Abstract
Recent investigations of COVID-19 have largely focused on the effects of this novel virus on the vital organs in order to efficiently assist individuals who have recovered from the disease. In the present study we used hippocampal tissue samples extracted from people who died after COVID-19. Utilizing histological techniques to analyze glial and neuronal cells we illuminated a massive degeneration of neuronal cells and changes in glial cells morphology in hippocampal samples. The results showed that in hippocampus of the studied brains there were morphological changes in pyramidal cells, an increase in apoptosis, a drop in neurogenesis, and change in spatial distribution of neurons in the pyramidal and granular layer. It was also demonstrated that COVID-19 alter the morphological characteristics and distribution of astrocyte and microglia cells. While the exact mechanism(s) by which the virus causes neuronal loss and morphology in the central nervous system (CNS) remains to be determined, it is necessary to monitor the effect of SARS-CoV-2 infection on CNS compartments like the hippocampus in future investigations. As a result of what happened in the hippocampus secondary to COVID-19, memory impairment may be a long-term neurological complication which can be a predisposing factor for neurodegenerative disorders through neuroinflammation and oxidative stress mechanisms.
Keywords: COVID-19, Hippocampus, Degeneration, Glial cells, Sholl analysis
Introduction
More than one year since the emergence of COVID-19, this disease has caused millions of deaths and despite efforts including vaccine and therapeutic development, the number of casualties is still significant [1]. Initially, the main concern of clinicians was to manage the acute complications of the disease; however, as long-term consequences of SARS-CoV-2 infection have emerged, management of these sequelae has become a priority. Although COVID-19 is largely known for its respiratory symptoms, multiple organ damage, including to the nervous system, kidneys, heart, etc., occurs–especially in the severe forms of the disease—and can impose permanent sequelae or even lead to death [2].
The prevalence of central nervous system (CNS) manifestations in COVID-19 is about 25% [3, 4], with neurological symptoms including learning deficits (in both adults and children), hippocampal and cortical memory and attention impairment (either acute or chronic), and delirium [5–7]. Cognitive deficits have been reported in COVID-19 patients both acutely and after recovery [2, 8]. According to evidence garnered from animal and human studies, SARS-CoV-2 can invade the brain, including the brainstem, directly through the olfactory nerves even without any prior lung involvement [9, 10]. This might be explained by the presence of ACE2 (angiotensin-converting enzyme 2) receptors in the brain, which can bind to viral spike glycoprotein. These receptors are widely found in the brain, from cardiorespiratory centers in the medulla, to dopamine neurons of striatum [11–13].
So far, some investigations [14, 15] have found direct evidence for SARS-CoV-2 neurotropism. To gain access to human cells, both SARS-CoV and SARS-CoV-2 bind to ACE2 receptor [7]. Many SARS-CoV-mediated pathologies may also be the same as SARS-CoV-2 due to similarities in target receptors and structure. Numerous papers have largely found documentations for neurotropism and neurovirulence of SARS-CoV [16]. Some case reports revealed that CSF samples from a patient with SARS infection along with tonic–clonic seizures indicated positive tests for SARS-CoV, implying SARS-CoV infection transmission through CNS [17]. Moreover, Jun et al. have isolated SARS-CoV from a brain tissue sample from a SARS patient, indicating neuronal glial cell hyperplasia and necrosis [18]. According to Berardis study, the spike protein of SARS-CoV-2 can moderate virus entry into cells via ACE-2 receptors and is triggered by a serine two protease transmembrane (TMPRSS2). Both TMPRSS2 and ACE-2 are abundant in the brain, particularly in critical areas for psychiatric disorders such as the hippocampus and prefrontal cortex. Furthermore, microglia is considered a target for the SARS-CoV-2 via the same mechanisms causing its activation, which harms the brain via a local cytokine storm syndrome similar to mild autoimmune and viral encephalitis. Also, CD8-positive T cells have been found to have roles in the CNS infiltrations [19]. These findings of neurovirulence and neuroinvasion in SARS-CoV could provide strong circumstantial evidence of SARSCoV-2's neurotropic properties [18].
Infectious disease-associated encephalopathy is an umbrella term used to describe neurological manifestations in infections—in this instance, SARS-CoV-2—which are assumed to have different pathophysiological mechanisms than encephalopathy of a non-infectious origin [20]. Several hypotheses have now been proposed to explain the mechanism of acute and long-term SARS-CoV-2-associated cognitive impairment; including extensive systemic inflammation (e.g. microglia activation [21, 22] and cytokine signaling [23, 24]), viral neurotropism, and psychological burden of the pandemic. Unfortunately, it has been shown that CNS dysfunction can lead to poorer prognosis in COVID-19 patients [25].
Emerging research points out to the potential role of COVID-19 in exacerbation of the clinical spectrum of neurological diseases. Additionally, it has been recently hypothesized that the novel coronavirus plays a role in the future development of neurological diseases. Therefore, the relationship between SARS-CoV-2 infection and neurological diseases and their manifestations has recently become a matter of great interest. It have already been established that coronaviruses can be detectable in the CNS of patients with neurodegenerative diseases such as Alzheimer disease (AD), Parkinson disease (PD), and multiple sclerosis (MS) [12, 26]. AD, as one of the most common neurodegenerative disorders, is known to affect the memory and learning and alter the behavior and cognitive performance of the patients. According to the pathophysiology of the disease, deposition of amyloid beta (Aβ) or neurofibrillary tangles (NFT) in certain brain regions (especially the hippocampus), which are responsible for the process of learning and memory, leads to these behavioral and cognitive impairments [4, 27].
As mentioned earlier, the most eminent pathophysiology of SARS-CoV-2 is rooted in the inflammatory response of the immune system and “cytokine storm (CS)” is its hallmark. CS is described as a state in which the elevation of pro-inflammatory cytokines and chemokines including interleukins (specifically IL-6 and IL-1β), chemokine (C–C-motif) ligand 2 (CCL2), tumor necrosis factor-alpha (TNF-α), and granulocyte colony stimulating factor (G-CSF) occurs [23, 28–31]. Previous studies have revealed that inflammation and peripheral immune response can lead to (or in some cases exacerbate) both acute and chronic neuro-inflammation [32–39]. For instance, mast cells are immune cells implicated in the process of inflammation (specifically neuro-inflammation) and psychological stress [25, 40].
Multiple and interacting causes have been proposed to explain the mechanism of SARS-CoV-2 infection-associated sub-clinical cognitive impairment and neurological symptoms, including both indirect causes due to non-CNS systemic impairment or psychological trauma, and direct causes due to damage caused by the virus to the cortical and adjacent subcortical structures (e.g. brainstem) [41]. As stated earlier, the involvement of the nervous system structures, particularly the brainstem, by SARS-CoV-2 is possible [10]. This has previously been observed in other coronaviruses which demonstrated ability to invade the peripheral-nerve terminals and retrograde progression toward the CNS, via synapse-connected routes [42–45]. This capacity is also well established in other viruses, such as Herpes Simplex and Herpes Zoster, which lay dormant and later start to migrate via peripheral neurons to the CNS (or sensory ganglia) and cause extensive damage [46–48]. Interestingly, another single-stranded RNA beta coronavirus, named hemagglutinating encephalomyelitis virus (HEV), has also been found to be able to disseminate in the primary motor cortex of infected rats [44, 49].
Altogether, the present study aims to elucidate the link between SARS-CoV-2 infection and hippocampal-related neurodegenerative disorders in order to further illuminate potential long-term sequelae of COVID-19 for the scientific and medical communities.
Materials and methods
Ethics and informed consent
The study was approved by the ethics committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran (ethics committee No. IR.SBMU.RETECH.REC.1400.476).
Sample and data collection
This study was conducted on four patients with COVID-19 and four age- and sex-matched healthy control groups. Control data were obtained from people in the age range of 30 − 45 years, with the following exclusion criteria applied: history of neurological complications or addiction, cause of death including cardiac arrest, head trauma, and internal bleeding. In the COVID-19 group, the subjects were selected from patients admitted to the intensive care unit of a major university-affiliated hospital between March 26, 2020 (the outbreak of the epidemic in Iran) and April 17, 2020. Four patients with clinical features including anosmia and respiratory symptoms compatible with COVID-19 were intubated due to respiratory distress. The diagnosis of COVID-19 was confirmed by RT-PCR. Additionally, a computed tomography scan showed pneumonia in the COVID-19 group. After death, it took about 4 to 5 h to transfer the dead body to the dissection room of the legal medicine. With previous permission from the patients’ families, the brains were removed from the skull, and the hippocampus was dissected and immersed into the 10% formalin. The specimen of the hippocampal formation was taken at a mid-anterior–posterior level of the hippocampus. Then, the specimens were routinely processed under standard biosafety measures for further investigation. Tissue specimens were then dehydrated in graded ethanol, embedded in paraffin and serially cut in 5-µm coronal sections (Fig. 1).
