Abstract
Background:
While mucosal healing (MH) and transmural healing (TH) predict relevant clinical outcomes in Crohn’s disease (CD), little is known about the real significance and clinical impact of deep remission (DR).
Objectives:
To better explore the concept of DR, toward a direct correlation between MH, TH, and biomarkers.
Design:
Real-world observational longitudinal study to evaluate the rate of clinical remission (CR), MH and TH, and the fecal calprotectin (FC)/C-reactive protein (CRP) levels in all consecutive CD patients on biologics.
Methods:
A receiver operating characteristic (ROC) curve was constructed to define the best FC and CRP cut-offs associated with MH and TH. Finally, patients achieving CR, MH, and TH, in association with the target FC/CRP values, were considered in DR.
Results:
Among 118 CD patients, CR, MH, and TH were achieved in 62.7, 44.1, and 32.2%, respectively. After 2 years, the mean FC levels decreased from 494 ± 15.4 μg/g to 260 ± 354.9 μg/g (p < 0.01). Using the ROC curve analysis, an FC cut-off value of 94 μg/g was associated with both MH [sensitivity: 94.2%, specificity: 84.8%, positive predictive value (PPV): 83.05%, negative predictive value (NPV): 94.92%, area under the curve (AUC): 0.95] and TH (sensitivity: 92.1%, specificity: 70%, PPV: 64.4%, NPV: 94.9%, AUC: 0.88). CRP < 5 mg/L was associated with both MH (sensitivity: 96.1%, specificity: 62.1%, PPV: 66.7%, NPV: 95.35%, AUC: 0.85) and TH (sensitivity: 97.4%, specificity: 52.5%, PPV: 52%, NPV: 95.35%, AUC: 0.78). When considering CD patients with concomitant CR, MH, and TH associated with an FC < 94 μg/g and CRP < 5 mg/L, this association was found identified in 33 patients (27.9%).
Conclusion:
An FC < 94 μg/g and a normal CRP are associated with CR, MH, and TH and could be included in the definition of DR in association. So by definition, DR could be achieved in approximately 30% of CD patients during maintenance treatment with biologics.
Keywords: biologics, Crohn’s disease, deep remission, fecal calprotectin, mucosal healing, transmural healing
Introduction
Crohn’s disease (CD) is a transmural, chronic, and life-threatening inflammatory disease, which often leads to surgery due to complications such as strictures, fistulae, and abscesses.1–3 To prevent such complications and the subsequent irreversible bowel damage, the cornerstone of the treatment is the disease remission, which should ideally comprise clinical, biochemical, mucosal, and transmural healing (TH), although the ultimate therapeutic target in CD is still a matter of debate. 4
Practically, Abundant evidence has previously shown that mucosal healing (MH), defined as the endoscopic healing of any inflamed tract of the mucosal layer, is a pivotal outcome in CD able to predict lower request for steroids, hospitalization, and surgery in the years following treatment.5–9
More recently, some studies have ‘explored the deep’, demonstrating that TH, defined as the normalization of the bowel wall thickness (BWT) of all inflamed segments involved in CD,3,10–16 is associated with improved long-term outcomes in CD by reducing clinical relapses, hospitalization, and surgery rates, even better than MH.
Thanks to this new evidence, in the last few years, the concept of deep remission (DR) has changed from clinical and endoscopic remission (MH) 17 to combined clinical, endoscopic (MH), and TH. 4
The current definition of DR does not take into account the role of biochemical markers, such as C-reactive protein (CRP) and fecal calprotectin (FC), which have demonstrated a prognostic significance. In particular, FC is able to predict long-term clinical outcomes when measured 12 weeks after the onset of medical treatment. 18 The STRIDE-II systematic review supported using an FC cut-off value of 150 mg/g to identify MH. 4 Although the correlation between FC and endoscopic findings has been well-explored, very few studies report the relationship between FC and TH.
Similarly, CRP, which is widely used in clinical practice but is outperformed by FC, is characterized by high specificity but low sensitivity19,20 and has been associated with response to treatment and risk of relapse. 21
Due to the importance of the treat-to-target strategy, further studies are urgently needed to evaluate the new endpoints (i.e. TH, DR) that take into account the entire complexity of the disease.
The aim of the present study was to better explore the concept of DR in CD, toward the correlation between MH, TH, and biochemical markers, especially FC.
Materials and methods
Study population and study design
From 2018 to 2021, we carried out a real-world observational, longitudinal, prospective study to evaluate the rate of clinical remission (CR), MH, and TH in all consecutive adult patients with CD undergoing maintenance treatment with biologics (infliximab, adalimumab, vedolizumab, and ustekinumab) for 2 years at our tertiary Inflammatory Bowel Disease (IBD) Unit of ‘Federico II’ University Medical School in Naples, Italy. For each included subject, we investigated the CRP and FC levels associated with CR, MH, and TH in order to better assess the concept of DR.
The CD diagnosis was made in accordance with current European Crohn’s and Colitis Organization (ECCO) guidelines. 22 The indications for the use of biologics in CD patients had followed ECCO and Italian guidelines.22,23
All patients underwent clinical assessment, laboratory exams, including CRP and FC evaluation, at our centralized laboratory, and bowel sonography (BS) and endoscopy before and after a 2-year treatment period with biologics, as routinely performed at our center. All these evaluations were performed within 14 days. Based on the combined clinical, endoscopic, and sonographic findings, all patients that participated in the study were divided into three groups: (a) the TH group, which included patients who had achieved TH plus MH; (b) the MH group, which comprised patients who showed MH only; and (c) the CR group, which included patients with CR, but who displayed pathological BS and endoscopy.
