Abstract
Environment chemicals can interfere with the endocrine axis hence they are classified as endocrine disrupting chemicals (EDCs). Bisphenol S (BPS) is used in the manufacture of consumer products because of its superior thermal stability and is thought to be a safe replacement chemical for its analog bisphenol A (BPA). However, the safety profile of these compounds alone or in the presence of other EDCs is yet to be fully investigated. Also, the estrogenic chemical 17α-ethinyl estradiol (EE2) and a constituent of female oral contraceptives for women is present in water supplies. To simulate concurrent exposure of the population to chemical mixtures, we investigated the effects of BPA, BPS, EE2, and their combinations on sex steroid secretion in the growing male rat gonad. Prepubertal and pubertal male rats at 21 and 35 days of age were provided test chemicals in drinking water (parts per billion) for 14 days. At termination of exposure, some individual chemical effects were modified by exposure to chemical combinations. Single chemical exposures markedly decreased androgen secretion but their combination (e.g., BPA+BPS+EE2) caused the opposite effect, i.e., increased Leydig cell T secretion. Also, the test chemicals acting alone or in combination increased testicular and Leydig cell 17β-estradiol (E2) secretion. Chemical-induced changes in T and E2 secretion were associated with altered testicular expression of the cholesterol side-chain cleavage (Cyp11a1) and 17 β-hydroxysteroid dehydrogenase (Hsd17β) enzyme protein. Additional studies are warranted to understand the mechanisms by which single and chemical combinations impact function of testicular cells and disrupt their paracrine regulation.
Keywords: xenoestrogen, testis, endocrine disruptor, bisphenol compounds, 17α-ethinyl estradiol
1.0. INTRODUCTION
Exposure of the population to chemicals in the environment has increased significantly in the past 100 years due to their use in the manufacture of plastic materials, pesticides, herbicides, medications and other industrial products (1,2). This is a public health concern because there is evidence that many chemicals have the capacity to interfere with the endocrine axis hence, they are designated endocrine disrupting chemicals (EDCs) (3). Xenoestrogens are chemicals that mimic the activity of endogenous estrogens and are among the most common EDCs (4). Importantly, the population is concurrently exposed to mixtures of compounds in food, air, and water (5) Thus, the growing concept of “chemical mixture effects” seems reasonable because individual chemical actions may underestimate the effects of combined chemical actions. In the present study, we have focused on singular and chemical mixture effects due to three environmentally relevant xenoestrogens, namely, bisphenol A (BPA), bisphenol S (BPS), and 17α-ethinyl estradiol (EE2) in the rodent male gonad.
Despite restrictions on use of BPA as constituent of infant products, BPA and its analogs are still used in the manufacture of many household and consumer items (6–8), including electronics, automobile parts, water storage tanks, thermal papers, and luggage tags (9,10). BPS (bis (4-hydroxyphenyl) sulfone) is considered to be a potential replacement chemical for BPA as the anticorrosive agent in epoxy resins, infant feeding bottles and several household and industrial products (7,9–11). Exposure of the population through daily contact with products containing BPA and BPS is widespread (12). On the other hand, EE2 is a synthetic estrogen used in the treatment of estrogen insufficiencies and as a constituent of oral contraceptives for women. A substantial amount of EE2 is excreted unchanged in urine and fecal matter (13). Although bacterial degradation and photodegradation in sewage treatment plants are used to remove EE2, significant amounts of EE2 remain in water supplies due to its high lipophilic capacity (12,14,15). Thus, EE2 is considered a potent estrogen contaminant of treated water and an EDC with public health implications (16–18).
Our previous studies showed that perinatal BPA exposures of female rats at 2.5 and 25 μg/kg body weight (bw) increased expression of Ar, Esr1, Esr2, Wnt4, beta-Catenin, and p-Erk in testes of pubertal and adult male offspring (19). We also demonstrated that perinatal BPA exposures disrupted seminiferous tubule development in male prepubertal rats (20). Other studies showed that gavage administration of BPS at 50 μg/kg/day for 28 days decreased daily sperm production and increased sperm DNA damage in adult rats (21). BPS was found to activate the GPR30 estrogen receptor, induced oxidative stress, and increased DNA fragmentation in sperm cells and HepG2 cells in vitro (21,22). Similarly, exposures to EE2 was associated with anomalies of sexual development (23,24), altered sexual maturation (25), reduced fecundity (26), disruption of spermatogenesis (27) and reproductive tract anomalies in aquatic animals (28). Perinatal exposures of male rats to EE2 at 5 μg/kg/bw from prenatal day seven to postnatal day 18 decreased testis and seminal vesicle weights in adult animals while exposure at a greater dose (50 μg/kg bw) reduced sperm production in adulthood (29). Therefore, BPA, BPS and EE2 are known to have the capacity to cause adverse effects in male reproductive tract tissues.
Assessment of chemical mixture effects is critical to risk assessment of the populations that are simultaneously exposed to multiple chemicals. Strategies for conducting mixture effects are the subject of continuing debate (30–34). However, there is a general agreement that combined EDC exposures could either alleviate or intensify effects due to single chemicals. For example, exposures of pregnant dams to mixtures of di (2-ethyhexyl) phthalate (DEHP), vinclozolin, prochloraz and finasteride at suboptimal doses caused malformation of the external genitalia greater than due to individual chemicals in male offspring (35). A multi-component mixture of ten xenoestrogens at below individual “no-observed-effect” concentrations enhanced activation of the Esr in an additive manner (32). Similarly, a mixture of five phthalates, [benzyl butyl phthalate (BBP), di (n) butyl phthalate (DBP), diethyl hexyl phthalate (DEHP), di isobutyl phthalate (DiBP), and dipentyl phthalate (DPP)], with a common mode of action but different active metabolites, decreased fetal T production in a cumulative dose-additive manner (36). Although health effects due to BPA have been studied extensively, studies of exposure effects due to BPS and EE2 in the mammalian male gonad are limited. Given that EE2 is a common contaminant of domestic water sources, it is perhaps reasonable to speculate that concurrent exposures of the population to EE2, BPA and its BPS analog are not uncommon. Additionally, many of the effects of BPA and BPS reported in the literature are associated with estrogen signaling pathways in a similar manner to EE2 (37). To address the lack of data on the combined effects of xenoestrogens, we performed experiments to determine the singular effects of BPA, BPS, EE2 and their combinations at environmentally relevant concentrations in the growing male rat gonad.
2.0. MATERIALS AND METHODS
2.1. Chemicals
Bisphenol A (Lot # 1065060 13706030), Bisphenol S (lot # BCBV2462), and 17α- ethinylestradiol (lot # WXBC6630V) were purchased from Sigma-Aldrich (St. Louis, MO). Trypsin inhibitor, EDTA, HEPES, BSA, bovine lipoprotein, sodium bicarbonate (NaHCO3), DMEM nutrient mixture [Ham’s F-12 (DMEM/F-12; 1:1 mixture without phenol red)], albumin, gentamicin were purchased from Sigma Chemical Company (St. Louis, MO). Dulbecco’s PBS, medium 199, and 10 × Hanks’ balanced salt solution were obtained from Life Technologies, Inc. (Grand Island, NY). Ovine luteinizing hormone (LH) was provided by the National Hormone and Pituitary Program (NIDDK, Bethesda, MD).
2.2. Ethics Statement
All animal and euthanasia procedures were performed in accordance with a protocol approved by the Auburn University Institutional Animal Care and Use Committee (IACUC) and are based on recommendations of the panel on Euthanasia of the American Veterinary Medical Association.
2.3. Animal Studies
Male prepubertal (n=36) and pubertal (n=36) Long-Evans rats were obtained from Harlan- Teklad, (Madison WI). After acclimatization for three days at the College of Veterinary Medicine Division of Laboratory Animal Health (DLAH) Facility, animals were randomly placed in groups of 1–3 per cage (length, 0.47 m; width, 0.25 m; height, 0.22 m) (Snyder Manufacturing Company; Centennial, CO). Water was provided in glass water bottles ad libitum. The housing of animals in plastic cages and use of glass bottles were designed to minimize background exposure to estrogens and bisphenol compounds as may occur with resin- containing cages (38). Due to the presence of phytoestrogens in soybeans and their capacity to interfere with the male reproductive system (39,40), animals were fed a soy-free X2020 diet (Harlan-Teklad, Madison WI) throughout the experimental period. Animals were maintained under constant conditions of light (12L: 12D) and temperature (20–23.38°C) with free access to pelleted food.
Test chemical concentrations were selected based on their presence in the environment as reported in the literature (7,9,41,42). However, a major limitation is that small variations in composition or concentration of individual chemicals may lead to significant changes in observed effects (5,43). Thus, small differences in chemical concentrations may result in an exaggerated or diminished effect on biological parameters and thereby confound analysis of observed effects. To overcome this limitation, chemical concentrations were kept constant at 5 μg/L of drinking water either alone or in combination. The administration of test chemicals in drinking was designed to mimic the natural route of exposure and minimize activation of the pituitary-adrenal axis (44).
In the first set of experiments, prepubertal and pubertal male rats at 21 and 35 days of age were randomly assigned by weight to six groups: Control, BPA, BPS, EE2, BPA+EE2, and BPS+EE2 (n=6 animals/group). Test chemicals were dissolved in 0.001% of DSMO in drinking water and fed to animals at 5 μg/L alone or in combination as appropriate for 14 days. The control group was provided only with water containing 0.001% DSMO for the same period. In the second experiment designed to analyze chemical effects in Leydig cells which analysis was not included in the first experiment, we used prepubertal male rats constituted into control, BPA, BPS, EE2, BPA+BPS, BPA+BPS+EE2 groups (n=9 animals/group). Single chemicals were fed in drinking water at 5 μg/L but dual and triple chemical mixtures were fed each at 2.5 μg/L and 1.7 μg/L for 14 days, i.e., from PND 21–35. In all cases, the control group was provided only with water containing 0.001% DSMO. At the end of each experiment, animals were sacrificed to obtain blood and testicular tissues for hormonal and protein expression analyses. Measurements of steroid hormone concentrations may be confounded differences in the numbers of androgen secreting Leydig cells. Therefore, Leydig cells were isolated from testes pooled from animals in the same treatment group to measure steroid hormone secretion.
