Abstract
Forty-seven strains representing 14 different Bacillus species isolated from clinical and food samples were grown in reconstituted infant milk formulae (IMF) and subsequently assessed for adherence to, invasion of, and cytotoxicity toward HEp-2 and Caco-2 cells. Cell-free supernatant fluids from 38 strains (81%) were shown to be cytotoxic, 43 strains (91%) adhered to the test cell lines, and 23 strains (49%) demonstrated various levels of invasion. Of the 21 Bacillus cereus strains examined, 5 (24%) were invasive. A larger percentage of clinically derived Bacillus species (20%) than of similar species tested from the food environment were invasive. Increased invasion occurred after growth of selected Bacillus species in reconstituted IMF containing glucose. While PCR primer studies revealed that many different Bacillus species contained DNA sequences encoding the hemolysin BL (HBL) enterotoxin complex and B. cereus enterotoxin T, not all of these isolates expressed these diarrheagenic genes after growth in reconstituted IMF. Of the 47 Bacillus isolates examined, 3 isolates of B. cereus and 1 isolate of B. subtilis produced the HBL enterotoxin after 18 h of growth in brain heart infusion broth. However, eight isolates belonging to the species B. cereus, B. licheniformis, B. circulans, and B. megaterium were found to produce this enterotoxin after growth in reconstituted IMF when assessed with the B. cereus enterotoxin (diarrheal type) reversed passive latex agglutination (RPLA) kit. It is concluded that several Bacillus species occurring occasionally in clinical specimens and food samples are of potential medical significance due to the expression of putative virulence factors.
The bacterial genus Bacillus comprises a very large and diverse group whose members are found ubiquitously (2, 10, 22). With the exception of Bacillus anthracis and B. cereus, other Bacillus species are generally perceived as inconsequential and of little clinical significance (8). Due to the endospore-forming ability of members of this genus, these bacteria tolerate adverse conditions better than most bacterial enteropathogens. They occur frequently in hospital foods (21) and domestically prepared foods (22).
Most food poisoning incidents attributed to Bacillus species are associated with B. cereus; this bacterium is known to cause a variety of nongastrointestinal diseases as well as two different types of food poisoning (for reviews, see references 7, 10, 13, and 14), which are characterized by either diarrhea or emesis. The diarrheal type is attributed to heat-labile enterotoxins that cause cytotoxicity, fluid accumulation in the ligated ileal loop of experimental animals, and dermonecrosis and is lethal for mice (14, 17). Two protein complexes from B. cereus strains, hemolysin BL (HBL) and nonhemolytic enterotoxin (NHE), and an enterotoxic protein, enterotoxin T (BceT), with these diarrheagenic properties have been previously characterized (3, 11, 14, 25). The emetic type is caused by a heat-stable dodecadepsipeptide, cereulide (14). The relevance of other Bacillus species as food poisoning organisms is being increasingly recognized, with recent epidemiological evidence linking B. licheniformis, B. subtilis, B. pumilus, and B. thuringiensis with incidents of food-borne illness (8). Previous research has shown that a variety of different Bacillus species isolated from the dairy environment may have the potential to produce diarrheal enterotoxins (2). Beattie and Williams (2) recently showed that supernatant fluids from isolates of B. thuringiensis, B. circulans, B. licheniformis, B. lentus, B. laterosporus, and B. mycoides reacted positively with the commercially available Bacillus diarrheal enterotoxin (BDE) visual immunoassay (Tecra VIA; International Bioproducts Inc., Redmond, Wash.) and the B. cereus enterotoxin (diarrheal type) reversed passive latex agglutination (RPLA) kit (Oxoid Ltd., Basingstoke, England).
Many members of the genus Bacillus have also been shown to be the etiological agents in local, deep-tissue, and systemic infections (8, 15). Nongastrointestinal infections have been primarily seen in individuals who are intravenous drug abusers or immunocompromised as a consequence of human immunodeficiency virus infection, chemotherapy, or malignancy (2). Due to the marked involvement of the liver and spleen with a brevity of gastrointestinal symptoms, the possibility that these systemic infections may have resulted from bacterial translocation from the gastrointestinal tract has been raised (8). While Bacillus spp. have been associated with human illnesses, the virulence status of many members of this genus has yet to be defined.
We report on the ability of different clinical and food isolates of Bacillus to adhere to, invade, and produce a cytotoxic effect in human HEp-2 and Caco-2 epithelial cells after growth in commercially produced baby foods. We also report that the ability of selected Bacillus isolates to express diarrheal enterotoxins HBL and BceT after growth in baby foods was influenced by the nutritional compositions of the products.
MATERIALS AND METHODS
Bacterial strains and growth media.
The Bacillus strains (Table 1) used in this study were, if not otherwise indicated, obtained from the Department of Bacteriology, Glasgow Royal Infirmary, Glasgow, Scotland (GRI); from Yorkhill Hospital, Glasgow, Scotland (YH); from the Food Safety Microbiology Laboratory, Central Public Health Laboratory, Colindale, United Kingdom (PHLS); from the Hannah Dairy Research Institute, Ayr, Scotland (HDRI); and from the National Collection of Type Cultures, Central Public Health Laboratory, Colindale, United Kingdom (NCTC). Other Bacillus strains were isolated from reconstituted infant milk formulae (IMF), pasteurized milk, enteral feeds, and high-energy buildup foods. Listeria monocytogenes NCTC 11994 was used as a positive control for bacterial adherence and invasion studies. The identity of each Bacillus isolate was confirmed by performing a sequence of characteristic morphological and physiological tests as described previously (22) and by use of miniaturized biochemical API 50CHB and API 20E galleries (bioMérieux, Marcy l'Etiole, France).
TABLE 1.