We excluded all subjects without FC and/or CRP evaluation, who were treated with biologics for less than 2 years and who refused endoscopic examination. Furthermore, we excluded all subjects who underwent biologic treatment for prophylaxis of post-operative recurrence.
Stool samples were collected at the patients’ homes, refrigerated at 2°C–8°C, then sent to our centralized laboratory to be processed and analyzed according to the manufacturer’s instructions (Calprest; Eurospital Spa, Trieste, Italy). FC levels were expressed as µg/g of feces. An FC value >120 µg/g was considered ‘positive’ or indicative of active inflammation; a CRP value <5 mg/L was considered ‘negative’, while a result higher than 5 mg/L was considered ‘positive’.
The reporting of this study conforms to the STROBE statement. 24
Clinical remission
In accordance with ECCO statements, 22 steroid-free CR was identified in the presence of Harvey–Bradshaw index <5 in patients who did not need treatment with systemic steroids or budesonide.
Mucosal healing
Ileocolonoscopy was used to assess MH. It was performed with a conventional colonoscope (Olympus Exera CV-190) by two expert operators (NI and AR) who were blinded to the outcome of the other diagnostic procedures, after standard bowel cleansing with a split dose of 4-L solution of polyethylene glycol. The endoscopic activity of CD and occurrence of MH both at the start of the study and during the follow-up were assessed using the Simple Endoscopic Score for Crohn’s Disease (SES-CD).9,25,26 MH was identified in the presence of endoscopic remission in the entire bowel by a per-patient evaluation (SES-CD ⩽ 2). 9 An esophagogastroduodenoscopy was performed in case of involvement of the proximal gastrointestinal tract by CD.
Transmural healing
BS was used to evaluate TH. It was performed at the start of the study and every 3 months during the follow-up period after overnight fasting using a Logiq S7 ultrasound system with linear and convex probes (5–9 MHz). Neither special preparation nor contrast and/or paralytic agents were administered. The ultrasound procedure was performed by two experienced gastroenterologists (FC and AT) who were blinded to the outcomes of other diagnostic procedures. Each patient underwent a systematic scanning of the abdomen.
BWT was considered normal for values ⩽3 mm. 27 Positive BS was defined as the occurrence of concentric and regular increased BWT > 3 mm. 28 The BS definition of complicated disease was defined in accordance with the literature. 28 In particular, strictures were diagnosed in the presence of thickened (>4 mm) intestinal wall, narrowed intestinal lumen, and fluid-distended or echogenic content-filled loops just above the thickened intestinal tract. Furthermore, enterocutaneous, enteroenteric, and enteromesenteric fistulas were scored when hypoechoic duct-like structures with fluid or air content were seen between skin and intestinal loops, between two intestinal loops, or between intestinal loop and mesentery, respectively. 28 TH was identified and recorded with BS by a global per-patient evaluation. In accordance with existing evidence, a decrease in BWT to values ⩽3 mm was considered diagnostic of TH.28,29 Moreover, sonography was able to identify antral or duodenal inflamed or healed segments in all enrolled cases.
Statistical analysis
Data were analyzed using the Statistical Package for Social Sciences (SPSS software v.15.0, Chicago, IL, United States) for Windows and the StatsDirect Statistical Software (v.3.0). The descriptive statistics used included determination of mean values and standard deviations (SDs), or medians and interquartile ranges, of the continuous variables, and of percentages and proportions of the categorical variables.
Statistical analysis was performed using chi-square, the Mann–Whitney U-test, Student’s t-test, or analysis of variance (ANOVA), as appropriate.
A receiver operating characteristic (ROC) curve was constructed to define the best FC and CRP cut-off values associated with MH and TH. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the curve (AUC), with the respective 95% confidence interval (95% CI), were calculated. Finally, CD patients who achieved CR, MH, or TH, in association with the target FC and CRP values, were considered to have achieved DR. In addition, the agreement (k) between MH/FC and TH/FC, and between FC and CRP, was calculated. A p-value < 0.05 was considered significant.
Results
The study included 118 patients with CD (males 65.3%; mean age 36.1 ± 15.1) who enrolled in our Unit and completed a 2-year treatment phase. Seventy-nine of them (66.9%) were treated with anti-tumor necrosis factor (TNF) alpha (infliximab or adalimumab), 18 subjects (15.3%) with vedolizumab, and 15 (12.7%) with ustekinumab. Six subjects (5.1%) received a combination treatment with anti-TNF alpha plus azathioprine. Furthermore, 18 out of 85 patients (21.2%) treated with anti-TNF alpha agents and 6 out of 18 subjects (33.3%) treated with vedolizumab, were escalated during the first 6 months of therapy. Their baseline features of all patients are shown in Table 1.
Table 1.
Baseline features of study population.
| Crohn’s disease (118) | |
|---|---|
| Male gender, n (%) | 77 (65.3) |
| Age at enrollment (years), mean ± SD | 36.1 ± 15.1 |
| Disease duration (months), mean ± SD | 123.4 ± 104.5 |
| Age at diagnosis, n (%) | |
| A1 | 32 (27.1) |
| A2 | 67 (56.8) |
| A3 | 19 (16.1) |
| Disease location, n (%) | |
| L1 | 23 (19.5) |
| L2 | 8 (6.8) |
| L3 | 84 (71.2) |
| L4 | 3 (2.5) |
| Disease behavior, n (%) | |
| B1 | 46 (39) |
| B2 | 52 (44.1) |
| B3 | 20 (16.9) |
| Perianal disease, n (%) | 30 (25.4) |
| Smoking habits, n (%) | |
| Yes | 36 (30.5) |
| Not | 59 (50) |
| Ex | 23 (19.5) |
| Familial history, n (%) | 23 (19.5) |
| Need for steroids at diagnosis, n (%) | 60 (50.8) |
| Previous surgery, n (%) | 58 (49.2) |
| Previous thiopurines exposure, n (%) | 47 (39.8) |
| Extra-intestinal manifestations, n (%) | 48 (40.7) |
| Biologic, n (%) | |
| IFX | 10 (8.5) |
| ADA | 75 (63.6) |
| VDZ | 18 (15.3) |
| UST | 15 (12.7) |
| Combination therapy, n (%) | 6 (5.1) |
Combination therapy: the concomitant association of anti-TNF alpha and immunomodulators such as azathioprine or 6-mercaptopurine.