2.4. Measurement of Serum Hormones
Serum was separated from trunk blood collected at sacrifice. Testicular explants (~100 mg) were incubated in microcentrifuge tubes containing DMEM/F12 culture medium buffered with 14 mm NaHCO3, 15 mm HEPES, 0.1% BSA, and 0.5 mg/mL bovine lipoprotein. Incubations were conducted without (basal) and with a maximally stimulating dose of ovine LH (100 ng/mL, NIDDK; LH-stimulated) for 3 h at 34°C. Steroid hormone concentrations were assayed in aliquots of serum and spent media using a tritium-based RIA (45). Tritium-labeled T and E2 were obtained from Perkin Elmer (Cat #s NET 370250UC and NET 013250UC, Lot #s 2570512 and 2393224), T and E2 antibodies from the Andrology and Reproduction Laboratory at Colorado State University (Fort Collins, CO), and T and E2 standards from Southeastern Biochemicals (Morristown, TN) and Sigma (St. Louis, MO; Cat # E2758–250, Lot # SLCF1943). The T-assay has a sensitivity of 270 pg/mL and inter-assay and intra-assay coefficients of variation range from 3.38% to 9.56% and 5.69% to 9.84%, respectively (46). The E2-assay has no cross-reactivity with EE2 and has a sensitivity of 10 pg/mL with inter- and intra-assay coefficients of variation from 1.38% to 2.56% and 3.8% to 7.24%, respectively (47). Hormone production was normalized to nanograms (ng) per ml for serum, ng/mg testes and ng/106 Leydig cells.
2.5. Procedure for Isolation of Leydig Cells
Animals were killed by CO2 asphyxiation after which testes were collected and digested in a dissociation buffer containing 0.25 mg/ml collagenase, 46 μg/ml dispase, and 6 μg/ml DNase for 1 h in a shaking water bath at 34°C. Seminiferous tubules were removed by passing testicular fractions through a nylon mesh with a pore size of 0.2 μm (Spectrum Laboratories, New Brunswick NJ). The supernatant was centrifuged at 2500 rpm for 15 min at 4°C. The cell fractions were loaded onto a Percoll gradient (Sigma-Aldrich) and centrifuged at 13500 rpm for 60 min at 4°C. Leydig cells were isolated from the Percoll gradient tube at 1.069–1.073 density (48–50). Leydig cell yields were estimated using a hemocytometer and the purity of Leydig cell fractions was assessed by histochemical staining for 3βHSD using 0.4 mM etiocholan-3β-ol-17- one as the enzyme-substrate (Sigma-Aldrich) (50).
2.6. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blot Analysis
Expression of key enzymes of the steroidogenic pathway was analyzed using western blot procedures. For example, Cyp11A1 catalyzes the conversion of cholesterol to pregnenolone which the rate-limiting step in steroidogenesis while Hsd17β mediates the final step in the conversion of androstenedione to T(51). For example, Cyp11A1 catalyzes the conversion of cholesterol to pregnenolone which is the rate-limiting step in steroidogenesis while the enzyme Hsd17β mediates the final step in the conversion of androstenedione to T (51). We assayed for steroidogenic enzyme protein expression which, unlike steady-state mRNA levels, directly correlates with enzyme activity and function (52). Furthermore, the Anti-Müllerian hormone (Amh) is a glycoprotein hormone secreted by Sertoli cells. Amh is a marker for Sertoli cell maturation (53) and is a regulator of the hypothalamic-pituitary-gonadal (HPG) axis and supports development of puberty in the male (54). The Desert hedgehog protein (Dhh), a signaling molecule also secreted by Sertoli cells, is supports germ cell maturation (55,56) and differentiation of Leydig cells and Sertoli cells (57). Both Amh and Dhh were analyzed in western blots of testes from all experimental groups. Briefly, tissues were homogenized in T- PER lysis buffer (Pierce Biotechnology, Rockford, IL) that was freshly supplemented with a protease inhibitor cocktail (Catalog #78410; Pierce Biotechnology). Tubes were centrifuged at 3000 rpm for 14 minutes at 4°C to remove cellular debris. Protein concentrations were determined using the Bio-Rad protein assay with BSA as standard (Bio-Rad Laboratories, Hercules, CA). Aliquots (50 μL) of whole-cell lysates were dissolved in an equal volume of Laemmli buffer containing 5% β-mercaptoethanol and boiled for 5 min at 95°C. All samples were resolved on varying percentages of Tris-HCl acrylamide gels by SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Catalog #1620147; Bio-Rad Laboratories) and subsequently incubated in blocking buffer (5% whole milk in 0.1% Tween 20 PBS) for 1 h at room temperature to reduce nonspecific binding by antibodies. Membranes were then incubated in a blocking buffer containing appropriate primary antibodies overnight at 4°C. Parameters of primary antibodies used in the present study are provided in Table 1. On the next day, blots were washed three times in 0.1% Tween 20 PBS to remove any unbound primary antibody before incubation with the appropriate horseradish peroxidase-conjugated secondary antibody. Afterward, membranes were washed four times with 0.1% Tween 20 PBS and then scanned using a LI-COR Odyssey Infrared Scanner (Lincoln, NE). All protein measurements were normalized to β-actin.
TABLE 1.
Characteristics of antibodies used
| Target | Antibody sequence | Name of Antibody | Manufacturer, Catalog Number | Polyclonal or Monoclonal | Dilution Used |
|---|---|---|---|---|---|
| Amh (MIS) | Genetic locus: AMH (human) mapping to 19 p13.3; AMH (mouse) mapping to 10C1 | MIS (C-20) | Santa Cruz Biotechnologies, Sc-6886 MW (AMH) 70/74 kDa | Mouse monoclonal IgG | 1:2000 |
| Dhh | Genetic locus: DHH (human) mapping to 12q13.12; Dhh (mouse) mapping to 15F1 | Dhh (F9): sc-2711688 | Santa Cruz Biotechnologies, sc-2711688 MW (Dhh) 42 kDa | Mouse monoclonal IgG | 1:1000 |
| Cyp11A1 | Recombinant full-length protein corresponding to Human CYP11A1 aa 40–320 mapping to 10q24.32 | Anti-Cypl 1A1 antibody | Abcam, Ab175408 MW (Cyp11A1) 55 kDa | Rabbit polyclonal IgG | 1:1000 |
| 17ßHSD | Genetic locus: HSD17B1 (human) mapping to 17q21.2 | 17ß-HSD (A5) | Santa Cruz Biotechnologies, Sc-376719 MW (17ß-HSD) 35 kDa | Mouse monoclonal IgG | 1:2000 |
| ACTB | Epitope mapping at the C\ terminus of actin of human origin | Beta Actin antibody (GT5512) | GeneTex, GTX629630 | Mouse monoclonal IgG | 1:2000 |
Abbreviations: ACTB= actin; Amh (MIS) = Mullerian inhibiting substance; CPY11A1 = Cytochrome P450 side cleavage enzyme; Dhh= Desert hedgehog; HSD= hydroxysteroid dehydrogenase; MW, molecular weight
2.6. Statistical Analysis
Analysis of data used the “whole mixture approach” i.e., each chemical mixture (BPA+EE2, BPS+EE2 and BPA+BPS+EE2) was treated as a single agent (5,31). Data are presented as the mean ± SEM. Within each expperiment, data were analyzed by one-way ANOVA followed by the Dunnett’s test for multiple group comparisons, (GraphPad Prism software. San Diego. Ca). Differences of p ≤ 0.05 were considered significant.
3.0. RESULTS
3.1. General Observations
No deaths were recorded from any treatment group in this study. Exposure of animals to xenoestrogens had no effects on body weights, paired testicular weights and gonadosomatic index (data not shown).
3.2. Effect of single and combined chemical exposures on serum and testicular steroid hormone concentrations
Results from the first experiment showed that exposure of prepubertal animals to BPA and its combination with EE2 (BPA+EE2) decreased (P<0.05) serum T concentrations (Fig. 1A), whereas exposures to BPS, EE2, and BPS+EE2 had no effect (Fig. 1B) compared to control. Basal testicular T concentrations were decreased by individual chemicals and their combinations compared to control (Fig. 1C, D) and more so (P<0.0001) in BPA-, EE2- and BPA+EE2-treated animals (Fig. 1C) than in the BPS (P<0.001) and BPS+EE2 (P<0.05) groups (Fig. 1D). Interestingly, LH-stimulated testicular T production was decreased (P < 0.05) only in animals treated with BPA and its combination with EE2 (Fig. 1E, F) compared to control. Interestingly, exposure of pubertal animals to test chemicals caused a similar pattern of effects on T concentrations as in prepubertal animals. For example, serum T concentrations and basal testicular T production were decreased only in BPA- and BPA+EE2-treated animals compared to control (P<0.05; Fig. 2A-D). LH-stimulated testicular T production was similar in all groups (Fig. 2E, F) as in prepubertal animals.
Fig. 1: Effect of BPA, BPS, EE2 and their combinations on serum and testicular testosterone (T) concentrations in prepubertal rats.