Fig. 1.
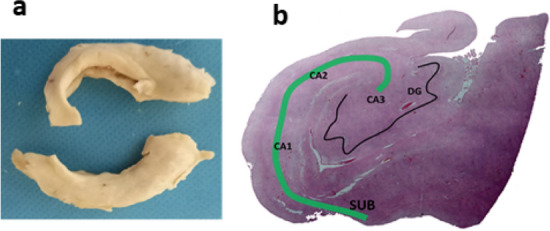
a A general view of excracted hippocampus of postmorterm subjects. b H&E staining of hppocampal areas: subiculum (SUB); dentate gyrus (DG) and cornu ammonis (CA)
Immunohistochemistry
Hippocampal tissues were first dehydrated in a series of graded ethanol baths, and then infiltrated with paraffin using the tissue-embedding machine. Samples were sectioned by a microtome and 5 μm of the formalin-fixed paraffin-embedded tissue samples were deparaffinized and rehydrated. To block nonspecific background staining, tissues were incubated with the protein block for 5 min at room temperature and washed in a buffer. Sections were incubated in the primary antibodies against Iba-1, Ki67, cleaved caspase-3 and glial fibrillary acidic protein (GFAP) overnight at 4 °C. The following morning, tissues were washed four times in the buffer before the application of Streptavidin Peroxidase. They were then incubated for 10 min at room temperature, and rinsed again four times in the buffer. Afterwards, 20 μl of 3, 3′-Diaminobenzidine (DAB) chromogen was added to 1 ml of DAB substrate and mixed by swirling before being applied to the tissue sample. For immunofluorescence detection, secondary antibodies (TRITC or FITC) were used. Finally, tissues were incubated for 10 min, and washed in the buffer. After the immunohistochemical reaction, tissues were counterstained with hematoxylin or DAPI, and examined under a microscope.
Investigation of microglia and astrocyte morphology and distribution
Microglial cells were stained with iba-1 antibody (specific microglia marker) and Astrocyte cells were stained with GFAP antibody (specific astrocyte marker). Thirty individual iba-1+ and GFAP+ cells were selected and captured by 40 × objective lens for reconstruction [50]. Figure was imported to ImageJ (Java. NIH, USA) after setting the image scale, the nucleus of each microglia and astrocyte were marked as the center. Soll analysis and total process length was performed based on previous descriptions [51, 52]. Total number of branches was calculated by the ‘Analyze Skeleton’ menu in ImageJ [53]. To measure soma size, each image was imported to ImageJ. After setting the image scale by ‘Wand’ tools the border of each soma was selected, nearest neighbor distance (NND) was determined, and a script for Fiji (ImageJ) developed by Mao [54] was used as described in previous studies [55]. To calculate soma roundness following formula was used:
where A is the area of the soma and M is the length of the major axis [55].
Regularity index (RI) was calculated by following formula:
where is the average NND of a population and is the standard deviation of that population [55, 56]. To calculate arbor area, a polygon was drawn manually by connecting the endpoints of the appendages using the imageJ ‘Polygon’ tools [55].
Investigation of the spatial distribution of hippocampal neurons
Following tissue processing, the serial coronal sections of 5 μm in thickness were prepared and stained with hematoxylin and eosin (H&E) (0.1%). The spatial distribution of neurons in the pyramidal layer of CA1 and granular layer of dentate gyrus was investigated by Voronoi tessellation method. Each polygon represents the space that a cell occupies [57]. The neurons were mapped by the Image J Voronoi Plugin (Java. NIH, USA). Each polygon was drawn around the cell body of neurons. To do this, brain section images were captured by a 40 × objective lens (Nikon Eclipse E-200). Each image imported to Image j, after set scale, by Voronoi plugin, and polygons were drawn by clicking on the nuclei. Area of the polygon by running analyze → measure was calculated [58, 59]. The area variation of the polygons was analyzed by calculating the variance. To determine the spatial distribution of neurons, percentage coefficient of variation (CV) calculated (standard deviation of the polygon areas/mean × 100). CV of 33%–64% is associated with a random distribution of the neurons; CVs less than 33% have a regular pattern, and those more than 64% are considered as a clustered distribution [60].
Investigation of dendrites of hippocampal pyramidal neurons by scholl analysis
To stain pyramidal neurons and these processes, hippocampal tissues were immersed in Golgi-Cox solution [61]. After processing the tissue, 60 µm-thick serial coronal sections were prepared. The microscopic images for analysis were captured by a 40 × objective lens (Nikon Eclipse E-200). Thirty individual Golgi cells were selected for reconstruction [61]. Nucleus of pyramidal neurons was marked as the center Scholl analysis and total dendrite length was done according to the previous part.
Data analysis
All statistical analyses were performed using SPSS23 software. The graphs were designed by Graph Pad Prism 7. Data are expressed as mean ± SEM. Differences between experimental groups were measured using the independent sample T-test. P-values < 0.05 were considered as significant.
Results
COVID-19 changed morphological characteristics and distribution of microglia cells
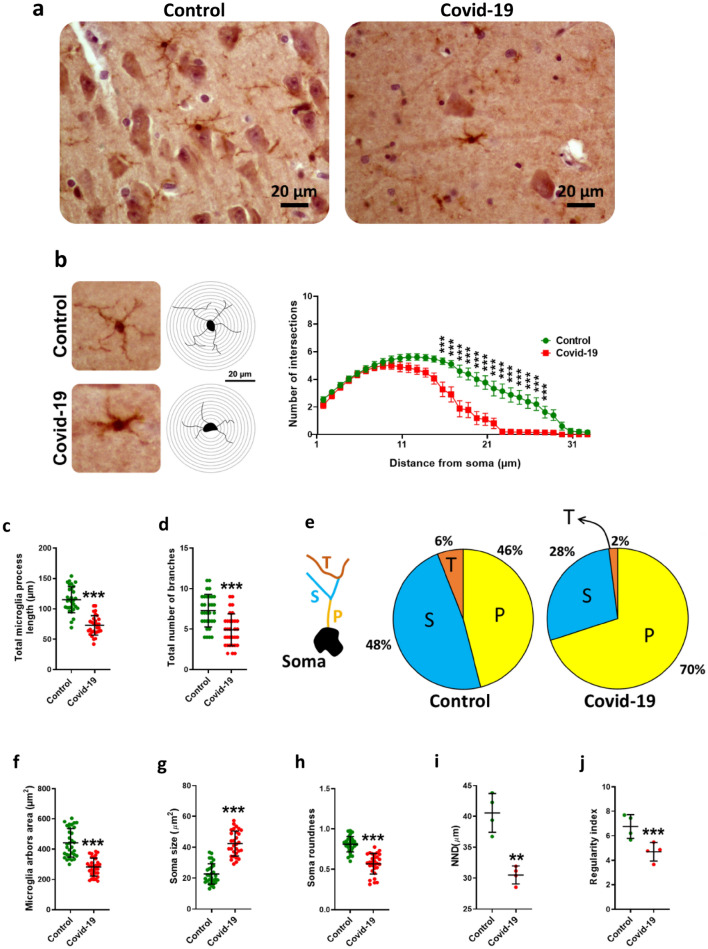
Immunohistochemistry was done against Iba-1 as a marker of microglia (Fig. 2a). Our results showed that SARS-CoV-2 infection significantly reduced morphological complexity of microglia processes (Fig. 2b), reducing their length (P < 0.001) (Fig. 2c) as well as their number (P < 0.001) (Fig. 2d). Analysis of branching structure showed that in infected descendants, the percentage of primary arbores increased (70%) but secondary and tertiary arbores decreased (2%) (Fig. 2e). Our studies have also shown that the area of microglia arbores is significantly reduced due to COVID-19 (P < 0.001) (Fig. 2f). Measurement of cell body area showed an increase in individuals with COVID-19 (P < 0.001) (Fig. 2g). Examination of the cell body showed that infection significantly reduced cell body roundness (P < 0.001) (Fig. 2h). Investigation of microglial scattering showed that nearest neighbor distance (NDD) (P < 0.01) (Fig. 2i) and regularity index significantly decreased in COVID-19 patients (P < 0.001) (Fig. 2j).