ADA, adalimumab; SD, standard deviation; IFX, Infliximab; n, number; TNF, tumor necrosis factor; UST, ustekinumab; VDZ, vedolizumab.
All patients who participated in the study underwent clinical exam, laboratory investigation for CRP and FC, and sonographic and endoscopic evaluation both before starting the use of biologics and 2 weeks to completion of the 2-year treatment course with biological drugs. These assessments revealed a CR rate of 62.7% (74 subjects out of 118); the prevalence of MH was 44.1% (52 out of 118 subjects), whereas TH was found in 38 patients (32.2%). All patients with TH also showed MH on endoscopic evaluation.
At baseline, the mean FC level of the entire population was 494 ± 515.4 μg/g, whereas after 2 years the FC level decreased to 260 ± 354.9 μg/g (p < 0.01). More specifically, the FC level decreased from 307.5 ± 248.2 μg/g to 45.4 ± 31.3 μg/g in the TH group (p < 0.01), from 384.5 ± 355.8 μg/g to 44.9 ± 27.8 μg/g in the MH group (p < 0.01), and from 523.4 ± 591.7 μg/g to 105.9 ± 156.9 μg/g in the CR group (p < 0.01) (Table 2). No differences were seen in FC levels between the TH and MH groups 2 years from the onset of treatment with biologics, but subjects with CR displayed higher FC levels than those with MH or TH (p < 0.01) (Table 3).
Table 2.
Clinical, laboratory, endoscopic, and sonographic findings at baseline and after 2 years.
| Baseline | After 2 years | p | |
|---|---|---|---|
| Clinical | |||
| HBI, mean ± SD | 6.69 ± 1.32 | 5.19 ± 2.11 | <0.001 |
| Laboratory | |||
| CRP levels (mg/L), mean ± SD | 11.75 ± 14.84 | 10.11 ± 18.78 | 0.63 |
| FC levels (μg/g), mean ± SD | 493.83 ± 515.45 | 260.15 ± 354.93 | <0.001 |
| Endoscopy | |||
| SES-CD, mean ± SD | 9.65 ± 4.84 | 5.45 ± 5.27 | <0.001 |
| Bowel sonography | |||
| BWT at BS (mm), mean ± SD | 6.57 ± 1.66 | 4.81 ± 2.39 | <0.001 |
| Disease extension at BS (cm), mean ± SD | 15.47 ± 7.79 | 9.4 ± 10.1 | <0.001 |
Significant differences have been highlighted in bold.
BS, bowel sonography; BWT, bowel wall thickness; CRP, C-reactive protein; HBI, Harvey-Bradshaw index; FC, fecal calprotectin; SD, standard deviation; SES-CD, Simple Endoscopic Score for Crohn’s Disease.
Table 3.
Fecal calprotectin and CRP levels according with TH, MH, and CR.
| Baseline FC | After 2 years FC | p | |
|---|---|---|---|
| TH (n = 38) | 307.53 ± 248.23 | 45.45 ± 31.26 | <0.001 |
| MH (n = 52) | 384.48 ± 355.86 | 44.92 ± 27.78 | <0.001 |
| CR (n = 74) | 523.4 ± 591.76 | 105.9 ± 156.89 | <0.01 |
| Baseline CRP | After 2 years CRP | p | |
| TH (n = 38) | 11.1 ± 15.58 | 2.5 ± 1.76 | <0.001 |
| MH (n = 52) | 11.3 ± 14.04 | 2.4 ± 1.66 | <0.001 |
| CR (n = 74) | 13.2 ± 17.85 | 5 ± 13.32 | <0.01 |
Significant differences have been highlighted in bold.
CR, clinical remission; CRP, C-reactive protein, results are expressed as mg/L; FC, fecal calprotectin, results are expressed as μg/g; MH, mucosal healing; TH, transmural healing.
Moreover, patients with colonic or ileocolonic CD showed higher but insignificant FC levels than isolated ileal CD patients both at baseline (530.4 ± 561.3 μg/g versus 402.7 ± 364.1 μg/g, p = 0.3) and during follow-up (274.3 ± 384.5 μg/g versus 217.1 ± 251.7 μg/g, p = 0.5), without differences in the rate of CR, MH, and TH according to the disease location.
Similarly, the CRP level decreased from 11.7 ± 14.8 mg/L at baseline in the entire study group to 10.1 ± 18.7 mg/L after 2 years, although this decrease was not significant (p = 0.6) (Table 2). However, the CRP level significantly decreased from 11.1 ± 15.6 mg/L to 2.5 ± 1.7 mg/L in the TH group (p < 0.01), from 11.3 ± 14.04 mg/L to 2.4 ± 1.6 mg/L in the MH group (p < 0.01), and from 13.2 ± 17.8 mg/L to 5 ± 13.3 mg/L in the CR group (p < 0.01) (Table 3). Table 4 summarizes the differences between FC and CRP levels in patients that reached or did not reach any outcome (TH, MH, or CR).
Table 4.