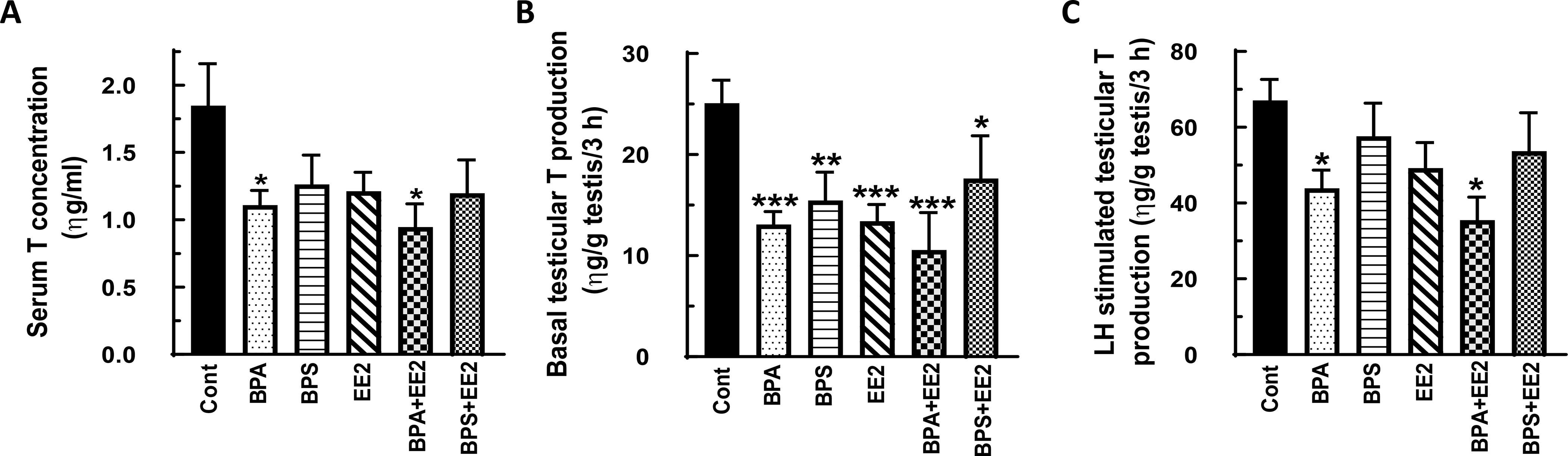
Male Long-Evans rats at 21 days of age were fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. Aliquots of serum and spent media were analyzed to measure T concentrations by RIA (n = 6; *p < 0.05, **p < 0.001, ***p < 0.0001).
Fig. 2: Effect of BPA, BPS, EE2 and their combinations on serum and testicular testosterone (T) concentrations in pubertal rats.
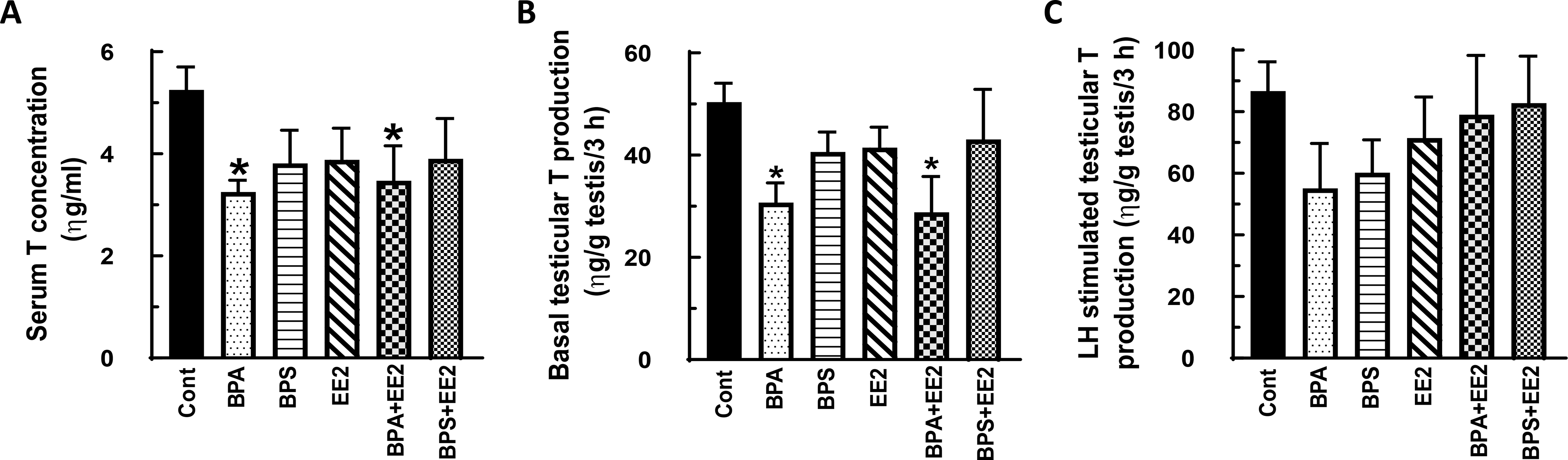
Male Long-Evans rats at 35 days of age were fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. Aliquots of serum and spent media were analyzed to measure T concentrations by RIA (n = 6; *p < 0.05).
In general, E2 secretion was increased by exposure to test chemicals administered alone or in combination. For example, Serum E2 concentrations were increased (P<0.05) in prepubertal male rats in the BPS+EE2 treatment and was similar in control and other groups (Fig. 3A, B). On the other hand, basal (Figs. 3C, D) and LH-stimulated (Figs. 3E, F) testicular E2 production were increased (P<0.05) after exposure to BPS, EE2, BPA+EE2 and BPS+EE2 compared to control, but this effect was absent in BPA-exposed animals (P>0.05). Exposure of pubertal male rats to test chemicals had no effect on serum E2 concentrations (Figs. 4A, B). However, measurement of basal (Fig. 4C, D) and LH-stimulated (Fig. 4E, F) testicular E2 production were increased (P<0.05) in pubertal animals exposed to BPS, EE2, BPA+EE2, and BPS+EE2, but not BPA, compared to control (Figs. 4C-F).
Fig. 3: Effect of BPA, BPS, EE2 and their combinations on serum and testicular 17β-estradiol (E2) concentrations in prepubertal rats.
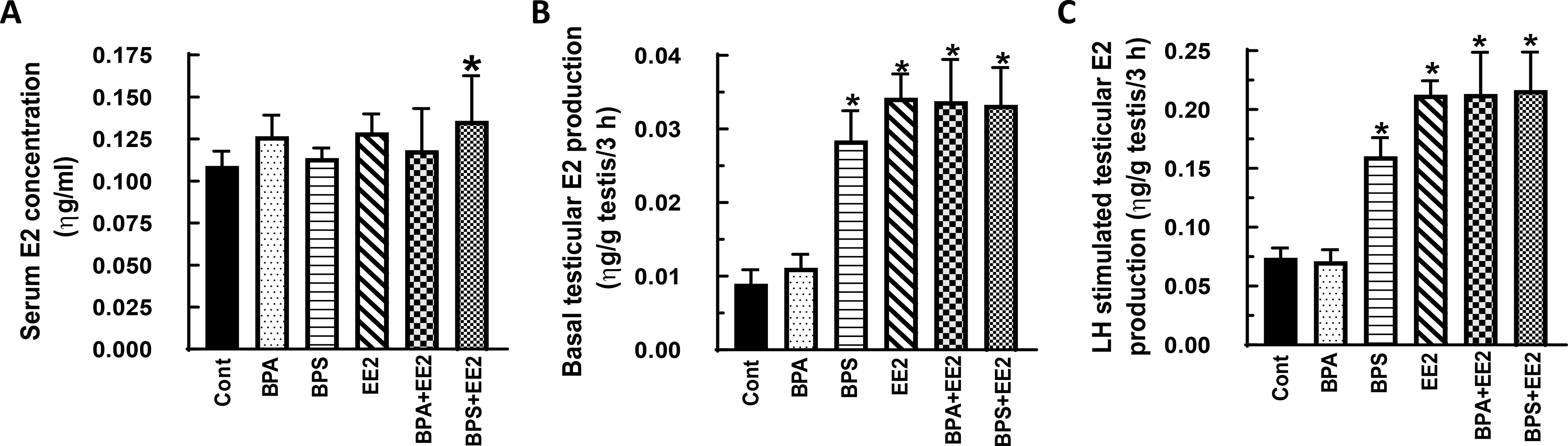
Male Long-Evans rats at 21 days of age were fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. Aliquots of serum and spent media were analyzed to measure E2 concentrations by RIA (n = 6; *p < 0.05).
Fig. 4: Effect of BPA, BPS, EE2 and their combinations on serum and testicular 17β-estradiol (E2) concentrations in pubertal rats.
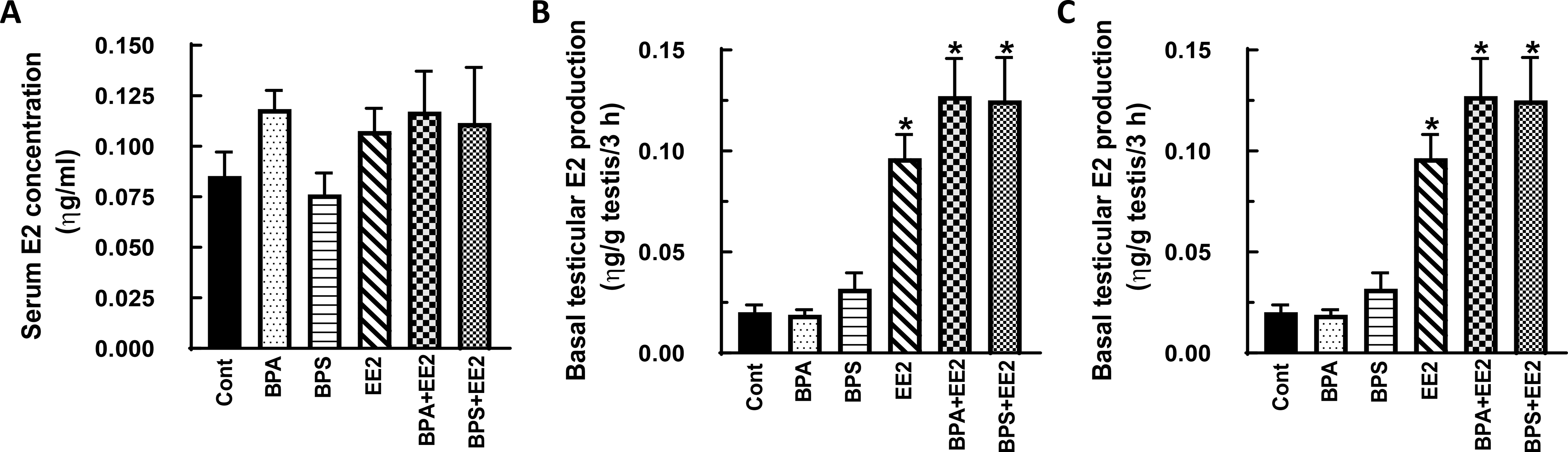
Male Long-Evans rats at 35 days of age were fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. Aliquots of serum and spent media were analyzed to measure E2 concentrations by RIA (n = 6; *p < 0.05).
