Summary
Background
Extreme temperatures are associated with the risk of preterm birth (PTB), but evidence on the effects of different clinical subtypes and across different regions is limited. We aimed to evaluate the effects of maternal exposure to extreme temperature on PTB and its clinical subtypes in China, and to identify effect modification of regional factors in dimensions of population, economy, medical resources and environmental factors.
Methods
This was a prospective population-based cohort of 210,798 singleton live births from 16 counties in eight provinces across China during 2014-2018. We used an extended Cox regression with time-varying variables to evaluate the effects of extreme heat and cold on PTB and its subtypes in the entire pregnancy, each trimester, the last gestational month and week. Meta-analysis and meta-regression were conducted to estimate the pooled effects of each city and effect modification by regional characteristics.
Findings
Exposure to heat and cold during the entire pregnancy significantly increased the risk of PTB. The effects varied with subtypes, for medically indicated and spontaneous PTB, hazard ratios were 1·84 (95% CI: 1·29, 2·61) and 1·50 (95% CI: 1·11, 2·02) for heat, 2·18 (95% CI: 1·83, 2·60) and 2·15 (95% CI: 1·92, 2·41) for cold. The associations were stronger for PTB less than 35 weeks than those during weeks 35-36. The effects varied across locations, and GDP per capita (β=−0·16) and hospital beds per 1000 persons (β=−0·25) were protective factors for the effects.
Interpretation
Extreme temperature can increase the risk of medically indicated and spontaneous PTB, and higher regional socio-economic status may moderate such effects. In the context of climate change, such findings may have important implications for protecting the health of vulnerable groups, especially newborns.
Funding
National Key R&D Program of China (2018YFA0606200), National Natural Science Foundation of China (42175183), Strategic Priority Research Program of the Chinese Academy of Sciences (XDA20030302), National Natural Science Foundation of China (42071377).
Keywords: Extreme temperature, Climate change, Preterm birth, Clinical subtype, China
Research in context.
Evidence before the study
We searched PubMed for studies published until January 2022, using the key terms “preterm birth”, “gestational age”, “climate change”, “temperature”, and “weather”.
Previous epidemiologic studies have reported that maternal exposure to extreme temperature may increase the risk of preterm birth. Evidence from experimental studies have further indicated that extreme temperature may induce different clinical subtypes of preterm birth through different pathways. Therefore, clarifying the effects of extreme temperature on preterm birth subtypes may help understand the underlying mechanisms. However, the evidence from epidemiological studies still remains limited. In addition, the impacts of extreme temperature on preterm birth may vary across regions because of multiple climatic characteristics, socio-economic status and etc. However, most studies were conducted in a single region. Therefore, identifying regional heterogeneity and potential modifying factors for the impacts of extreme temperature on preterm birth will help promote regional management strategies to reduce the risk of preterm birth, which is imperative for China, a large country with obvious regional climate and economic disparities.
Added value of this study
To the best of our knowledge, this is the first study to investigate the impact of extreme temperature on preterm birth and its clinical subtypes in China. In this large-scale and population-based multi-center prospective cohort, we identified positive associations between the risk of preterm birth, medically indicated preterm birth, and spontaneous preterm birth and extreme heat during the entire pregnancy, with the HRs of 1·63, 1·84 and 1·50, respectively. Similar increased risks were found for extreme cold, with the HRs of 2·16, 2·18 and 2·15, respectively. Additionally, the effects varied across cities (I2>50%), and higher GDP per capita (β=−0·16), as well as more hospital beds per 1000 persons (β=−0·25) contributed to mitigate such effects.
Implications of all the available evidence
Our findings highlight the importance of clarifying the effects on PTB subtypes in informing plausible mechanisms of PTB, and also the significance of identifying region-specific risk and potential effect modifiers for the development of more targeted and effective intervention strategies.
Alt-text: Unlabelled box
Introduction
Preterm birth (PTB), defined as the birth of an infant less than 37 completed weeks, is a complex syndrome and a significant global public health problem. The global number of PTB in 2014 is approximately 14·84 million, while China ranks second with 1·17 million PTBs (6·9% of all births).1 PTB and its complications are the leading cause of neonatal death,1 and have been associated with neurodevelopmental disability, social and emotional problems as well as learning difficulties persisting into adult life,2 which ultimately lead to a heavy psychological and financial burden for the family and society.3 While the severe consequences of PTB are of great concern and have been extensively studied, the etiology of PTB is not fully understood. Potential risk factors that have been identified include genetic, sociodemographic, behavioral, psychological, socio-economic, and environment factors.4,5
Recently, there has been an emerging interest in examining the effects of ambient temperature on the risk of PTB. The most recent systematic review,6 combining 47 studies from all over the world, found that the risk of PTB increased 5% per 1°C increase in temperature and 16% during heatwaves (using various context-specific definitions). It also indicated that PTB was more often associated with heat than cold with 40 out of the 47 studies identifying an association with hot temperatures.6 While the frequency and intensity of extreme temperature are increasing rapidly due to climate change, more epidemiological studies are needed to better understand the relationship between extreme temperature exposure and PTB and to help find preventive methods.
Based on clinical presentation, preterm birth can be classified into medically indicated preterm birth (MI-PTB) and spontaneous preterm birth (S-PTB).7 MI-PTB was medically indicated induction of labor or cesarean delivery,8 while S-PTB was PTB with premature rupture of membranes (PROM) or preterm labor.9 Maternal exposure to extreme temperature may trigger PTB through different pathways. Extreme temperature may induce MI-PTB by affecting potential indications for intervention such as preeclampsia,10 fetal growth restriction,11 etc. Besides, extreme temperature may also elevate the secretions of oxytocin or stimulate rupture of the membranes to induce spontaneous preterm labor.12,13 However, epidemiological evidence on the effects of maternal exposure to extreme temperature on different clinical subtypes of PTB remains limited.
Additionally, most previous studies on the effects of extreme temperature on PTB used time-series or case-crossover designs rather than cohort study based on individual data.12,14,15 Thus, some important individual factors (for example, pre-pregnancy BMI, etc.) were not included, which may cause potential biases. Besides, potential effect heterogeneity may exist across geographical contexts because of climatic characters and socioeconomic levels.16 Most previous studies were conducted in a single site and selected study population from specific areas,12,15,17,18 which may cause sample selection biases and restrict the external validity.
Given the knowledge gap, the present study sought to estimate the association between maternal exposure to extreme temperature and preterm birth in a population-based birth cohort with detailed individual characteristics in eight cities of China. The primary objective of the study was to clarify the effects of extreme temperature on different clinical subtypes of preterm birth. The study also aimed to identify potential effect modification of regional population, economy, medical resource, and environment determinants.