Bacterial strains used
| Bacterial species | Isolate | Provider | Reference no. | Source | Associated disease |
|---|---|---|---|---|---|
| Bacillus cereus | KD4 | GRI | 85895.F | Human blood | Sepsis (burn unit) |
| KD5 | GRI | 20191.V | Human blood | Sepsis (special care baby unit) | |
| KD6 | GRI | 33247.K | Human blood | Sepsis | |
| KD10 | GRI | 45221.W | Human blood | Sepsis (general medicine) | |
| KD11 | GRI | 59180.X | Human blood | Sepsis (general medicine) | |
| KD12 | GRI | 32465.X | Human blood | Sepsis (orthopedic unit) | |
| Et | NCTC | 11145 | Stool | Diarrheal food poisoning | |
| Em | NCTC | 11143 | Vomit | Emetic food poisoning | |
| DC1 | GRI | SU112/99 | Enteral feed | None reported | |
| DC2 | GRI | SU113/99 | Enteral feed | Diarrheal food poisoning | |
| NR11 | Rowan et al. (22) | SU11/95 | IMF | None reported | |
| NR14 | YH | SU14/95 | Pasteurized milk | None reported | |
| NR30 | Rowan et al. (22) | SU30/95 | Enteral feed | None reported | |
| NR42 | Rowan et al. (22) | SU42/95 | IMF | None reported | |
| NR46 | Rowan et al. (22) | SU46/95 | IMF | None reported | |
| NR50 | Rowan et al. (22) | SU50/95 | IMF | None reported | |
| NR53 | Rowan et al. (22) | SU53/95 | Enteral feed | None reported | |
| NR62 | Rowan et al. (22) | SU62/96 | IMF | None reported | |
| NR91 | Rowan et al. (22) | SU91/96 | UHT milka | None reported | |
| NR93 | Rowan et al. (22) | SU93/96 | IMF | None reported | |
| B-4ac | DSM4384 | Pea soup | Diarrheal food poisoning | ||
| Bacillus licheniformis | KD1 | GRI | 102469.S | Human blood | Sepsis |
| KD8 | GRI | 33263.Y | Human blood | Sepsis | |
| KD17 | PHLS | R2512/98 | Knee fluid | Septic arthritis | |
| H6 | HDRI | Dairy environment | None reported | ||
| Bacillus subtilis | KD19 | PHLS | R422/98 | Human blood | Sepsis |
| NR 106 | Rowan et al. (22) | SU106/96 | IMF | None reported | |
| H7 | HDRI | Dairy environment | None reported | ||
| Bacillus brevis | H4 | HDRI | Dairy environment | None reported | |
| NR83 | Rowan et al. (22) | SU83/96 | UHT milk | None reported | |
| Bacillus megaterium | KD16 | PHLS | B17/97 | Finger | Pyrexia and sepsis |
| NR73 | Rowan et al. (22) | SU73/95 | IMF | None reported | |
| Bacillus circulans | KD9 | GRI | 79491.N | Human blood | Sepsis |
| KD15 | PHLS | R7106/97 | Human blood | Lymphoma | |
| H3 | HDRI | Dairy environment | None reported | ||
| Bacillus firmus | KD2 | GRI | 88773.T | Human blood | Sepsis |
| KD7 | GRI | 88022.Q | Human blood | Sepsis | |
| Bacillus sphaericus | KD18 | PHLS | R3794/98 | Human blood | Sepsis |
| NR66 | Rowan et al. (22) | SU66/95 | Pasteurized milk | None reported | |
| Bacillus pumilus | KD14 | PHLS | R7106/97 | Necrotic tissue aspirate | Spreading fasciitis |
| NR103 | Rowan et al. (22) | SU103/95 | Enteral feed | None reported | |
| Bacillus mycoides | H1 | HDRI | Dairy environment | None reported | |
| NR98 | YH | SU98/96 | Pasteurized milk | None reported | |
| Bacillus thuringiensis | KD3 | GRI | 78420.S | Human blood | Sepsis |
| Bacillus polymyxa | H2 | HDRI | Dairy environment | None reported | |
| Bacillus lentus | H5 | HDRI | Dairy environment | None reported | |
| Bacillus coagulans | KD13 | SU114/99 | IMF | None reported | |
| Listeria monocytogenes | NR1 | NCTC | 11994 | Cerebrospinal fluid | Adult meningitis |
UHT, ultra-high-temperature treated.
Bacillus cells were grown in brain heart infusion (BHI) broth (Oxoid) or in reconstituted IMF (tyndallized to sterility; three consecutive days of steaming for 30 min) at 37°C with agitation. The reconstituted IMF used in this study were Farley's Follow-On milk (designated IMF 1; containing maltodextrin), SMA WYSOY (designated IMF 2; containing glucose), and SMA Gold and SMA White (designated IMF 3 and IMF 4, respectively; containing neither glucose nor maltodextrin). IMF were used as test food matrices in this study, as they are frequently contaminated with acceptably low numbers of Bacillus spores and have supported the growth of these organisms (22). For adherence and invasion assays, the bacteria were harvested after 5 and 18 h of growth and were washed three times by centrifugation (MSE Centaur 1) at 3,000 rpm for 10 min each time in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Life Technologies Ltd., Paisley, Scotland). The bacteria were stored at −70°C (Microbank System, Pro-Lab Diagnostics, Ontario, Canada) to prevent the loss of virulence characteristics.
Adherence and invasion assays.
The ability of Bacillus test strains to adhere to and invade HEp-2 and Caco-2 cells was determined by previously described procedures (19), with minor modifications. HEp-2 and Caco-2 cell monolayers were grown overnight in a 5% CO2 atmosphere at 37°C in DMEM supplemented with 10% fetal calf serum (FCS; Gibco BRL) in 24-well tissue culture plates seeded with approximately 105 cells per well. Prior to assays, the monolayers were washed three times with DMEM, inoculated with 1 ml of bacterial culture (in DMEM with 10% FCS) in triplicate, and incubated for 2 h at 37°C in a 5% CO2 atmosphere. After incubation, the monolayers were washed three times with DMEM to remove any nonadherent cells, and then 1 ml of DMEM containing 10% FCS was added to each well of one of the test plates and incubated for 2 h. For the invasion assays, 1 ml of DMEM containing 10% FCS and 100 μg of gentamicin ml−1 was added to each well of the other 24-well plate and incubated for 2 h. The monolayers were then washed three times with DMEM, and the tissue culture cells were lysed with 1 ml of 1% Triton X-100 (vol/vol in distilled water) for 5 min at 37°C. Samples (0.1 ml) of lysate from each tissue culture plate were serially diluted in 0.9 ml of sterile distilled water, with subsequent enumeration by plating of 20 μl of appropriate 10-fold dilutions on BHI agar plates.
Cell cytotoxicity assay
Assessment of cytotoxic effects was made by measuring total cellular metabolic activity using the tetrazolium salt 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, Poole, United Kingdom). The cell cytotoxicity assay of Coote and Arian (5) was used, with minor modifications. HEp-2 and Caco-2 cell monolayers were grown overnight at 37°C in a 5% CO2 atmosphere in DMEM supplemented with 10% FCS in 96-well microplates seeded with approximately 5 × 104 cells per well. Bacterial cultures were grown for 18 h as described above, and 0.1-ml samples were filter sterilized (0.2-μm-pore-size membranes; Sarstedt, Nümbrecht, Germany). Samples were added in triplicate to the test plate immediately, after heating of the supernatant at 95°C for 10 min, or after enzymatic treatment with 0.1% trypsin. Positive and negative assay controls were 1% Triton X-100 (Sigma) and phosphate-buffered saline, respectively. Tissue culture monolayers containing bacterial culture supernatants were incubated overnight at 37°C in a 5% CO2 atmosphere, followed by the addition of phosphate-buffered saline containing 0.5% MTT to each well and incubation for 4 h at 37°C. The suspension in the wells was then removed, and the formazan product was solubilized by the addition of 100 μl of 0.04 HCl in dimethyl sulfoxide (Sigma). The contents of the plates were measured spectrophotometrically at 540 nm with a microplate reader (Labsystems EMS Reader). The toxic effects of the cell-free bacterial culture supernatants on the HEp-2 and Caco-2 cell lines were calculated from the following equation: (1 − optical density of test sample/optical density of negative control) × 100. Cytotoxic effects produced in HEp-2 and Caco-2 cells were also confirmed by light microscopy.