In the second experiment, serum T concentrations were increased in the BPA+BPS treatment group (p<0.05) but basal testicular T secretion was decreased by BPA treatment as was LH-stimulated testicular T secretion in the BPA and BPA+BPS+EE2 groups compared to control (p<0.05) (Figs. 4A-C). Both basal and LH-stimulated Leydig cell T secretion was decreased (p<0.05) in all treatment groups compared to control except that the BPA+BPS+EE2 group showed increased (p<0.05) Leydig cell T secretion (Figs. 4D, E). On the other hand, elevated serum E2 concentrations (p<0.05) were measured only in the EE2 group (Fig. 5A). Similarly, basal testicular E2 secretion was increased to varying degrees in the EE2, BPA+BPS and BPA+BPS+EE2 treatment groups as was LH-stimulated testicular E2 secretion in the BPA+BPS group compared to control (Figs. 5B-C). Basal Leydig cell E2 secretion was increased in all but the BPS treatment group and more so in the BPA (p<0.001), BPA+BPS (p<0.01) and BPA+BPS+EE2 groups (p<0.05) (Fig. 5D). The pattern of LH-stimulated Leydig cell E2 secretion was similar to basal secretion and was markedly increased (p<0.001) in the BPA and BPA+BPS treatment groups compared to control (Fig. 5E).
Fig. 5: Effect of BPA, BPS, EE2 and their combinations on serum, testicular and Leydig cell testosterone (T) concentrations in prepubertal male rats.
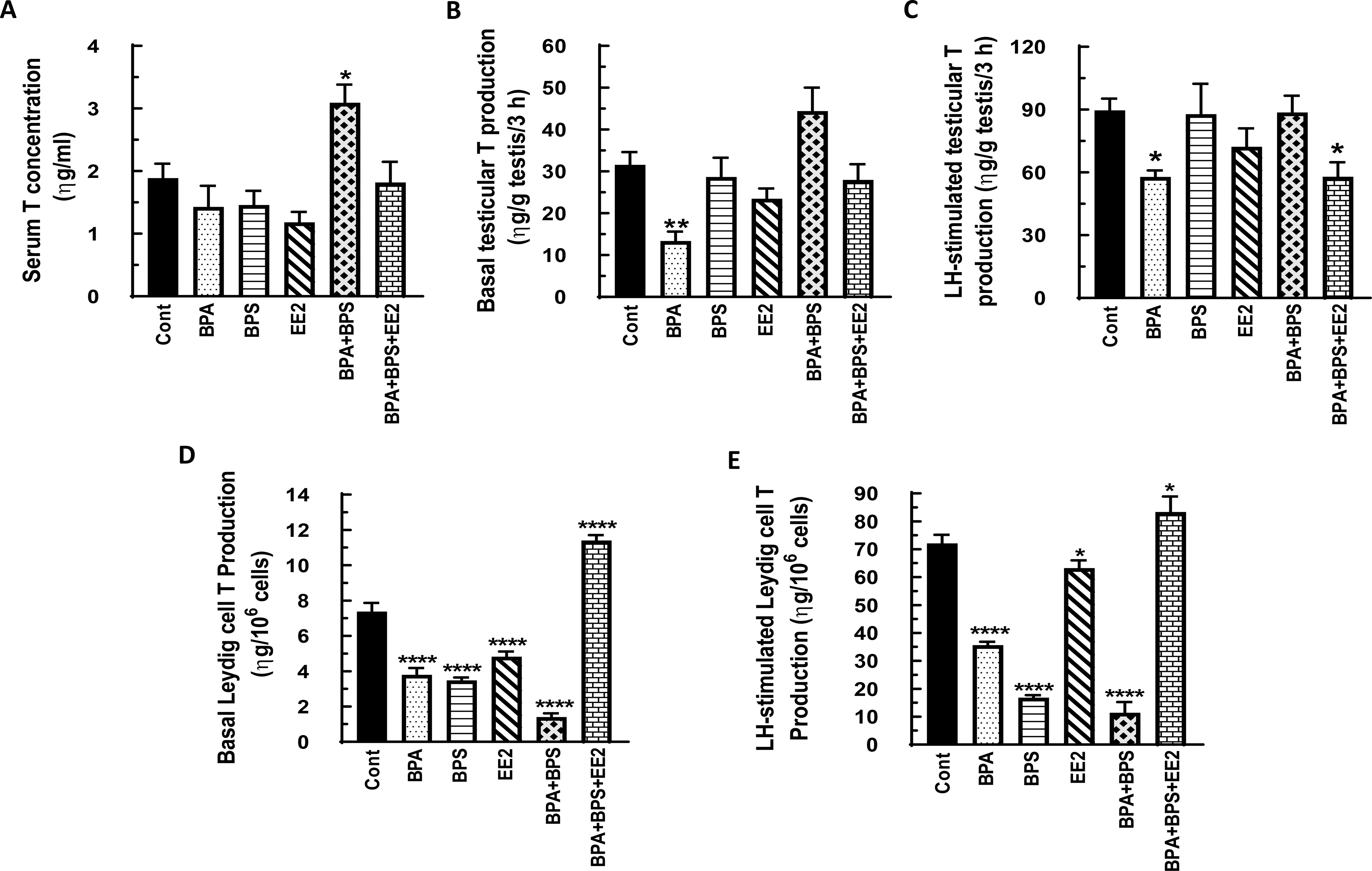
Long-Evans male rats at 21 days of age (n=48) were fed drinking water containing BPA, BPS, or EE2 (5 μg/L) or their combinations BPA+BPS (2.5 μg/L) each and BPA+BPS+EE2 (1.7 μg/L) each for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. In addition Leydig cells were isolated and incubated in DMEM/Ham’s F-12 culture medium without (basal, D) or with 100 ng/ml ovine LH (NIDDK, NIH) (LH-stimulated, E) for 3 h. Aliquots of serum and spent media were analyzed to measure T concentrations by RIA (n = 8; *p < 0.05, **p <0.001, ***p < 0.0001).
3.3. Effect of single and combined chemical exposures on testicular protein gene expression
We attempted to validate observations on steroid hormone concentrations by evaluation of gonadal steroidogenic capacity in chemical-exposed animals. Pubertal male rats at 35 days of age were used for these assays because they represent the intermediate stage of reproductive development and exhibit robust gonadal steroidogenic enzyme capacity for androgen secretion. We evaluated steroidogenic capacity by analysis of enzyme protein expression levels which reflect steroidogenic enzyme capacity better than measurements of steady-state mRNA levels (21). Analysis of western blots showed that testicular expression of the Cyp11A enzyme was subject to regulation by estrogenic chemicals and was decreased in all treatment groups compared to control (P<0.05; Fig. 7A, B). However, expression of the Hsd17β enzyme was increased (P< 0.05) in BPA- and BPA+EE2-treated animals (Fig. 7C) but decreased in EE2- and BPS-treated animals compared to control (P<0.05; Figs. 7C, D). Expression of the Sertoli cell- factor Amh was not affected by exposure to BPA (P>0.05) but was decreased (P<0.05) in the EE2, BPS, BPA+EE2, and BPS+EE2 groups compared to control (Figs. 8A, B). On the other hand, testicular expression of the Dhh protein was increased (P<0.05) after exposure to BPA, EE2, and BPS+EE2 while the BPA+EE2 group was similar (P>0.05) to control (Figs. 8C, D).
Fig. 7: Effect of BPA, BPS, EE2 and their combinations on testicular steroidogenic enzyme protein expression.