Fig. 2.
Sholl analysis findings in hippocampal microglia. a The immunohistochemical images in both groups to show a number of microglia in the microscopy view. b In contrast to control group, COVID -19 reduced the complexity of microglial processes. c COVID -19 administration significantly decreased the microglia process length and their number d. In COVID -19 infection the percentage of the primary arbores, P, increased (70%) but secondary, S, and tertiary (T) arbores decreased (2%) (e). The area of microglia arbores is significantly reduced due to COVID-19 infection (f). Measurement of cell body area showed that it increased due to COVID-19 infection (g). Examination of the cell body showed that COVID-19 infection significantly reduced the cell body roundness (h). Investigation of the microglia scattering showed that nearest neighbor distance (NND) (i) and regularity index significantly decreased in COVID-19 infection (j). Asterisk (*) shows the difference between the COVID-19 and the control groups (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM
COVID-19 changed morphological characteristics and distribution of astrocytes cells
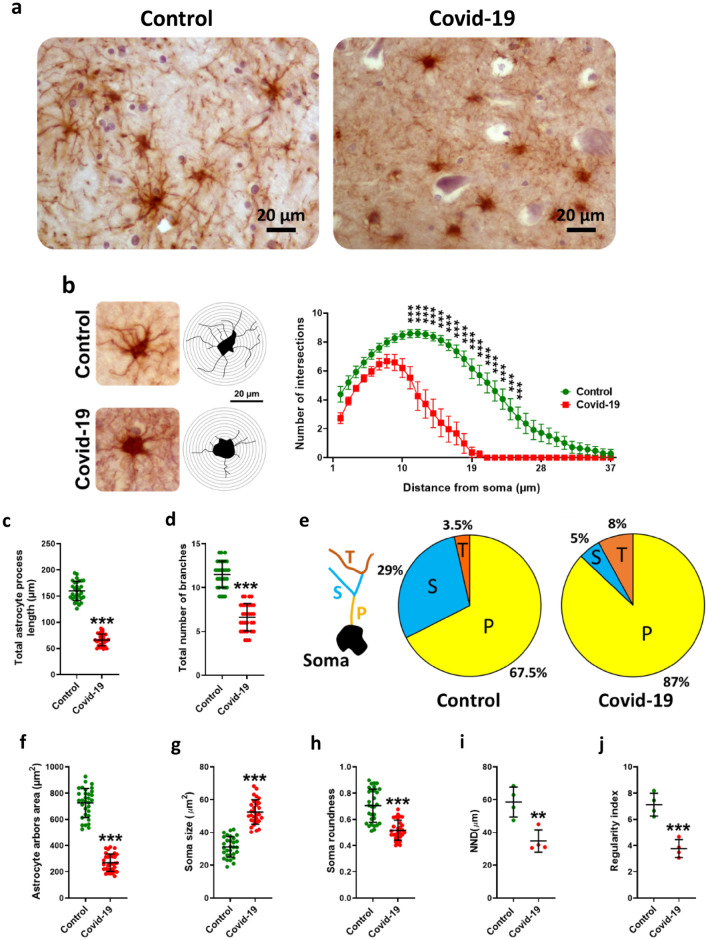
Immunohistochemistry was done against GFAP as a marker of astrocyte (Fig. 3a). Similar to the changes that occurred in astrocyte cells, COVID-19 significantly reduced the complexity (Fig. 3b), length (Fig. 3c) and total number (P < 0.001) (Fig. 3d) of astrocyte processes, determined by analysis of branching structure. The percentage of primary (87%) and tertiary arbores (8%) increased but secondary arbores decreased (5%) (Fig. 3e). Furthermore, the area of astrocyte arbores significantly decreased (P < 0.001) (Fig. 3f). The soma size, as in astrocytes, significantly increased due to infected patients (P < 0.001) (Fig. 3g). Soma roundness (P < 0.001) (Fig. 3h), NND (P < 0.01) (Fig. 3i) and regularity index (P < 0.001) (Fig. 3j) also increased significantly in individuals with COVID-19.
Fig. 3.
Sholl analysis findings in hippocampal astrocytes. a The immunohistochemical images in both groups to show several number of astrocytes in the microscopy field. b In contrast to control group, COVID -19 reduced the complexity of astrocyte processes. c COVID -19 administration significantly decreased the astrocyte process length and their number d. In COVID -19 infection the percentage of the primary arbores (P) increased (70%) but secondary (S) and tertiary (T) arbores decreased (2%) (e). The area of astrocytic arbores is significantly reduced due to COVID-19 infection (f). Measurement of cell body area showed that it increased due to COVID-19 infection (g). Examination of the cell body showed that COVID-19 infection significantly reduced the cell body roundness (h). Investigation of the astrocyte scattering showed that nearest neighbor distance (NND) (i) and regularity index significantly decreased in COVID-19 infection (j). Asterisk (*) shows the difference between the COVID-19 and the control group (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM
COVID-19 altered the spatial distribution of neurons in the hippocampal granular layer
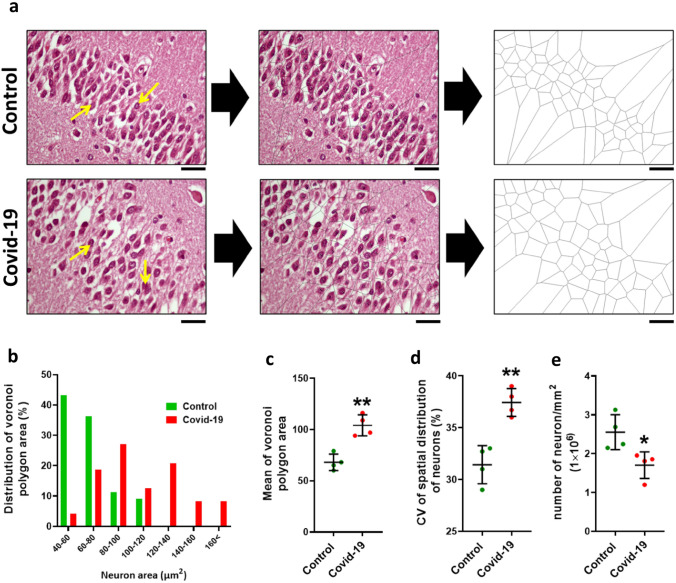
Voronoi tessellation of the dentate gyrus (granular layer) in the control and COVID-19 groups was performed (Fig. 4a). Our data showed that 43.18% Voronoi polygons in the granular layer in the control group were in the range of 40–60 μm2, however, in COVID-19 only 4.16% polygons areas of the neurons were within this range, while most of polygon areas (27%) were in the range of 80–100 μm2 (Fig. 4b). Additionally, the mean area of the polygons in the COVID group was significantly increased compared to the control group (P < 0.01) (Fig. 4c). Based on the (CV) classification, the mean CV of polygon areas in both groups located in a random range (33%–64%) while in COVID-19 group significantly increased, indicating the distribution of neurons in the granular layer is more regular than COVID-19 group (P < 0.01) (Fig. 4d). Also, our results show that the number of neurons in the granular layer is significantly reduced in COVID-19 patients compared to the control group (P < 0.05) (Fig. 4e).
Fig. 4.
