Abstract
Background
Decreased visual acuity (VA) is reported to be a risk factor for dementia. However, the association between VA and cortical thickness has not been established. We investigated the association between VA and cortical thickness in cognitively normal adults.
Method
We conducted a cross-sectional, single-center cohort study with cognitively normal adults (aged ≥ 45) who received medical screening examinations at the Health Promotion Center at Samsung Medical Center. Subjects were categorized as bad (VA ≤ 20/40), fair (20/40 < VA ≤ 20/25), and good (VA > 20/25) VA group by using corrected VA in the Snellen system. Using 3D volumetric brain MRI, cortical thickness was calculated using the Euclidean distance between the linked vertices of the inner and outer surfaces. We analyzed the association between VA and cortical thickness after controlling for age, sex, hypertension, diabetes, dyslipidemia, intracranial volume, and education level.
Results
A total of 2756 subjects were analyzed in this study. Compared to the good VA group, the bad VA group showed overall thinner cortex (p = 0.015), especially in the parietal (p = 0.018) and occipital (p = 0.011) lobes. Topographical color maps of vertex-wise analysis also showed that the bad VA group showed a thinner cortex in the parieto-temporo-occipital area. These results were more robust in younger adults (aged 45 to 65) as decreased VA was associated with thinner cortex in more widespread regions in the parieto-temporo-occipital area.
Conclusion
Our results suggest that a thinner cortex in the visual processing area of the brain is related to decreased visual stimuli.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-022-01045-0.
Keywords: Cortical thickness, Visual acuity, Dementia
Introduction
Visual disturbance is one of the most common complaints in aging. According to a global survey, an estimated 285 million people have visual problems, and two-thirds of them are aged 50 or older [1]. Several studies have suggested that visual acuity (VA) is associated with cognitive impairment. Previous studies showed that decreased VA is a risk factor for dementia [2–4]. Another study in the USA also demonstrated that VA was related to dementia severity [5].
Ample evidence supports the idea that decreased sensory stimuli lead to the degeneration of relevant areas in the brain. A previous study showed that hearing loss [6–9] or central vision loss due to macular degeneration [10–12] could cause neurodegeneration in the brain. On the other hand, reduced visual input in early life may lead to poor development in the visual processing area of the brain which may result in a thinner cortex [13]. Reversely, an inherently thinner visual cortex or dysmaturation of the visual cortex in the developmental phase may lead to decreased VA [14, 15]. However, the association between VA and cortical thickness among individuals without known ocular disease has not been established yet. Currently, the degree of cortical thickness can be measured on brain magnetic resonance imaging (MRI) [16–18]. As cortical atrophy precedes dementia symptoms and individuals with a thinner cortex are at higher risk for dementia [18–21], cortical thickness is an important biomarker and is used as a proxy for neurodegeneration.
Therefore, we assessed the association between VA and cortical thickness in a large cohort of cognitively normal adults (aged ≥ 45). We hypothesized that reduced VA is associated with a thinner cortex, especially in the primary visual cortex as well as in the areas related to visual processing and visual memory. We also expected that the relationship between decreased VA and cortical thickness would be more prominent in younger adults (aged 45–60), because the effect of decreased VA on thinner cortex may be masked by physiological neurodegeneration related to aging in older adults (aged > 65) [22].
Methods
Study design and participants
We retrospectively collected data on 2975 subjects aged 45 years or older who underwent a medical screening examination at the Health Promotion Center of Samsung Medical Center (Seoul, Korea) between September 2008 and December 2014. All of them underwent brain MRI including three-dimensional volume images and visual acuity assessment. We excluded the following participants: (1) subjects with significant cognitive impairment defined by an MMSE score below the 16th percentile of age- and education-matched norms (n = 169); (2) subjects with incomplete information on hypertension, diabetes, or dyslipidemia (n = 1); and (3) subjects whose cortical thickness measurement failed due to head motion artifact (n = 49). We finally included data from 2756 subjects for analysis in this study.
Standard protocol approvals, registrations, and patient consents
This study was approved by the Institutional Review Board at Samsung Medical Center. All methods were conducted in accordance with the approved guidelines. The need for informed consent was waived by the Institutional Review Board at Samsung Medical Center, as we collected retrospective and de-identified data.
Health screening examination
Health screening examination was conducted by trained health professionals according to the standard protocols at Samsung Medical Center. This included physical examination, laboratory analysis, brain MRI, and VA measurement. A standardized questionnaire was used to collect information related to demographic characteristics including hypertension, diabetes, dyslipidemia, and total duration of formal education, as previously described [23]. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure > 90 mmHg, a self-reported history of hypertension, or current use of antihypertensive medication. Diabetes was defined as a fasting serum glucose level greater than 126 mg/dL, self-reported diabetes history, or use of diabetes medications. Dyslipidemia was defined as a fasting total cholesterol level > 200 mg/dL, low-density lipoprotein level > 100 mg/dL, high-density lipoprotein level < 50 mg/dL, triglyceride level > 200 mg/dL, self-reported history of dyslipidemia, or current use of lipid-lowering medication.
Acquisition of brain MRI data
All participants underwent a 3D volumetric brain MRI. Previous studies have described the details of MRI protocols and the process of cortical thickness measurements [24]. In brief, an Achieva 3.0T MRI scanner (Philips, Best, The Netherlands) was applied to obtain 3D T1 turbo field echo MRI data with the following imaging parameters: sagittal slice thickness of 1.0 mm with 50% overlap, no gap, repetition time of 9.9 ms, echo time of 4.6 ms, flip angle of 8°, and matrix size of 240 × 240 pixels reconstructed to 480 × 480 over a field view of 240 mm. Radiologists initially inspected all MR images for evidence of brain tumors, lobar infarctions (except lacunar infarctions), and hemorrhages (observed as low intensity on T2-weighted images).