Methods
Study design and participants
We obtained data from the National Maternal and Newborn Health Monitoring Project.19 This project was conducted by the National Center for Women and Children's Health, Chinese Center for Disease Control and Prevention, mainly aiming at monitoring the quality of women's and children's health care prospectively and dynamically, and to understand and improve the health of mothers and infants. It systematically collected a wide range of prospective data, including information on maternal characteristics, prenatal care, delivery as well as information on newborns. Based on the selection criteria including climatic character, socioeconomic level, and management of maternal and perinatal health care, 16 communities in eight cities (Anshan, Shijiazhuang, Huanggang, Yueyang, Heyuan, Xiamen, Zigong, Yuxi) that can well represent China were selected (Figure 1). All pregnant women living in the 16 communities were prospectively monitored throughout their pregnancy from March 6, 2013, at the first antenatal care, until the delivery date of December 31, 2018. A total of 271,720 pairs of “pregnant women and baby” were included in our analyses.
Figure 1.
Spatial distribution of 16 study sites in eight provinces across China.
Since the monitoring project was in the process of continuous improvements at the initial stages of the study period, we selected the study period when the database was relatively complete, from March 11, 2014 to December 31, 2018. More than 220,000 pairs of “pregnant women and baby” were included, and then we established the prospective birth cohort. After excluding stillbirths (n=314), multiple births (n= 6803), women with gestational age <20 or >44 weeks (n=123) and women aged <13 years or >50 years (n=4409) (Figure S1), 210,798 singleton live births remained in the study sample (Figure S1).
Maternal information including maternal residence address, age, education level, behavioral risk factors (an indicator of any of the factors including smoking, drinking, drugs, toxic and harmful substances, radiation, and others) during pregnancy, parity, last menstrual period (LMP) were collected by qualified nurses using structured questionnaires in face-to-face interviews. Other characteristics of women, such as weight and height before pregnancy as well as gestational week, were collected through physical examinations and ultrasound examinations. Neonates’ information including date of birth, delivery mode, infant sex were obtained by follow-up investigation for all participants. A strict quality control on monitoring data was carried out, including specialized training for healthcare personnel, standardized questionnaire survey, standardized operation procedures of examinations and calibration of measurement tools, and double questionnaire data entry.
This study was approved by the Institutional Review Board of the School of Public Health, Sun Yat-sen University, and informed consent was provided by all participants at enrollment in the project.
Outcome definition
Preterm birth was defined as birth less than 37 completed weeks of gestation. Gestational age at birth was determined according to an ultrasound examination in the first or second trimester. If the ultrasound examination was unavailable, the date of the last menstrual period (LMP) was used to calculate the gestational age. In this study, there were 202470 (96%) births determining gestational age by ultrasonography, 8328 (4%) births by LMP.
Besides, we classified preterm birth into two clinical subtypes,7,8 including medically indicated preterm birth (MI-PTB) and spontaneous preterm birth (S-PTB). Additionally, we further categorized PTB into four mutually exclusive groups according to gestational age-less than 34 completed weeks, 34 to before 35 completed weeks, 35 to before 36 completed weeks, and 36 to before 37 completed weeks.
Exposure assessment
We collected daily mean (Tmean), maximum (Tmax) and minimum (Tmin) temperature as well as daily relative humidity (RH) between 2014 and 2018 from the 680 weather stations of the China Meteorological Data Service Center (http://data.cma.cn/). We then used the Inverse Distance Weighting (IDW) interpolation technique to determine the daily temperature and relative humidity for communities that were not covered by the weather stations at a resolution of 1km × 1km. In consideration of spatial autocorrelation among exposure observations, IDW assumes that the value of a given location affected by observations from surrounding monitoring stations, and the effects decrease with increasing distance. Thus, based on the distance between a given location and monitoring stations, IDW estimates the value of a given location by weighted average of observations from monitoring stations within a specific search window.20 Finally, we assigned exposures of daily temperature and relative humidity for each pregnant woman according to residential addresses.
We used the estimated date of conception to determine the start and end date of each gestational week for each pregnancy. Exposures were assessed using average Tmean in the following six exposure windows: the first trimester (week 1-12), second trimester (week 13-27), third trimester (week 28-delivery for PTB, week 28-37 for term birth), the entire pregnancy (week 1-delivery for PTB, week 1-37 for term birth), one week prior to delivery, and four weeks prior to delivery.21 We also used the same approach to estimate exposures to relative humidity.
To reflect regional acclimation, we categorized our temperature exposure using local temperature distributions among study participants for each pregnancy window.16 For each city and for each exposure window, we created a temperature distribution, then defined exposures based on the following cut-offs: cold (< 5th percentile of Tmean), hot (> 95th percentile of Tmean), and non-extreme temperatures (5–95th percentile of Tmean).
We also used IDW to interpolate daily concentrations of particulate matter<2·5 μm in aerodynamic diameter (PM2·5), PM<10 μm in aerodynamic diameter (PM10), carbon monoxide (CO), nitrogen dioxide (NO2), sulfur dioxide (SO2), and ozone (O3) (daily maximum 8-h average level) at the same resolution of 1km × 1km based on the data at 1,597 stations from the China National Environmental Monitoring Centre (http://www.cnemc.cn/). We then estimated average values of air pollutants for each woman in the following six exposure windows, including (1-3) three trimesters, (4) the entire pregnancy, (5) one week before delivery and (6) four weeks before delivery.
Regional characteristics
To identify potential regional effect modifiers, we considered four dimensions, including population, economy, medical resource, and environment determinants. In each dimension, several city-level factors were obtained from the Statistical Bulletin on National Economic and Social Development in each city during the period. The population dimension included population number (ten thousand people) and population density (people/km2). For the economy, factors including GDP per capita (CNY), Engel coefficient (%), and unemployment rate (%) were obtained. We selected the number of health institutions (n), hospital beds per 1000 persons (n), and physicians per 1000 persons (n) to represent levels of medical resource. Good air quality (% of excellent or good air quality days per year) was selected to reflect environmental conditions. Besides, we also assessed greenness exposure of each participant by using the NDVI on every sixteen-day MODIS products with a spatial resolution of 250m × 250m, which was obtained from the Geospatial Data Cloud (http://www.gscloud.cn). We selected a buffer of 250m around each maternal residential address to extract the average of NDVI as previous studies did.22,23 Then we calculated city-level exposures by averaging the NDVI measurements for each participant during the entire pregnancy.
Statistical analyses
We applied an extended Cox regression with time-varying variables separately to examine the effects of maternal exposure to extreme temperature in each trimester, the entire pregnancy, one week before delivery, and four weeks before delivery on PTB. We also stratified the effects in PTB subtypes and gestational age. We then examined and pooled the effects of each city to evaluate heterogeneity. Further, meta-regression was used to identify potential regional modifiers.