Voronoi analysis on dentate gyrus. a A micrograph of neurons and schematic of Voronoi tessellation in the dentate gyrus for control and COVID-19 groups. b The most distribution of Voronoi polygon area is in the range 80–100 µm2. c Mean ± Standard deviation of Voronoi polygon area in both groups, d Coefficient of Variation (CV) of distribution of neurons within the dentate gyrus, e number of neurons per mm2 of dentate gyrus. Asterisk (*) shows the difference between the COVID and the control group (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM. The values were expressed as means ± SEM
COVID-19 changed spatial distribution of neurons in the hippocampal pyramidal layer
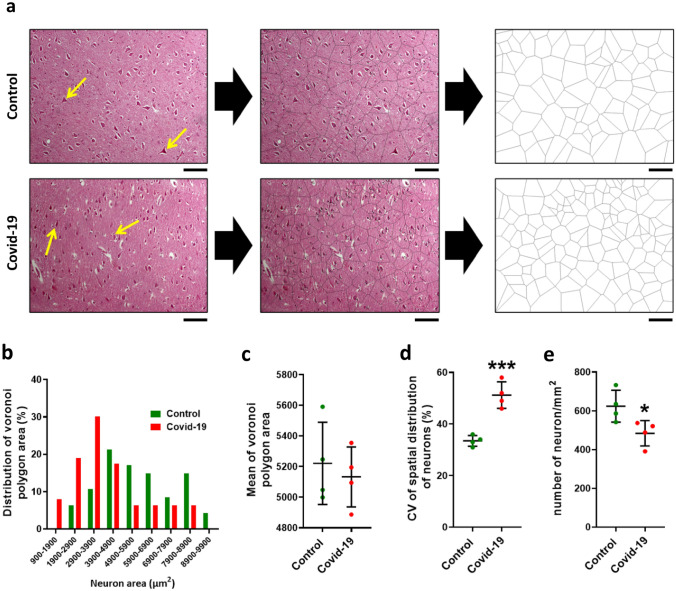
Voronoi tessellation of the CA1 neurons (pyramidal layer) in the control and COVID-19 groups was performed (Fig. 5a). Examination of the pyramidal layer in the control group showed that 21.2% polygons areas of the neurons were in the range of 3900–4900 μm2 while in COVID-19 group 30% polygons areas of the neurons were in the range of 200–3900 μm2 (Fig. 5b). Mean area of the polygons in the Covid group was significantly increased compared to the control group (Fig. 5c). Based on the (CV) classification, the mean CV of polygon areas in both groups located in a random range (33%–64%) while in COVID-19 group significantly increased that this indicates distribution neurons in pyramidal layer is more regular than COVID-19 group (P < 0.001) (Fig. 5d). Also, our results show that the number of neurons in the pyramidal layer is significantly reduced in COVID-19 infection (P < 0.05) (Fig. 5e).
Fig. 5.
Voronoi analysis in pyramidal layer. a A micrograph of neurons and schematic of Voronoi tessellation in the hippocampal pyramidal layer for control and COVID-19 groups. b The most distribution of Voronoi polygon area is in the range 80–100 µm2. c Mean ± Standard deviation of Voronoi polygon area in both groups, d Coefficient of Variation (CV) of distribution of neurons within the hippocampus, e number of neurons per mm2 of hippocampus. Asterisk (*) shows the difference between the COVID and the control group (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM. The values were expressed as means ± SEM
COVID-19 increases apoptosis and decreases neurogenesis in hippocampus
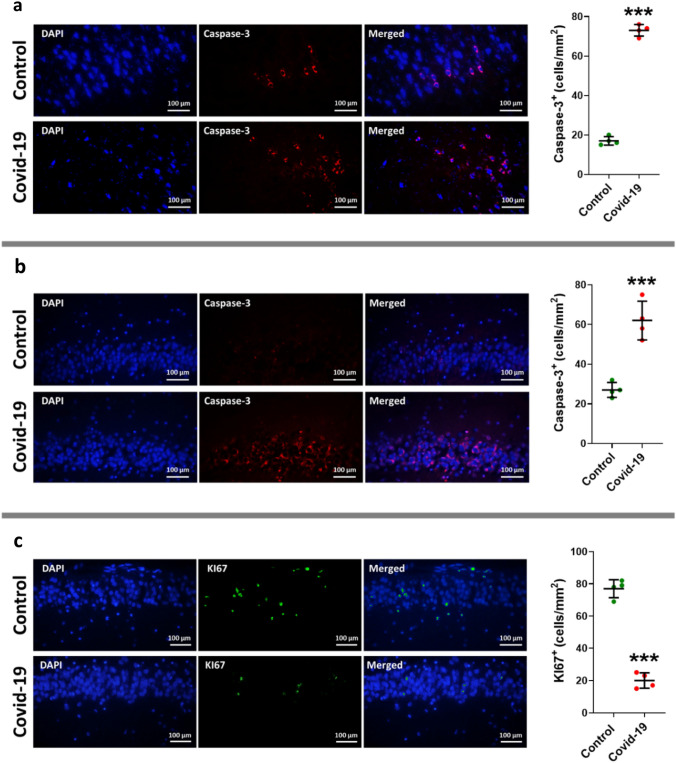
IHC was applied to measure two agents: the apoptosis factor of cleaved caspase-3 and the neurogenesis factor Ki67. Our results showed a surge in the cleaved caspase-3 in the CA1 of the hippocampus and dentate gyrus group of COVID-19 group in comparison to the control group (P < 0.001) (Fig. 6 a, b). On the other hand, a significant drop was observed in the Ki67 in the dentate gyrus of COVID-19 group (P < 0.001) (Fig. 6c).
Fig. 6.
Immunofluorescence staining of cleaved caspases-3 in tissue sections taken from the hippocampal pyramidal layer (a) and granular layer (b) of dentate gyrus, and neurogenesis factor Ki67 (c) in dentate gyrus of two groups of cases including control and COVID-19. The antibody in detecting cleaved caspases-3, and Ki67 are shown in right side (red is cleaved caspase-3 staining, green is KI67, and total nuclei stained with DAPI are blue) (a and b), and the left graphs illustrate in the mean and standard error of cleaved caspase-3 and Ki67 marker expression in dentate gyrus. Asterisk (*) shows the difference between the COVID and the control group (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM. The values were expressed as means ± SEM
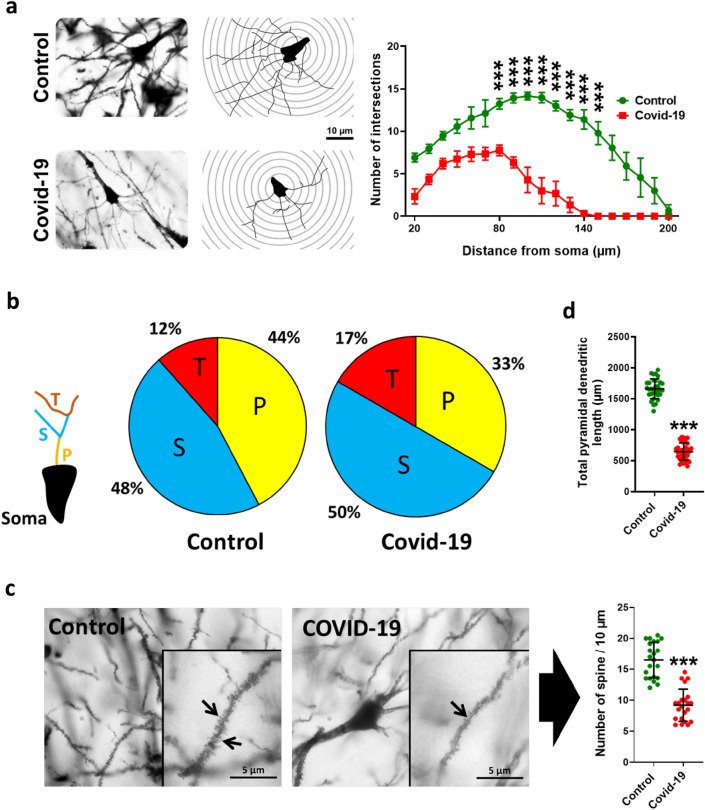
COVID-19 changed morphological characteristics pyramidal cells
Our results showed that morphological complexity of dendritic of pyramidal cells in COVID-19 infection was significantly reduced (P < 0.001) (Fig. 7a). Branching analysis showed that the percentage of primary arbores decreased (33%) while secondary and tertiary arbores increased (50% and 17% respectively) (Fig. 7b). Total dendritic length in COVID-19 infection significantly decreased (P < 0.001) (Fig. 7c). Investigation of dendrites with high magnification showed that the number of dendritic spin in COVID-19 infection significantly decreased (P < 0.001) (Fig. 7d).
Fig. 7.
Hippocampal Golgi images and the changes in morphological complexity of dendritic of pyramidal cells in COVID-19 subjects compared with control ones (a). COVID-19 changed the percentage of primary, secondary and tertiary arbors (b). Also, there is a significant decrease in number of spine (c) and pyramidal dendritic length (d) as a result of COVID-19. Asterisk (*) shows the difference between the COVID and the control group (*P < 0.05; **P < 0.01; ***P < 0.001). The values were expressed as means ± SEM. The values were expressed as means ± SEM
Discussion
Due to the increase of CNS complications in COVID-19 patients, we anticipate potential enhancement in incidence of longer-term cognitive disorder affecting the ability to perform daily activities. Recovery after COVID-19 is mainly evaluated based on the improvement of respiratory symptoms; however, both animal and clinical investigations have demonstrated that coronaviruses can have a presence in the nervous system [41]. Therefore, at this time, studying potential long-term sequelae of COVID-19 is a priority.