Cortical thickness measurement
T1-weighted MRIs were automatically analyzed with the standard Montreal Neurologic Institute image processing software (CIVET 2.1.0, http://www.bic.mni.mcgill.ca/ServicesSoftware/CIVET). The technique used in this study was previously described [25]. In summary, native MR images were registered into a standardized stereotaxic space using an affine transformation [26]. Using the artificial neural net classifier, the registered and corrected volumes were classified as white matter (WM), gray matter (GM), cerebrospinal fluid (CSF), and background [27]. Using the Laplacian-based automated segmentation with proximities algorithm, we extracted the surfaces of the inner and outer cortices by deforming the spherical mesh onto the gray-white boundaries of each hemisphere. Cortical thickness was calculated using the Euclidean distance between the linked vertices of the inner and outer surfaces, after applying an inverse transformation matrix to the surface of the cortex and reconstructing it in the native space [28]. We used classified tissue information and a skull mask acquired from the T1-weighted image and computed intracranial volume (ICV) to control for brain size [29]. ICV was defined as the sum of the volume of the GM, WM, and CSF, considering the voxel dimension. GM, WM, CSF, and the background within the mask were transformed back into the individual native space. The thicknesses were registered spatially on an unbiased iterative group template by matching the sulcal folding pattern using surface-based registration involving sphere-to-sphere warping to compare the thickness of the corresponding regions among individuals [30]. Global and regional analyses involving the frontal, temporal, parietal, and occipital lobes were performed with the SUMA program (http://afni.nimh.nih.gov) [7, 31].
Visual acuity (VA) assessment
VA was measured using the Snellen system. For individuals who wear spectacles, spectacle-corrected VA was recorded. The study subjects were classified into 3 groups based on VA in the better-seeing eye: bad (VA ≤ 20/40), fair (20/40 < VA ≤ 20/25), and good (VA > 20/25) VA. The World Health Organization uses a threshold of 20/60 for visual impairment, while 20/40 is used as the standard of visual impairment for research purposes [32–34]. VA ≤ 20/25 (loss of 1 line) indicates mild poor vision, which means the individual may have mild difficulty in daily life without spectacles [35, 36].
Statistical analysis
We compared demographic factors across the three groups (bad, fair, and good VA) using chi-square testing or analysis of variance.
To evaluate the association between VA and the cortical thickness of each lobe, we performed a multivariable linear regression analysis. VA group was analyzed as an ordinary variable, and the good VA was used as the reference standard. In addition, multivariable linear regression analysis was conducted using VA as a continuous variable. A subgroup analysis was performed after stratifying subjects into younger adults (aged 45 to 65) and older adults (aged > 65). All analyses were performed with adjustment for age, sex, hypertension, diabetes, dyslipidemia, education, and ICV. We selected these factors as the covariates for the multivariable linear regression based on the clinical relevance.
Analyses were performed using STATA version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC). Reported p-values were calculated as two-sided, and the significance level was set at 0.05.
The MATLAB-based toolbox 2014b (https://kr.mathworks.com/products/matlab.html) was used to evaluate the topography of cortical thickness related to VA. A detailed method was previously reported [37]. In brief, after diffusion smoothing to blur each cortical thickness map (leading to increased signal-to-noise ratio and statistical power), linear regression was performed vertex-by-vertex after controlling for age, sex, hypertension, diabetes, dyslipidemia, education, and ICV. Then, we analyzed the localized differences and the statistical map of cortical thickness on the surface model. The thresholds of the statistical maps were set using a false discovery rate (FDR) with a q-value of 0.05 after pooling the p-values from regression analysis.
Results
Demographics of subjects
Among the 2756 subjects, 159 subjects were classified into the bad VA group, 1256 subjects were classified into the fair VA group, and 1341 subjects were classified into the good VA group. Compared to subjects in the bad VA or fair VA groups, subjects in the good VA group were younger, more likely to be male, and had higher education levels and larger ICVs (Table 1). The distribution of VA according to age was plotted in Additional file 1: Fig. S1.
Table 1.