Differences in FC and CRP levels after 2 years of treatment according with any achieved outcome.
| TH yes (38/118) | TH not (80/118) | p | |
|---|---|---|---|
| FC (μg/g), mean ± SD | 45.45 ± 31.26 | 391.68 ± 362.12 | <0.001 |
| CRP (mg/L), mean ± SD | 2.5 ± 1.76 | 21.91 ± 13.7 | 0.002 |
| MH yes (52/118) | MH not (66/118) | p | |
| FC (μg/g), mean ± SD | 44.92 ± 27.78 | 429.73 ± 399.8 | <0.001 |
| CRP (mg/L), mean ± SD | 2.4 ± 1.66 | 23.41 ± 16.2 | <0.001 |
| CR yes (74/118) | CR not (44/118) | p | |
| FC (μg/g), mean ± SD | 156.89 ± 105.9 | 519.56 ± 437.24 | <0.001 |
| CRP (mg/L), mean ± SD | 13.32 ± 5 | 23.21 ± 18.7 | <0.001 |
Significant differences have been highlighted in bold.
CR, clinical remission; CRP, C-reactive protein; FC, fecal calprotectin; MH, mucosal healing; TH, transmural healing.
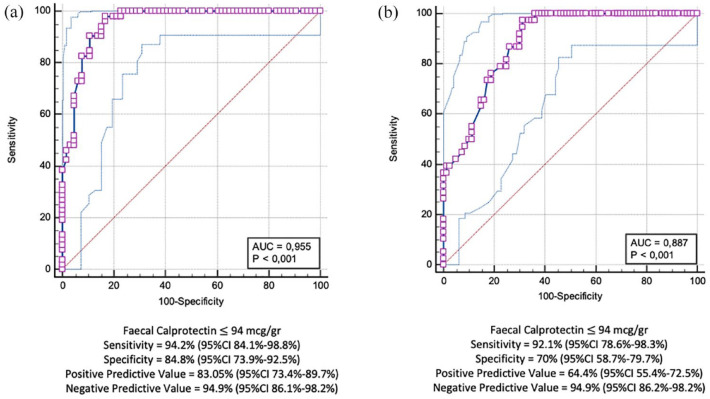
The ROC curve analysis showed that an FC cut-off value of 94 μg/g was associated with both MH (sensitivity: 94.2%, 95% CI: 84.1–98.8%, specificity: 84.8%, 95% CI: 73.9–92.5%, PPV: 83.05%, 95% CI: 73.4–89.7%, NPV: 94.92%, 95% CI: 86.1–98.2%, AUC: 0.95) (Figure 1(a)) and TH (sensitivity: 92.1%, 95% CI: 78.6–98.3%, specificity: 70%, 95% CI: 58.7–79.7%, PPV: 64.4%, 95% CI: 55.4–72.5%, NPV: 94.9%, 95% CI: 86.2–98.2%, AUC: 0.88) (Figure 1(b)). The MH/FC and TH/FC k of agreement was 0.81 (p < 0.01) and 0.58 (p < 0.01), respectively.
Figure 1.
Receiver operating characteristic (ROC) curve showing the best fecal calprotectin cut-off value for MH (a) and TH (b).
AUC, area under the curve; 95% CI, 95% confidence interval; MH, mucosal healing; TH, transmural healing.
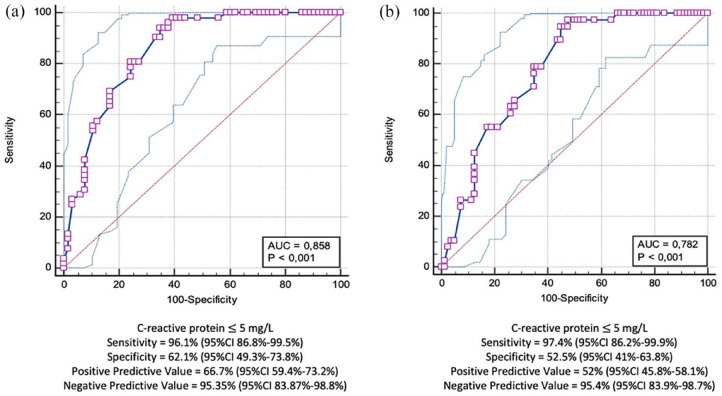
Moreover, a CRP cut-off value of 5 mg/L (also named ‘negative’ CRP) was associated with both MH (sensitivity: 96.1%, 95% CI: 86.8–99.5%, specificity: 62.1%, 95% CI: 49.3–73.8%, PPV: 66.7%, 95% CI: 59.4–73.2%, NPV: 95.35%, 95% CI: 83.87–98.78%, AUC: 0.85) (Figure 2(a)) and TH (sensitivity: 97.4%, 95% CI: 86.2–99.9%, specificity: 52.5%, 95% CI: 41–63.8%, PPV: 52%, 95% CI: 45.8–58.1%, NPV: 95.35%, 95% CI: 83.9–98.7%, AUC: 0.78) (Figure 2(b)). The MH/CRP and TH/CRP k of agreement was 0.63 (p < 0.01) and 0.54 (p < 0.01), respectively.
Figure 2.
Receiver operating characteristic (ROC) curve showing the best CRP cut-off value for MH (a) and TH (b).
AUC, area under the curve; 95% CI = 95% confidence interval; MH, mucosal healing; TH, transmural healing.
The FC/CRP k of agreement was 0.82 (p < 0.01) for MH and 0.74 (p < 0.01) for TH.
When considering CD patients with concomitant CR, MH, and TH associated with an FC level <94 μg/g and negative CRP (<5 mg/L), this association was identified in 33 patients (27.9%) (Figure 3).
Figure 3.