An extended Cox regression with time-varying variables was applied to determine the hazard ratios (HRs) and 95% confidence intervals (CIs) of PTB associated with extreme temperature exposures during the above exposure windows, adjusting for the following covariates. Eq. (1) summarizes the model fitted:
| (1) |
where, GA is the gestational age, PTB is the outcome variable (PTB=1, TB =0); h0(GA) is the baseline hazard function, indicating the hazard function for an individual with all variables equal to zero; is a categorical variable indicating exposure to extreme temperature (extreme heat=1, extreme cold=2, optimal temperature=0) in the window t. Xi is defined as the values of the time-varying covariates, including long-term gestational air pollution and relative humidity exposure, defined as the exposure across the entire pregnancy.12,14,18 Due to the high correlations between PM2·5 and other pollutants except for O3, finally, we only controlled for PM2·5 and O3 in the model to avoid collinearity. Besides, for effects in the week before delivery and in four weeks before delivery, we controlled for long-term average temperature as a natural cubic spline with three degrees of freedom (dfs), defined as the exposure across the entire pregnancy except for the last week or month.15
X1 refers to the values of time-independent covariates during pregnancy, mainly including maternal and fetal characteristics: maternal age, mother's education (≤6 years, 7-9 years, 10-12 years, >12 years), parity (primiparous, multiparous), pre-pregnancy body mass index (BMI) (<18·5, 18·5-23·9, >23·9 kg/m2), season of conception (spring, summer, fall, winter), delivery mode (vaginal, cesarean), maternal behavioral risk factors during pregnancy (yes, no; history of exposure to smoking, alcohol drinking, drugs, toxic and harmful substances, radiation, or others), infant sex (male, female), and the community where participants resided. According to the recommendations of the Guidelines for Pre-pregnancy and Pregnancy Health care (2018) in China,24 the Adequacy of Prenatal Care Utilization index (APNCU) was determined for each woman. The APNCU index was the ratio of actual prenatal care visits to recommended prenatal care visits, and it was divided into four categories: Inadequate (< 50%), Intermediate (50-79%), Adequate (80-109%) and Adequate Plus (≥110%). The APNCU index was also included in the model.14
We then implemented stratified models for the two types of PTB (MI-PTB and S-PTB), and for gestational age (less than 34 completed weeks, 34 to before 35 completed weeks, 35 to before 36 completed weeks, and 36 to before 37 completed weeks).
Meta-analysis methods have been used to pool results in previous multi-city studies.25 Similarly, we applied a Cox regression model for each city to examine the effects of MI-PTB and S-PTB, and then used meta-analysis to generate the pooled estimates and investigated heterogeneity across cities. The coefficient of inconsistency (I2) describes the percentage of heterogeneity across cities and was tested by using Cochran's Q χ2 test. Results are presented as hazard ratios (HR) and associated 95 percent confidence intervals (CI).
The impact of the extreme temperature may vary across cities due to the variation in regional characteristics, resulting in heterogeneity of findings. Thus, a meta-regression was implemented to identify factors that could potentially affect the magnitude of the effects across cities. We incorporated each factor in the above four dimensions of regional characteristics separately into the meta-regression model. Eq. (2) summarises the model fitted:
| (2) |
where, Yi is the estimated log HR for city i. Xki is the value of factor k for city i, and βk is the regression coefficient of factor k. β0 is the coefficient of the intercept. εi refers to the residual term of the model.
Sensitivity analyses
We performed several sensitivity analyses to ensure the robustness of our findings. These analyses involved: a) defining exposures based on the different cut-offs and examining the effects: cold days (lower than 1th, or 10th, or 25th percentile of Tmean), hot days (higher than 99th, or 90th, or 75th percentile of Tmean), and non-extreme days (1th–99th, or 10th–90th, or 25th–75th percentile of Tmean); b) using Tmax and Tmin to define exposures based on the same cut-offs with Tmean and examining the effects; c) evaluating the effects of extreme hot (95th-Tmean) and cold (5th-Tmean) on the risk of preterm birth by excluding births determining gestational age only by LMP.
This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (STROBE-checklist). All analyses were conducted in R 3·4·2 with the “survival” package and “meta” package (R Foundation for Statistical Computing, Vienna, Austria).
Role of the funding source
The funding source had no role in study design, data collection, data analysis, data interpretation, or manuscript preparation. The corresponding authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
The demographic characteristics of the study population are shown in Table 1. A total of 210,798 singleton live births were included in this study. Among them, 8587 (4·07%) were PTB, 4050 (1·92%) were MI-PTB, and 4537 (2·15%) were S-PTB. Compared with the mothers of S-PTB, those of MI-PTB had a slightly higher percentage in higher maternal age, higher pre-pregnancy BMI, and education of high school and above (Table S3). Besides, mothers of MI-PTB tended to be multiparous and had a slightly higher percentage with APNCU up to 50% (Table S3). The distributions of the season of conception, maternal behavioral risk factors were similar between MI-PTB and S-PTB (Table S3). Table S1 describes the distribution of temperature and relative humidity by pregnancy windows. In the whole pregnancy, the median of Tmean, Tmax and Tmin were 17·02°C, 21·87°C and 13·42°C, respectively (Table S1).
Table 1.
Summary statistics of all live births, preterm births, and subtypes of preterm birth in 16 study sites of eight provinces across China (2014-2018).