Two groups of cells form the central nervous system: glial cells and neurons [62]. Glial cells, such as oligodendrocytes, microglia, and astrocytes, regulate the neuronal function [62, 63]. Astrocytes and microglia perform a variety of activities like innate immune reactions in the brain [64]. Results of Sholl analysis revealed a significant microglial activation in the brain of COVID-19 group, which was characterized by decreased number of microglial branches, NND, arbors area, regularity index, and process length, in addition to increased soma size. It was also determined that the number of primary branches of astrocytes had increased, while secondary and tertiary branches had reduced. Microglia will be activated following the pathophysiology of the disease and then the microglia exhibit neurotoxic or neuroprotective activity, which indicates the staging of the disease [65].
The M1 phenotype activation can be stimulated by IFN-γ and lipopolysaccharide (LPS) [66], and functions as a defense system against tumor growth and pathogens; so, pro-inflammatory cytokines, free radicals, reactive oxygen species (ROS), and interleukins IL-12, IL-1β, STAT3, TNF-α, IL-23, IL-6, are generated along with the loss of neurons. M2 alternative/phenotype activation releases anti-inflammatory cytokines IL-13, IL-10, TGF-β, IL-4, causing tissue remodeling and angiogenesis, and pro-inflammatory cytokine generation is prevented [67, 68].
Similar to glial activation, according to the results of Sholl analysis, the astrocytic activation occurred in the brain of COVID-19 group, which was characterized by decreased number of astrocytic branches, NND, arbor area, regularity index, and process length, in addition to increased soma size. It was also observed that the number of primary and tertiary branches of astrocytes had increased. Astrocytes can generate immunoregulatory or pro-inflammatory mediators based on the polarization phenotype as well as microglia [68].
Astrocytes deform in conditions like CNS disorders or injury and are altered to reactive astrogliosis. This status is defined by elevated astrocytic structural protein expression of vimentin and protein of glial fibrillary. Morphological alterations include the proliferation and hypertrophy of the cell body and help the formation of astrocyte scar in the injured tissues [69, 70]. Alterations in molecular morphology and expression of astrocytes measured by glial fibrillary acid protein (GFAP) may show the reactive astrogliosis severity, as a sign of CNS pathology [70]. Astrocytes defect in the early stage of injury like autoimmune encephalomyelitis and spinal cord injury that are associated with neuronal loss, exacerbated clinical results, blood brain barrier change, and neuroinflammation [71].
Inflammatory mediators produced by pro-inflammatory microglia, like TNF-α, IL-1α, C1q, and IL-1β, can activate pro-inflammatory astrocytes and stimulate a secondary inflammatory reaction [72, 73]. Harmful astrocytic signaling routes can be made by some other neurotrophins, cytokines, and sphingolipids (LacCer and sphingosine 1-phosphate) [71].
During neuroinflammation, astrocytes can upregulate tropomyosin receptor kinase B (TrkB) and the receptors for IL-17. Linking IL-17 to its receptors can lead to the generation of pro-inflammatory cytokines and uptake the nuclear factor κB (NFκB) activator 1 (Act1) [74]. TrkB-free conditioned mice may be safe against EAE-induced nerve damage, whereas TrkB stimulation by brain-derived neurotrophic factor (BDNF) has destructive impacts on nerve cells in the brain [64, 75]. However, astrocytes and microglia have numerous reactive phenotypes that have an association with the stage and type of regional location and neurological diseases [76–78]. In addition, alterations in astrocyte and microglia phenotypes, absence of neuroprotective activities, and increased neurotoxicity are complex processes and may vary with the severity and stage of neurodegenerative disorders. Thus, simple bipolar classification may not indicate the different phenotypes of astrocytes and microglia [76].
Immunohistochemical analysis against the cleaved caspase-3 marker in the CA1 field of the hippocampus indicated the death of pyramidal cells in this field in the COVID-19 group. Similarly, apoptosis of granular cells in this gyrus in the COVID-19 group was observed. It has been shown that neuroinflammation (following microglial and astrocytic activation) lead to ROS and oxidative stress are produced that begins the expression of IL-1β, phosphorylated-nuclear factor kappa B (p-NF-kB), and TNF-α, affecting the activity of hippocampal neuronal, causing lack of memory and learning, and neuronal apoptosis [79–81].
Furthermore, it has been revealed that neuroinflammation during AD leads to death of microglial cells from cleaved caspase-dependent apoptotic cells [69].
As a result, stereological analysis counting the number of CA1 neurons in the pyramidal layer of the hippocampus showed a significant drop in the COVID-19 group. The same drop was also observed in granular neurons of dentate in the COVID-19 group compared to the control. These findings point out to the detrimental effect of SARS-CoV-2-associated glial activation and further neuroinflammation and cell death.
Analysis of the Voronoi tessellation to examine the spatial distribution of cells in the pyramidal layer of the CA1 hippocampus depicted an alteration in the spatial distribution of cells in this hippocampal field. These alterations were as follows: due to the death of granular layer neurons, the specific space for each cell, mean of Voronoi polygon area, and spatial distribution of the neurons increased. Similar to CA1 neurons, analysis of the Voronoi tessellation in the granular layer of the dentate gyrus also depicted an alteration in the spatial distribution of these cells. These findings are justified by higher rates of apoptosis due to COVID-19 infection and further stereological disturbances observed in these patients.
Adult neurogenesis is considered a multi-step, complicated process involving new neuron formation in mammalian brain from residential neural stem cells (NSCs) in two of the neurogenic niches in CNS: subgranular zone (SGZ) of the dentate gyrus (DG) of hippocampal formation and subventricular zone (SVZ) in lateral ventricles (LVs). Previous studies have shown that new neurons produced through this activity have an important role in hippocampus-dependent memory and learning such as orientation and spatial memory and also neuronal injury improvement [82]. Immunohistochemical analysis was performed against the Ki67 marker, a marker of neurogenesis, in dentate gyrus. The dentate gyrus contains a population of neural stem cells that, by dividing and differentiating themselves, cause neurogenesis and memory.
Moreover, our analysis showed that neurogenesis was reduced in the COVID-19 group and that these neural stem cells may have undergone cell death. Adult neurogenesis might be affected by many extrinsic and intrinsic factors. Neurotrophins and growth factors, including BDNF, can stimulate neurogenesis, maturation, differentiation increasing, and survival of NSCs proliferation. Neuroinflammation, which are caused by microglial activation and the release of inflammatory cytokines such as TNF-α, IL-6, and IL-1β, has been demonstrated to prevent neurogenesis and decrease NSCs proliferation. The behavioral consequence of this process is a cognitive defect, caused by detriment to spatial memory and learning [83, 84]. Neural stem cell death as a result of viral infections has been also reported in the olfactory epithelium (OE) and olfactory bulb (OB) [85]. Experimental investigations in rodents offer that the NSC mediated neurogenesis in OB and olfactory epithelium (OE) is vital for smell [86, 87], while the lack of olfactory neurogenesis as a result of pathogenic stimulus like oxidative stress and neuroinflammation is related to smell loss [88, 89]. It is noteworthy that neurogenesis regulation in OB changes negatively during viral infections and different neuropathogenic disorders such as PD [89–91]. Furthermore, Bassan et al. have demonstrated an association between adult neurogenesis and spatial memory neuroinflammation, in the STZ-ICV SAD model. Animals injected with streptozotocin demonstrated abnormalities in short-term spatial memory in Y and OLT maze and disturbances in long-term spatial memory during the contextual fear ventilation test 30 days after toxin injection. Some papers have demonstrated enhancement in neuroinflammation in the STZ SAD model, which is caused by reactive gliosis and also an elevation in the proinflammatory markers related to the cognitive decline [92–95].
Previous investigations have also exhibited a reduction in neurogenesis in the STZ model related to amyloid pathology [96], or oxidative stress [97], however the effect of neuroinflammation on neurogenesis and subsequent cognitive decline in the STZ model has not been well defined [83].