Baseline characteristics of participants in each visual acuity (VA) groupa (total n = 2756)
| Bad (n = 159) | Fair (n = 1256) | Good (n = 1341) | pb | ||||
|---|---|---|---|---|---|---|---|
| Overall | Bad vs fair | Bad vs good | Fair vs good | ||||
| Age (years) | 67.4 ± 7.6 | 64.9 ± 7.0 | 61.4 ± 6.6 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Sex (male, %) | 54 (34.0) | 543 (43.2) | 786 (58.6) | < 0.001 | 0.026 | < 0.001 | < 0.001 |
| Education (years) | 10.2 ± 5.1 | 12.2 ± 4.4 | 13.6 ± 3.6 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Hypertension (%) | 82 (51.6) | 620 (49.4) | 547 (40.8) | < 0.001 | 0.600 | 0.009 | < 0.001 |
| Diabetes (%) | 34 (21.4) | 198 (15.8) | 206 (15.4) | 0.143 | 0.071 | 0.050 | 0.777 |
| Dyslipidemia (%) | 41 (25.8) | 418 (33.3) | 420 (31.3) | 0.132 | 0.057 | 0.153 | 0.286 |
| ICV (mL) × 105 | 13.3 ± 1.5 | 13.4 ± 1.2 | 13.8 ± 1.2 | < 0.001 | 0.136 | < 0.001 | < 0.001 |
| MMSEc | 26.4 ± 0.3 | 27.6 ± 0.1 | 28.2 ± 0.1 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| VA (logMAR) | 0.49 ± 0.13 | 0.16 ± 0.07 | − 0.02 ± 0.05 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
| Total cortical thickness (× 10−1mm) | 60.16 ± 2.42 | 60.81 ± 2.29 | 61.16 ± 2.13 | < 0.001 | 0.001 | < 0.001 | < 0.001 |
ICV intracranial volume, MMSE Mini-Mental state examination, logMAR -log (Snellen visual acuity)
aGrouped by VA in better-seeing eye: bad = VA ≤ 20/40, fair = 20/40 < VA ≤ 20/25, and good = VA > 20/25 (VA was presented in the Snellen system). Numerical continuous parameters were described as mean ± standard deviation, and categorical parameters were described as total numbers (percentages)
bResults from analysis of variance (ANOVA) with Bonferroni post hoc test for continuous variables (total cortical thickness, age, education, ICV, MMSE) and Pearson’s chi-squared test for binary variable (sex, hypertension, diabetes, dyslipidemia)
cMMSE has a missing value: 11 subjects in the bad VA group, 54 subjects in the fair VA group, and 58 subjects in the good VA group
Association between VA and cortical thickness
Compared to the good VA group, subjects in the bad VA group showed reduced cortical thickness with statistical significance (ß = − 0.46, 95% confidence interval [CI] − 0.84 to − 0.09) especially in the parietal (ß = − 0.57, 95% CI − 1.05 to − 0.10) and occipital (ß = − 0.54, 95% CI − 0.96 to − 0.12) lobes in the multivariable linear regression analysis after adjusting for age, sex, hypertension, diabetes, hyperlipidemia, education, and ICV. p for trend also showed a statistically significant correlation between VA and global cortical thicknesses, as well as parietal and occipital lobe thickness (p = 0.036, 0.023, and 0.025, respectively). However, there was no statistical difference in cortical thickness between the good and fair VA groups (Table 2). The results of the subgroup analysis of corrected VA and uncorrected VA are presented in Additional file 2: Table S1.
Table 2.
Analysis of the relationship between cortical thickness (x 10-1mm) and visual acuity (VA) groupsa
| Global cortical thickness | Frontal lobe | Temporal lobe | Parietal lobe | Occipital lobe | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ß (95%CI) | pb | ß (95%CI) | pb | ß (95%CI) | pb | ß (95%CI) | pb | ß (95%CI) | pb | |
| All age | ||||||||||
| Bad | − 0.46 (− 0.84, − 0.09) | 0.015 | − 0.31 (− 0.70, 0.09) | 0.131 | − 0.50 (− 1.06, 0.06) | 0.079 | -0.57 (-1.05, -0.10) | 0.018 | -0.54 (-0.96, -0.12) | 0.011 |
| Fair | 0.01 (− 0.16, 0.19) | 0.893 | < 0.001 (− 0.19, 0.18) | 0.958 | − 0.04 (− 0.30, 0.22) | 0.780 | 0.08 (-0.14, 0.30) | 0.497 | 0.02 (-0.17, 0.22) | 0.827 |
| Good | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| pc | 0.036 | 0.296 | 0.210 | 0.023 | 0.025 | |||||
| pd | 0.296 | 0.280 | 0.050 | 0.050 | ||||||
| Age ≤ 65 | ||||||||||
| Bad | − 0.71 (− 1.24, − 0.17) | 0.010 | − 0.46 (− 1.03, 0.10) | 0.108 | − 0.63 (− 1.46, 0.19) | 0.130 | − 0.83 (− 1.54, − 0.13) | 0.021 | − 0.99 (− 1.60, − 0.39) | 0.001 |
| Fair | − 0.04 (− 0.25, 0.17) | 0.696 | − 0.05 (− 0.27, 0.18) | 0.689 | − 0.20 (− 0.52, 0.13) | 0.235 | 0.12 (− 0.16, 0.40) | 0.397 | − 0.04 (− 0.28, 0.19) | 0.722 |
| Good | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| pc | 0.035 | 0.273 | 0.209 | 0.030 | 0.005 | |||||
| pd | 0.273 | 0.273 | 0.060 | 0.002 | ||||||
| Age > 65 | ||||||||||
| Bad | − 0.14 (− 0.68, 0.40) | 0.615 | − 0.09 (− 0.68, 0.50) | 0.769 | − 0.05 (− 0.82, 0.72) | 0.897 | − 0.36 (− 1.01, 0.29) | 0.278 | − 0.08 (− 0.69, 0.53) | 0.803 |
| Fair | 0.15 (− 0.15, 0.46) | 0.327 | 0.10 (− 0.24, 0.43) | 0.572 | 0.31 (− 0.12, 0.74) | 0.158 | 0.03 (− 0.33, 0.40) | 0.858 | 0.18 (− 0.16, 0.53) | 0.299 |
| Good | Ref. | Ref. | Ref. | Ref. | Ref. | |||||
| pc | 0.385 | 0.726 | 0.278 | 0.445 | 0.449 | |||||
| pd | 0.726 | 0.599 | 0.599 | 0.599 | ||||||
CI confidential interval, presented as ([lower value], [upper value])
aGrouped by VA in better-seeing eye: bad = VA ≤ 20/40, fair = 20/40 < VA ≤ 20/25, and good = VA > 20/25 (VA was presented in the Snellen system)
bResult from multivariable linear regression adjusted for age, sex, hypertension, diabetes, dyslipidemia, intracranial volume, and education
cp for trends
dAdjusted p using Benjamini and Hochberg’s method for the multiple tests