| Characteristics | All live births | PTB | MI-PTB | S-PTB | |
|---|---|---|---|---|---|
| Mean (SD)/ n (%) | Mean (SD)/ n (%) | Mean (SD)/ n (%) | Mean (SD)/ n (%) | ||
| N | 210,798 (100) | 8,587 (4.07) | 4,050 (1.92) | 4,537 (2.15) | |
| Gestational age (weeks) | 39.0 (1.46) | 34.7 (1.94) | 34.8 (1.83) | 34.7 (2.03) | |
| Maternal age (years) | 34.0 (6.54) | 34.9 (7.04) | 36.4 (6.74) | 33.7 (7.08) | |
| Pre-pregnancy BMI (kg/m2) | 22.0 (3.35) | 22.4 (3.58) | 23.2 (3.78) | 21.7 (3.23) | |
| Mother's education | Primary and below | 6,106 (2.90) | 277 (3.23) | 115 (2.84) | 162 (3.57) |
| Junior high school | 65,824 (31.23) | 2,719 (31.66) | 1,220 (30.12) | 1,499 (33.04) | |
| High school | 64,805 (30.74) | 2,368 (27.58) | 1,160 (28.64) | 1,208 (26.63) | |
| College and above | 66,953 (31.76) | 3,003 (34.97) | 1,457 (35.98) | 1,546 (34.08) | |
| Unknown | 7,110 (3.37) | 220 (2.56) | 98 (2.42) | 122 (2.68) | |
| Maternal behavioral | Yes | 7,711 (3.66) | 335(3.90) | 164 (4.05) | 171 (3.77) |
| risk factorsa | No | 203,083 (96.34) | 8,252 (96.10) | 3,886 (95.95) | 4,366 (96.23) |
| Unknown | 4 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Parity | Primiparity | 116,831(55.42) | 4,586 (53.40) | 2,083 (51.43) | 2,503 (55.17) |
| Multiparity | 93,967 (44.58) | 4,001 (46.59) | 1,967 (48.57) | 2,034 (44.83) | |
| Unknown | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | |
| Delivery mode | Vaginal | 120,094 (56.97) | 4,384 (51.05) | 0 (0.00) | 4,384 (96.63) |
| Cesarean | 90,355 (42.86) | 4,186 (48.75) | 4,050 (100.00) | 136 (3.00) | |
| Unknown | 349 (0.17) | 17 (0.20) | 0 (0.00) | 17 (0.37) | |
| Season of conception | Spring (3-5) | 53,108 (25.19) | 1,975 (23.00) | 895 (22.10) | 1,080 (23.80) |
| Summer (6-8) | 55,943 (26.54) | 2,347 (27.33) | 1,085 (26.79) | 1,262 (27.82) | |
| Fall (9-11) | 53,178 (25.23) | 2,172 (25.29) | 1,062 (26.22) | 1,110 (24.47) | |
| Winter (12-2) | 48,569 (23.04) | 2,093 (24.37) | 1,008 (24.89) | 1,085 (23.91) | |
| APNCUb | <50% | 56,906 (27.00) | 946 (11.02) | 395 (9.75) | 551 (12.15) |
| 50%-79% | 70,706 (33.54) | 2,013 (23.44) | 977 (24.12) | 1,036 (22.83) | |
| 80%-109% | 41,985 (19.92) | 2,782 (32.40) | 1,403 (34.64) | 1,379 (30.39) | |
| ≥110% | 41,201 (19.55) | 2,846 (33.14) | 1,275 (31.48) | 1,571 (34.63) | |
| Infant Sex | Males | 111,514 (52.90) | 4,808 (55.99) | 2,217 (54.74) | 2,591 (57.11) |
| Females | 99,247 (47.08) | 3,778 (44.00) | 1,833 (45.26) | 1,945 (42.87) | |
| Unknown | 37 (0.02) | 1 (0.01) | 0 (0.00) | 1 (100.00) |
Abbreviation: SD, standard deviation. BMI, body mass index. APNCU, Adequacy of Prenatal Care Utilization.
PTB, preterm birth. MI-PTB, medically induced preterm birth. S-PTB, spontaneous preterm birth.
maternal behavioral risk factors: the history of exposure to smoking, drinking, drugs, toxic and harmful substances, radiation, or others during pregnancy.
APNCU: According to the recommendations of the Guidelines for Pre-pregnancy and Pregnancy Health care (2018), APNCU Index is divided into four categories: Inadequate (< 50%), Intermediate (50-79%), Adequate (80-109%) and Adequate Plus (≥110%).
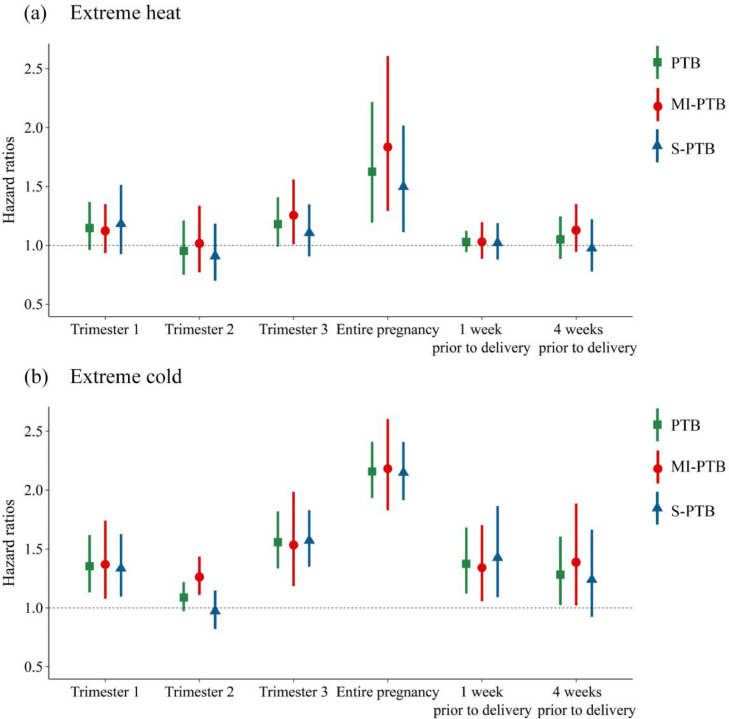
We found exposure to extreme heat in the entire pregnancy was associated with the risk of preterm birth (PTB), medically indicated preterm birth (MI-PTB), and spontaneous preterm birth (S-PTB) (Figure 2). The HRs for PTB, MI-PTB and S-PTB were 1·63 (95% CI: 1·19, 2·22), 1·84 (95% CI: 1·29, 2·61) and 1·50 (95% CI: 1·11, 2·02), respectively. An increased risk was also found for MI-PTB in the 3rd trimester (HR 1·26, 95% CI: 1·01, 1·56).
Figure 2.
Hazard ratios of preterm birth and its subtypes associated with extreme heat and cold by pregnancy windows.
Abbreviation: PTB, preterm birth. MI-PTB, medically induced preterm birth. S-PTB, spontaneous preterm birth. Pre1w, one week before delivery. Pre4w, four weeks before delivery.
Exposures to extreme cold in the early (the 1st trimester), late (the 3rd trimester and the week prior to delivery), and entire pregnancy were associated with the risk of PTB and its subtypes (Figure 2). The largest effects were detected in the entire pregnancy, with HRs of 2·16 (95% CI: 1·93, 2·41), 2·18 (95%CI: 1·83, 2·60) and 2·15 (95%CI: 1·92, 2·41) for PTB, MI-PTB and S-PTB, respectively. Cold exposures in the 2rd trimester and four weeks prior to delivery were also associated with an increased risk of MI-PTB, with HRs of 1·26 (95% CI: 1·11, 1·43) and 1·39 (95% CI: 1·02, 1·89), respectively.