Golgi staining in the CA1 field of the hippocampus for pyramidal neurons revealed that the length of dendrites decreased in the CA1 pyramidal neurons in the COVID-19 group. On the other hand, the number of dendritic spines has decreased in the COVID-19 group. This decreased dendritic length and number of dendritic spines indicates a reduction in synaptic plasticity and consequently memory impairment in the COVID-19 group. In addition, results of Sholl analysis showed a reduction in the number of cellular branches of pyramidal neurons. This finding is justifiable by downregulation of ACE2 as a result of SARS-CoV-2 infection. In the case of the brain ACE2 system, the main impacts in the brain could be associated with the Ang (1–7)/Mas system. Most of the mediating effects of Ang (1–7) in the brain are probably based on the Mas receptor activation, because to the best of our knowledge, Mas is mainly expressed in the CNS in various adult brains [98]. These regions include regions related to olfaction or associated with the limbic system like the amygdala or the hippocampal formation. Mas has been reported to influence neuronal excitability in the brain [99], and the elimination of Mas has an effect on behavior [100]. In 2005, Ang(1–7) hippocampal long-term potentiation (LTP) was found to be increased by the Mas receptor [101], exhibiting that Ang(1–7) / Mas signaling affects neuronal plasticity. In addition, Mas receptor defects influence adult neurogenesis in the hippocampus, and a morphological association of hippocampal neuronal plasticity processes is related to memory and learning [102].
Moreover, it has been demonstrated that Ang (1–7)/Mas dependent alterations in neuronal plasticity may lead to behavioral changes. For example, it has been exhibited that the integrity of Ang (1–7)/Mas axis is needed to express the memory of object recognition, since Mas removal or occlusion in the CA1 region within the hippocampus, this type of memory will be disrupted in mice [103]. Ang (1–7) also affect anxiety-related behaviors. One study explained central injection of Ang (1–7) causes anxiolytic-like impacts in the enhanced plus maze [104]. In another investigation, a reduction in anxiety-like manner has been found in transgenic rats with chronic overgeneration of Ang (1–7) [105], the ACE2 system plays a special role in neuronal plasticity and neuronal excitability in limbic system which is associated with changed mechanisms in behavior, memory, and learning [106].
In summary, it can be concluded that memory impairment may be a long-term neurological complication of COVID-19, which can be a predisposing factor for neurodegenerative disorders such as AD or other types of dementia. This memory impairment is mainly caused by neuroinflammation and further oxidative stress, which are mainly due to microglial and astrocytic activation. Such long-term neurological sequelae may also occur as a result of other viral infections and through a similar mechanism. For instance, the risk of parkinsonism has been reported to be higher in the survivors of influenza, which, similar to SARS-CoV-2, was responsible for a huge pandemic in the last century [107]. In order to establish immune barriers, it is necessary to encourage people to get vaccinated. When supplies are limited, distributing doses to the same number of people is a better strategy for limiting SARS-CoV-2 transmission. Despite vaccination, non-pharmaceutical intervention (NPI) is still needed for the prevention of worldwide outbreaks [108]. In sum, it seems necessary to prioritize research aimed at the study of long-term neurological sequelae, in order to rapidly develop preventive strategies.
Acknowledgements
The authors thank all the officials and staff in Clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran, for their enormous and collaborative efforts in this project. We appreciate the Iranian Legal Medicine organization for giving us the opportunity to have access to the patient registries and biological samples. Also, we should appreciate Emily Rudman from Massachusetts General Hospital, Boston, USA for editing the language of this manuscript.
Abbreviations
- COVID-19
Coronavirus Disease of 2019
- ACE2
Angiotensin-Converting Enzyme 2
- CNS
Central Nervous System
- TNFα
Tumor Necrosis Factor-alpha
- IL-6
Interleukin-6
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- Aβ
Amyloid beta
- NFT
Neurofibrillary Tangles
- CS
Cytokine Storm
- NND
Nearest neighbor distance
- CA1
Cornu Ammonis
- TrkB
Tropomyosin receptor kinase B
- DG
Dentate Gyrus
- Ang
Angiotensin
Author Contributions
AHB and HA contributed equally. AHB. and E.M. had a critical role in the acquisition of data and revised the manuscript. MHM, VE, MF, KV, GRM, and MF had a role in the acquisition of data. MEB, ZN, HAA and AA revised the manuscript. MAA had a role in the design of the study, conceptualized it, and drafted the manuscript for intellectual content. AA designed and conceived study, analyzed and interpreted the data, and revised the manuscript for intellectual content.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Amir-Hossein Bayat and Helia Azimi contributed equally to this work.
Contributor Information
Abbas Aliaghaei, Email: aghaei60@gmail.com.
Mohammad-Amin Abdollahifar, Email: m_amin58@yahoo.com.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ali Awan H, Najmuddin DM, Aamir A, Ali M, Di Giannantonio M, Ullah I, et al. SARS-CoV-2 and the Brain: What Do We Know about the Causality of ‘Cognitive COVID? J Clin Med. 2021;10(15):3441. doi: 10.3390/jcm10153441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman MA, Islam K, Rahman S, Alamin M. Neurobiochemical cross-talk between COVID-19 and Alzheimer’s disease. Mol Neurobiol. 2021;58(3):1017–1023. doi: 10.1007/s12035-020-02177-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach SR, Praschan NC, Hogan C, Dotson S, Merideth F, Kontos N, et al. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valenzano A, Scarinci A, Monda V, Sessa F, Messina A, Monda M, et al. The social brain and emotional contagion: COVID-19 effects. Medicina. 2020;56(12):640. doi: 10.3390/medicina56120640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varatharaj A, Thomas N, Ellul MA, Davies NW, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiat. 2020;7(10):875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaywant A, Vanderlind WM, Alexopoulos GS, Fridman CB, Perlis RH, Gunning FM. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology. 2021 doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferini-Strambi L, Salsone M. COVID-19 and neurological disorders: are neurodegenerative or neuroimmunological diseases more vulnerable? J Neurol. 2021;268(2):409–419. doi: 10.1007/s00415-020-10070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez-Perez AI, Garrido-Gil P, Pedrosa MA, Garcia-Garrote M, Valenzuela R, Navarro G, et al. Angiotensin type 2 receptors: Role in aging and neuroinflammation in the substantia nigra. Brain Behav Immun. 2020;87:256–271. doi: 10.1016/j.bbi.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 14.De Oliveira FAA, Palmeira DCC, Rocha-Filho PAS. Headache and pleocytosis in CSF associated with COVID-19: case report. Neurol Sci. 2020;41(11):3021–3022. doi: 10.1007/s10072-020-04694-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gialluisi A, De Gaetano G, Iacoviello L. New challenges from Covid-19 pandemic: an unexpected opportunity to enlighten the link between viral infections and brain disorders? Neurol Sci. 2020;41(6):1349–1350. doi: 10.1007/s10072-020-04444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsai L, Hsieh S, Chang Y. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwanica. 2005;14(3):113. [PubMed] [Google Scholar]
- 17.Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yachou Y, El Idrissi A, Belapasov V, Ait BS. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41(10):2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Berardis D. How concerned should we be about neurotropism of SARS-Cov-2? A brief clinical consideration of the possible psychiatric implications. CNS Spectr. 2020;27(3):258–325. doi: 10.1017/S1092852920002175. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa-Silva MC, Lima MN, Battaglini D, Robba C, Pelosi P, Rocco PR, et al. Infectious disease-associated encephalopathies. Crit Care. 2021;25(1):1–14. doi: 10.1186/s13054-021-03659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol. 2004;78(7):3398–3406. doi: 10.1128/JVI.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messina G, Polito R, Monda V, Cipolloni L, Di Nunno N, Di Mizio G, et al. Functional role of dietary intervention to improve the outcome of COVID-19: a hypothesis of work. Int J Mol Sci. 2020;21(9):3104. doi: 10.3390/ijms21093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye Q, Wang B, Mao J. Cytokine storm in COVID-19 and treatment. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kempuraj D, Selvakumar GP, Ahmed ME, Raikwar SP, Thangavel R, Khan A, et al. COVID-19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26(5–6):402–414. doi: 10.1177/1073858420941476. [DOI] [PubMed] [Google Scholar]