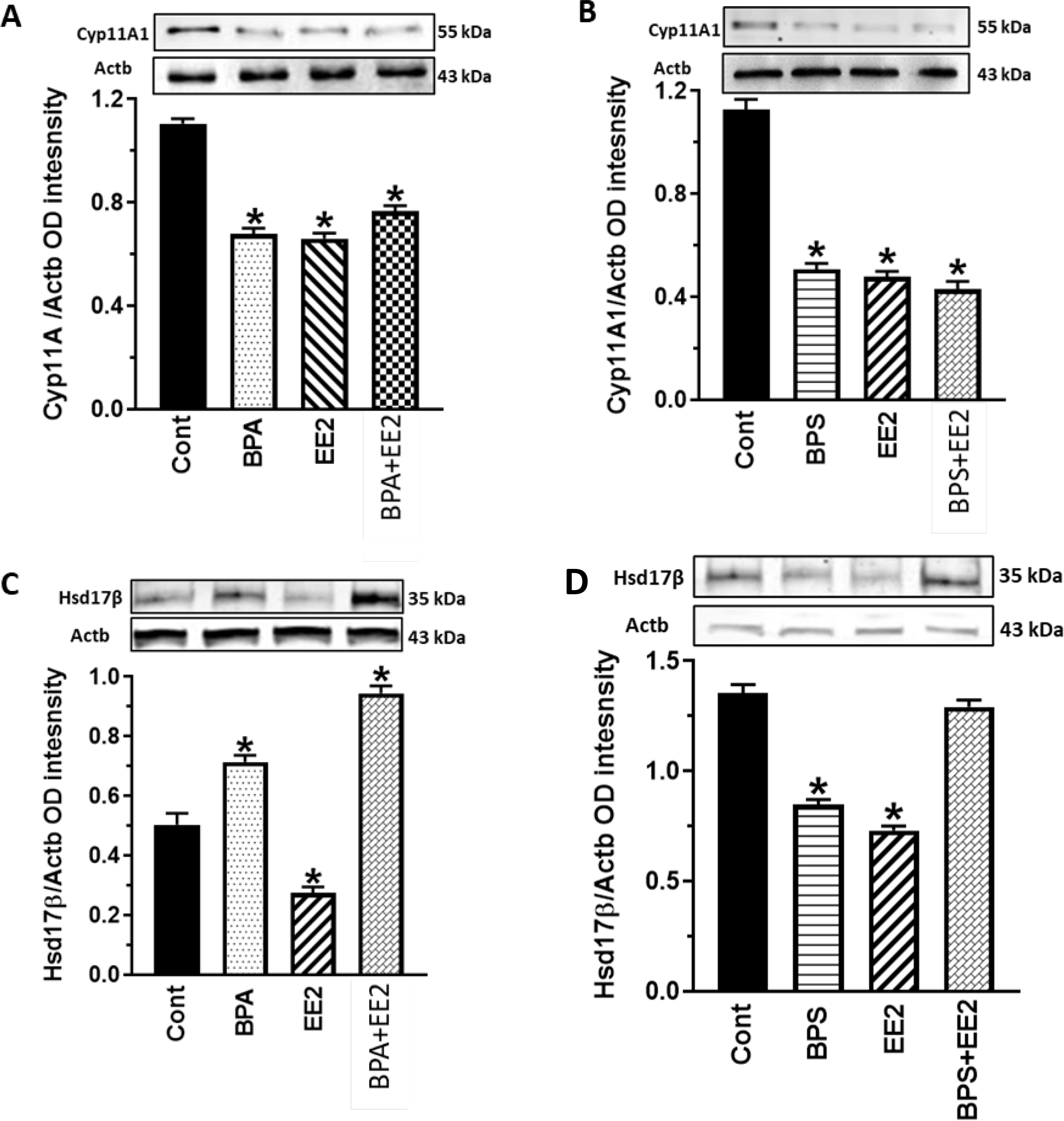
Testes were obtained from pubertal male Long-Evans rats (PND 34–49) fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. Tissues were processed for western blot analysis to analyze 17β-hydroxysteroid dehydrogenase (Hsd17β) and cytochrome P45011A1 (Cyp11A1). Proteins were normalized to actin (ACTB). Tissues were obtained from three animals per group and western blot was repeated at least three times. Hsd17β=35 kDa, Cyp11A1=55 kDa, ACTB=43 kDa *, P<0.05 vs. control.
Fig. 8: Effect of BPA, BPS, EE2 and their combinations on testicular gene protein expression.
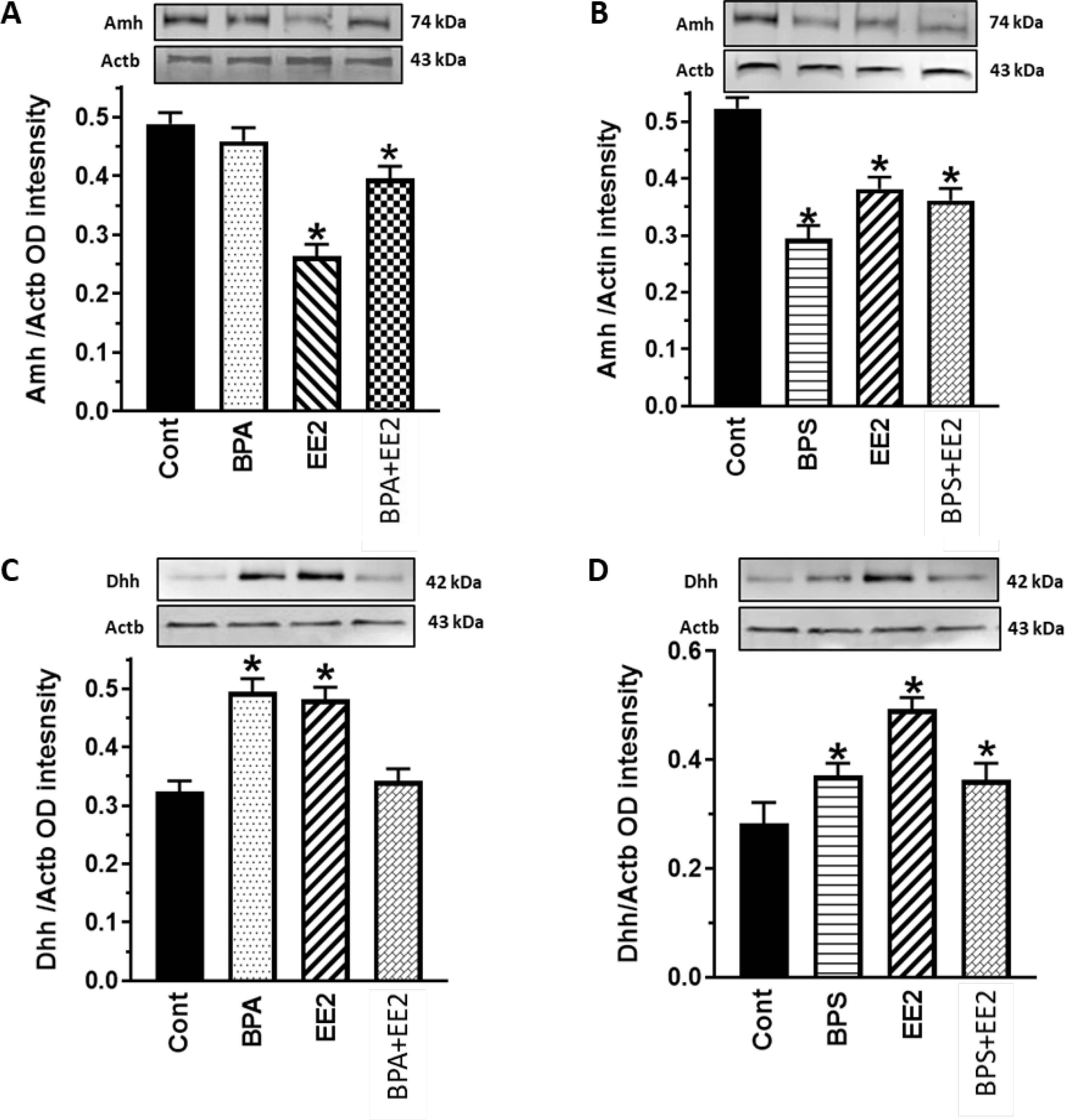
Testes were obtained from pubertal male Long-Evans rats (PND 34–49) fed drinking water containing BPA, BPS, EE2 (5 μg/L) or their combinations (BPA+EE2, BPS+EE2, each at 2.5 μg/L) for 14 days. Tissues were processed for western blot analysis to analyze Anti-Mϋllerian hormone (Amh) and Dhh protein expression. Proteins were normalized to actin (ACTB). Tissues were obtained from three animals per group and western blot was repeated at least three times. Amh=74 kDa, Dhh=42 kDa, Actb=43 kDa *, P<0.05 vs. control.
4.0. DISCUSSION
The results of the present study demonstrated that developmental exposures of male rats to environmentally relevant concentrations of estrogenic chemicals and their combinations alter sex steroid hormone production in the male rat gonad. Similar to the present findings, we reported previously that perinatal (20,58,59) and prepubertal (60) exposures to BPA decreased testicular T biosynthesis in male rats. The present findings also indicated that BPA is a more potent inhibitor of testicular T biosynthesis in prepubertal than in pubertal male rats. However, other studies have shown that BPS administered at 50 μg/kg/day for 28 days decreased intratesticular T biosynthesis in adult rats (61,62) but with a diminished capacity for Esr activation compared to BPA (63,64). Although BPS may exert lesser toxicity in the male gonad, it nevertheless has the capacity to regulate testicular cells. Therefore, exposure of the population to the BPS compound remains a public health concern (65). In addition, exposure to EE2 decreased serum T and intratesticular T concentrations to a lesser degree in pubertal animals than in prepubertal animals suggesting age-dependent sensitivity of male rats to EE2. These observations are similar to other reports of dose-dependent inhibition of Leydig cell T biosynthesis by EE2 (66). Although BPA, BPS, and EE2 each acting alone had an inhibitory effect on androgen biosynthesis and secretion, their combinations (BPS+EE2, BPA+BPS+EE2) had a diminished inhibitory effect on basal testicular T secretion, implying that interaction among chemicals in a mixture may influence their activity in testicular cells. Unlike measurements of testicular T concentrations which are based on unit mass, Leydig cell T secretion was normalized to Leydig cell numbers in order to remove any confounding effects arising from differences in Leydig cell numbers. Thus, our observation of increased Leydig cell T secretion in the BPA+BPS+EE2 treatment group compared to the inhibitory action of the individual chemicals suggests that interactions of chemicals in the mixture increased steroid hormone secretion capacity. Further studies are needed to investigate the androgen biosynthetic pathway in Leydig cells after exposure to single and chemical combinations.