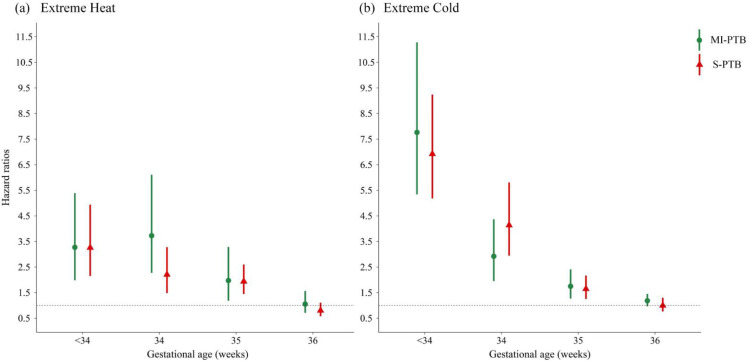
The effects of extreme temperature on both S-PTB and MI-PTB decreased with increased gestational age (Figure 3). The largest effects occurred in gestational age less than 34 weeks for S-PTB, with HRs of 3·26 (95% CI: 2·15, 4·94) for extreme heat and 6·92 (95% CI: 5·18, 9·24) for extreme cold. For MI-PTB, there was an increased risk for extreme heat at 34-35 gestational weeks (HR=3·73, 95% CI: 2·28, 6·11). The effects of extreme cold for MI-PTB were the largest in gestational age less than 34 weeks, with HR of 7·76 (95% CI: 5·34, 11·28).
Figure 3.
Hazard ratios of subtypes of preterm birth associated with extreme heat and cold in the entire pregnancy by gestational weeks.
Abbreviation: MI-PTB, medically induced preterm birth. S-PTB, spontaneous preterm birth.
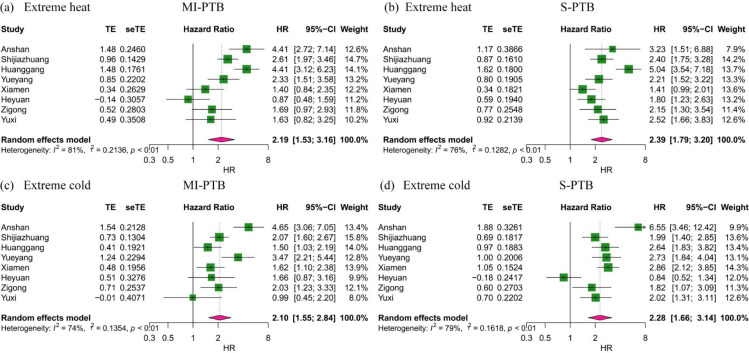
City-stratified effects between extreme temperature and PTB subtypes varied across cities (I2>50%) (Figure 4). For the impact of extreme heat on MI-PTB, we identified harmful effects in Anshan, Shijiazhuang, Huanggang, and Yueyang, and the largest adverse effects were found in Anshan and Huanggang with HRs of 4·41(95% CI: 2·72, 7·14) and 4·41(95% CI: 3·12, 6·23), respectively. Adverse effects of extreme heat on S-PTB were also detected in almost all cities except for Xiamen, and the largest adverse effects were found in Huanggang with HR of 5·04 (95% CI: 3·54, 7·18). For exposure to extreme cold, we found positive and precise associations with MI-PTB in Anshan, Shijiazhuang, Huanggang, Yueyang, Xiamen and Zigong, while effects on S-PTB were detected in all cities except Heyuan. The greatest effects were seen in Anshan (MI-PTB: HR=4·65, 95% CI: 3·06, 7·05; S-PTB=6·55, 95% CI: 3·46, 12·42).
Figure 4.
Hazard ratios of subtypes of preterm birth associated with extreme heat and cold in the entire pregnancy in eight provinces of China.
Abbreviation: HR, hazard ratios. CI, confidence interval. MI-PTB, medically induced preterm birth. S-PTB, spontaneous preterm birth.
The meta-regression indicated that several factors in the dimensions of economy and medical resources modified the effects of extreme temperature (Table 2). Regarding the economy dimension, we found that increase in GDP per capita decrease the effect of extreme heat on S-PTB (β=−0·16, 95% CI: -0·30, -0·01). We also found medical resources captured via the number of hospital beds per 1000 persons decrease the association between extreme cold and MI-PTB (β=−0·25, 95% CI: -0·50, -0·01).
Table 2.
Effect modification by city-level characteristics for the association between extreme temperature and preterm birth.
| Extreme heat |
Extreme cold |
|||
|---|---|---|---|---|
| Potential factors | MI-PTB | S-PTB | MI-PTB | S-PTB |
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | |
| Population | ||||
| Resident population (10,000 people) | 0.22 (-0.34, 0.77) | 0.09 (-0.18, 0.35) | 0.06 (-0.13, 0.24) | 0.09 (-0.10, 0.28) |
| Population density (people/km2) | 0.11 (-0.20, 0.42) | 0.05 (-0.40, 0.50) | 0.06 (-0.11, 0.22) | 0.07 (-0.10, 0.24) |
| Economy | ||||
| GDP per capita (CNY) | -0.13 (-0.43, 0.17) | -0.16 (-0.30, -0.01) | -0.06 (-0.24, 0.12) | -0.05 (-0.57, 0.47) |
| Engel coefficient (%) | 0.03 (-0.41, 0.48) | 0.11 (-0.32, 0.53) | 0.09 (-0.25, 0.42) | 0.10 (-0.38, 0.59) |
| Unemployment rate (%) | 0.02 (-0.49, 0.53) | -0.07 (-0.70, 0.57) | 0.07 (-0.15, 0.29) | 0.13 (-0.26, 0.52) |
| Medical resource | ||||
| Number of health institutions (n) | 0.05 (-0.56, 0.66) | -0.05 (-0.56, 0.47) | 0.07 (-0.17, 0.32) | -0.07 (-0.32, 0.18) |
| Hospital beds per 1000 people (n) | -0.12 (-0.47, 0.23) | -0.06 (-0.25, 0.13) | -0.25 (-0.50, -0.01) | -0.06 (-0.57, 0.46) |
| Physicians per 1000 people (n) | -0.10 (-0.44, 0.24) | -0.05 (-0.20, 0.09) | -0.09 (-0.38, 0.21) | 0.13 (-0.26, 0.51) |
| Environment | ||||
| NDVI | -0.06 (-0.59, 0.47) | -0.32 (-0.14, 0.75) | -0.08 (-0.45, 0.29) | -0.11 (-0.28, 0.05) |
| Air quality (%) | -0.11 (-0.31, 0.09) | -0.08 (-0.24, 0.08) | -0.05 (-0.21, 0.11) | -0.06 (-0.28, 0.15) |
Abbreviation: MI-PTB, medically induced preterm birth. S-PTB, spontaneous preterm birth. NDVI, Normalized Difference Vegetation Index.