Measurement of other virulence factors.
All the isolates were tested for lecithinase (phosphatidylinositol-specific phospholipase C) activity after overnight growth on nutrient agar supplemented with 8% egg yolk (Oxoid) and by overlaying 1% l-d-phosphatidylinositol substrate (Sigma) in 0.7% agarose on overnight cultures of the bacteria on L agar plates. Lecithinase-positive strains produced a halo of precipitation (insoluble diacylglycerol) around the bacterial colonies. Production of catalase was assayed for by using an ID Color Catalase testing kit (bioMérieux). The ability of the isolates to induce hemolysis of 7% horse erythrocytes on blood agar was examined. In addition, hemolysis of 10% horse erythrocytes in BHI broth by the isolates was determined spectrophotometrically at 640 nm. The presence of diarrheal enterotoxin was measured using the B. cereus enterotoxin (diarrheal type) RPLA kit according to the manufacturer's instructions.
Screening of Bacillus spp. for the presence of bceT, hblA, hblC, and hblD enterotoxin DNA sequences by PCR.
Chromosomal DNA was isolated from the test Bacillus spp. by a previously described procedure (4). The DNA sequences of the diarrheagenic genes bceT (1), hblC and hblA (23), and hblD (11) were used to design primers that would amplify segments of the genes, if present, in a selection of the above-mentioned test Bacillus strains. Amplification was carried out with a DNA thermal cycler for 36 cycles of 30 s at 94°C; 1 min at 54°C, 60°C, 62°C, and 65°C for the hblD, bceT, hblC, and hblA genes, respectively; and 1 min at 72°C. PCR products of 429, 617, 399, and 873 bp were detected when the following pairs of oligonucleotide primers were used, respectively: HBLD-N (5′-AATCAAGAGCTGTCACGAAT-3′) and HBLD-C (5′-CACCAATTGACCATGCTAAT-3′), BCET6-N (5′-CATATGAAAGAGTTAGTTTCA-3′) and BCET5-C (5′-CGGATGAGGTGAGAAATGAAC-3′), HBLA-N (5′-GCTAATGTAGTTTCACCTAGCAAC-3′) and HBLA-C (5′-AATCATGCCACTGCGTGGACATATAA-3′), and HBLC-N (5′-AATAGGTACAGATGGAACAGG-3′) and HBLC-C (5′-GGCTTTCATCAGGTCATACTC-3′).
Statistical analysis.
All studies were performed in triplicate, and averages and standard errors were determined. Differences in bacterial adherence, invasion, and cytotoxicity were examined with human epithelial HEp-2 and Caco-2 cells at the 95 or 99.9% confidence interval using analysis of variance (one-way or balanced model) with Minitab software, release 11 (Minitab Inc., State College, Pa.).
RESULTS
Forty-seven Bacillus isolates representing 14 different species from clinical and food environments were used in this study (Table 1). The human diseases associated with the clinical Bacillus isolates ranged from severe infections, such as spreading fasciitis (e.g., B. pumilus KD14), to less serious food-borne illnesses.
Confirmation of the identity of some of the Bacillus isolates to species level was problematic, as they showed atypical characteristics for a number of the physiological and biochemical tests. For instance, the clinical isolates B. licheniformis KD1 and KD8 and B. pumilus KD14 produced lecithinase, yet these species were previously reported to be unable to produce this phospholipase. Interestingly, B. licheniformis KD8 produced lecithinase at 37°C but not at the lower culture temperature of 30°C. All Bacillus species tested were shown to be catalase positive and, with the exception of B. firmus, were also shown to be hemolytic. Irrespective of the source of the organism, the commercially available API 50CHB system was unable to differentiate between B. licheniformis and the closely related B. subtilis.
Ability of Bacillus test isolates to adhere to, invade, and produce cytotoxic effects in epithelial cells after growth in laboratory-based culture media.
The ability of the Bacillus test isolates to adhere to, invade, and produce cytotoxic effects in Caco-2 and HEp-2 cells was assessed after 18 h of growth in BHI broth. While the results showed that there were species-to-species variations in the levels of cytotoxicity produced, the culture supernatant fluids from 38 Bacillus isolates (81%, representing all 14 Bacillus species) had cytotoxic effects in both epithelial cell lines (Table 2). Clinical Bacillus isolates were significantly more cytotoxic to HEp-2 cells (P < 0.05); the mean toxicities of 19 clinical and food Bacillus isolates were 70.7% ± 12% and 34.8% ± 24%, respectively (Table 2). While both cell lines were susceptible to the culture supernatant fluids from many Bacillus isolates, some Bacillus spp. were cytotoxic to one cell line only. Other species, such as B. megaterium NR73, B. subtilis NR106, B. cereus DC1, and B. mycoides H1, were shown to be noncytotoxic. Separate heat and trypsin treatments of culture supernatant fluids either reduced or eliminated toxicity in HEp-2 and Caco-2 cells, suggesting that the cytotoxic activity was attributable to the proteinaceous fraction of the culture supernatant fluids (Table 3).
TABLE 2.