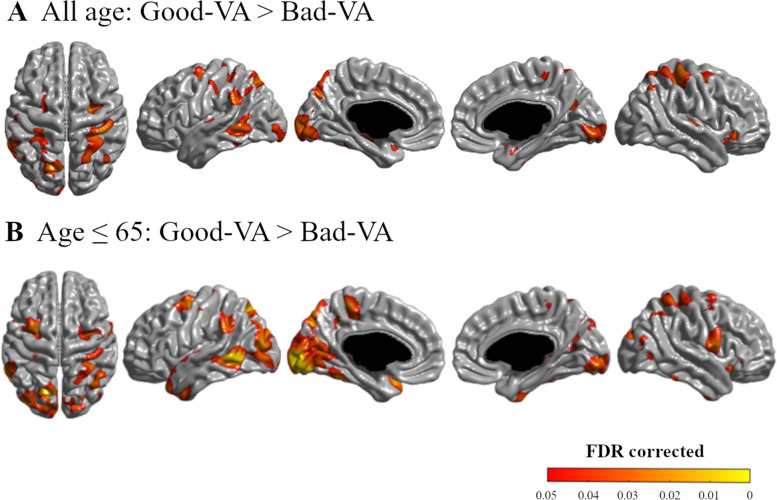
The statistical topographic map also showed that VA was associated with cortical thickness in the colored areas in Fig. 1. Compared to the good VA group, subjects in the bad VA group had significantly thinner cortices, mainly in the parieto-temporo-occipital areas (bilateral superior parietal lobule, bilateral cuneus, and left lateral temporal areas) and in a focal region in the bilateral medial temporal areas (FDR corrected, q < 0.05) (Fig. 1A). However, the uncolored areas showed no significant difference between the good VA group and bad VA group (Fig. 1A).
Fig. 1.
Statistical topography of the relationship between cortical thickness and visual acuity (VA). Compared to the good VA group, the bad VA group showed a thinner cortex in the bilateral temporo-parieto-occipital lobes (A). In the analysis of the subjects who were equal or under 65 years old, a similar result was found with a larger extent and greater significance (B). False discovery rate corrected (q < 0.05). This result was adjusted for age, sex, hypertension, diabetes mellitus, hyperlipidemia, education, and intracranial volume. The MATLAB-based toolbox 2014b (https://kr.mathworks.com/products/matlab.html) was used for the drawing and the configuration of this figure
Association between VA and cortical thickness in younger and older adults
We also analyzed the association between VA and cortical thickness after stratifying the subjects into younger (45 ≤ age ≤ 65) and older (age > 65) adults. In younger adults, the bad VA group showed a thinner cortex in the global area (ß = − 0.71, 95% CI − 1.24 to − 0.17), as well as the parietal (ß = − 0.83, 95% CI − 1.54 to − 0.13) and the occipital (ß = − 0.99, 95% CI − 1.60 to − 0.39) lobes compared to the good VA group. p for trend showed a significant relationship between cortical thickness and VA in the same area (p = 0.035, .030, and 0.005, respectively). However, in older adults, the cortical thickness of the bad VA group and fair VA group did not differ significantly from that of the good VA group (Table 2).
On the statistical map of younger adults, the bad VA group showed a thinner cortex compared to the good VA group mainly in the parieto-temporo-occipital areas, including the bilateral lateral parietal area; left precuneus, bilateral cuneus, and left lateral temporal areas; and also a focal region in the bilateral medial temporal areas (FDR corrected, q < 0.05) (Fig. 1B). However, the uncolored areas showed no significant difference between the good VA group and bad VA group in younger adults (Fig. 1B). In older adults, however, the statistical map of cortical thickness in the bad VA group and fair VA group did not differ from that of the good VA group.
Discussion
We evaluated the association between VA and cortical thickness in a large cohort of cognitively normal adults. We found that decreased VA was related to a thinner cortex mostly in the posterior regions—specifically, the parieto-temporo-occipital areas. This relationship was more robust in younger adults but was not seen in older adults. Our results imply that structural alterations in the visual processing area of the brain are related to decreased VA in cognitively normal healthy adults.
The major finding in this study was that decreased VA was associated with thinner cortex in cognitively normal adults. Although the criteria for the bad VA group in this study did not reach the visual impairment standard defined by the WHO (visual acuity ≤ 20/60), decreased VA was significantly correlated with a thinner cortex. The topography showed that this correlation was most significant in the posterior regions, specifically in the parieto-temporo-occipital area. This area is known to be responsible for processing visual information. Visual information primarily reaches the occipital lobe and then follows two streams to process this information: the dorsal stream (known as the “where pathway”) and the ventral stream (known as the “what pathway”). The “what pathway,” which spreads from the primary visual cortex to the temporal lobe, performs serial processing of perception and recognition of shape, size, objects, faces, and text [38]. On the other hand, the “where pathway,” which spreads from the primary visual cortex to the parietal lobe, processes spatial layout such as location, distance, relative position, position in egocentric space, and motion [38]. Our results suggest that the degree of visual stimuli might affect the cortical thickness in corresponding areas. We also observed that the bad VA group had a thinner cortex in the bilateral medial temporal areas compared to the good VA group. We expected the right medial temporal lobe, which is responsible for visual memory, to be more associated with decreased VA as previously suggested [39]. However, our results showed that both medial temporal lobes were associated with decreased VA. Such results suggested that brain structural alteration related to decreased VA is not limited to visual processing areas, but extends to regions related to visual and verbal memory. A previous Japanese study also showed a possible link between VA and cognitive impairment, even in cognitive domains unrelated to visuospatial function or visual memory [40].