In the sensitivity analysis, when using 95th percentiles of Tmax and Tmin to define heat exposures, similar significant effects during the entire pregnancy were found for PTB, MI-PTB and S-PTB. Additionally, when heat was more extreme, the effects were larger. For example, when using 90th or 99th percentiles of Tmean to define heat, the HRs for PTB were 3·48 (95%CI: 2·19, 5·51) and 1·16 (95%CI: 0·94, 1·42), respectively (Table S5). For sensitivity analysis of cold effects, when using 5th percentiles of Tmax or Tmin to define cold, similar susceptible windows including the 1st and 3rd trimester, entire pregnancy, one and four weeks before delivery were found. Additionally, the effects were larger in the entire pregnancy when cold defined with smaller cut-offs. For example, when using 1th or 10th percentiles of Tmean to define cold, the HRs for PTB were 3·48 (95%CI: 2·19, 5·51) and 1·81 (95%CI: 1·55, 2·13), respectively (Table S6).
Sensitivity analysis was conducted by excluding births determining gestational age only based on LMP. The results were similar to those of all births determining gestational age based on LMP or ultrasonography. Increased risks of preterm birth were detected for heat exposure in the entire pregnancy, and for cold exposure in the 1st trimester, the 3rd trimester, the entire pregnancy and one week before delivery. (Table S7).
Discussion
In this large-scale and population-based multi-center prospective cohort study, we evaluated the effects of maternal exposure to extreme temperature on the risk of PTB and its clinical subtypes in China. We identified positive associations between the risk of preterm birth (PTB), medically indicated preterm birth (MI-PTB), and spontaneous preterm birth (S-PTB) and extreme heat during the entire pregnancy, as well as with extreme cold during all exposure windows except the 2nd trimester. Additionally, we found that the effects of extreme temperature on PTB subtypes decrease with the increase of gestational age. Furthermore, the effects varied across cities, and that higher GDP per capita, as well as more hospital beds per 1000 persons contributed to mitigate such effects.
Exposure to extreme heat in the entire pregnancy was associated with increased risks of PTB in our study. Consistent with our findings, similar adverse effects in the entire pregnancy14,26,27 have been found in China,26 the United States14 and Korea.27 Additionally, susceptible windows were also detected in late pregnancy,17,25 early pregnancy16,28 or even in the three months before pregnancy.26 The potential mechanisms by which exposure to extreme heat may influence the risk of PTB remain unclear. One possible mechanism is that heat exposure may cause dehydration, in turn increasing blood viscosity, elevating cholesterol levels and leading to constriction in uterine blood flow, which can induce uterine contraction and onset of labor.29 Animal models suggest that heat may lead to an increase in the secretion of prostaglandin F2α (PGF2α), oxytocin and that can induce labor.30
Our study found adverse effects of exposure to extreme cold in early, late, and the entire pregnancy, while similar adverse effects were found in Guangzhou China,17 the United States16 and Iran.31 A possible explanation may be that cold exposure could increase blood viscosity and vascular constriction, which can induce the onset of labor. Additionally, cold weather may increase exposure to pathogenic agents and result in complications during pregnancy, further increasing the risk of PTB.8 However, protective effects of extreme cold during pregnancy were also found in some studies from the USA25 and China.26 It speculated that pregnant women might take more precautions in early or late pregnancy, such as using central heating or altering their behavior in response to cold to limit exposure. Changes in physiological conditions associated with pregnancy, such as an increase in fat deposition and a decrease in the ratio of body surface area to body mass, could make pregnant women less susceptible to cold.25 Future studies comparing different geographical contexts and quantifying the drivers of such heterogeneity will be helpful to understand the inconsistent results of cold effects on PTB. Moreover, the Urban Heat Island (UHI), a well-studied phenomenon that temperatures in urban centers are generally higher than in surrounding suburban and rural areas, can directly exacerbate health effects during heat waves and bring health benefits to urban areas during cold months.32 But in this study, we did not consider the health effects of UHI, which may need to be quantitatively estimated in future research.
Similar to the results for PTB, harmful effects of exposure to extreme heat on both MI-PTB and S-PTB in the entire pregnancy were detected. For exposure to extreme cold, susceptive windows for MI-PTB and S-PTB were the same with PTB, including the early, late, and entire pregnancy. Although the pathway by which extreme temperature induces PTB is not fully understood, the mechanism may vary with clinical subtypes of PTB. For MI-PTB, maternal exposure to extreme temperature may promote oxidative stress or inflammatory response,33,34 damage vascular endothelial cells,35 and affect the regulation of systemic vascular tone, thus inducing preeclampsia.10 In addition, it may also affect nutrition and oxygen delivery from the placenta to the fetus, thereby causing fetal growth restriction11 and ultimately inducing MI-PTB. For S-PTB, exposure to extreme temperature may increase the risk of premature rupture of membranes.12 Besides, it can also induce the hypersecretion of antidiuretic hormone and oxytocin, decrease uterine blood flow and cause fetal intrauterine hypoxia, thus triggering preterm labor.13
So far, only three studies from Israel36,37 and the United States12 clarified the impacts of extreme temperature on PTB subtypes. A time-series study in Israel found that exposure to extreme heat increased the risk of S-PTB by 7% but not for MI-PTB.36 It speculated that MI-PTB was generally associated with prevalent risk factors among childbearing women such as in vitro fertilization (IVF), older maternal age, and higher maternal body mass index (BMI). Therefore, no effect of extreme temperature on MI-PTB was observed. Another retrospective cohort study in Israel37 only focused on S-PTB and found that preterm premature rupture of membranes (PROM) was more sensitive to heat than preterm labor. Similar harmful effect for PROM was also detected in a case-crossover study in the United States,12 and a 1°C increase was associated with 5% (95% CI: 3%, 6%) PTB risk in the week prior to delivery during the warm season. It speculated that for PROM, physical and physiological changes, as well as external mechanical collision will lead to rupture of membranes.38 In addition, genital tract infection and acute stimulation of extreme temperature,12 may aggravate the above natural process, thus triggering PROM. Clarifying effects by subtypes may contribute to the research on the mechanism of extreme temperature on PTB. More studies related to the effects of extreme temperature on subtypes are expected in the future.
The effect of extreme temperature on both S-PTB and MI-PTB decreased with increase in gestational age. Similar results were reported from a cohort study in Guangzhou, China17 and a multi-center cohort study in the United States.16 Besides, we also detected larger effects of cold on PTB with gestational age less than 34 weeks. A possible explanation may be that PTB especially with smaller gestational age are immature in respiratory, circulatory system, etc., making them more susceptible to ambient environments. Besides, infection is one of the most critical risk factors for PTB, especially for early PTB. Maternal exposure to extreme cold may aggravate infection through oxidative stress and inflammation,34 and may also aggravate maternal complications,39 thus ultimately trigger early PTB. However, studies conducted in Guangzhou,15 China, found the extreme temperature effects increased as the gestational age increased. The authors speculated that environmental factors might be more important for late PTB.
