Abstract
Osteoporosis is a common skeletal disease with marked bone loss, deterioration of the bone microstructure and bone fragility. An abnormal bone remodelling cycle with relatively increased bone resorption is the crucial pathophysiological mechanism. Bone remodelling is predominantly controlled by osteoblasts and osteoclasts, which are specialized cell types that are regulated by a variety of osteogenic and osteoclastic factors, including cytokines expressed within the bone microenvironment under local or systemic inflammatory conditions. Signal transducer and activator of transcription 3 (STAT3) plays a prominent role in the communication between cytokines and kinases by binding downstream gene promotors and is involved in a wide range of biological or pathological processes. Emerging evidence suggests that STAT3 and its network participate in bone remodelling and the development of osteoporosis, and this factor may be a potent target for osteoporosis treatment. This review focuses on the role and molecular mechanism of the STAT3 signalling pathway in osteogenesis, osteoclastogenesis and osteoporosis, particularly the bone-related cytokines that regulate the osteoblastic differentiation of bone marrow stromal cells and the osteoclastic differentiation of bone marrow macrophages by initiating STAT3 signalling. This review also examines the cellular interactions among immune cells, haematopoietic cells and osteoblastic/osteoclastic cells.
Video abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-022-00924-1.
Keywords: Osteoporosis, STAT3, Osteogenesis, Osteoclastogenesis
Background
As a serious public health problem, osteoporosis is a systemic bone metabolic disease characterized by reduced bone mass, low bone density and damaged bone microstructure and is accompanying an increased probability of fragility fractures [1, 2]. The incidence of osteoporosis is 70% in people aged over 80 years and 15% in people over 50 years [3]. At present, there are more than 200 million patients suffering from osteoporosis worldwide; as life expectancy increases globally, osteoporosis will lead to increased financial and medical burdens in most countries, and it is expected that the global cost of osteoporosis may increase to $131.5 billion by 2050 [4]. Fragility fractures caused by low-energy trauma, particularly hip fracture, are common complications of osteoporosis that result in poor health-related quality of life and a high risk of disability and mortality [5, 6]. Previous studies reported that more than 2 million hip fractures occurred worldwide in 2010, and 51% of these fractures could have been prevented if osteoporosis could be effectively treated [7]. The treatment for osteoporosis, thus far, mainly targets the balance between bone formation by osteoblasts and bone resorption by osteoclasts [8]. However, anti-resorption drugs such as bisphosphonates and the receptor activator of nuclear factor-κB ligand (RANKL) inhibitor denosumab or teriparatide, which is the only anabolic drug approved by the Food and Drug Administration (FDA), have or contribute to complicated side effects [9]. Thus, thoroughly clarifying the pathogenesis of osteoporosis, particularly the key molecules and underlying signalling pathways, and identifying new effective therapeutic targets for osteoporosis, is still challenging but important task.
The signal transducer and activator of transcription (STAT) family consists of seven proteins: STAT1, STAT2, STAT3, STAT4, STAT5α, STAT5β and STAT6 [10, 11]. These proteins are conserved among eukaryotes and are involved in a wide range of functions, such as cell differentiation, cell proliferation, the immune response, cell survival, apoptosis, and angiogenesis [12, 13]. Among this family of latent cytoplasmic protein members, STAT3 plays a prominent role in the communication between cytokines and kinases. Normal STAT3 signalling is tightly controlled during the standard cellular response. Suppressors of cytokine signalling (SOCS) proteins and protein tyrosine phosphatases (PTPs) provide negative feedback to the receptor and prevent continuous signalling by switching off the signalling cascade under physiological conditions [14]. Currently, the phosphorylation of STAT3 has been proven to be a crucial event in several specific signalling networks that regulate osteogenic and osteoclastic processes.
This review focuses on the role of STAT3 in regulating the balance of bone remodelling in osteoporosis, particularly the differentiation and function of osteoblasts and osteoclasts. We also discuss recent advances in how STAT3 links other molecules to regulate osteoblastic differentiation in bone marrow stromal cells (BMSCs) and osteoclastic differentiation in bone marrow macrophages (BMMs), thereby affecting bone remodelling, as well as the interactions among immune cells, haematopoietic cells and bone cells.