Adhesion, invasion, and cytotoxic abilities of Bacillus species for HEp-2 and Caco-2 cells after bacterial cultivation in BHI broth at 37°C for 18 ha
| Bacterial species | Isolate | % of the following property for the indicated cells:
|
|||||
|---|---|---|---|---|---|---|---|
| Cell death
|
Adherence
|
Invasion
|
|||||
| HEp-2 | Caco-2 | HEp-2 | Caco-2 | HEp-2 | Caco-2 | ||
| B. licheniformis | KD1 | 67 ± 5 | 75 ± 2 | 2.5 ± 0.25 | 0.23 ± 0.04 | 0.02 ± 0.005 | 0.20 ± 0.03 |
| KD8 | 70 ± 6 | 24 ± 2 | 0.78 ± 0.04 | 0.44 ± 0.1 | 0.02 | 0.06 ± 0.01 | |
| KD17 | 68 ± 1 | 62 ± 10 | 0.28 ± 0.08 | 2.7 ± 0.35 | 0.05 ± 0.001 | 0.03 | |
| H6 | 20 ± 2 | 36 ± 3 | 0.86 ± 0.15 | 1.15 ± 0.06 | 0.03 ± 0.01 | 0.09 ± 0.02 | |
| B. subtilis | KD19 | 49 ± 10 | 72 ± 5 | 0.96 ± 0.08 | 0.08 ± 0.01 | 0.02 | 0.03 ± 0.01 |
| H7 | 85 ± 8 | 62 ± 4 | 0.63 ± 0.2 | 0.35 ± 0.05 | — | — | |
| NR106 | — | — | — | — | — | — | |
| B. brevis | NR83 | 83 ± 6 | 63 ± 5 | — | — | — | — |
| H4 | 29 ± 10 | 15 ± 3 | 0.12 ± 0.02 | 0.19 ± 0.14 | 0.03 | 0.02 | |
| B. megaterium | KD16 | 56 ± 13 | 35 ± 4 | 0.45 ± 0.1 | 0.43 ± 0.09 | 0.1 ± 0.01 | 0.16 ± 0.03 |
| NR73 | — | — | 4.0 ± 0.25 | 0.13 ± 0.04 | 2.1 ± 0.12 | 0.09 ± 0.01 | |
| B. circulans | H3 | 26 ± 2 | 5 ± 1 | 0.07 | 0.16 ± 0.02 | 0.05 ± 0.01 | 0.05 ± 0.01 |
| KD15 | 72 ± 2 | 45 ± 5 | 0.56 ± 0.1 | 0.53 ± 0.1 | 0.13 ± 0.02 | 0.01 | |
| KD9 | 57 ± 6 | 89 ± 2 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.01 | |
| B. firmus | KD2 | 80 ± 11 | 42 ± 5 | 0.97 ± 0.22 | 1.07 ± 0.3 | — | 0.86 ± 0.05 |
| KD7 | 63 ± 6 | 71 ± 5 | 2.73 ± 1.1 | 0.63 ± 0.06 | — | — | |
| B. cereus | KD4 | 76 ± 5 | 66 ± 3 | 0.38 ± 0.05 | — | 0.02 ± 0.002 | — |
| KD5 | 62 ± 11 | 84 ± 9 | 0.02 ± 0.005 | 0.17 ± 0.03 | 0.005 | 0.02 | |
| KD6 | 69 ± 8 | 32 ± 1 | — | — | — | — | |
| KD10 | 83 ± 7 | 91 ± 7 | 1.43 ± 0.17 | 0.02 ± 0.01 | — | — | |
| KD11 | 86 ± 5 | 83 ± 5 | 0.03 ± 0.01 | 0.40 ± 0.04 | — | — | |
| KD12 | 85 ± 2 | 72 ± 6 | 3.4 ± 0.3 | 0.26 ± 0.07 | — | — | |
| NR11 | 65 ± 3 | 71 ± 10 | 0.15 ± 0.04 | 0.22 ± 0.04 | — | — | |
| NR14 | 63 ± 5 | 49 ± 3 | 1.5 ± 0.16 | 0.9 ± 0.09 | — | — | |
| NR30 | 67 ± 5 | 63 ± 2 | 0.09 ± 0.03 | 0.03 | — | — | |
| NR42 | 45 ± 7 | 71 ± 5 | 1.0 ± 0.15 | 1.3 ± 0.11 | — | — | |
| NR50 | 86 ± 5 | 80 ± 5 | 0.46 ± 0.04 | 0.51 ± 0.07 | — | — | |
| NR53 | 63 ± 1 | 83 ± 4 | — | — | — | — | |
| DC1 | — | — | 1.11 ± 0.23 | 0.90 ± 0.16 | 0.32 ± 0.06 | 0.19 ± 0.03 | |
| DC2 | 5 ± 1 | — | 0.93 ± 0.31 | 1.4 ± 0.36 | 0.36 ± 0.1 | 0.22 ± 0.01 | |
| Et | 85 ± 2 | 85 ± 3 | 0.03 ± 0.005 | 0.04 ± 0.01 | — | — | |
| Em | 83 ± 4 | 75 ± 1 | 0.2 ± 0.06 | 0.01 | — | 0.04 ± 0.01 | |
| B. sphaericus | KD18 | 61 ± 9 | 52 ± 5 | 2.7 ± 0.3 | 2.67 ± 0.5 | 0.15 ± 0.02 | — |
| B. pumilus | KD14 | 48 ± 10 | 50 ± 4 | 1.13 ± 0.14 | 3.72 ± 0.34 | 0.02 | 0.03 |
| NR103 | 12 ± 3 | — | 0.15 ± 0.06 | 0.23 ± 0.05 | — | — | |
| B. mycoides | H1 | — | — | 1.19 ± 0.2 | 1.16 ± 0.2 | 0.07 ± 0.01 | 0.1 |
| B. thuringiensis | KD3 | 66 ± 11 | 41 ± 5 | 3.3 ± 0.3 | 4.72 ± 0.72 | 0.13 ± 0.03 | 0.83 ± 0.11 |
| B. polymyxa | H2 | 59 ± 4 | 79 ± 3 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.2 ± 0.04 | 0.13 ± 0.02 |
| B. lentus | H5 | 11 ± 2 | 17 ± 4 | 1.14 ± 0.41 | 0.8 ± 0.15 | 0.2 ± 0.1 | 0.09 |
| B. coagulans | KD13 | 15 ± 3 | — | 1.97 ± 0.2 | 1.15 ± 0.3 | — | — |
| L. monocytogenes | NR1 | 72 ± 5 | 82 ± 9 | 3.88 ± 0.37 | 3.45 ± 0.2 | 1.15 ± 0.23 | 1.2 ± 0.04 |
Sterilized BHI broth was used as a control and had no cytotoxic influence (—) on the cell lines. Values shown are representative of triplicate samples from two separate trials and are reported as means and standard errors.
TABLE 3.