City-stratified associations showed the effects of extreme temperature on both S-PTB and MI-PTB varied across cities. Furthermore, higher GDP per capita and more hospital beds per 1000 persons were found to significantly mitigate the harmful effects of extreme temperatures on PTB subtypes. GDP per capita and hospital beds per 1000 persons are important indicators of regional socio-economic status (SES). The potential mechanisms whereby SES affects PTB have been previously published,27,40, 41, 42, 43 and previous studies have suggested that low regional SES is a marker for poor pregnancy outcomes.40, 41, 42 It suggested that in areas with low SES, a greater risk of job loss40 and subsequent amplified biological and psychosocial stress were often found,42 which may be associated with a higher risk of PTB. Besides, social and economic barriers could also increase the risk of inadequate prenatal care,44 which may also be associated with higher PTB risk. For example, transportation costs to the hospital and the cost of receiving medical care may be a sufficient burden that restricts prenatal care in pregnant women living in areas with low SES.43
A few limitations should be considered when interpreting the findings from our study. First, as an inherent limitation of environmental epidemiology studies, the study could only clarify an association between extreme temperature and preterm birth, but could not prove causality. Second, exposure assessment in our study was established at a resolution of 1km × 1km and matched to the residential address for each pregnant woman. Exposure misclassification could exist due to lack of information on the maternal residence mobility, work address and its mobility, activity pattern, use of air conditioning or heating, and parameters affecting heat transfer such as metabolism, and type of clothing. Third, due to data unavailability, we cannot control for other personal risk factors related to the risk of PTB, such as prior history of preterm birth, medical complications, maternal physical activity and dietary intake during pregnancy. Future studies are recommended to consider these factors.
In conclusion, our study found that maternal exposure to extreme heat in the entire pregnancy may increase the risks of preterm birth and its subtypes, while extreme cold in the early, late, and entire pregnancy may increase the risk. In addition, socioeconomic conditions may affect the effects in different regions. These findings may have important implications in a comprehensive understanding of the health effects of extreme temperatures, and protecting the health of vulnerable populations, particularly newborns, in the context of climate change.
Contributors
Cunrui Huang conceived and designed the study; Meng Ren, Jiangli Di, Qiong Wang, Wei Zhao and Huanqing Hu collected and cleaned the data; Zhoupeng Ren provided technical guidance on exposure measurement; Meng Ren performed the data analysis and drafted the manuscript; Huanhuan Zhang, Bin Jalaludin, Tarik Benmarhnia, Ying Wang, John S. Ji and Wannian Liang helped revise the manuscript. All authors read and approved the final manuscript.
Data sharing statement
The individual, de-identified participant data that underline the results reported in this article (text, tables, figures, and appendices) are available on reasonable request from the corresponding authors under certain conditions (with the consent of all participating centers and with a signed data access agreement).
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of Interests
The authors declare no competing financial interest.
Footnotes
Note: Chinese translation of abstract is available in appendix section.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100496.
Appendix. Supplementary materials
References
- 1.Chawanpaiboon S, Vogel JP, Moller A-B, et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi: 10.1016/S2214-109X(18)30451-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson S, Marlow N. Early and long-term outcome of infants born extremely preterm. Arch Dis Child. 2017;102(1):97–102. doi: 10.1136/archdischild-2015-309581. [DOI] [PubMed] [Google Scholar]
- 3.Pokras S, Pimenta J, Merinopoulou E, Lambrelli D. Short and long-term costs among women experiencing preterm labour or preterm birth: The German experience. BMC Pregnancy Childbirth. 2018;18(1):284. doi: 10.1186/s12884-018-1912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koullali B, Oudijk MA, Nijman TA, Mol BW, Pajkrt E. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med. 2016;21(2):80–88. doi: 10.1016/j.siny.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 5.He Y, Tam CH, Yuen LY, Catalano PM, Ma RC, Tam WH. Optimal gestational weight gain for Chinese women - analysis from a longitudinal cohort with childhood follow-up. Lancet Reg Health West Pac. 2021;13 doi: 10.1016/j.lanwpc.2021.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chersich MF, Pham MD, Areal A, et al. Associations between high temperatures in pregnancy and risk of preterm birth, low birth weight, and stillbirths: Systematic review and meta-analysis. BMJ. 2020;371:m3811. doi: 10.1136/bmj.m3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamath-Rayne BD, DeFranco EA, Chung E, Chen A. Subtypes of preterm birth and the risk of postneonatal death. J Pediatr. 2013;162(1):28–34. doi: 10.1016/j.jpeds.2012.06.051. .e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch AM, Wagner BD, Hodges JK, et al. The relationship of the subtypes of preterm birth with retinopathy of prematurity. Am J Obstet Gynecol. 2017;217(3):354.e351–354.e358. doi: 10.1016/j.ajog.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Finken MJJ, van der Voorn B, Hollanders JJ, et al. Programming of the hypothalamus-pituitary-adrenal axis by very preterm birth. Ann Nutr Metab. 2017;70(3):170–174. doi: 10.1159/000456040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu H, Khalil RA. Vascular mechanisms and molecular targets in hypertensive pregnancy and preeclampsia. Am J Physiol Heart Circ Physiol. 2020;319(3):H661–H681. doi: 10.1152/ajpheart.00202.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krause BJ, Carrasco-Wong I, Caniuguir A, Carvajal J, Farias M, Casanello P. Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta. 2013;34(1):20–28. doi: 10.1016/j.placenta.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Ha S, Liu DP, Zhu YY, Sherman S, Mendola P. Acute associations between outdoor temperature and premature rupture of membranes. Epidemiology. 2018;29(2):175–182. doi: 10.1097/EDE.0000000000000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dadvand P, Basagana X, Sartini C, et al. Climate extremes and the length of gestation. Environ Health Perspect. 2011;119(10):1449–1453. doi: 10.1289/ehp.1003241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloog I, Melly SJ, Coull BA, Nordio F, Schwartz JD. Using satellite-based spatiotemporal resolved air temperature exposure to study the association between ambient air temperature and birth outcomes in Massachusetts. Environ Health Perspect. 