The present data indicated that chemical inhibition of androgen biosynthesis was associated with decreased testicular expression of Cypllal similar to previous reports (67,68). We showed previously that perinatal exposures to BPA increased expression of Hsd17β in neonatal male offspring (20) but decreased expression in the adult testis (58,59). It is possible that BPA interferes with many steroidogenic enzymes because it affected Hsd17β, Cyp17a1, Hsd3β, and Cypllal in the rat as in the human testes (61). Reports on BPS effects are limited, but studies in mice showed that this compound inhibited T secretion with no effect on the CypllAl enzyme (65,69,70). On the other hand, decreased Hsd17β mRNA levels were found in testes of Zebrafish exposed to BPS (71). In the present study, we observed that EE2 inhibition of androgen biosynthesis was related to a decrease in testicular expression of both CypllAl and Hsd17β protein. Our finding related to EE2 effects on Cypllal aligns with a previous report demonstrating that EE2 inhibited Cypllal activity in Leydig cells (66). Thus, it is likely that testicular Cypllal is a primary target for EDCs with estrogenic properties and may be a marker for xenoestrogen exposure.
Moreover, we reported previously that perinatal BPA exposures did not affect E2 biosynthesis in adult male offspring (59) but in vitro assays demonstrated that E2 secretion was decreased after incubation of Leydig cells with BPA (60). In the present study, single and chemical combinations increased testicular E2 secretion in both prepubertal and pubertal male rats similar to previous studies in mice (69). Because BPA acting alone did not affect E2 biosynthesis, the effect of increased E2 secretion seen in the BPA+EE2, BPS+EE2 and BPA+BPS+EE2 groups possibly result from the actions of EE2 and BPS and/or their interactions. Although differences in E2 secretion may be due to test chemical action in a variety of testicular cells, including Leydig cells, Sertoli cells (72,73) and germ cells (74), the present study confirmed that BPA and its combination with BPS and EE2 stimulated E2 secretion by Leydig cells. However, it is to be noted that serum E2 may be contributed in part by extra-testicular sources, e.g., adipocytes (75). Because germ cells express ESRs (76), changes in intratesticular E2 concentrations have implications for germ cell development and sperm function. We and others reported previously that BPA (60,77), BPS (78) and EE2 (79) regulated pituitary gonadotropin release and circulating LH concentration in male rats. Thus, the finding of increased circulating or testicular E2 concentrations has implications for E2-mediated feedback regulation of the HPG axis.
Chemical exposure effects on testicular development were evident in altered expression of Sertoli cell-produced factors. For example, Amh protein was decreased but Dhh was increased in all chemical-exposed animals. These are important observations because the Amh protein is a marker of Sertoli cell differentiation, whereas Dhh is required for progression of germ cells through the process of spermiogenesis (80). Male mice deficient in Dhh exhibited functionally immature sperm and a decrease in the number of Leydig cells (81). Other studies demonstrated that Dhh overexpression impaired Dhh signaling, affected Sertoli cell function, disrupted spermatogenesis and decreased the number of primary spermatocytes (82). Thus, the finding of decreased Amh expression and increased Dhh expression due to BPA, BPS, EE2, and BPS+EE2 implies that the test chemicals acting alone and in combination have the capacity to interfere with testicular development.
In summary, we observed that, in many instances, chemical combinations modified individual chemical effects on steroid hormone secretion at the low dose exposure paradigms. Altogether, our results demonstrated that single chemical exposures (BPA, BPS, or EE2) markedly decreased androgen secretion but their combination caused the opposite effect (i.e., increased Leydig cell T secretion), and test chemicals acting alone or in combination caused an increase in testicular and Leydig cell E2 secretion. Chemical-induced changes in androgen secretion were associated at least in part to altered steroidogenic enzyme protein. Although BPA increased but EE2 decreased testicular expression of the Hsd17β enzyme protein, their combined effect was similar to BPA’s stimulatory action. Also, BPS and EE2 both decreased Hsd17β protein expression when acting alone but their combination removed this inhibitory effect. These results showed that environmentally relevant concentrations of BPA, BPS, and EE2 either acting alone or in combination regulated steroidogenic capacity in the rodent male gonad. Further studies are warranted to investigate mechanisms associated with differential effects of single and chemical mixtures in biological systems. Furthermore, chemical exposures in the present study were normalized to parts per billion of drinking water. Ongoing and future studies will validate these findings using equimolar chemical concentrations in in vitro assays.
Fig. 6: Effect of BPA, BPS, EE2 and their combinations on serum, testicular and Leydig cell 17β- estradiol (E2) concentrations in prepubertal male rats.
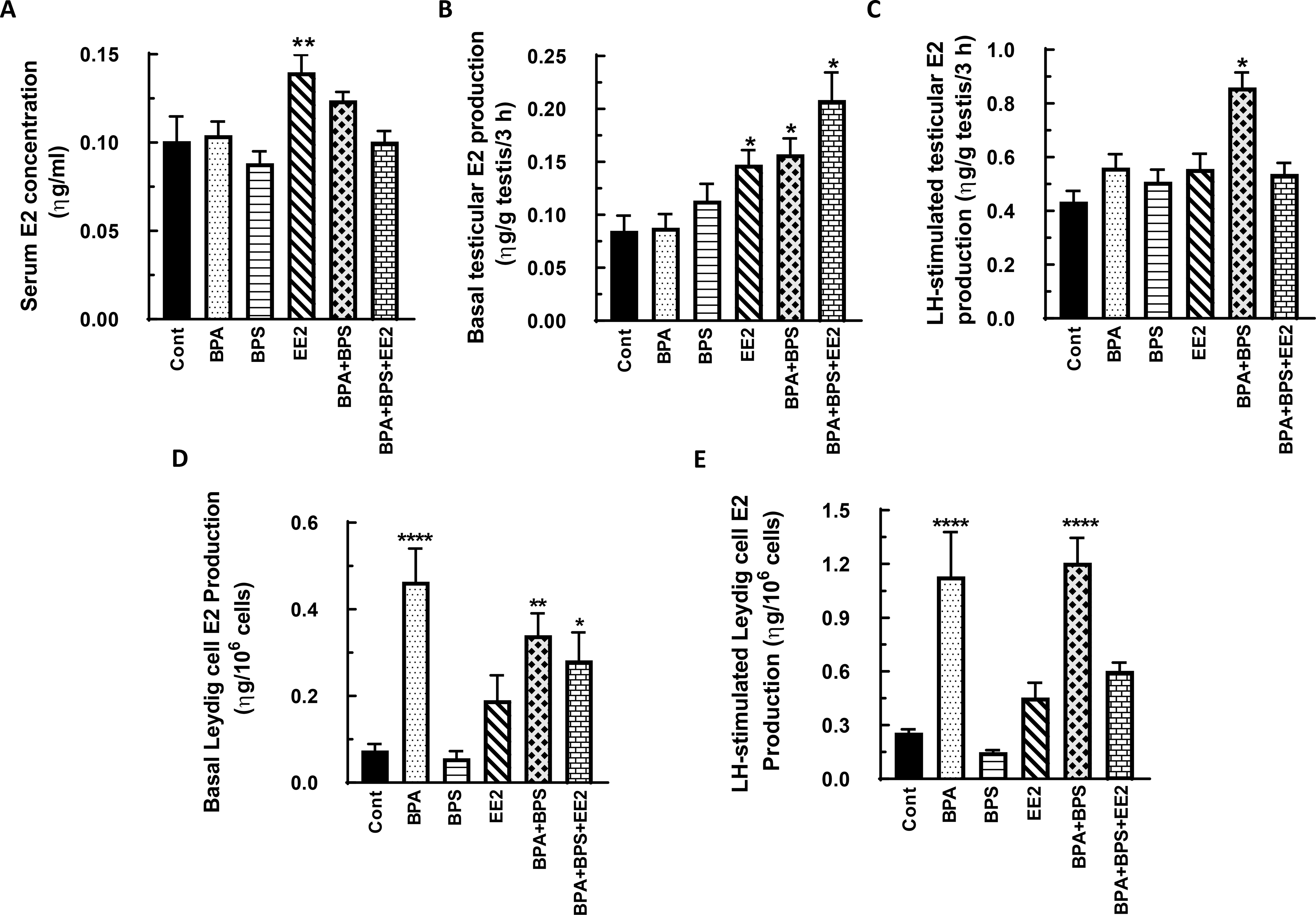
Long-Evans male rats at 21 days of age (n=48) were fed drinking water containing BPA, BPS, or EE2 (5 μg/L) or their combinations BPA+BPS (2.5 μg/L) each and BPA+BPS+EE2 (1.7 μg/L) each for 14 days. At sacrifice, blood was processed to obtain serum (A). Testicular explants were obtained and incubated in DMEM/Ham’s F-12 culture medium in triplicate without (basal, B) or with 100 ηg/ml ovine LH (NIDDK, NIH) (LH-stimulated, C) for 3 h. Furthermore, Leydig cells were isolated and incubated in DMEM/Ham’s F-12 culture medium without (basal, D) or with 100 ng/ml ovine LH (NIDDK, NIH) (LH-stimulated, E) for 3 h. Aliquots of serum and spent media were analyzed to measure E2 concentrations by RIA (n = 8; *p < 0.05, **p < 0.001, ***p < 0.0001).
HIGHLIGHTS.
Single chemical exposures (BPA, BPS, EE2) markedly decreased androgen secretion but their combination caused the opposite effect, i.e., increased Leydig cell T secretion
Chemicals, whether acting alone or as mixtures, increased testicular and Leydig cell E2 secretion
BPA increased and EE2 decreased testicular expression of the Hsd17β enzyme but their combination increased enzyme protein
BPA and EE2, when acting alone, increased testicular Dhh protein expression but this effect was abrogated by exposure to their combination
Acknowledgements:
This work was supported by the Animal Health and Disease Research Program of Auburn University College of Veterinary Medicine and in part by NIEHS grant ES 05886-02 (to BTA).