Pathological mechanism of osteoporosis
Postmenopausal osteoporosis and senile osteoporosis are two main pathophysiological processes that are related to significant bone loss [15, 16]. While there are various causes of osteoporosis [17], the uncoupling of the bone remodelling cycle and increased bone resorption relative to formation are common underlying pathophysiological mechanisms. Bone remodelling starts with the destruction of old bone by osteoclasts followed by the deposition of osteoids by osteoblasts within basic multicellular units (BMUs), which are composed of osteoclasts, osteoblasts and the capillary blood supply. Subsequently, the organic extracellular matrix is mineralized [16, 18]. This process, which occurs throughout life, may replace microdamage and ensure structural integrity. However, imbalanced bone remodelling with increased resorption relative to formation could lead to osteopenia or even osteoporosis [16, 19]. In this context, several factors have been shown to impact bone remodelling and reduce bone mass: (1) changes in the differentiation of mesenchymal stem cells with more adipocytes and fewer osteoblasts [20]; (2) vitamin D deficiency leading to secondary hyperparathyroidism, which in turn enhances osteoclastic bone resorption [21, 22]; (3) altered immune cell activity and elevated inflammatory cytokines such as IL-1, IL-6 and IL-11 [23]; and (4) a decline in oestrogen levels, which stimulates the expression of RANKL, which activates osteoclasts, especially in postmenopausal women [24]. Interestingly, under oestrogen-deficient conditions, the crosstalk between oestrogen and bone cell-produced cytokines tends to cause bone loss; for example, IL-6 stimulates bone resorption, whereas oestrogen blocks osteoblast synthesis of IL-6 and antagonizes the IL-6 receptor [25]. These factors contribute to bone loss and deterioration of the bone microarchitecture, while the underlying mechanism, particularly the cellular and molecular details of the interactions among these factors, remains unclear.
Structure and signalling cascades of STAT3
STAT3, which has a relative molecular weight of 92 kDa, consists of approximately 770 amino acids and includes six functional domains: the N-terminal domain, coiled-coil domain, DNA binding domain, C-terminal transactivation domain, linker domain, and Src homology 2 (SH2) domain. Each domain which plays a different role in signal transduction and the activation of gene transcription [26, 27]. The N-terminal domain is involved in STAT3 dimer nuclear translocation, synergistic DNA binding, protein–protein interactions to form various dimer complexes, and subsequent negative regulatory processes [27]. The coiled-coil domain is responsible for mediating the interaction of STAT3 with other proteins. The DNA binding domain recognizes specific DNA sequences and provides a binding interface between proteins and DNA to form STAT3-DNA complexes [13]. The linker domain is involved in interactions with the transcriptional system [11]. Because STAT3 is a regulatory module for intracellular signalling cascades, the SH2 domain is critical for STAT3 binding to kinase receptor residues and dimerization. The SH2 domain acts in a sequence-specific and strictly phosphorylation-dependent manner through highly affinity interactions with phosphorylated tyrosine-containing target peptides that are on the cytoplasmic side of cytokine receptors, resulting in subsequent tyrosine kinase phosphorylation of STAT3 [11]. Phosphorylated STAT3 can form homodimers and STAT1-STAT3 heterodimers through interactions between the phospho-tyrosine motif of one and the SH2 domain of the other [28]. The STAT3 SH2 domain contains three important solvent-accessible subpockets, which can be targeted by small-molecule inhibitors and are potential therapeutic targets [29, 30]. In addition, STAT3 has two phosphorylation sites: tyrosine 705 (Tyr705) and serine 727 (Ser727). The transcriptional role of STAT3 is canonically regulated by tyrosine phosphorylation [31]. Tyr705 phosphorylation is essential for p-STAT dimerization, which in turn mediates nuclear translocation, DNA binding, and target gene expression [12, 32]. The phosphorylation of Tyr705 is mainly mediated by receptor-associated JAK kinases, Src family kinases, or receptor type tyrosine kinases, and tyrosine-phosphorylated STAT3 forms a stable dimer by reciprocal interactions between the phospho-Tyr705 (pY705)-containing region and the SH2 domain of the other STAT3 molecule [33]. Ser727 phosphorylation has been considered to be a secondary event after Tyr705 phosphorylation. The C-terminal Ser727 is phosphorylated by multiple Ser/Thr kinases, and phospho-Ser727 (pS727) has been shown to enhance STAT3 transcriptional activity. Interestingly, in mitochondria, STAT3 requires pS727 but not pY705 to enhance electron respiratory chain activity and Ras-dependent tumorigenesis [34, 35] (Fig. 1).
Fig. 1.