A thinner cortex related to decreased VA was more robust in younger adults in this study. Because aging is the major factor that affects brain cortical atrophy [41], we stratified by age to minimize the effect of aging on cortical atrophy. Consequently, the relationship between VA and cortical thickness was more significant in younger adults. In older adults, there was no significant association. It is possible that reduced VA itself may not be a significant factor of cortical atrophy in older adults. It is also possible that, in older adults, other factors might have comprehensively affected cortical atrophy and may have masked the effect of decreased VA although we adjusted for possible confounders [42–49]. Another possible explanation would be neuroplasticity. That is, better VA might induce a thicker cortex especially in younger adults who are less affected by aging.
This study is the first report that included a topographical analysis of the relationship between a thinner cortex and decreased VA in cognitively normal adults. Similar studies have been conducted on patients with visual problems with known ocular diseases such as glaucoma, retinal disease, or macular disease [10, 11, 50–56]. These studies suggested that decreased VA from ocular diseases also could cause structural alteration in the brain, and this was pathologically confirmed in a glaucoma patient [57]. Subjects with acquired blindness were reported to have a thinner primary visual cortex [10, 11, 50–55, 58]. Our study showed that cortical atrophy patterns related to decreased VA were similar to those from studies on acquired blindness. These studies imply that decreased visual stimuli from various conditions can be related to structural changes in the brain. Considering that most of the participants in this study had no specific ocular diseases such as glaucoma or macular disease, our study inferred that a thinner cortex was related to decreased VA per se.
The pathophysiologic mechanism of the relationship between a thinner cortex and decreased VA remains unclear. One possible mechanism is desensitization. Decreased VA, which decreases visual stimuli to the brain, can cause anterograde trans-synaptic degeneration. This has been reported in visual cortices of patients with retinal disease/damage [59]. Retrograde trans-synaptic degeneration has also been observed: when the brain cortex was damaged, the number of retinal ganglion cells was reduced in regions that projected nerve fibers to the damaged cortex [51–53, 60, 61]. Furthermore, decreased visual stimuli also can reduce functional connectivity between the visual cortex and somatosensory area and between the visual cortex and temporal cortex, which was seen in a study of subjects with recently acquired blindness [62].
Due to the retrospective and cross-sectional nature of the study, our data does not provide a causal relationship between decreased VA and thinner cortex. Decreased VA might have affected neurodegeneration in the brain, or decreased VA might have affected poor development in the visual processing area of the brain, or reversely, an inherently thinner visual cortex or dysmaturation of the visual cortex in the developmental phase might have caused decreased VA.
Limitations
Our study has several limitations. Firstly, due to the retrospective and cross-sectional nature of our study, we could not elucidate whether the association between visual impairment and thinner cortex had a causal relationship. The lack of serum or CSF biomarkers of neurodegeneration limits the interpretation of our results as to whether a thinner cortex was due to neurodegenerative change. A further longitudinal prospective study is needed to see whether decreased VA at baseline causes a thinner cortex over time or whether longitudinal VA change is related to a thinner cortex. Secondly, we could not completely exclude individuals who had ocular diseases such as glaucoma or macular degeneration. Although we acknowledge the association between neurodegeneration and ocular diseases, the prevalence of these diseases is relatively small in the general population [63, 64]. Thus, we suspect that the significant result of a thinner cortex in this study was mostly derived from the effect of low VA itself. Third, the number of the participants in bad VA group was much smaller than the fair or good VA group. This imbalance might have led to artifactual differences related to unknown confounders. Lastly, a multi-center study in various regions or ethnicity is required to see consistent results for generalization.
Conclusion
Our results suggest that a thinner cortex in the visual processing area of the brain is related to decreased visual stimuli in cognitively normal adults.
Supplementary Information
Additional file 1: Fig. 1. The distribution of visual acuity according to the age of the study participants. Grouped by VA in better-seeing eye: bad = VA ≤ 20/40, fair = 20/40 < VA ≤ 20/25, good = VA > 20/25 (VA was presented in Snellen system).
Additional file 2: Table S1. Subgroup analysis of the relationship between cortical thickness (x 10-1mm) and visual acuity (VA) groups divided by VA types.
Acknowledgements
G.H. and J.S.K. contributed equally as the first authors. H.J.K. and D.H.L. are the co-corresponding authors of this study.
Abbreviations
- VA
Visual acuity
- WM
White matter
- GM
Gray matter
- CSF
Cerebrospinal fluid
- ICV
Intracranial volume
- logMAR
Logarithm of the minimum angle of resolution
- MMSE
Mini-Mental State Examination
Authors’ contributions
DHL and HJK: study design and conduct. GH, JSK, and YHP: data analysis. GH and JSK: writing of the manuscript. GH, JSK, and YHP: statistical analysis. JSK, SHK, H-RK, SH, and HYS: acquisition of the data. T-YC, DLN, and SWS: interpretation of the findings. DLN, SWS, DHL, and HJK: critical review of the manuscript. The authors read and approved the final manuscript.