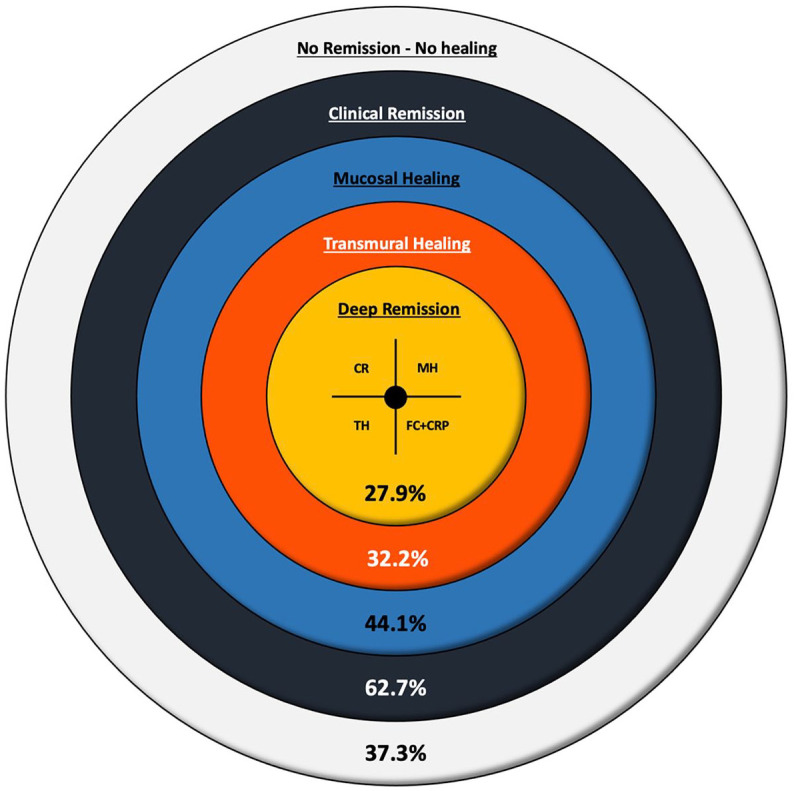
The new concept of DR, expressed as evolution of outcomes from CR to MH and TH in accordance with the treat-to-target strategy.
CR, clinical remission; DR, deep remission; MH, mucosal healing; TH, transmural healing.
No significant differences were seen in terms of effectiveness of a specific treatment and the rates of concomitant clinical, biochemical remission, MH, and TH (Table 5).
Table 5.
Treatment received by 33 subjects achieving concomitant CR, biochemical remission (FC level <94 μg/g and CRP < 5 mg/L), MH, and TH.
| No. of patients treated (entire population) | No. of patients achieving DR | |
|---|---|---|
| Adalimumab | 75 | 21 (28%) |
| Infliximab | 10 | 3 (30%) |
| Vedolizumab | 18 | 5 (27.7%) |
| Ustekinumab | 15 | 4 (26.7%) |
CR, clinical remission; CRP, C-reactive protein; DE, Deep remission; FC, fecal calprotectin; MH, mucosal healing; TH, transmural healing.
Discussion
The treatment strategies for CD have evolved in the last few years, moving from a clinical-based management to a treat-to-target approach, which includes objective monitoring and tight disease control. 4 Recent evidence shows that a decision-making strategy based on accurate and tight evaluation with even more stringent endpoints (i.e., TH) is associated with better long-term outcomes, reducing hospitalization, the rate of surgery, progressive bowel damage, and disease burden and disability.10,11,15 From this perspective, biomarkers such as CRP and FC have emerged as non-invasive useful tools for monitoring disease activity. In particular, FC is able to predict long-term clinical outcomes when measured 12 weeks after the onset of medical treatment 18 ; the STRIDE-II systematic review supported using an FC cut-off value of 150 μg/g to identify MH. 4 Despite this evidence, the current definition of DR does not take into account the role of biochemical markers, such as CRP and FC. 30 In effect, current evidences, although encouraging, are too limited to support the correlation between radiological findings and biomarkers. 30
In the present study, with the aim to establish a direct correlation between MH and TH with biochemical markers (FC and CRP), we found that an FC cut-off value of 94 μg/g was associated with both MH (sensitivity: 94.2%, specificity: 84.8%, PPV: 83.05%, NPV: 94.92%) and TH (sensitivity: 92.1%, specificity: 70%, PPV: 64.4%, NPV: 94.9%). For CRP, a cut-off of 5 mg/L was associated with both MH (sensitivity: 96.1%, specificity: 62.1%, PPV: 66.7%, NPV: 95.35%) and TH (sensitivity: 97.4%, specificity: 52.5%, PPV: 52%, NPV: 95.35%). When considering CD patients with concomitant CR, MH, and TH associated with an FC level <94 μg/g and negative CRP (<5 mg/L), this association was found in 33 patients and by this definition DR was achieved in 27.9% of treated CD population.
FC is considered an excellent biomarker associated with both clinical and endoscopic disease activity. 31 In fact, most data commonly reveal that FC is significantly reduced in IBD subjects with clinical and endoscopic remission and that values higher than 150–200 μg/g are mainly associated with disease relapse.31,32 Recently, the STRIDE-II has identified an FC cut-off value of 150 μg/g associated with MH, 4 but has considered a gray zone for FC values ranging from 150 to 250 μg/g. A post hoc analysis from the CALM study 33 demonstrated that an FC cut-off <250 μg/g was a useful surrogate marker for MH in CD, with a sensitivity of 70–74%, a specificity of 62–64%, and an AUC of 0.63–0.68. In accordance with this previous evidence, we found that an FC cut-off value of 94 μg/g was strongly and significantly associated with MH (sensitivity: 94.2%, specificity: 84.8%, PPV: 66.7%, NPV: 95.35%, AUC: 0.95), with an excellent FC/MH agreement (k = 0.81).
Some studies have explored using the combined cut-off values of FC and CRP to predict MH.