- 26.Matías-Guiu J, Gomez-Pinedo U, Montero-Escribano P, Gomez-Iglesias P, Porta-Etessam J, Matias-Guiu J. Should we expect neurological symptoms in the SARS-CoV-2 epidemic? Neurologia. 2020;35(3):170–175. doi: 10.1016/j.nrleng.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–186. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 28.Azkur AK, Akdis M, Azkur D, Sokolowska M, Van De Veen W, Brüggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75(7):1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debuc B, Smadja DM. Is COVID-19 a new hematologic disease? Stem Cell Rev. 2021;17(1):4–8. doi: 10.1007/s12015-020-09987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson MP, Jack AS. Coronavirus disease 2019 (COVID-19) in neurology and neurosurgery: a scoping review of the early literature. Clin Neurol Neurosurg. 2020;193:105866. doi: 10.1016/j.clineuro.2020.105866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrera-Pastor A, Llansola M, Montoliu C, Malaguarnera M, Balzano T, Taoro-Gonzalez L, et al. Peripheral inflammation induces neuroinflammation that alters neurotransmission and cognitive and motor function in hepatic encephalopathy: underlying mechanisms and therapeutic implications. Acta Physiol. 2019;226(2):e13270. doi: 10.1111/apha.13270. [DOI] [PubMed] [Google Scholar]
- 33.Kempuraj D, Ahmed ME, Selvakumar GP, Thangavel R, Dhaliwal AS, Dubova I, et al. Brain injury–mediated neuroinflammatory response and Alzheimer’s disease. Neuroscientist. 2020;26(2):134–155. doi: 10.1177/1073858419848293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempuraj D, Mentor S, Thangavel R, Ahmed ME, Selvakumar GP, Raikwar SP, et al. Mast cells in stress, pain, blood-brain barrier, neuroinflammation and Alzheimer’s disease. Front Cell Neurosci. 2019;13:54. doi: 10.3389/fncel.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kempuraj D, Thangavel R, Selvakumar GP, Zaheer S, Ahmed ME, Raikwar SP, et al. Brain and peripheral atypical inflammatory mediators potentiate neuroinflammation and neurodegeneration. Front Cell Neurosci. 2017;11:216. doi: 10.3389/fncel.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kustrimovic N, Marino F, Cosentino M. Peripheral immunity, immunoaging and neuroinflammation in Parkinson’s disease. Curr Med Chem. 2019;26(20):3719–3753. doi: 10.2174/0929867325666181009161048. [DOI] [PubMed] [Google Scholar]
- 37.Magrone T, Magrone M, Russo MA, Jirillo E. Peripheral immunosenescence and central neuroinflammation: a dangerous liaison-a dietary approach. Endocr Metab Immune Disord Drug Targets. 2020;20(9):1391–1411. doi: 10.2174/1871530320666200406123734. [DOI] [PubMed] [Google Scholar]
- 38.Skaper SD, Facci L, Zusso M, Giusti P. Neuroinflammation, mast cells, and glia: dangerous liaisons. Neuroscientist. 2017;23(5):478–498. doi: 10.1177/1073858416687249. [DOI] [PubMed] [Google Scholar]
- 39.Stüve O, Zettl U. Neuroinflammation of the central and peripheral nervous system: an update. Clin Exp Immunol. 2014 doi: 10.1111/cei.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Theoharides TC. Effect of stress on neuroimmune processes. Clin Ther. 2020;42(6):1007–1014. doi: 10.1016/j.clinthera.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Ritchie K, Chan D, Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain commun. 2020;2(2):fcaa069. doi: 10.1093/braincomms/fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andries K, Pensaert M. Immunofluorescence studies on the pathogenesis of hemagglutinating encephalomyelitis virus infection in pigs after oronasal inoculation. Am J Vet Res. 1980;41(9):1372–1378. [PubMed] [Google Scholar]
- 43.Li Y-C, Bai W-Z, Hirano N, Hayashida T, Hashikawa T. Coronavirus infection of rat dorsal root ganglia: ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012;163(2):628–635. doi: 10.1016/j.virusres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YC, Bai WZ, Hirano N, Hayashida T, Taniguchi T, Sugita Y, et al. Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J Comp Neurol. 2013;521(1):203–212. doi: 10.1002/cne.23171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsuda K, Park C, Sunden Y, Kimura T, Ochiai K, Kida H, et al. The vagus nerve is one route of transneural invasion for intranasally inoculated influenza a virus in mice. Vet Pathol. 2004;41(2):101–107. doi: 10.1354/vp.41-2-101. [DOI] [PubMed] [Google Scholar]
- 46.Dasilva A, Dossantos M. The role of sensory fiber demography in trigeminal and postherpetic neuralgias. J Dent Res. 2012;91(1):17–24. doi: 10.1177/0022034511411300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dworkin RH, Gnann JW, Jr, Oaklander AL, Raja SN, Schmader KE, Whitley RJ. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain. 2008;9(1):37–44. doi: 10.1016/j.jpain.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 48.Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92(1–2):139–145. doi: 10.1016/S0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- 49.Dossantos MF, Devalle S, Aran V, Capra D, Roque NR, Coelho-Aguiar JDM, et al. Neuromechanisms of SARS-CoV-2: a review. Front Neuroanat. 2020;14:37. doi: 10.3389/fnana.2020.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langhammer CG, Previtera ML, Sweet ES, Sran SS, Chen M, Firestein BL. Automated Sholl analysis of digitized neuronal morphology at multiple scales: whole cell Sholl analysis versus Sholl analysis of arbor subregions. Cytometry A. 2010;77(12):1160–1168. doi: 10.1002/cyto.a.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boroujeni ME, Simani L, Bluyssen HA, Samadikhah HR, Zamanlui BS, Hassani S, et al. Inflammatory response leads to neuronal death in human post-mortem cerebral cortex in patients with COVID-19. ACS Chem Neurosci. 2021 doi: 10.1021/acschemneuro.1c00111. [DOI] [PubMed] [Google Scholar]
- 52.Moghaddam MH, Bayat A-H, Eskandari N, Abdollahifar M-A, Fotouhi F, Forouzannia A, et al. Elderberry diet ameliorates motor function and prevents oxidative stress-induced cell death in rat models of Huntington disease. Brain Res. 2021;1762:147444. doi: 10.1016/j.brainres.2021.147444. [DOI] [PubMed] [Google Scholar]
- 53.Wang S, Tanzi RE, Li A. Quantitative analysis of neuronal dendritic arborization complexity in Drosophila. JoVE. 2019;143:e57139. doi: 10.3791/57139. [DOI] [PubMed] [Google Scholar]
- 54.Mao Y (2016) Nearest neighbor distances calculation with ImageJ
- 55.Davis BM, Salinas-Navarro M, Cordeiro MF, Moons L, De Groef L. Characterizing microglia activation: a spatial statistics approach to maximize information extraction. Sci Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-01747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wäussle H, Grüunert U, Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;332(4):407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- 57.Torquato S, Haslach H., Jr Random heterogeneous materials: microstructure and macroscopic properties. Appl Mech Rev. 2002;55(4):B62–B63. doi: 10.1115/1.1483342. [DOI] [Google Scholar]
- 58.Safaeian N, David T. A computational model of oxygen transport in the cerebrocapillary levels for normal and pathologic brain function. Cereb Blood Flow Metab. 2013;33(10):1633–1641. doi: 10.1038/jcbfm.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Horssen P, Van Den Wijngaard JP, Brandt M, Hoefer IE, Spaan JA, Siebes M. Perfusion territories subtended by penetrating coronary arteries increase in size and decrease in number toward the subendocardium. Am J Physiol Heart Circ Physiol. 2014;306(4):H496–H504. doi: 10.1152/ajpheart.00584.2013. [DOI] [PubMed] [Google Scholar]
- 60.Duyckaerts C, Godefroy G. Voronoi tessellation to study the numerical density and the spatial distribution of neurones. J Chem Neuroanat. 2000;20(1):83–92. doi: 10.1016/S0891-0618(00)00064-8. [DOI] [PubMed] [Google Scholar]
- 61.Rosoklija GB, Petrushevski VM, Stankov A, Dika A, Jakovski Z, Pavlovski G, et al. Reliable and durable Golgi staining of brain tissue from human autopsies and experimental animals. J Neurosci Methods. 2014;230:20–29. doi: 10.1016/j.jneumeth.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019 doi: 10.1016/j.semcdb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14(5):311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kwon HS, Koh S-H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):1–12. doi: 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64(1):110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boche D, Perry V, Nicoll J. Activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39(1):3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- 67.Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev Neurosci. 2011;33(3–4):199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 68.Stephenson J, Nutma E, Van Der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154(2):204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaur D, Sharma V, Deshmukh R. Activation of microglia and astrocytes: a roadway to neuroinflammation and Alzheimer’s disease. Inflammopharmacology. 2019;27(4):663–677. doi: 10.1007/s10787-019-00580-x. [DOI] [PubMed] [Google Scholar]