Footnotes
Financial Disclosure: Authors have nothing to disclose.
Declaration of competing interest: The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Carpenter DO, Arcaro K, Spink DC. Understanding the human health effects of chemical mixtures. Environmental health perspectives. 2002;110(suppl 1):25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimental D, Tort M, D’Anna L, Krawic A, Berger J, Rossman J, Mugo F, Doon N, Shriberg M, Howard E. Ecology of increasing disease, population growth, and environmental degradation. 1998. [Google Scholar]
- 3.Keith LH. Environmental endocrine disruptors. Pure and Applied Chemistry. 1998;70(12):2319–2326. [Google Scholar]
- 4.Degen G, Bolt H. Endocrine disruptors: update on xenoestrogens. International archives of occupational and environmental health. 2000;73(7):433–441. [DOI] [PubMed] [Google Scholar]
- 5.Agency UEP. Guidelines for the health risk assessment of chemical mixtures. Fed Reg. 1986;51(185):34014–34025. [Google Scholar]
- 6.Tsang H. BPA bans and restrictions in food contact materials. Accessed online at https://www.sgs.com/en/news/2018/10/bpa-bans-and-restrictions-in-food-contact-materials. October 7, 2018.
- 7.Liao C, Liu F, Kannan K. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environmental science & technology. 2012;46(12):6515–6522. [DOI] [PubMed] [Google Scholar]
- 8.FDA. Bisphenol A (BPA): Use in Food Contact Application. Accessed online at https://www.fda.gov/food/food-additives-petitions/bisphenol-bpa-use-food-contact-application. 2018.
- 9.Liao C, Kannan K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Additives & Contaminants: Part A. 2014;31(2):319–329. [DOI] [PubMed] [Google Scholar]
- 10.Wu L-H, Zhang X-M, Wang F, Gao C-J, Chen D, Palumbo JR, Guo Y, Zeng EY. Occurrence of bisphenol S in the environment and implications for human exposure: A short review. Science of the Total Environment. 2018;615:87–98. [DOI] [PubMed] [Google Scholar]
- 11.Ullah A, Pirzada M, Jahan S, Ullah H, Shaheen G, Rehman H, Siddiqui MF, Butt MA. Bisphenol A and its analogs bisphenol B, bisphenol F, and bisphenol S: Comparative in vitro and in vivo studies on the sperms and testicular tissues of rats. Chemosphere. 2018;209:508–516. [DOI] [PubMed] [Google Scholar]
- 12.Baronti C, Curini R, D’Ascenzo G, Di Corcia A, Gentili A, Samperi R. Monitoring natural and synthetic estrogens at activated sludge sewage treatment plants and in a receiving river water. Environmental Science & Technology. 2000;34(24):5059–5066. [Google Scholar]
- 13.D’ascenzo G, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, Samperi R. Fate of natural estrogen conjugates in municipal sewage transport and treatment facilities. Science of the Total Environment. 2003;302(1–3):199–209. [DOI] [PubMed] [Google Scholar]
- 14.Colucci MS, Bork H, Topp E. Persistence of estrogenic hormones in agricultural soils: I. 17β - estradiol and estrone. Journal of Environmental Quality. 2001;30(6):2070–2076. [DOI] [PubMed] [Google Scholar]
- 15.Buxton HT, Kolpin DW. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams. Water Encyclopedia. 2005;5:605–608. [Google Scholar]
- 16.Routledge E, Desbrow C, Brighty G, Waldock M. Identification of estrogenic chemicals in STW effluent. Chemical fractionation and in vitro biological screening. Environ Sci Technol. 1998;32:1549–1558. [Google Scholar]
- 17.Cargouet M, Perdiz D, Mouatassim-Souali A, Tamisier-Karolak S, Levi Y. Assessment of river contamination by estrogenic compounds in Paris area (France). Science of the total environment. 2004;324(1–3):55–66. [DOI] [PubMed] [Google Scholar]
- 18.Desbrow C, Routledge E, Brighty G, Sumpter J, Waldock M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and in vitro biological screening. Environmental science & technology. 1998;32(11):1549–1558. [Google Scholar]
- 19.Abdel-Maksoud F, Knight R, Waler K, Yaghoubi-Yeganeh N, Olukunle J, Thompson H, Panizzi J, Akingbemi B. Exposures of male rats to environmental chemicals [bisphenol A and di (2-ethylhexyl) phthalate] affected expression of several proteins in the developing epididymis. Andrology. 2018;6(1):214–222. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Maksoud FM, Ali FAZ, Akingbemi BT. Prenatal exposures to bisphenol A and di (2- ethylhexyl) phthalate disrupted seminiferous tubular development in growing male rats. Reproductive Toxicology. 2019;88:85–90. [DOI] [PubMed] [Google Scholar]
- 21.Ullah H, Ambreen A, Ahsan N, Jahan S. Bisphenol S induces oxidative stress and DNA damage in rat spermatozoa in vitro and disrupts daily sperm production in vivo. Toxicological & Environmental Chemistry. 2017;99(5–6):953–965. [Google Scholar]
- 22.Fic A, žegura B, Dolenc MS, Filipič M, Mašič LP. Mutagenicity and DNA damage of bisphenol A and its structural analogues in HepG2 cells. Archives of Industrial Hygiene and Toxicology. 2013;64(2):189–200. [DOI] [PubMed] [Google Scholar]
- 23.Länge R, Hutchinson TH, Croudace CP, Siegmund F, Schweinfurth H, Hampe P, Panter GH, Sumpter JP. Effects of the synthetic estrogen 17α-ethinylestradiol on the life-cycle of the fathead minnow (Pimephales promelas). Environmental Toxicology and Chemistry: An International Journal. 2001;20(6):1216–1227. [DOI] [PubMed] [Google Scholar]
- 24.Örn S, Holbech H, Madsen TH, Norrgren L, Petersen GI. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquatic toxicology. 2003;65(4):397–411. [DOI] [PubMed] [Google Scholar]
- 25.Nash JP, Kime DE, Van der Ven LT, Wester PW, Brion F, Maack G, Stahlschmidt-Allner P, Tyler CR. Long-term exposure to environmental concentrations of the pharmaceutical ethynylestradiol causes reproductive failure in fish. Environmental health perspectives. 2004;112(17):1725–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenske M, Maack G, Schäfers C, Segner H. An environmentally relevant concentration of estrogen induces arrest of male gonad development in zebrafish, Danio rerio. Environmental Toxicology and Chemistry: An International Journal. 2005;24(5):1088–1098. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Yang J, Wang Y, Jiang Q, Chen H, Song H. Exposure to 17α-ethynylestradiol impairs reproductive functions of both male and female zebrafish (Danio rerio). Aquatic Toxicology. 2008;88(1):1–8. [DOI] [PubMed] [Google Scholar]
- 28.Balch GC, Mackenzie CA, Metcalfe CD. Alterations to gonadal development and reproductive success in japanese medaka (Oryzias latipes) exposed to 17 α-ethinylestradiol. Environmental Toxicology and Chemistry: An International Journal. 2004;23(3):782–791. [DOI] [PubMed] [Google Scholar]
- 29.Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE Jr. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicological Sciences. 2008;102(2):371–382. [DOI] [PubMed] [Google Scholar]
- 30.Safe SH. Hazard and risk assessment of chemical mixtures using the toxic equivalency factor approach. Environmental health perspectives. 1998;106(suppl 4):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kortenkamp A Ten years of mixing cocktails: a review of combination effects of endocrine- disrupting chemicals. Environmental health perspectives. 2007;115(Suppl 1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environmental health perspectives. 2002;110(9):917–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howdeshell KL, Rider CV, Wilson VS, Furr JR, Lambright CR, Gray LE Jr. Dose addition models based on biologically relevant reductions in fetal testosterone accurately predict postnatal reproductive tract alterations by a phthalate mixture in rats. Toxicological Sciences. 2015;148(2):488–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kortenkamp A, Altenburger R. Synergisms with mixtures of xenoestrogens: a reevaluation using the method of isoboles. Science of the Total Environment. 1998;221(1):59–73. [DOI] [PubMed] [Google Scholar]
- 35.Christiansen S, Scholze M, Dalgaard M, Vinggaard AM, Axelstad M, Kortenkamp A, Hass U. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environmental health perspectives. 2009;117(12):1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howdeshell KL, Wilson VS, Furr J, Lambright CR, Rider CV, Blystone CR, Hotchkiss AK, Gray LE Jr. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicological sciences. 2008;105(1):153–165. [DOI] [PubMed] [Google Scholar]
- 37.Program NT. NTP research report on the CLARITY-BPA core study: a perinatal and chronic extended-dose-range study of bisphenol A in rats. 2018. [PubMed] [Google Scholar]
- 38.Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, Vom Saal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ Health Perspect. 2003;111(9):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeminiwa BO, Knight RM, Braden TD, Cruz-Espindola C, Boothe DM, Akingbemi BT. Regulation of the neuroendocrine axis in male rats by soy-based diets is independent of age and due specifically to isoflavone actiont. Biology of reproduction. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Laboratory investigation; a journal of technical methods and pathology. 2001;81(5):735–747. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Caldwell DJ, Suri RP. In vitro estrogenic activity of representative endocrine disrupting chemicals mixtures at environmentally relevant concentrations. Chemosphere. 2019;215:396–403. [DOI] [PubMed] [Google Scholar]