Bondjemah et al. 34 found that in adult active CD, FC < 200 μg/g or CRP < 5 mg/L could predict CDEIS < 6 with 83% sensitivity and 71% specificity. When assessing 355 patients with CD in CR, the combination of CRP < 10 mg/L and FC < 200 μg/g predicted CDEIS < 3 with 78% sensitivity and 58% specificity. In our study, we found that all subjects with FC < 94 μg/g also had CRP < 5 mg/L, with an excellent/good FC/CRP agreement for both MH (k = 0.82) and TH (k = 0.74). Thus, the ‘normal’ value of CRP should be included in the new definition of DR.
Although FC has been extensively evaluated in ulcerative colitis, and in association with MH in IBD in general, little is known about the relationship between FC and TH in CD.
A recent systematic review 35 explored the association between TH and biomarkers: among five studies that included a total of 742 patients, a normal CRP level was statistically associated with TH in two studies, and a good concordance (p = 0.02) between CRP levels and TH was observed in two other studies. In effect, only two studies (one of which was conducted in a pediatric setting 36 and the other in an adult setting 37 ) evaluated the association between TH and FC.
With regard to the relationship between FC and active transmural disease, previous studies have shown that FC correlates with transmural inflammation measured by magnetic resonance enterography (MRE), with a correlation coefficient range of 0.46–0.72.38–40 Domachevsky et al. 41 and Bertani et al. 42 arbitrarily chose an FC cut-off >150 mg/g to associate FC with inflammation documented by PET/MRE and MRE, respectively. Cerrillo et al. 43 demonstrated that an FC cut-off of 166.5 mg/g predicted transmural inflammation (i.e., MaRIA score ⩾7), with an AUROC of 0.914.
Current knowledge about TH and FC is even more limited. In a recent multicenter Italian study by Calabrese et al. 44 to evaluate the role of ultrasonographic tight control and monitoring in CD during biological therapies, it was found that after 3, 6, and 12 months a significant proportion of patients achieved CR and normalization of CRP and FC compared with the baseline. After 12 months, patients who achieved clinical and biochemical remission had a higher rate of TH or improved lesions than the unchanged/worsened lesion group, although specific FC or CRP cut-off values were not found in the study.
A recent pediatric study 36 evaluating the role of biomarkers (FC and CRP) in predicting MH and TH found that an FC cut-off value <100 μg/g predicted deep healing (MH + TH), with a sensitivity of 71%, a specificity of 92%, and an AUC of 0.93. Moreover, the best cut-off value of CRP to predict deep healing was <3 mg/L (72% sensitivity, 68% specificity, and 0.81 AUC).
Noh et al. 37 evaluated the usefulness of FC for predicting deep healing (MH + TH) in 268 adult Korean patients with CD receiving anti-TNF therapy, and found that subjects with deep healing (achievable in 28.7% of anti-TNF-treated patients) displayed lower FC values than those with ‘only’ MH (56.5 versus 160.5 mg/kg, p < 0.01). The FC cut-off value of 81.1 mg/kg showed 62.3% sensitivity, 81.7% specificity, 57.8% PPV, and 84.3% NPV for predicting deep healing, with an AUC of 0.767.
In the present study, we found that the FC cut-off value of 94 μg/g was associated with TH (sensitivity 92.1%, specificity 70%, PPV 64.4%, NPV 94.9%, AUC 0.88), with a TH/FC agreement of 0.58 (p < 0.01), even though we did not find any significant difference in FC concentration between the TH and MH groups. By contrast, CD patients with only CR had significant higher FC levels than patients with MH/TH, confirming and strengthening the few previous results.
In accordance with Noh et al., 37 we would like to underline the high NPV of FC < 94 μg/g in predicting both MH (94.92%) and TH (94.9%), confirming the hypothesis that FC is an excellent biomarker that should be taken into account in the assessment of DR in CD.
To the best of our knowledge, this is the first prospective study that is directly focused on the association of FC levels and both MH and TH, with the aim of incorporating this biochemical marker in the definition of DR, so that the definition would combine clinical, biochemical, endoscopic, and transmural remission. This new endpoint, achievable in about 30% of CD patients treated with biologics, follows the concept of the treat-to-target strategy, in order to guide clinicians in the management of CD, as demonstrated in the CALM trial. 45 Further studies are needed to compare the long-term outcome of CD patients with DR and that of patients with only MH or TH. Similarly, current data are scarce to make suggestions on when, if ever, biologic therapy can be stopped/de-escalated in patients with IBD achieving TH. Therefore, the decision to discontinue or de-escalate a biologic drug is typically made case by case on the basis of considerations around benefits, risks, and cost-effectiveness. 10 Further studies with therapeutic strategic finality are needed to better define the potential advantages to stop or de-escalate biologic therapy in CD patients achieving all combined outcome of DR.
Our study has some limitations. First, it should be considered as a ‘per-protocol’ study with a consequent ‘positive’ selection bias and probable overestimation of the rates of CR, MH, and TH. Moreover, TH was assessed only by BS, and a cross-sectional evaluation (MRE) of TH was not routinely performed. However, previous studies have demonstrated an excellent agreement between BS and other imaging techniques (about 90%), such as magnetic resonance and computed tomography enterography, in the detection of bowel lesions.2,3
Although a formal definition of sonographic TH is not currently available, most previous studies used BWT alone to define TH, whereas some combined BWT with doppler signal. The recent consensus by Ilvemark et al. 46 suggests including the negative color doppler signal alongside the BWT, even though the prognostic role of negative doppler plus BWT versus BWT alone has yet to be demonstrated.
We also recognized that a BWT ⩽ 4 mm of the colon can be normal for some patients, especially if diverticula are present. However, very recent consensus 46 recommends defining TH as BWT ⩽ 3 mm for both small and large bowel.