Effect of heat or trypsin treatment on cytotoxicity of cell-free supernatant fluids from selected Bacillus species for HEp-2 and Caco-2 epithelial cellsa
| Bacterial species | Isolate | % of the following cells dead after the indicated treatment:
|
|||||
|---|---|---|---|---|---|---|---|
| HEp-2
|
Caco-2
|
||||||
| Normal | HT | TT | Normal | HT | TT | ||
| B. cereus | KD4 | 76 ± 5 | 13 ± 1 | 11 ± 3 | 66 ± 3 | 10 ± 1 | 16 ± 3 |
| KD5 | 62 ± 11 | — | 5 ± 1 | 84 ± 9 | 80 ± 8 | 10 ± 2 | |
| KD10 | 83 ± 7 | 46 ± 3 | 22 ± 3 | 91 ± 7 | 60 ± 5 | — | |
| KD11 | 86 ± 5 | 35 ± 3 | 10 ± 1 | 83 ± 5 | — | 1 | |
| KD12 | 85 ± 2 | 6 | 8 ± 1 | 72 ± 6 | — | — | |
| Et | 85 ± 2 | 57 ± 5 | 3 ± 1 | 85 ± 7 | 2 | — | |
| Em | 83 ± 4 | 12 ± 3 | — | 75 ± 1 | — | — | |
| NR30 | 67 ± 5 | 15 ± 1 | — | 63 ± 2 | 15 ± 5 | — | |
| NR53 | 63 ± 1 | 22 ± 2 | — | 83 ± 4 | 4 | — | |
| NR11 | 65 ± 3 | — | — | 71 ± 10 | — | — | |
| B4ac | 65 ± 10 | 15 | — | 75 ± 7 | 28 ± 3 | 1 | |
| DC1 | — | — | — | — | — | — | |
| DC2 | 5 ± 1 | — | — | — | — | — | |
| B. licheniformis | KD1 | 67 ± 5 | — | — | 75 ± 2 | — | — |
| KD8 | 70 ± 6 | 8 ± 1 | — | 24 ± 2 | — | — | |
| KD17 | 68 ± 1 | 5 | 1 | 62 ± 10 | — | — | |
| H6 | 20 ± 2 | 3 | — | 36 ± 3 | — | — | |
| B. subtilis | KD19 | 49 ± 49 | 27 ± 3 | 1 | 72 ± 5 | 22 ± 2 | — |
| H7 | 85 ± 8 | 5 ± 1 | — | 62 ± 4 | — | — | |
| B. megaterium | KD16 | 56 ± 13 | 20 ± 5 | 3 | 35 ± 4 | — | — |
| NR73 | — | — | — | — | — | — | |
| B. sphaericus | KD18 | 61 ± 9 | 5 ± 2 | 1 | 52 ± 5 | 15 ± 3 | — |
| B. thuringiensis | KD3 | 66 ± 11 | 1 | — | 41 ± 5 | — | — |
| B. coagulans | KD13 | 15 ± 3 | 35 ± 6 | 9 ± 3 | — | — | 2 |
| B. circulans | KD9 | 57 ± 6 | 5 | — | 89 ± 2 | — | — |
| KD15 | 72 ± 2 | 35 ± 2 | 2 | 45 ± 5 | — | — | |
| H3 | 26 ± 2 | — | — | 5 ± 1 | — | — | |
| B. firmus | KD2 | 80 ± 11 | — | — | 42 ± 5 | — | — |
| KD7 | 63 ± 6 | — | — | 71 ± 5 | — | — | |
| B. brevis | H4 | 29 ± 10 | — | — | 15 ± 3 | — | — |
| B. lentus | H5 | 11 ± 2 | 3 | — | 17 ± 4 | — | — |
| B. mycoides | H1 | — | — | — | 5 | — | — |
| B. pumilus | KD14 | 48 ± 10 | 54 ± 3 | 12 ± 1 | 50 ± 4 | — | — |
| B. polymyxa | H2 | 59 ± 4 | 5 ± 1 | — | 79 ± 3 | — | — |
Supernatant fluids were untreated (Normal), heated (HT), or trypsin treated (TT) prior to assessment of toxicity to HEp-2 and CaCo-2 cells. Values shown are means and standard errors from triplicate trials where samples were examined in triplicate. —, no effect.
Forty-three Bacillus isolates (91%) encompassing all 14 species adhered to both cell lines, with 23 isolates (46%) demonstrating various levels of invasion (Table 2). While B. megaterium NR73, B. cereus KD12, and B. thuringiensis KD3 showed levels of adherence similar to that achieved by L. monocytogenes NR1, these Bacillus isolates were shown to be less invasive (P < 0.05) (Table 2). With the exception of B. coagulans, isolates of the other 13 different Bacillus species were capable of invading epithelial cells. B. cereus KD6 and NR53 and B. subtilis NR106 were incapable of adhering to these cell lines under the test conditions. Of the 21 B. cereus isolates examined, 5 (24%) were invasive. Twenty percent more clinical isolates than similar types of Bacillus species tested from the food environment were invasive (Table 2).
Putative virulence factor expression by Bacillus species after growth in reconstituted IMF.
The cytotoxicity, adherence, and invasion potentials of a selection of Bacillus species were assessed after 18 h of growth at 37°C in a variety of commonly used reconstituted IMF (Table 4). All of the Bacillus isolates tested were capable of growth in a variety of reconstituted IMF that differed in nutritional compositions. IMF 1 contained the starch derivative maltodextrin and lactose, IMF 2 contained glucose syrup and lactose, and IMF 3 and IMF 4 contained lactose. The results showed that the cell-free culture supernatants from many of the selected Bacillus isolates produced various levels of cytotoxicity in HEp-2 cells (Table 4). While some Bacillus species showed similar levels of toxicity after growth in all four IMF and in BHI broth (such as B. cereus KD4 and B. megaterium KD16), most Bacillus species varied considerably (P < 0.05) in their ability to elicit cytotoxicity in HEp-2 cells. Some Bacillus species (such as B. licheniformis KD1, B. circulans KD9, and B. thuringiensis KD3) were cytotoxic after growth in BHI broth but not after growth in certain IMF (Table 4). Not all of the IMF used for growth of the cytotoxigenic Bacillus species resulted in toxicity in HEp-2 cells.
TABLE 4.
