this scope among many institutions. Secondly, the risk of inaccurate documentation is always a factor with retrospective studies. Our understanding of patient-specific dosing considerations were limited to available documentation in the EMR. There may have been inconsistencies in medication administration by nursing or among dosing decisions and calculations by individual pharmacists that were beyond our ability to assess. Calculations for pharmacokinetic evaluation and dosing can be found in Figure 2. The equations were built into a Microsoft Excel spreadsheet for convenience. Thirdly, patients in the trough cohort may have demonstrated subtherapeutic steady state concentrations due to institutional policy that previously limited loading doses to 2000 mg prior to AUC-based dosing. Our target attainment results may not have been statistically significant had our institution already allowed for a maximum loading dose of 2500 mg for more rapid attainment of therapeutic drug concentrations. Based on this hypothesis, higher rates of nephrotoxicity in the trough cohort would likely be due to a secondary factor aside from vancomycin. Although we may demonstrate correlation with nephrotoxicity in this study, this cannot lead to definitive conclusions about causality. Additionally, in our study drug exposure was determined to be similar among both cohorts yet the AUC cohort demonstrated a decreased incidence of nephrotoxicity. Although the reason may be difficult to elucidate why, the inability of population based pharmacokinetic parameter estimates to accurately describe the volume of distribution of the obese patient may be a factor. It is possible that, even with a therapeutic trough, a smaller than expected volume of distribution could lead to significantly elevated peak serum concentrations and subsequent AUCs greater than the defined therapeutic range.
Figure 2.
Equations used for pharmacokinetic evaluation.
Given the fact that the baseline demographics did not demonstrate any statistical differences among cohorts, there may have been an additional factor correlated with nephrotoxicity that our study did not consider. As this study is limited to descriptive statistics, no further differentiation between confounding factors such as nephrotoxic medications or age could be assessed. Additionally, due to the timeframe of our facility’s transition to AUC dosing, the number of trough-based patient samples far exceeds the number of AUC-based patient samples. Due to limited statistical resources within our facility, no propensity score matching could be accomplished for more involved statistical analysis.
Additional limitations include potential bias with extending inclusion criteria to 48 hours of vancomycin administration rather than 72 hours. As vancomycin-associated acute kidney injury generally develops within 4 days of therapy, our results could be underestimated in either cohort due to this shortened time interval. As our study was retrospective in nature, desired outcomes such as all-cause mortality at a specific time period after vancomycin administration or even 30-day readmission rates would be difficult to collect as patients may not have further follow-up at our institution. Prospective studies would need to be completed in order to assess these outcomes. Further, the number of patients meeting RIFLE criteria may be underestimated as many were discharged prior to evaluating if their SCr increase persisted for 24 hours. As total daily dose was based on the amount of vancomycin documented in the MAR, overall average total daily dose could also be underestimated as patients who were discharged in the morning or early afternoon did not receive a full 24 hours of therapy on their last day of therapy.
Overall, our institution illustrated that our current practices in utilizing AUC-based dosing as compared to trough-based dosing correlated with a reduction in acute kidney injury despite an overall higher loading dose exposure. Of note, although not statistically significant, patients in the AUC cohort received lower overall total daily dose exposure with maintenance doses as compared to the trough cohort which would theoretically decrease the risk of acute kidney injury as expected per previously discussed studies. This provides relevant information to indicate the need for further research on the use of AUC-based TDM with obese patients and the risk of nephrotoxicity.
Conclusion
AUC-based dosing was associated with a statistically significant reduction in AKI occurrence despite overall higher loading dose exposure as compared to the trough cohort. Though maintenance dose exposure was similar between both cohorts, patients in the AUC cohort maintained therapeutic concentrations at a higher percentage than the trough cohort. Further studies are warranted to illustrate the potential association of decreased drug exposure and nephrotoxicity when utilizing AUC-based pharmacokinetics in obese patients receiving vancomycin.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amanda Wolfe  https://orcid.org/0000-0001-7195-087X
https://orcid.org/0000-0001-7195-087X
References
- 1. Mylan Institutional LLC. Vancomycin hydrochloride [package insert]. Mylan Institutional LLC; 1958. [Google Scholar]
- 2. Elting LS, Rubenstein EB, Kurtin D, et al. Mississippi mud in the 1990s: risks and outcomes of vancomycin-associated toxicity in general oncology practice. Cancer. 1998;83(12):2597-2607. doi: [DOI] [PubMed] [Google Scholar]
- 3. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;71(11):835-864. doi: 10.1093/cid/ciaa303 [DOI] [PubMed] [Google Scholar]
- 4. Suzuki Y, Kawasaki K, Sato Y, et al. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant Staphylococcus aureus pneumonia. Chemotherapy. 2012;58(4):308-312. doi: 10.1159/000343162 [DOI] [PubMed] [Google Scholar]
- 5. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. doi: 10.1128/AAC.01293-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kellum JA, Lameire N, Aspelin P, et al. Kidney Disease: Improving Global Outcomes (KDIGO). Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. doi: 10.1038/kisup.2012.1 [DOI] [Google Scholar]
- 7. Carreno JJ, Kenney RM, Lomaestro B. Vancomycin-associated renal dysfunction: where are we now? Pharmacotherapy. 2014;34(12):1259-1268. doi: 10.1002/phar.1488 [DOI] [PubMed] [Google Scholar]
- 8. Blouin RA, Bauer LA, Miller DD, Record KE, Griffen Wo., Jr. Vancomycin pharmacokinetics in normal and morbidly obese subjects. Antimicrob Agents Chemother. 1982;21(4):575-580. doi: 10.1128/AAC.21.4.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2018-2018. NCHS Data Brief No. 360. Centers for Disease Control and Prevention. 2020. Accessed August 29, 2020. https://www.cdc.gov/nchs/products/databriefs/db360.htm [PubMed] [Google Scholar]
- 10. Cross A. Adult obesity in Kentucky reaches all-time high of 36.6%, fifth in U.S. Kentucky Health News. 2019. Accessed August 29, 2020. https://ci.uky.edu/kentuckyhealthnews/2019/09/16/adult-obesity-in-ky-reaches-all-time-high-of-36-6-fifth-in-u-s-doctor-says-insurance-needs-to-start-covering-obesity-prevention/
- 11. Bosso JA, Nappi J, Rudisill C, et al. Relationship between vancomycin trough concentrations and nephrotoxicity: a prospective multicenter trial. Antimicrob Agents Chemother. 2011;55(12):5475-5479. doi: 10.1128/AAC.00168-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Prybylski JP. Vancomycin trough concentration as a predictor of clinical outcomes in patients with Staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy. 2015;35:889-898. doi: 10.1002/phar.1638 [DOI] [PubMed] [Google Scholar]
- 13. Langton MM, Ahern JW, MacDougall J. An AUC target simulation for vancomycin in patients with class III obesity. J Pharm Pract. 2021;34:577-580. doi: 10.1177/0897190019885241 [DOI] [PubMed] [Google Scholar]
- 14. Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004;8:R204-R212. doi: 10.1186/cc2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sinha Ray A, Haikal A, Hammoud KA, Yu AS. Vancomycin and the risk of AKI: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2016;11(12):2132-2140. doi: 10.2215/CJN.05920616 [DOI] [PMC free article] [PubMed] [Google Scholar]