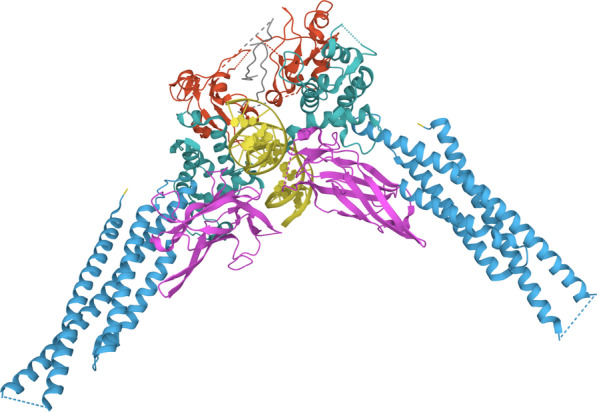
Crystal structure of a STAT3128–715, monomer as follows: blue CCD (138–320), magenta DBD (321–465), green LD (466–554), red SH2 (586–688), and partially TAD in grey (689–715). Missing residues are displayed as dash lines. The PDB entry is 6QHD
The canonical STAT3 signalling pathway involves JAKs/STAT3, including the glycoprotein (gp130) receptor (also called IL-6 Rβ), Janus kinases (JAKs) and STAT3. The signalling is triggered by the binding of IL-6 to the IL-6 receptor (IL-6R), which forms the IL-6/IL6R complex that activates a homodimer of gp130, which is the signal transducer for all cytokines in the IL-6 family [36, 37]. Following receptor dimerization, receptor-coupled JAKs are close to each other and activated by tyrosine phosphorylation. The activated JAKs phosphorylate multiple tyrosine residues on gp130 receptors and activate the STAT1/STAT3, phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT and Src homology region 2 (SH-2) domain-containing phosphatase 1 and 2 (SHP2)/renin-angiotensin system (RAS)/extracellular signal-regulated kinase (ERK) signalling cascade, as well as STAT3-mediated induction of the negative regulator SOCS3 [38]. The phosphorylated tyrosine residue, which is a docking site, recruits cytoplasmic STAT3 and binds inactive STAT3 through the SH2 domain. After binding to gp130, STAT3 is phosphorylated by JAK at Tyr705, resulting in STAT3 dimerization and an increase in nuclear concentrations. Subsequently, the activated dimer exposes a nuclear localization signal and translocates into the nucleus via import systems such as importin-a/importin-b1-Ran, after which it binds to the promoters of target genes to modulates their transcription. These target genes are involved in cell differentiation, proliferation and survival, as well as apoptosis, angiogenesis and inflammation [31, 39–42].
STAT3 function is tightly regulated by endogenous inhibitors such as SOCS3 and PTP proteins and inhibitors of activated STATs (PIASs). SOCS3, which belongs to the SOCS family, consists of an SH2 domain and a segment called the SOCS box near the C-terminus and can regulate signal transduction by inhibiting the components of the JAK/STAT3 pathway. The SH2 domain of SOCS3 binds to SHP-2-binding domains of the gp130 receptor, accessing the activation loop of JAK through its kinase inhibitor region domain to inhibit JAK activity [43]. Dephosphorylation of the tyrosine residue by PTPs is the main negative regulatory mechanism of STAT3. Currently, seven PTPs have been shown to dephosphorylate STAT3 and regulate STAT3 signalling: PTP receptor-type D, T and K (PTPRD, PTPRT, PTPRK), SHP1, SHP2, MEG2/PTP nonreceptor type 9 (PTPN9) and T-cell PTP (TC-PTP)/PTP nonreceptor type 2 (PTPN2) [44]. PTPRD, PTPRT, PTPRK and PTPN9 negatively regulate STAT3-mediated signalling by directly dephosphorylating STAT3 at Tyr705. SHP1 reduces STAT3 phosphorylation in coordination with SHP2 and TC-PTP [45, 46]. Furthermore, in response to cytokine stimulation, protein inhibitor of activated STAT3 (PIAS3) binds to activated STAT3 and prevents it from binding to DNA [47].
Thus, the structure-based function and modification of STAT3 is crucial, and its involvement in a series of biological and pathological processes has drawn increasing attention. Interestingly, STAT3 can play different or even opposite roles in various conditions, depending on cell type, developmental status and stimulus. The most prominent and well-studied role of STAT3 in cancer is as a proto-oncogene. Aberrant STAT3 activity has been detected in a variety of solid and haematological tumours [48, 49]. However, Grabner et al. demonstrated that STAT3 played a -suppressive role in V-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue (KRAS) mutant lung adenocarcinoma [50]. The dual roles of STAT3 have also been identified in glioblastoma, in which STAT3 exerts both tumour-enhancing and tumour-suppressive effects depending on the genomic mutational profile [51]. Notably, the expression of the STATS family has been identified in bone tissues, especially STAT3, which has been reported to be involved in cell proliferation, apoptosis and the motility of osteoblasts and osteoclasts, the crosstalk between which plays a crucial role in bone remodelling and related bone diseases.
STAT3 signalling pathway and osteogenesis
BMSCs may differentiate into osteogenic, adipogenic, myogenic, and chondrogenic lineages. The commitment of BMSCs to becoming osteoblasts is crucial for osteogenesis; when immature osteoblasts mature and become functional, they begin to embed in the bone as osteocytes and regulate matrix mineralization and bone quality [52]. The balance between osteoblast proliferation and apoptosis determines the number of active osteoblasts and thus affects the homeostasis of bone formation [53].