It is known that FC can significantly vary in individual patients on a day-to-day basis or even in two different samples taken on the same day. 47 We never use FC alone in clinical practice for IBD management: the FC value of 94 μg/g has to be associated with predictive values of TH and MH.
Furthermore, this study was not designed to evaluate a specific treatment or therapeutic strategy, but rather to assess outcomes and associate them to biomarkers, regardless of the medication used. Similarly, it is important in clinical practice to establish the prognostic role of such new definition: a study evaluating the prognostic role of DR versus TH alone or MH alone is ongoing.
Moreover, the baseline CRP level is significantly influenced by the underlying coding gene. The CRP non-producers may exist in 15–20% of IBD population. In other words, CRP may not be universally used as an inflammation marker and may not be applicable to everyone. 48
Conclusion
In conclusion, an FC < 94 μg/g and a normal CRP are associated with CR, MH, and TH and could be included in the definition of DR in association. So, by definition, if applying the combination of our outcomes, DR could be achieved in a little percentage of CD patients treated with biologics for at least 2 years (particularly when considering the ‘per protocol’ nature of the study). However, this definition of DR could be considered a new and more inclusive concept of clinical, biochemical, endoscopic, and transmural remission.
Acknowledgments
None.
Footnotes
ORCID iDs: Nicola Imperatore  https://orcid.org/0000-0003-3230-6832
https://orcid.org/0000-0003-3230-6832
Olga Maria Nardone  https://orcid.org/0000-0002-9554-4785
https://orcid.org/0000-0002-9554-4785
Contributor Information
Fabiana Castiglione, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy, Via S. Pansini 5, Naples 80131, Italy.
Nicola Imperatore, Gastroenterology and Endoscopy Unit, AORN Antonio Cardarelli, Naples, Italy.
Anna Testa, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Roberto de Sire, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Olga Maria Nardone, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Simona Ricciolino, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Imma Di Luna, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Marta Patturelli, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Guido Daniele Villani, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Oriana Olmo, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Antonio Rispo, Gastroenterology Unit, Department of Clinical Medicine and Surgery, ‘Federico II’ School of Medicine, Naples, Italy.
Declarations
Ethics approval and consent to participate: Since this was an observational real-life study, a formal approval by Ethics board was not needed; however, all patients gave their consent to include their own de-identified data in the study.
Consent for publication: All patients gave their consent to publish their own de-identified data in the study.
Author contribution(s): Fabiana Castiglione: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – review & editing.
Nicola Imperatore: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Anna Testa: Data curation; Methodology; Supervision; Validation; Writing – review & editing.
Roberto de Sire: Data curation; Validation.
Olga Maria Nardone: Validation.
Simona Ricciolino: Investigation; Validation.
Imma Di Luna: Validation; Visualization.
Marta Patturelli: Validation; Visualization.
Guido Daniele Villani: Validation; Visualization.
Oriana Olmo: Validation; Visualization.
Antonio Rispo: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing Interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm Bowel Dis 2011; 17: 471–478. [DOI] [PubMed] [Google Scholar]
- 2. Rispo A, Imperatore N, Testa A, et al. Bowel damage in Crohn’s disease: direct comparison of ultrasonography-based and magnetic resonance-based Lemann index. Inflamm Bowel Dis 2017; 23: 143–151. [DOI] [PubMed] [Google Scholar]
- 3. Castiglione F, Mainenti P, Testa A, et al. Cross-sectional evaluation of transmural healing in patients with Crohn’s disease on maintenance treatment with anti-TNF alpha agents. Dig Liver Dis 2017; 49: 484–489. [DOI] [PubMed] [Google Scholar]
- 4. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021; 160: 1570–1583. [DOI] [PubMed] [Google Scholar]
- 5. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. New Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 6. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis 2009; 15: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 7. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468. [DOI] [PubMed] [Google Scholar]
- 8. Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn’s disease: time for a change. Gut 2011; 60: 1754–1763. [DOI] [PubMed] [Google Scholar]
- 9. Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology 2012; 142: 1102–1111.e2. [DOI] [PubMed] [Google Scholar]
- 10. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019; 49: 1026–1039. [DOI] [PubMed] [Google Scholar]
- 11. Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by ultrasonography as target of biological treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2020; 18: 2030–2037. [DOI] [PubMed] [Google Scholar]
- 12. Van Assche G, Herrmann KA, Louis E, et al. Effects of infliximab therapy on transmural lesions as assessed by magnetic resonance enteroclysis in patients with ileal Crohn’s disease. J Crohns Colitis 2013; 7: 950–957. [DOI] [PubMed] [Google Scholar]
- 13. Ripollés T, Paredes Arquiola JM, Moreno-Osset E. Ultrasonography and transmural healing in Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1549–1551. [DOI] [PubMed] [Google Scholar]
- 14. Eder P, Katulska K, Krela-Kaźmierczak I, et al. The influence of anti-TNF therapy on the magnetic resonance enterographic parameters of Crohn’s disease activity. Abdom Imaging 2015; 40: 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buisson A, Hordonneau C, Deepak P. Transmural healing and MRI remission: new promising therapeutic targets in Crohn’s disease. Inflamm Bowel Dis 2017; 23: E44–E45. [DOI] [PubMed] [Google Scholar]
- 16. Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn’s disease. Gastroenterology 2014; 146: 374–382.e1. [DOI] [PubMed] [Google Scholar]