Screening of Bacillus strains for DNA sequences containing genes associated with the HBL complex and B. cereus BceT
| Bacterial species | Isolate | Cytotoxicity (% cell death) in the following mediuma:
|
Detection of the following diarrheagenic gene by PCRb:
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BHI broth | IMF 1 | IMF 2 | IMF 3 | IMF 4 | bceT | hblC | hblA | hblD | ||
| B. cereus | KD4 | 76 ± 5 | 80 ± 5 | 83 ± 12 | 52 ± 5 | 50 ± 10 | − | + | + | + |
| KD5 | 62 ± 11 | 40 ± 3 | 72 ± 7 | 55 ± 3 | 47 ± 8 | − | + | + | + | |
| KD10 | 83 ± 7 | — | 38 ± 3 | 53 ± 1 | 56 ± 3 | + | + | + | + | |
| KD11 | 86 ± 5 | 66 ± 7 | — | 75 ± 6 | 88 ± 3 | − | + | + | + | |
| NR11 | 85 ± 2 | 71 ± 9 | 38 ± 3 | 54 ± 5 | 17 ± 1 | + | + | + | + | |
| NR14 | 63 ± 5 | 56 ± 7 | 74 ± 5 | 51 ± 3 | 62 ± 2 | − | + | + | + | |
| NR42 | 45 ± 7 | 40 ± 5 | 55 ± 5 | 61 ± 4 | 56 ± 3 | − | + | + | + | |
| NR50 | 86 ± 5 | 81 ± 4 | 75 ± 8 | 69 ± 4 | 61 ± 2 | + | + | + | + | |
| NR93 | 81 ± 4 | 62 ± 5 | 73 ± 5 | 54 ± 8 | 66 ± 8 | + | + | + | + | |
| B-4ac | 75 ± 9 | 82 ± 3 | 64 ± 2 | 62 ± 5 | 55 ± 6 | + | + | + | + | |
| Em | 83 ± 4 | 45 ± 3 | 59 ± 5 | 62 ± 3 | 64 ± 4 | − | − | − | − | |
| B. licheniformis | KD1 | 67 ± 5 | 41 ± 5 | 29 | — | — | + | + | + | + |
| KD8 | 70 ± 6 | 64 ± 2 | 45 ± 3 | 55 ± 3 | 22 ± 3 | + | + | + | + | |
| KD17 | 68 ± 1 | 57 ± 6 | 63 ± 2 | 60 ± 3 | 73 ± 8 | − | − | − | ||
| H6 | 20 ± 2 | 65 ± 3 | 52 ± 5 | 66 ± 9 | 68 ± 1 | − | + | + | + | |
| B. subtilis | KD19 | 49 ± 10 | 37 ± 3 | 30 ± 1 | — | — | + | + | + | + |
| H7 | 85 ± 8 | 77 ± 8 | 26 ± 4 | 70 ± 1 | 82 ± 3 | − | − | − | − | |
| B. pumilus | KD14 | 48 ± 10 | 53 ± 10 | 73 ± 5 | 43 ± 4 | 56 ± 1 | − | − | − | − |
| B. circulans | KD9 | 57 ± 6 | 26 ± 3 | — | 29 ± 2 | 28 ± 1 | − | + | + | + |
| KD15 | 72 ± 2 | 5 ± 1 | 41 ± 3 | 45 ± 1 | 38 | − | + | + | + | |
| H3 | 26 ± 2 | 47 ± 5 | 21 ± 4 | 8 ± 5 | 16 ± 3 | + | − | − | − | |
| B. thuringiensis | KD3 | 66 ± 11 | 55 ± 5 | 73 ± 8 | — | — | + | − | − | − |
| B. megaterium | KD16 | 56 ± 13 | 44 ± 5 | 38 ± 3 | 42 ± 3 | 43 ± 2 | + | + | + | + |
| NR73 | — | 54 ± 6 | 43 ± 6 | 59 ± 3 | 65 ± 5 | − | − | − | − | |
| B. sphaericus | KD18 | 61 ± 13 | 38 ± 5 | 61 ± 5 | 64 ± 5 | 40 ± 5 | − | − | + | + |
Values are reported as means and standard errors. —, no effect. Samples that tested positive for diarrheal toxin production in the RPLA test kit are shown in bold.
+, gene was detected; −, gene was not detected.
Analysis of cell-free culture supernatants for HBL diarrheal enterotoxin production using the commercially available RPLA test system revealed four toxin producers after growth in BHI broth (i.e., three isolates of B. cereus and one isolate of B. subtilis) (Table 4). However, RPLA analysis of culture supernatants from IMF samples revealed a further eight Bacillus isolates, belonging to B. cereus, B. licheniformis, B. subtilis, and B. megaterium, that were capable of producing HBL enterotoxin. With the exception of B. cereus KD4, all other Bacillus species tested were incapable of producing HBL enterotoxin in IMF products that contained lactose as the sole carbon source (Table 4). Reconstituted IMF 1, containing maltodextrin (a derivative of starch hydrolysis), and IMF 2, containing glucose, permitted a larger number of Bacillus species to produce diarrheal enterotoxin (Table 4). B. licheniformis, a known maltodextrin utilizer, was capable of HBL enterotoxin production in both IMF 1 and IMF 2. However, only B. cereus KD4 produced enterotoxin in IMF 1, suggesting that not all diarrheagenic B. cereus isolates have the appropriate genetic material to utilize maltodextrin for toxin production or that these bacteria have the necessary genes but require a specific environmental signal(s) for transcriptional activation.
Molecular analysis of a selection of different Bacillus species revealed that all diarrheagenic enterotoxin producers had the hblA, hblC, and hblD genes, which encode the HBL toxin complex (Table 4). Some of these diarrheagenic Bacillus isolates were also shown to have the bceT gene, which is associated with B. cereus BceT (Table 4). Some Bacillus isolates did not produce diarrheal toxins even though they were shown to contain DNA sequences for the above-mentioned diarrheagenic genes, while other Bacillus isolates were devoid of these enterotoxin-encoding genes (Table 4). There was good agreement between PCR analysis of Bacillus isolates for the diarrheagenic hblA, hblC, and hblD genes and detection of secreted HBL enterotoxin in culture supernatants by the RPLA test system (Table 4). Hansen and Hendriksen (11) detected genes for HBL, NHE, and BceT in 22 B. cereus and 41 B. thuringiensis strains by PCR (11). At least one gene for the protein complexes HBL and NHE was detected in all of the B. thuringiensis strains, while six B. cereus strains were devoid of all three HBL genes, three lacked at least two of the three NHE genes, and one lacked all three. The researchers did not mention whether the B. cereus or B. thuringiensis strains tested were obtained from clinical or food environments.
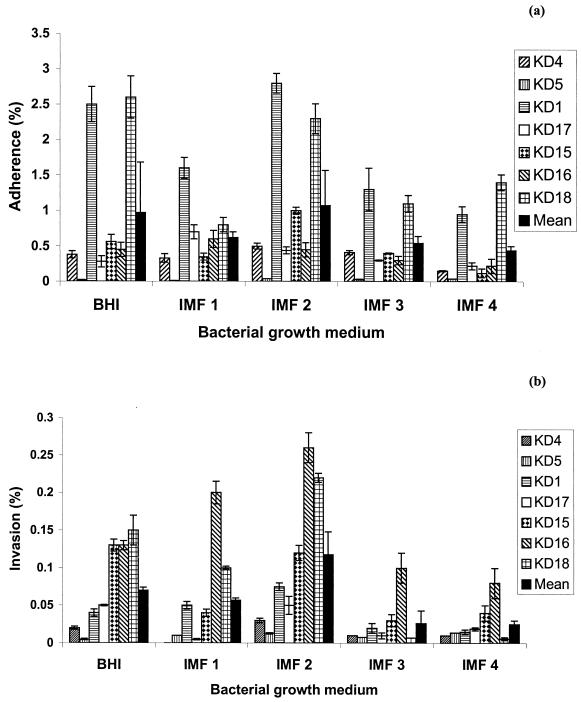
Greater levels of adherence to and invasion of HEp-2 cells were achieved by a selection of different Bacillus species after 18 h of growth at 37°C in IMF 2, containing glucose, than by the same species cultured in BHI broth or in IMF 3 or IMF 4, containing lactose (Fig. 1). Similar findings were observed when this study was repeated at the lower storage temperature of 30°C (data not shown).
FIG. 1.
Ability of selected Bacillus isolates to adhere to (a) and invade (b) HEp-2 cells after 18 h of growth in a variety of reconstituted IMF and in BHI broth.
DISCUSSION
This study constitutes the first demonstration that isolates of a wide variety of Bacillus spp., previously isolated from clinical specimens and food samples, are capable of adhering to, invading, and producing cytotoxic effects in human epithelial cells. Forty-seven Bacillus isolates were assessed for these putative virulence factors after growth in laboratory-based culture media and in baby foods. Production of the HBL enterotoxin complex was assessed using the B. cereus enterotoxin (diarrheal type) RPLA kit. Detection of DNA sequences encoding HBL enterotoxin and B. cereus BceT was achieved by PCR primer analysis.