- 42.Aris AZ, Shamsuddin AS, Praveena SM. Occurrence of 17 α-ethynylestradiol (EE2) in the environment and effect on exposed biota: a review. Environment international. 2014;69:104–119. [DOI] [PubMed] [Google Scholar]
- 43.Simmons JE. Chemical mixtures: challenge for toxicology and risk assessment. Toxicology. 1995;105(2–3):111–119. [DOI] [PubMed] [Google Scholar]
- 44.Vecsey CG, Wimmer ME, Havekes R, Park AJ, Perron IJ, Meerlo P, Abel T. Daily acclimation handling does not affect hippocampal long-term potentiation or cause chronic sleep deprivation in mice. Sleep. 2013;36(4):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cochran R, Ewing L, Niswender G. Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5 alpha-dihydrotestosterone, 5 alpha-androstane-3 alpha, 17 beta-diol, 5 alpha-androstane-3 beta, 17 beta-diol, and 17 beta-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Investigative urology. 1981;19(3):142–147. [PubMed] [Google Scholar]
- 46.Shrivastav TG, Kanaujia PK. Direct radioimmunoassay for the measurement of serum testosterone using 3H as label. Journal of Immunoassay and Immunochemistry. 2007;28(2):127–136. [DOI] [PubMed] [Google Scholar]
- 47.Korenman SG, Stevens RH, Carpenter LA, Robb M, Niswender GD, Sherman BM. Estradiol radioimmunoassay without chromatography: procedure, validation and normal values. The Journal of Clinical Endocrinology & Metabolism. 1974;38(4):718–720. [DOI] [PubMed] [Google Scholar]
- 48.Hardy MP. Differentiation of Leydig cell precursors in vitro: a role for androgen. Endocrinology. 1990;127(1):488–490. [DOI] [PubMed] [Google Scholar]
- 49.Risbridger GP, De Kretser DM. Percoll-gradient separation of Leydig cells from postnatal rat testes. Reproduction. 1986;76(1):331–338. [DOI] [PubMed] [Google Scholar]
- 50.Risbridger GP, Davies A. Isolation of rat Leydig cells and precursor forms after administration of ethane dimethane sulfonate. American Journal of Physiology-Endocrinology and Metabolism 1994;266(6):E975–E979. [DOI] [PubMed] [Google Scholar]
- 51.Bremer AA, Miller WL. Regulation of steroidogenesis. Cellular endocrinology in health and disease: Elsevier; 2014:207–227. [Google Scholar]
- 52.Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biology of reproduction. 2012;86(5):135, 131–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rey R, Lordereau-Richard I, Carel J-C, Barbet P, Cate RL, Roger M, Chaussain J-L, Josso N. Anti-müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development. The Journal of Clinical Endocrinology & Metabolism. 1993;77(5):1220–1226. [DOI] [PubMed] [Google Scholar]
- 54.Zhou B, Watts LM, Hutson JM. Germ cell development in neonatal mouse testes in vitro requires Müllerian inhibiting substance. The Journal of urology. 1993;150(2):613–616. [DOI] [PubMed] [Google Scholar]
- 55.Kawai Y, Noguchi J, Akiyama K, Takeno Y, Fujiwara Y, Kajita S, Tsuji T, Kikuchi K, Kaneko H, Kunieda T. A missense mutation of the Dhh gene is associated with male pseudohermaphroditic rats showing impaired Leydig cell development. Reproduction (Cambridge, England). 2011;141(2):217–225. [DOI] [PubMed] [Google Scholar]
- 56.Sansone A, Kliesch S, Isidori AM, Schlatt S. AMH and INSL3 in testicular and extragonadal pathophysiology: what do we know? Andrology. 2019;7(2):131–138. [DOI] [PubMed] [Google Scholar]
- 57.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biology of reproduction. 2000;63(6):1825–1838. [DOI] [PubMed] [Google Scholar]
- 58.Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biology of reproduction. 2012;86(5):135, 131–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nanjappa MK, Ahuja M, Dhanasekaran M, Coleman ES, Braden TD, Bartol FF, Bird RC, Wanders D, Judd RL, Akingbemi BT. Bisphenol A regulation of testicular endocrine function in male rats is affected by diet. Toxicology letters. 2014;225(3):479–487. [DOI] [PubMed] [Google Scholar]
- 60.Akingbemi BT, Sottas CM, Koulova AI, Klinefelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology. 2004;145(2):592–603. [DOI] [PubMed] [Google Scholar]
- 61.Ye L, Zhao B, Hu G, Chu Y, Ge R-S. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicology letters. 2011;207(2):137–142. [DOI] [PubMed] [Google Scholar]
- 62.Ullah H, Jahan S, Ain QU, Shaheen G, Ahsan N. Effect of bisphenol S exposure on male reproductive system of rats: A histological and biochemical study. Chemosphere. 2016;152:383–391. [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Jiao Z, Shi J, Li M, Guo Q, Shao B. Effects of bisphenol analogues on steroidogenic gene expression and hormone synthesis in H295R cells. Chemosphere. 2016;147:9–19. [DOI] [PubMed] [Google Scholar]
- 64.Molina-Molina J-M, Amaya E, Grimaldi M, Sáenz J-M, Real M, Fernandez MF, Balaguer P, Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicology and applied pharmacology. 2013;272(1):127–136. [DOI] [PubMed] [Google Scholar]
- 65.Eladak S, Grisin T, Moison D, Guerquin M-J, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R. A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertility and sterility. 2015;103(1):11–21. [DOI] [PubMed] [Google Scholar]
- 66.Kuo T-H, Wang K-L, Lin P-H, Chen Y-A, Chang C-H, Wang S-W, Wang PS. Inhibitory Effects of 17 Alpha-Ethynylestradiol on the Production of Testosterone by Rat Leydig Cells. Oxford University Press; 2012. [Google Scholar]
- 67.Nakamura D, Yanagiba Y, Duan Z, Ito Y, Okamura A, Asaeda N, Tagawa Y, Li C, Taya K, Zhang S-Y. Bisphenol A may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicology letters. 2010;194(1–2):16–25. [DOI] [PubMed] [Google Scholar]
- 68.Yang Q, Sui X, Cao J, Liu C, Zheng S, Bao M, Huang Y, Wu K. Effects of exposure to Bisphenol A during pregnancy on the pup testis function. International journal of endocrinology. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi M, Sekulovski N, MacLean JA II, Hayashi K. Prenatal Exposure to Bisphenol A Analogues on Male Reproductive Functions in Mice. Toxicological Sciences. 2018;163(2):620–631. [DOI] [PubMed] [Google Scholar]
- 70.Shi M, Sekulovski N, MacLean II JA, Hayashi K. Effects of bisphenol A analogues on reproductive functions in mice. Reproductive toxicology. 2017;73:280–291. [DOI] [PubMed] [Google Scholar]
- 71.Ji K, Hong S, Kho Y, Choi K. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environmental science & technology. 2013;47(15):8793–8800. [DOI] [PubMed] [Google Scholar]
- 72.Haverfield JT, Ham S, Brown KA, Simpson ER, Meachem SJ. Teasing out the role of aromatase in the healthy and diseased testis. Spermatogenesis. 2011;1(3):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carreau S, De Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Advances in Medical Sciences (De Gruyter Open). 2008;53(2). [DOI] [PubMed] [Google Scholar]
- 74.Janulis L, Bahr JM, Hess RA, Janssen S, Osawa Y, Bunick D. Rat testicular germ cells and epididymal sperm contain active P450 aromatase. Journal of andrology. 1998;19(1):65–71. [PubMed] [Google Scholar]
- 75.Kalicinska E, Wojtas K, Majda J, Zacharski M, Skiba J, Sliwowski J, Banasiak W, Ponikowski P, Jankowska EA. Expression of sex steroid receptors and aromatase in adipose tissue in different body regions in men with coronary artery disease with and without ischemic systolic heart failure. The aging male : the official journal of the International Society for the Study of the Aging Male. 2018:1–13. [DOI] [PubMed] [Google Scholar]
- 76.HEMSELL DL, Grodin J, Brenner P, Siiteri P, MacDonald P. Plasma precursors of estrogen. II. Correlation of the extent of conversion of plasma androstenedione to estrone with age. The Journal of Clinical Endocrinology & Metabolism. 1974;38(3):476–479. [DOI] [PubMed] [Google Scholar]
- 77.Wisniewski P, Romano RM, Kizys MM, Oliveira KC, Kasamatsu T, Giannocco G, Chiamolera MI, Dias-da-Silva MR, Romano MA. Adult exposure to bisphenol A (BPA) in Wistar rats reduces sperm quality with disruption of the hypothalamic–pituitary–testicular axis. Toxicology. 2015;329:1–9. [DOI] [PubMed] [Google Scholar]
- 78.Ullah A, Pirzada M, Jahan S, Ullah H, Razak S, Rauf N, Khan M, Mahboob S. Prenatal BPA and its analogs BPB, BPF, and BPS exposure and reproductive axis function in the male offspring of Sprague Dawley rats. Human & experimental toxicology. 2019;38(12):1344–1365. [DOI] [PubMed] [Google Scholar]
- 79.Lin P-H, Kuo T-H, Chen C-C, Jian C-Y, Chen C-W, Wang K-L, Kuo Y-C, Shen H-Y, Hsia S-M, Wang PS. Downregulation of testosterone production through luteinizing hormone receptor regulation in male rats exposed to 17α-ethynylestradiol. Scientific reports. 2020;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Morales CR, Fox A, El-Alfy M, Ni X, Argraves WS. Expression of patched-1 and smoothened in testicular meiotic and post-meiotic cells. Microscopy research and technique. 2009;72(11):809–815. [DOI] [PubMed] [Google Scholar]
- 81.Yao HH-C, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes & development. 2002;16(11):1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.La Sala G, Marazziti D, Di Pietro C, Golini E, Matteoni R, Tocchini-Valentini GP. Modulation of Dhh signaling and altered Sertoli cell function in mice lacking the GPR37-prosaposin receptor. The FASEB Journal. 2015;29(5):2059–2069. [DOI] [PubMed] [Google Scholar]