Funding
This research was supported by the MSIT(Ministry of Science and ICT), Korea, under the ICT Creative Consilience program(IITP-2021-2020-0-01821) supervised by the IITP(Institute for Information & communications Technology Planning & Evaluation); a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (HU21C0111, HI19C1132, HI19C0481, and HC19C0142); and a grant from National Research Foundation of Korea grant funded by the Korean government’s Ministry of Education (NRF-2021R1C1C1007795; Seoul, Korea). The funding body had no roles in this study.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding authors (DHL, HJK) on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board at Samsung Medical Center. All methods were conducted in accordance with the approved guidelines. The need for informed consent was waived by the Institutional Review Board at Samsung Medical Center, as we collected retrospective and de-identified data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gyule Han and Ji Sun Kim contributed equally as the first authors.
Contributor Information
Dong Hui Lim, Email: ldhlse@gmail.com.
Hee Jin Kim, Email: evekhj@gmail.com.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Marquie M, Castilla-Marti M, Valero S, Martinez J, Sanchez D, Hernandez I, et al. Visual impairment in aging and cognitive decline: experience in a memory clinic. Sci Rep. 2019;9(1):8698. doi: 10.1038/s41598-019-45055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee GI, Chi SA, Kim K, Seo SW, Kim HJ, Chung TY, et al. Visual impairment increases the risk of dementia, especially in young males in a 12-year longitudinal follow-up study of a national cohort. Sci Rep. 2021;11(1):11393. doi: 10.1038/s41598-021-91026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik JS, Ha M, Jung YH, Kim GH, Han KD, Kim HS, et al. Low vision and the risk of dementia: a nationwide population-based cohort study. Sci Rep. 2020;10(1):9109. doi: 10.1038/s41598-020-66002-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elyashiv SM, Shabtai EL, Belkin M. Correlation between visual acuity and cognitive functions. Br J Ophthalmol. 2014;98(1):129–132. doi: 10.1136/bjophthalmol-2013-304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha J, Cho YS, Kim SJ, Cho SH, Kim JP, Jung YH, et al. Hearing loss is associated with cortical thinning in cognitively normal older adults. Eur J Neurol. 2020;27(6):1003–1009. doi: 10.1111/ene.14195. [DOI] [PubMed] [Google Scholar]
- 7.Belkhiria C, Vergara RC, San Martin S, Leiva A, Marcenaro B, Martinez M, et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front Aging Neurosci. 2019;11:97. doi: 10.3389/fnagi.2019.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts L. The cerebral cortex and hearing. Ann Otol Rhinol Laryngol. 1960;69:830–848. doi: 10.1177/000348946006900312. [DOI] [PubMed] [Google Scholar]
- 9.Shiohama T, McDavid J, Levman J, Takahashi E. The left lateral occipital cortex exhibits decreased thickness in children with sensorineural hearing loss. Int J Dev Neurosci. 2019;76:34–40. doi: 10.1016/j.ijdevneu.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burge WK, Griffis JC, Nenert R, Elkhetali A, DeCarlo DK, ver Hoef LW, et al. Cortical thickness in human V1 associated with central vision loss. Sci Rep. 2016;6:23268. doi: 10.1038/srep23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanson RLW, Gale RP, Gouws AD, Airody A, Scott MTW, Akthar F, et al. Following the status of visual cortex over time in patients with macular degeneration reveals atrophy of visually deprived brain regions. Invest Ophthalmol Vis Sci. 2019;60(15):5045–5051. doi: 10.1167/iovs.18-25823. [DOI] [PubMed] [Google Scholar]
- 12.Prins D, Plank T, Baseler HA, Gouws AD, Beer A, Morland AB, et al. Surface-based analyses of anatomical properties of the visual cortex in macular degeneration. PLoS One. 2016;11(1):e0146684. doi: 10.1371/journal.pone.0146684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Lee JD, Kim EY, Park B, Oh MK, Lee S, et al. Morphological alterations in the congenital blind based on the analysis of cortical thickness and surface area. Neuroimage. 2009;47(1):98–106. doi: 10.1016/j.neuroimage.2009.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Kran BS, Lawrence L, Mayer DL, Heidary G. Cerebral/cortical visual impairment: a need to reassess current definitions of visual impairment and blindness. Semin Pediatr Neurol. 2019;31:25–29. doi: 10.1016/j.spen.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Philip SS, Dutton GN. Identifying and characterising cerebral visual impairment in children: a review. Clin Exp Optom. 2014;97(3):196–208. doi: 10.1111/cxo.12155. [DOI] [PubMed] [Google Scholar]