With the exception of B. anthracis and B. cereus, which can be identified using rapid molecular and immunological approaches (12, 16), identification of other Bacillus isolates to the species level remains arduous, as it largely depends on performing a series of biochemical and physiological tests (8, 21, 22). Identification is further complicated by the fact that many clinical isolates of a species occasionally do not provide characteristic reactions that are considered typical of that species. For instance, during this study, the clinical isolates B. licheniformis KD1 and KD8 and B. pumilus KD14 were shown to be atypically lecithinase positive. Despite these constraints, all Bacillus isolates were identified to the species level. While the miniaturized biochemical API 50CHB assay was used to confirm the identity of each Bacillus species, this method was unable to differentiate between B. licheniformis and B. subtilis (in such instances, additional physiological discriminatory tests were performed).
Cell-free culture supernatants of 90 and 81% of the 47 Bacillus isolates examined for cytotoxicity in this study showed toxicity in HEp-2 and Caco-2 epithelial cells, respectively. Others have shown that the tetrazolium salt MTT can be used to assess the cytotoxic effects of culture supernatant fluids of Bacillus species isolated from raw milk (2, 9). Only living eukaryotic cells are detected using this assay, as the tetrazolium ring of MTT is cleaved in the mitochondria of metabolically active cells. Beattie and Williams (2) showed that some isolates of B. circulans, B. laterosporus, B. lentus, B. licheniformis, B. mycoides, B. subtilis, B. cereus, and B. thuringiensis were toxigenic to Chinese hamster ovary (CHO) cells using this MTT assay. Tetrazolium salts have also been used to assess the cytotoxicity of other pathogens, such as Pasteurella haemolytica A1 leukotoxin (6) and the cytotoxin of Campylobacter jejuni (5), and they were recently used to investigate B. cereus toxicity (26). Findlay et al. (9) advocated the use of MTT, as the currently used HEp-2 cell vacuolation assay for Bacillus emetic toxin is laborious, subjective, and unreliable.
This study also showed that greater detection of toxigenic Bacillus isolates occurred with different cell lines, as the culture supernatant fluids of some Bacillus species did not produce a cytotoxic effect in each of the cell lines investigated. In this study and another study (2), a higher proportion of toxigenic strains was detected by cytotoxicological methods. The apparent lower detection rate with immunological methods (Tecra BDE and Oxoid RPLA diarrheal enterotoxin test kits) is likely to be attributable to their specificity for individual components in the toxin complexes. This study showed that the supernatant fluids from isolates of B. licheniformis, B. subtilis, B. circulans, and B. megaterium were positive in the B. cereus enterotoxin (diarrheal type) RPLA assay, which is specific for the L2 component of the HBL complex. This finding indicates that these isolates produced protein toxins that were very similar to those of B. cereus and that these species may also present a potential hazard in food products. HBL contains the protein components B (37.5 kDa), L1 (38.2 kDa), and L2 (43.5 kDa), and all three components are required to produce maximal biological activity. The toxic activities so far identified for HBL include hemolysis, vascular permeability and necrosis in rabbit skin, fluid accumulation in rabbit ileal loops, toxicity to a number of transformed cell lines, in vitro degradation of explanted rabbit retinal tissue, and in vivo ocular necrosis and inflammation in rabbits (14).
Schoeni and Wong (25) previously reported that HBL was secreted by over 200 B. cereus, B. thuringiensis, and B. mycoides strains tested. Beattie and Williams (2) showed that the supernatant fluids from isolates of B. thuringiensis, B. circulans, B. licheniformis, B. lentus, B. laterosporus, and B. mycoides reacted positively with both the BDE and the RPLA immunoassays. B. subtilis, B. licheniformis, B. pumilus, and B. thuringiensis were previously implicated in outbreaks of food-borne disease (13, 24). It has been shown that some B. cereus strains may contain multiple copies of the hbl genes that arose from duplication of a single gene (25). HBL has been mapped to a portion of the B. cereus chromosome that exhibits exceptional variability compared with other regions, and this variable region is sometimes located on large extrachromosomal DNA fragments that appear to be stable but may prove to be large mobile plasmids (25). These findings may explain why many different Bacillus species appear to have the B. cereus-associated HBL enterotoxin complex.
This study recognizes the existence of lecithinase-positive toxigenic B. licheniformis strains isolated from the clinical environment. Interestingly, of the 23 toxin-producing isolates of B. licheniformis previously reported, all were incapable of producing lecithinase (24). The production of lecithinase, other phospholipases, proteases, and enterotoxins is recognized as a putative virulence factor that is required by invasive bacterial pathogens to elicit successful systemic infections (8, 19). The identification of lecithinase activity in clinical isolates of B. licheniformis is significant (possibly arising from genetic exchanges with the ubiquitous B. cereus), as this finding suggests that toxigenic B. licheniformis isolates may also acquire the necessary virulence determinants to support infection; lecithinase-positive B. pumilus KD14 was associated with a patient who had spreading fasciitis.
While a number of Bacillus species have been occasionally associated with gastrointestinal illnesses, recent evidence suggests that many members of this genus may be the cause of serious systemic diseases, such as septicemia, endocarditis, peritonitis, ophthalmitis, liver failure, and meningitis (8, 15, 24). We have shown that 21 Bacillus isolates representing 14 different species can invade HEp-2 and Caco-2 epithelial cells. Interestingly, the nutritional composition of the growth medium influenced bacterial virulence factor expression, such that baby foods containing both glucose syrup and lactose supported growth and enhanced levels of invasion in HEp-2 cells. Environmental signals, such as the presence of a readily utilizable carbon source, have been previously shown to modulate virulence factor expression in other bacterial enteropathogens (18, 20). PrfA, the central virulence transcriptional activator in L. monocytogenes, is regulated by a variety of environmental cues (18). This study has also provided evidence for expressional cross talk between environmental signals and virulence in Bacillus species. For instance, many of the clinical and food isolates of Bacillus were shown to contain DNA sequences encoding the HBL complex, yet only certain Bacillus isolates expressed the necessary hblA, hblC, and hblD genes to produce this diarrheal toxin after growth in reconstituted IMF products and in BHI broth.
In summary, this study has shown that a variety of different Bacillus spp. isolated from clinical specimens and food samples were capable of adhering to, invading, and producing cytotoxic effects in epithelial cells after growth in reconstituted IMF. While many different Bacillus isolates were shown to possess DNA sequences associated with the bceT, hblA, hblC, and hblD diarrheal enterotoxin genes, the composition of the bacterial culture medium influenced the ability of these Bacillus isolates to express these genes. It should be noted that reconstituted IMF were used as test food matrices in this study, as these products are frequently contaminated with acceptably low numbers of Bacillus spores (22). Properly reconstituted IMF containing Bacillus spp. have not been previously associated with food-borne illness in infants.