- 70.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colombo E, Farina C. Astrocytes: key regulators of neuroinflammation. Trends Immunol. 2016;37(9):608–620. doi: 10.1016/j.it.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 72.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, et al. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17–dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 75.Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, et al. Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med. 2012;209(3):521–535. doi: 10.1084/jem.20110698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, et al. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Biase LM, Schuebel KE, Fusfeld ZH, Jair K, Hawes IA, Cimbro R, et al. Local cues establish and maintain region-specific phenotypes of basal ganglia microglia. Neuron. 2017;95(2):341–356e6. doi: 10.1016/j.neuron.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46(6):957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 79.Lee Y-J, Choi D-Y, Choi IS, Kim KH, Kim YH, Kim HM, et al. Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflamm. 2012;9(1):1–19. doi: 10.1186/1742-2094-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu B, Wang ZG, Ding J, Liu N, Wang DM, Ding LC, et al. Chronic lipopolysaccharide exposure induces cognitive dysfunction without affecting BDNF expression in the rat hippocampus. Exp Ther Med. 2014;7(3):750–754. doi: 10.3892/etm.2014.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lecca S, Melis M, Luchicchi A, Muntoni AL, Pistis M. Inhibitory inputs from rostromedial tegmental neurons regulate spontaneous activity of midbrain dopamine cells and their responses to drugs of abuse. Neuropsychopharmacology. 2012;37(5):1164–1176. doi: 10.1038/npp.2011.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bassani TB, Bonato JM, Machado MM, Cóppola-Segovia V, Moura EL, Zanata SM, et al. Decrease in adult neurogenesis and neuroinflammation are involved in spatial memory impairment in the streptozotocin-induced model of sporadic Alzheimer’s disease in rats. Mol Neurobiol. 2018;55(5):4280–4296. doi: 10.1007/s12035-017-0645-9. [DOI] [PubMed] [Google Scholar]
- 84.Vadodaria KC, Gage FH. SnapShot: adult hippocampal neurogenesis. Cell. 2014;156(5):1114–1114e1. doi: 10.1016/j.cell.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 85.Rethinavel HS, Ravichandran S, Radhakrishnan RK, Kandasamy M. COVID-19 and Parkinson’s disease: defects in neurogenesis as the potential cause of olfactory system impairments and anosmia. J Chem Neuroanat. 2021 doi: 10.1016/j.jchemneu.2021.101965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brann JH, Firestein SJ. A lifetime of neurogenesis in the olfactory system. Front Neurosci. 2014;8:182. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lledo P-M, Valley M. Adult olfactory bulb neurogenesis. Cold Spring Harb Perspect Biol. 2016;8(8):a018945. doi: 10.1101/cshperspect.a018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, et al. Anosmia—a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazarini F, Gabellec M-M, Moigneu C, De Chaumont F, Olivo-Marin J-C, Lledo P-M. Adult neurogenesis restores dopaminergic neuronal loss in the olfactory bulb. J Neurosci. 2014;34(43):14430–14442. doi: 10.1523/JNEUROSCI.5366-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kandasamy M, Rosskopf M, Wagner K, Klein B, Couillard-Despres S, Reitsamer HA, et al. Reduction in subventricular zone-derived olfactory bulb neurogenesis in a rat model of Huntington’s disease is accompanied by striatal invasion of neuroblasts. PLoS ONE. 2015;10(2):e0116069. doi: 10.1371/journal.pone.0116069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loseva E, Yuan T-F, Karnup S. Neurogliogenesis in the mature olfactory system: a possible protective role against infection and toxic dust. Brain Res Rev. 2009;59(2):374–387. doi: 10.1016/j.brainresrev.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chávez-Gutiérrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, et al. The mechanism of γ-secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Javed H, Khan M, Ahmad A, Vaibhav K, Ahmad M, Khan A, et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience. 2012;210:340–352. doi: 10.1016/j.neuroscience.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 94.Kraska A, Santin MD, Dorieux O, Joseph-Mathurin N, Bourrin E, Petit F, et al. In vivo cross-sectional characterization of cerebral alterations induced by intracerebroventricular administration of streptozotocin. PLoS ONE. 2012 doi: 10.1371/journal.pone.0046196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rajasekar N, Nath C, Hanif K, Shukla R. Intranasal insulin administration ameliorates streptozotocin (ICV)-induced insulin receptor dysfunction, neuroinflammation, amyloidogenesis, and memory impairment in rats. Mol Neurobiol. 2017;54(8):6507–6522. doi: 10.1007/s12035-016-0169-8. [DOI] [PubMed] [Google Scholar]
- 96.Sun P, Knezovic A, Parlak M, Cuber J, Karabeg M, M., Deckert J.,, et al. Long-term effects of intracerebroventricular streptozotocin treatment on adult neurogenesis in the rat hippocampus. Curr Alzheimer Res. 2015;12(8):772–784. doi: 10.2174/1567205012666150710112147. [DOI] [PubMed] [Google Scholar]
- 97.Qu Z-Q, Zhou Y, Zeng Y-S, Lin Y-K, Li Y, Zhong Z-Q, et al. Protective effects of a Rhodiola crenulata extract and salidroside on hippocampal neurogenesis against streptozotocin-induced neural injury in the rat. PLoS ONE. 2012;7(1):e29641. doi: 10.1371/journal.pone.0029641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Freund M, Walther T, Und Halbach OVB. Immunohistochemical localization of the angiotensin-(1–7) receptor mas in the murine forebrain. Cell Tissue Res. 2012;348(1):29–35. doi: 10.1007/s00441-012-1354-3. [DOI] [PubMed] [Google Scholar]
- 99.Halbach OVBU, Walther T, Bader M, Albrecht D. Interaction between mas and the angiotensin AT1 receptor in the amygdala. J Neurophysiol. 2000;83(4):2012–2021. doi: 10.1152/jn.2000.83.4.2012. [DOI] [PubMed] [Google Scholar]
- 100.Walther T, Voigt J-P, Fink H, Bader M. Sex specific behavioural alterations in Mas-deficient mice. Behav Brain Res. 2000;107(1–2):105–109. doi: 10.1016/S0166-4328(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 101.Hellner K, Walther T, Schubert M, Albrecht D. Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci. 2005;29(3):427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 102.Freund M, Walther T, Und HO, V. B. Effects of the angiotensin-(1–7) receptor Mas on cell proliferation and on the population of doublecortin positive cells within the dentate gyrus and the piriform cortex. Eur Neuropsychopharmacol. 2014;24(2):302–308. doi: 10.1016/j.euroneuro.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 103.Lazaroni TL, Raslan ACS, Fontes WR, De Oliveira ML, Bader M, Alenina N, et al. Angiotensin-(1–7)/Mas axis integrity is required for the expression of object recognition memory. Neurobiol Learn Mem. 2012;97(1):113–123. doi: 10.1016/j.nlm.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Bild W, Ciobica A. Angiotensin-(1–7) central administration induces anxiolytic-like effects in elevated plus maze and decreased oxidative stress in the amygdala. J Affect Disord. 2013;145(2):165–171. doi: 10.1016/j.jad.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 105.Kangussu LM, Almeida-Santos AF, Moreira FA, Fontes MA, Santos RA, Aguiar DC, et al. Reduced anxiety-like behavior in transgenic rats with chronically overproduction of angiotensin-(1–7): role of the Mas receptor. Behav Brain Res. 2017;331:193–198. doi: 10.1016/j.bbr.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 106.Von Bohlen Und Halbach, O. The angiotensin converting enzyme 2 (ACE2) system in the brain: possible involvement in Neuro-Covid. Histol. Histopathol. 2021:18356-18356 doi: 10.14670/hh-18-356 [DOI] [PubMed]
- 107.Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16(9):566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin YF, Li Y, Duan Q, Lei H, Tian D, Xiao S, et al. Vaccination strategy for preventing the spread of SARS-CoV-2 in the limited supply condition: a mathematical modelling study. J Med Virol. 2022 doi: 10.1002/jmv.27783. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.