- 16.Haidar H, Soul JS. Measurement of cortical thickness in 3D brain MRI data: validation of the Laplacian method. J Neuroimaging. 2006;16(2):146–153. doi: 10.1111/j.1552-6569.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 17.Kang SH, Park YH, Lee D, Kim JP, Chin J, Ahn Y, et al. The cortical neuroanatomy related to specific neuropsychological deficits in Alzheimer’s continuum. Dement Neurocogn Disord. 2019;18(3):77–95. doi: 10.12779/dnd.2019.18.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephen R, Liu Y, Ngandu T, Antikainen R, Hulkkonen J, Koikkalainen J, et al. Brain volumes and cortical thickness on MRI in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) Alzheimers Res Ther. 2019;11(1):53. doi: 10.1186/s13195-019-0506-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanc F, Colloby SJ, Philippi N, de Petigny X, Jung B, Demuynck C, et al. Cortical thickness in dementia with Lewy bodies and Alzheimer’s disease: a comparison of prodromal and dementia stages. PLoS One. 2015;10(6):e0127396. doi: 10.1371/journal.pone.0127396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im K, Lee JM, Seo SW, Yoon U, Kim ST, Kim YH, et al. Variations in cortical thickness with dementia severity in Alzheimer’s disease. Neurosci Lett. 2008;436(2):227–231. doi: 10.1016/j.neulet.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Ye BS, Jeon S, Ham JH, Lee JJ, Lee JM, Lee HS, et al. Dementia-predicting cognitive risk score and its correlation with cortical thickness in Parkinson disease. Dement Geriatr Cogn Disord. 2017;44(3-4):203–212. doi: 10.1159/000479057. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50-89 years: a cross-sectional study. Lancet Neurol. 2014;13(10):997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park HY, Lim SY, Hwang JH, Choi JH, Koh WJ, Sung J, et al. Lung function, coronary artery calcification, and metabolic syndrome in 4905 Korean males. Respir Med. 2010;104(9):1326–1335. doi: 10.1016/j.rmed.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim CH, Seo SW, Kim GH, Shin JS, Cho H, Noh Y, et al. Cortical thinning in subcortical vascular dementia with negative 11C-PiB PET. J Alzheimers Dis. 2012;31(2):315–323. doi: 10.3233/JAD-2012-111832. [DOI] [PubMed] [Google Scholar]
- 25.Kim SE, Lee JS, Woo S, Kim S, Kim HJ, Park S, et al. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology. 2019;93(11):e1045–e1e57. doi: 10.1212/WNL.0000000000008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. doi: 10.1097/00004728-199403000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 28.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27(1):210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 29.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 31.Im K, Lee JM, Lee J, Shin YW, Kim IY, Kwon JS, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31(1):31–38. doi: 10.1016/j.neuroimage.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 32.Chan T, Friedman DS, Bradley C, Massof R. Estimates of incidence and prevalence of visual impairment, low vision, and blindness in the United States. JAMA Ophthalmol. 2018;136(1):12–19. doi: 10.1001/jamaophthalmol.2017.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z, Liao H, Wang W, Scheetz J, Zhang J, He M. Visual impairment and major eye diseases in chronic kidney disease: the National Health and Nutrition Examination Survey, 2005-2008. Am J Ophthalmol. 2020;213:24–33. doi: 10.1016/j.ajo.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Ying GS, Fu X, Zhang R, Meng J, Gu F, et al. Prevalence of myopia and vision impairment in school students in Eastern China. BMC Ophthalmol. 2020;20(1):2. doi: 10.1186/s12886-019-1281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malkin AG, Ross NC, Chan TL, Protosow K, Bittner AK. U.S. Optometrists’ reported practices and perceived barriers for low vision care for mild visual loss. Optom Vis Sci. 2020;97(1):45–51. doi: 10.1097/OPX.0000000000001468. [DOI] [PubMed] [Google Scholar]
- 36.Li T, Xu X, Xu Y, Jin P, Chen J, Shi Y, et al. PPARG polymorphisms are associated with unexplained mild vision loss in patients with type 2 diabetes mellitus. J Ophthalmol. 2019;2019:5284867. doi: 10.1155/2019/5284867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Shin HY, Kim HJ, Jang YK, Jung NY, Lee J, et al. Combined effects of physical exercise and education on age-related cortical thinning in cognitively normal individuals. Sci Rep. 2016;6:24284. doi: 10.1038/srep24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Haan EH, Cowey A. On the usefulness of ‘what’ and ‘where’ pathways in vision. Trends Cogn Sci. 2011;15(10):460–466. doi: 10.1016/j.tics.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Meyer SRA, De Jonghe JFM, Schmand B, Ponds R. Visual associations to retrieve episodic memory across healthy elderly, mild cognitive impairment, and patients with Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2019;26(3):447–462. doi: 10.1080/13825585.2018.1475002. [DOI] [PubMed] [Google Scholar]
- 40.Mine M, Miyata K, Morikawa M, Nishi T, Okamoto N, Kawasaki R, et al. Association of visual acuity and cognitive impairment in older individuals: Fujiwara-kyo Eye Study. Biores Open Access. 2016;5(1):228–234. doi: 10.1089/biores.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madan CR, Kensinger EA. Cortical complexity as a measure of age-related brain atrophy. Neuroimage. 2016;134:617–629. doi: 10.1016/j.neuroimage.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makino N, Maeda T, Oyama J, Higuchi Y. Role of the environmental factors on aging. Nihon Rinsho. 2009;67(7):1332–1336. [PubMed] [Google Scholar]
- 43.Manasatchakun P, Chotiga P, Hochwalder J, Roxberg A, Sandborgh M, Asp M. Factors associated with healthy aging among older persons in northeastern Thailand. J Cross Cult Gerontol. 2016;31(4):369–384. doi: 10.1007/s10823-016-9296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manolio TA. Study designs to enhance identification of genetic factors in healthy aging. Nutr Rev. 2007;65(12 Pt 2):S228–S233. doi: 10.1301/nr.2007.dec.S228-S233. [DOI] [PubMed] [Google Scholar]