2015;123(10):1053–1058. doi: 10.1289/ehp.1308075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Li B, Benmarhnia T, et al. Independent and combined effects of heatwaves and PM₂.₅ on preterm birth in Guangzhou, China: A survival analysis. Environ Health Perspect. 2020;128(1):17006. doi: 10.1289/EHP5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha SD, Liu DP, Zhu YY, Kim SS, Sherman S, Mendola P. Ambient temperature and early delivery of singleton pregnancies. Environ Health Perspect. 2017;125(3):453–459. doi: 10.1289/EHP97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He JR, Liu Y, Xia XY, et al. Ambient temperature and the risk of preterm birth in Guangzhou, China (2001-2011) Environ Health Perspect. 2016;124(7):1100–1106. doi: 10.1289/ehp.1509778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Tong S, Williams G, Pan X. Exposure to heat wave during pregnancy and adverse birth outcomes: An exploration of susceptible windows. Epidemiology. 2019;30 Suppl 1:S115–S121. doi: 10.1097/EDE.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 19.Wu K, Hu H, Ren Z, et al. Effects of maternal exposure to fine particulate matter on birth weight in 16 counties across China: A quantile regression analysis. Environ Res Lett. 2021;16(5) [Google Scholar]
- 20.Zhang H, Wang Q, Benmarhnia T, et al. Assessing the effects of non-optimal temperature on risk of gestational diabetes mellitus in a cohort of pregnant women in Guangzhou. China. Environ Int. 2021;152 doi: 10.1016/j.envint.2021.106457. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Liang Q, Li C, et al. Interaction of air pollutants and meteorological factors on birth weight in Shenzhen. China. Epidemiology. 2019;30 Suppl 1:S57–S66. doi: 10.1097/EDE.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Sheridan P, Laurent O, et al. Associations between green space and preterm birth: Windows of susceptibility and interaction with air pollution. Environ Int. 2020;142 doi: 10.1016/j.envint.2020.105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Y, Ilango SD, Schwarz L, et al. Examining the joint effects of heatwaves, air pollution, and green space on the risk of preterm birth in California. Environ Res Lett. 2020;15(10) doi: 10.1088/1748-9326/abb8a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi H, Yang H. Guidelines for pre-pregnancy and pregnancy health care (2018) Chinese Journal of Obstetrics and Gynecology. 2018;53(01):7–13. [Google Scholar]
- 25.Sun SZ, Weinberger KR, Spangler KR, Eliot MN, Braun JM, Wellenius GA. Ambient temperature and preterm birth: A retrospective study of 32 million US singleton births. Environ Int. 2019;126:7–13. doi: 10.1016/j.envint.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T, Wang Y, Zhang H, et al. The association between ambient temperature and the risk of preterm birth in China. Sci Total Environ. 2018;613-614:439–446. doi: 10.1016/j.scitotenv.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 27.Son JY, Lee JT, Lane KJ, Bell ML. Impacts of high temperature on adverse birth outcomes in Seoul, Korea: Disparities by individual- and community-level characteristics. Environ Res. 2019;168:460–466. doi: 10.1016/j.envres.2018.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang YY, Li Q, Guo Y, et al. Ambient temperature and the risk of preterm birth: A national birth cohort study in the mainland China. Environ Int. 2020;142 doi: 10.1016/j.envint.2020.105851. [DOI] [PubMed] [Google Scholar]
- 29.Stan CM, Boulvain M, Pfister R, Hirsbrunner-Almagbaly P. Hydration for treatment of preterm labour. Cochrane Database Syst Rev. 2013;(11) doi: 10.1002/14651858.CD003096.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai S, Hagihara N, Kuse M, Kimura K, Okuda K. Heat stress affects prostaglandin synthesis in bovine endometrial cells. J Reprod Dev. 2018;64(4):311–317. doi: 10.1262/jrd.2018-051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammadi D, Naghshineh E, Sarsangi A, Zare Sakhvidi MJ. Environmental extreme temperature and daily preterm birth in Sabzevar, Iran: A time-series analysis. Environ Health Prev Med. 2019;24(1):5. doi: 10.1186/s12199-018-0760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SW, Brown RD. Urban heat island (UHI) intensity and magnitude estimations: A systematic literature review. Sci Total Environ. 2021;779 doi: 10.1016/j.scitotenv.2021.146389. [DOI] [PubMed] [Google Scholar]
- 33.Fu J, Liu CP, Zhang ZW, Xing MW, Xu SW. Influence of inflammatory pathway markers on oxidative stress induced by cold stress in intestine of quails. Res Vet Sci. 2013;95(2):495–501. doi: 10.1016/j.rvsc.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Ganesan S, Volodina O, Pearce SC, et al. Acute heat stress activated inflammatory signaling in porcine oxidative skeletal muscle. Physiol Rep. 2017;5(16):e13397. doi: 10.14814/phy2.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walfisch A, Kabakov E, Friger M, Sheiner E. Trends, seasonality and effect of ambient temperature on preterm delivery. J Matern Fetal Neonatal Med. 2017;30(20):2483–2487. doi: 10.1080/14767058.2016.1253063. [DOI] [PubMed] [Google Scholar]
- 37.Gat R, Kachko E, Kloog I, et al. Differences in environmental factors contributing to preterm labor and PPROM - population based study. Environ Res. 2021;196 doi: 10.1016/j.envres.2021.110894. [DOI] [PubMed] [Google Scholar]
- 38.Lannon SM, Vanderhoeven JP, Eschenbach DA, Gravett MG, Adams Waldorf KM. Synergy and interactions among biological pathways leading to preterm premature rupture of membranes. Reprod Sci. 2014;21(10):1215–1227. doi: 10.1177/1933719114534535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davidson KW, Barry MJ, Mangione CM, et al. Aspirin use to prevent preeclampsia and related morbidity and mortality: US preventive services task force recommendation statement. JAMA. 2021;326(12):1186–1191. doi: 10.1001/jama.2021.14781. [DOI] [PubMed] [Google Scholar]
- 40.Noelke C, Chen YH, Osypuk TL, Acevedo-Garcia D. Economic downturns and inequities in birth outcomes: Evidence from 149 million US births. Am J Epidemiol. 2019;188(6):1092–1100. doi: 10.1093/aje/kwz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Margerison CE, Luo Z, Li Y. Economic conditions during pregnancy and preterm birth: A maternal fixed-effects analysis. Paediatr Perinat Epidemiol. 2019;33(2):154–161. doi: 10.1111/ppe.12534. [DOI] [PubMed] [Google Scholar]
- 42.Margerison-Zilko CE, Li Y, Luo Z. Economic conditions during pregnancy and adverse birth outcomes among singleton live births in the United States, 1990-2013. Am J Epidemiol. 2017;186(10):1131–1139. doi: 10.1093/aje/kwx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim MK, Lee SM, Bae SH, et al. Socioeconomic status can affect pregnancy outcomes and complications, even with a universal healthcare system. Int J Equity Health. 2018;17(1):2. doi: 10.1186/s12939-017-0715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bruno SKB, Rocha HAL, Rocha S, et al. Prevalence, socioeconomic factors and obstetric outcomes associated with adolescent motherhood in Ceará, Brazil: A population-based study. BMC Pregnancy Childbirth. 2021;21(1):616. doi: 10.1186/s12884-021-04088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.