- 17. Peyrin-Biroulet L, Sandborn W, Sands BE. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 18. Haisma SM, Verkade HJ, Scheenstra R, et al. Time-to-reach target calprotectin level in newly diagnosed patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2019; 69: 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakarai A, Kato J, Hiraoka S, et al. Slight increases in the disease activity index and platelet count imply the presence of active intestinal lesions in C-reactive protein-negative Crohn’s disease patients. Intern Med 2014; 53: 1905–1911. [DOI] [PubMed] [Google Scholar]
- 20. Rispo A, Imbriaco M, Celentano L, et al. Noninvasive diagnosis of small bowel Crohn’s disease: combined use of bowel sonography and Tc-99m-HMPAO leukocyte scintigraphy. Inflamm Bowel Dis 2005; 11: 376–382. [DOI] [PubMed] [Google Scholar]
- 21. Gisbert JP, Marín AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther 2015; 42: 391–405. [DOI] [PubMed] [Google Scholar]
- 22. Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s Disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 23. Orlando A, Armuzzi A, Papi C, et al. The Italian Society of Gastroenterology (SIGE) and the Italian Group for the study of Inflammatory Bowel Disease (IG-IBD) clinical practice guidelines: the use of tumor necrosis factor-alpha antagonist therapy in inflammatory bowel disease. Dig Liver Dis 2011; 43: 1–20. [DOI] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 25. Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 26. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 414–22.e5. [DOI] [PubMed] [Google Scholar]
- 27. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology 2005; 236: 95–101. [DOI] [PubMed] [Google Scholar]
- 28. Parente F, Greco S, Molteni M, et al. Modern imaging of Crohn’s disease using bowel ultrasound. Inflamm Bowel Dis 2004; 10: 452–461. [DOI] [PubMed] [Google Scholar]
- 29. Daperno M, Castiglione F, de Ridder L, et al. Results of the 2nd part Scientific Workshop of the ECCO. II: Measures and markers of prediction to achieve, detect, and monitor intestinal healing in inflammatory bowel disease. J Crohns Colitis 2011; 5: 484–498. [DOI] [PubMed] [Google Scholar]
- 30. Wilkens R, Novak KL, Maaser C, et al. Relevance of monitoring transmural disease activity in patients with Crohn’s disease: current status and future perspectives. Therap Adv Gastroenterol 2021; 14: 17562848211006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jukic A, Bakiri L, Wagner EF, et al. Calprotectin: from biomarker to biological function. Gut 2021; 70: 1978–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toke N, Ramaswamy P, Panackel C, et al. IDDF2019-ABS-0346 Utility of inflammatory markers in the management of inflammatory bowel disease and their correlation with disease activity indices. Gut 2019; 68: A122.1–A122. [Google Scholar]
- 33. Reinisch W, Panaccione R, Bossuyt P, et al. Association of biomarker cutoffs and endoscopic outcomes in Crohn’s disease: a post hoc analysis from the CALM study. Inflamm Bowel Dis 2020; 26: 1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bondjemah V, Mary JY, Jones J, et al. P133 fecal calprotectin and CRP as biomarkers of endoscopic activity in Crohn’s disease: a meta-study. J Crohns Colitis 2012; 6: S63. [Google Scholar]
- 35. Geyl S, Guillo L, Laurent V, et al. Transmural healing as a therapeutic goal in Crohn’s disease: a systematic review. Lancet Gastroenterol Hepatol 2021; 6: 659–667. [DOI] [PubMed] [Google Scholar]
- 36. Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn’s disease. Clin Gastroenterol Hepatol 2018; 16: 1089–1097.e4. [DOI] [PubMed] [Google Scholar]
- 37. Noh SM, Oh EH, Park SH, et al. Association of faecal calprotectin level and combined endoscopic and radiological healing in patients with Crohn’s disease receiving anti-tumour necrosis factor therapy. J Crohns Colitis 2020; 14: 1231–1240. [DOI] [PubMed] [Google Scholar]
- 38. Zippi M, Al Ansari N, Siliquini F, et al. Correlation between faecal calprotectin and magnetic resonance imaging (MRI) in the evaluation of inflammatory pattern in Crohn’s disease. Clin Ter 2010; 161: e53–e56. [PubMed] [Google Scholar]
- 39. Parisinos CA, McIntyre VE, Heron T, et al. Magnetic resonance follow-through imaging for evaluation of disease activity in ileal Crohn’s disease: an observational, retrospective cohort study. Inflamm Bowel Dis 2010; 16: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 40. Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol 2014; 24: 277–287. [DOI] [PubMed] [Google Scholar]
- 41. Domachevsky L, Leibovitzh H, Avni-Biron I, et al. Correlation of 18F-FDG PET/MRE metrics with inflammatory biomarkers in patients with Crohn’s disease: a pilot study. Contrast Media Mol Imaging 2017; 2017: 7167292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bertani L, Ceccarelli L, de Bortoli N, et al. Mucosal and transmural healing during anti-TNF therapy. Is fecal calprotectin a marker of therapeutic response? In: Presented at the ECCO annual meeting, Barcelona, 17 February 2017, abstract P598. [Google Scholar]
- 43. Cerrillo E, Beltrán B, Pous S. Fecal calprotectin in ileal Crohn’s disease: relationship with magnetic resonance enterography and a pathology score. Inflamm Bowel Dis 2015; 21: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 44. Calabrese E, Rispo A, Zorzi F, et al. Ultrasonography tight control and monitoring in Crohn’s disease during different biological therapies: a multicenter study. Clin Gastroenterol Hepatol 2022; 20: e711–e722. [DOI] [PubMed] [Google Scholar]
- 45. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 46. Ilvemark JFKF, Hansen T, Goodsall TM, et al. Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement. J Crohns Colitis 2022; 16: 554–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. D’Amico F, Rubin DT, Kotze PG, et al. International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases. United Eur Gastroenterol J 2021; 9: 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones J, Loftus EV, Jr, Panaccione R, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2008; 6: 1218–1224. [DOI] [PubMed] [Google Scholar]