- 45.McFall GP, McDermott KL, Dixon RA. Modifiable risk factors discriminate memory trajectories in non-demented aging: precision factors and targets for promoting healthier brain aging and preventing dementia. J Alzheimers Dis. 2019;70(s1):S101–SS18. doi: 10.3233/JAD-180571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer JS, Akiyama H, Mortel KF, Konno S, Margishvili GM. Human aging: risk factors for cerebral atrophy. Ann N Y Acad Sci. 1997;826:483–489. doi: 10.1111/j.1749-6632.1997.tb48509.x. [DOI] [PubMed] [Google Scholar]
- 47.Nagano-Saito A, Bellec P, Hanganu A, Jobert S, Mejia-Constain B, Degroot C, et al. Why is aging a risk factor for cognitive impairment in Parkinson’s disease?-a resting state fMRI study. Front Neurol. 2019;10:267. doi: 10.3389/fneur.2019.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravaglia G, Forti P, Maioli F, Montesi F, Rietti E, Pisacane N, et al. Risk factors for dementia: data from the Conselice study of brain aging. Arch Gerontol Geriatr. 2007;44(Suppl 1):311–320. doi: 10.1016/j.archger.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 49.Smith DB. Human factors and aging: an overview of research needs and application opportunities. Hum Factors. 1990;32(5):509–526. doi: 10.1177/001872089003200502. [DOI] [PubMed] [Google Scholar]
- 50.Gauthier AC, Liu J. Neurodegeneration and neuroprotection in glaucoma. Yale J Biol Med. 2016;89(1):73–79. [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita T, Miki A, Goto K, Araki S, Takizawa G, Ieki Y, et al. Retinal ganglion cell atrophy in homonymous hemianopia due to acquired occipital lesions observed using Cirrus high-definition-OCT. J Ophthalmol. 2016;2016:2394957. doi: 10.1155/2016/2394957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keller J, Sanchez-Dalmau BF, Villoslada P. Lesions in the posterior visual pathway promote trans-synaptic degeneration of retinal ganglion cells. PLoS One. 2014;9(5):e97444. doi: 10.1371/journal.pone.0097444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132(Pt 3):628–634. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- 54.Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22(4):465–481. doi: 10.1016/S1350-9462(03)00026-0. [DOI] [PubMed] [Google Scholar]
- 55.Rutland JW, Delman BN, Huang KH, et al. Primary visual cortical thickness in correlation with visual field defects in patients with pituitary macroadenomas: a structural 7-Tesla retinotopic analysis [published online ahead of print, 2019 Oct 18]. J Neurosurg. 2019:1-11. 10.3171/2019.7.JNS191712. [DOI] [PMC free article] [PubMed]
- 56.Vottonen P, Paakkonen A, Tarkka IM, Kaarniranta K. Best-corrected visual acuity and retinal thickness are associated with improved cortical visual processing in treated wet AMD patients. Acta Ophthalmol. 2015;93(7):621–625. doi: 10.1111/aos.12774. [DOI] [PubMed] [Google Scholar]
- 57.Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90(6):674–678. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Q, Song M, Xu J, Qin W, Yu C, Jiang T. Cortical thickness development of human primary visual cortex related to the age of blindness onset. Brain Imaging Behav. 2017;11(4):1029–1036. doi: 10.1007/s11682-016-9576-8. [DOI] [PubMed] [Google Scholar]
- 59.Dinkin M. Trans-synaptic retrograde degeneration in the human visual system: slow, silent, and real. Curr Neurol Neurosci Rep. 2017;17(2):16. doi: 10.1007/s11910-017-0725-2. [DOI] [PubMed] [Google Scholar]
- 60.Beatty RM, Sadun AA, Smith L, Vonsattel JP, Richardson EP., Jr Direct demonstration of transsynaptic degeneration in the human visual system: a comparison of retrograde and anterograde changes. J Neurol Neurosurg Psychiatry. 1982;45(2):143–146. doi: 10.1136/jnnp.45.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitchell JR, Oliveira C, Tsiouris AJ, Dinkin MJ. Corresponding ganglion cell atrophy in patients with postgeniculate homonymous visual field loss. J Neuroophthalmol. 2015;35(4):353–359. doi: 10.1097/WNO.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 62.Yu C, Liu Y, Li J, Zhou Y, Wang K, Tian L, et al. Altered functional connectivity of primary visual cortex in early blindness. Hum Brain Mapp. 2008;29(5):533–543. doi: 10.1002/hbm.20420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park SJ, Kwon KE, Choi NK, Park KH, Woo SJ. Prevalence and incidence of exudative age-related macular degeneration in South Korea: a nationwide population-based study. Ophthalmology. 2015;122(10):2063–70 e1. doi: 10.1016/j.ophtha.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 64.Seo SJ, Lee YH, Lee SY, Bae HW, Hong S, Seong GJ, et al. Estimated prevalence of glaucoma in South Korea using the National Claims Database. J Ophthalmol. 2016;2016:1690256. doi: 10.1155/2016/1690256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. 1. The distribution of visual acuity according to the age of the study participants. Grouped by VA in better-seeing eye: bad = VA ≤ 20/40, fair = 20/40 < VA ≤ 20/25, good = VA > 20/25 (VA was presented in Snellen system).
Additional file 2: Table S1. Subgroup analysis of the relationship between cortical thickness (x 10-1mm) and visual acuity (VA) groups divided by VA types.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding authors (DHL, HJK) on reasonable request.