ACKNOWLEDGMENTS
This work was largely supported by the Chief Scientist Office, Scottish Executive, Edinburgh, Scotland (K/MRS/50/C2673). Thararat Chaithong thanks the government of Thailand for financial support.
We are grateful to V. Gill and M. Hughes for excellent technical assistance.
REFERENCES
- 1.Agata N, Ohta M, Arakawa Y, Mori M. The bceT gene of Bacillus cereus encodes an enterotoxic protein. Microbiology. 1995;141:983–988. doi: 10.1099/13500872-141-4-983. [DOI] [PubMed] [Google Scholar]
- 2.Beattie S H, Williams A G. Detection of toxigenic strains of Bacillus cereus and other Bacillus spp. with an improved cytotoxicity assay. Lett Appl Microbiol. 1999;28:221–225. doi: 10.1046/j.1365-2672.1999.00498.x. [DOI] [PubMed] [Google Scholar]
- 3.Beecher J D, Wong A C L. Tripartite haemolysin BL: isolation and characterization of two distinct homologous sets of components from a single Bacillus cereus isolate. Microbiology. 2000;146:1371–1380. doi: 10.1099/00221287-146-6-1371. [DOI] [PubMed] [Google Scholar]
- 4.Chaithong T. A molecular genetic investigation of enterotoxigenic factors in Bacillus cereus. Ph.D. thesis. Glasgow, Scotland: University of Strathclyde; 2000. [Google Scholar]
- 5.Coote J G, Arian T. A rapid, colorimetric assay for cytotoxin activity in Campylobacter jejuni. FEMS Immunol Med Microbiol. 1996;13:65–70. doi: 10.1111/j.1574-695X.1996.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 6.Craig F F, Dalgleish R, Sutherland A D. A colorimetric, microplate assay for the leucotoxin of Pasteurella haemolytica. Vet Microbiol. 1990;22:309–340. doi: 10.1016/0378-1135(90)90017-p. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich R, Fella C, Stich S, Märtlbauer E. Production and characterization of monoclonal antibodies against the hemolysin BL-enterotoxin complex produced by Bacillus cereus. Appl Environ Microbiol. 1999;65:4470–4474. doi: 10.1128/aem.65.10.4470-4474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drobniewski F A. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Findlay W J J, Logan N A, Sutherland A D. Semiautomated metabolic staining assay for Bacillus cereus emetic toxin. Appl Environ Microbiol. 1999;65:1811–1812. doi: 10.1128/aem.65.4.1811-1812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granum P E, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol Lett. 1997;157:223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- 11.Hansen B M, Hendriksen N B. Detection of enterotoxigenc Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl Environ Microbiol. 2001;67:185–189. doi: 10.1128/AEM.67.1.185-189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helgason E, Okstad O A, Caugant D A, Johansen H A, Fouet A, Mock M, Hegna I, Kolsto A-B. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl Environ Microbiol. 2000;66:2627–2630. doi: 10.1128/aem.66.6.2627-2630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson S G, Goodbrand R B, Ahmed R, Kasatiya S. Bacillus cereus and Bacillus thuringiensis isolated in a gastroenteritis outbreak investigation. Lett Appl Microbiol. 1995;21:103–105. doi: 10.1111/j.1472-765x.1995.tb01017.x. [DOI] [PubMed] [Google Scholar]
- 14.Kotiranta A, Lounatmaa K, Haapasalo M. Epidemiology and pathogenesis of Bacillus cereus infections. Microbes Infect. 2000;2:189–198. doi: 10.1016/s1286-4579(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 15.Mahler H, Pasi A, Kramer J M, Schulte P, Scoging A C, Baer W, Kraehenbuehl S. Fulminant liver failure associated with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336:1143–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 16.Mäntynen V, Lindström K. A rapid PCR-based DNA test for enterotoxic Bacillus cereus. Appl Environ Microbiol. 1998;64:1634–1639. doi: 10.1128/aem.64.5.1634-1639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notermans S, Batt C A. A risk assessment approach for food-borne Bacillus cereus and its toxins. J Appl Microbiol Symp Suppl. 1998;84:51S–61S. doi: 10.1046/j.1365-2672.1998.0840s151s.x. [DOI] [PubMed] [Google Scholar]
- 18.Rowan N J. Evidence that inimical food preservation barriers alter microbial resistance, virulence and cell morphology. Trends Food Sci Technol. 1999;10:261–272. [Google Scholar]
- 19.Rowan N J, Candlish A A G, Bubert A, Anderson J G, Kramer K, McLauchlin J. Virulent rough filaments of Listeria monocytogenes from clinical and food samples secreting wild-type levels of cell-free p60 protein. J Clin Microbiol. 2000;38:2643–2648. doi: 10.1128/jcm.38.7.2643-2648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowan N J, Anderson J G. Maltodextrin stimulates growth of Bacillus cereus and synthesis of diarrheal enterotoxin in infant milk formulae. Appl Environ Microbiol. 1997;63:1182–1184. doi: 10.1128/aem.63.3.1182-1184.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowan N J, Anderson J G. Growth and enterotoxin production by diarrhoeagenic Bacillus cereus in dietary supplements prepared for hospitalised HIV patients. J Hosp Infect. 1998;38:139–146. doi: 10.1016/s0195-6701(98)90067-6. [DOI] [PubMed] [Google Scholar]
- 22.Rowan N J, Anderson J G, Anderton A. Bacteriological quality of infant milk formulae examined under a variety of preparation and storage conditions. J Food Prot. 1997;60:1089–1094. doi: 10.4315/0362-028X-60.9.1089. [DOI] [PubMed] [Google Scholar]
- 23.Ryan P A, Macmillan J D, Zilinkas B A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J Bacteriol. 1997;179:2551–2556. doi: 10.1128/jb.179.8.2551-2556.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salkinoja-Salonen M S, Vourio R, Andersson M A, Kampfer P, Andersson M C, Honkanen-Buzalski T, Scoging A C. Toxigenic strains of Bacillus licheniformis related to food poisoning. Appl Environ Microbiol. 1999;65:4637–4645. doi: 10.1128/aem.65.10.4637-4645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeni J L, Wong A C L. Heterogeniety observed in the components of hemolysin BL, an enterotoxin produced by Bacillus cereus. Int J Food Microbiol. 1999;53:159–167. doi: 10.1016/s0168-1605(99)00158-0. [DOI] [PubMed] [Google Scholar]
- 26.von Dietrich R, Mauersberger K, Martlbauer E. Zur anwendbarkeit des MTT zytotoxizitatstests zum nachwies von B. cereus enterotoxin. Arch Lebensmittelhyg. 1997;48:73–96. [Google Scholar]