Crucial role of osteoblast lineage cell produced STAT3 in osteogenesis
Regarding the role of STAT3 in bone, a number of in vivo studies using cell-specific STAT3 transgenic mice were performed. Itoh et al. found a reduction in the bone formation rate in the osteoblast-specific STAT3-knockdown mice (α1(I)Cre/STAT3flox/−, heterozygous floxed STAT3 and expressing Cre under the α1(I) promoter), which indicates that STAT3 signalling is important for osteoblast function. Recently, Zhou et al. proved that ablation of STAT3 in BMSCs resulted in skeletal deformities, as osteoblast lineage-specific STAT3-knockout mice (Prx1Cre; STAT3fl/fl, homozygosis floxed STAT3 and expressing Cre under Prx1 promoter) were dwarfed relative to their control littermates, whereas in preosteoblast-specific STAT3-knockout mice (OsxCre; STAT3fl/fl, homozygosis floxed STAT3 and expressing Cre under the Osterix (Osx) promoter) exhibited an osteoporotic phenotype with reductions in bone volume/tissue volume (BV/TV), Tb.Th, Tb.N and cortical thickness (Ct.Th) but an increase in Tb.Sp [54] (Tables 1, 2).
Table 1.
Phenotypes of transgenic mice with cell-specific modified STAT3
| Cell | Transgenic strategy | Effect on STAT3 | Phenotypes | Reference |
|---|---|---|---|---|
| Osteoblast linage cells | α1(I)Cre/STAT3flox/− (10-week-old) | Disruption of STAT3 in osteoblast | Increased mineral apposition rate | [55] |
| Prx1 Cre; STAT3fl/+ mice (8-week-old) | Deletion of STAT3 in BMSCs | Dwarfed; defects and malformation in the skeleton of new-borns | [54] | |
| OsxCre; STAT3fl/+ (8-week-old) | Deletion of STAT3 in pre-osteoblast | Dwarfed; hypo mineralization | [54] | |
| Osteoclast linage cells | STAT3Ctsk (20-week-old) | Knockout STAT3 in osteoclast cells | Increased bone mass | [83] |
| STAT3Ctsk (8-week-old) | Knockout STAT3 in osteoclast cells | Lower BMD in female femurs | [84] | |
| Hematopoietic cells | Hematopoietic cell-specific disruption of the STAT3 | Tissue-specific STAT3 deletion in hematopoietic bone marrow cell lineages | Decreased bone mass and enhanced osteoclast function | [101] |
Table 2.
Phenotypes of osteoblast linage cells with modified STAT3 signaling pathway
| Origin | Signaling pathway | Regulating factors | Effect on STAT3 | Downstream target gene | Phenotypes | Reference |
|---|---|---|---|---|---|---|
| Mice BMSCs | JAK/STAT3 | LIF | Increased STAT3 phosphorylation | OCN, BSP, ALP, COLIαI, RUNX2, OSX | Suppressed osteogenic differentiation | [43] |
| SHP2KOBglap osteoblast cells | RUNX2/OSTERIX, SHP2/STAT3 | SHP2 | Increased STAT3 phosphorylation | RANKL | Inhibited cell maturation | [52] |
| gp130FXXQ/FXXQ mice osteoblast | YXXQ/STAT3-dependent | IL-6 | Decreased STAT3 phosphorylation | ALP | Decreased mineralization | [55] |
| gp130F759/F759 mice osteoblast | Y759/SHP-2-dependent negative regulatory | IL-6 | Increased STAT3 phosphorylation | ALP | Increased cell differentiation | [55] |
| Primary bone-derived cells | JAK2/STAT3 | IL-17A | Increased STAT3 phosphorylation | ALP | Increased cell differentiation and mineralized nodes | [56] |
| Human primary osteoblastic cells | JAK2/STAT3 | PC1-CT | Increased STAT3 phosphorylation; STAT3-DNA binding | RUNX2 | Increased cell differentiation | [60] |
| hFOB 1.19 | JAK2/STAT3 | EPO | Increased STAT3 phosphorylation | ALP, OCN, OPG, OPN | Stimulated osteoblast proliferation and differentiation | [70] |
| MC3T3-E1 cells | JAK3/STAT3 | NDRG2 | Increased STAT3 phosphorylation | RUNX2, OPG, OSX, ALP, OCN | Increased cell differentiation and | [62] |
| Osx::PKD1fl/fl calvarial preosteoblasts | STAT3 | PKD1 | Increased STAT3 phosphorylation | ALP, OSX, RUNX2, COL-α1 | Increased cell differentiation | [64] |
| Mice MSCs | STAT3 | HIF-1α | Increased STAT3 phosphorylation | COL1α1, RUNX2, ALP, OSX, OCN, VEGF | Increased cell differentiation and mineralization | [78] |
| MC3T3-E1 cells | SOCS3/STAT3 | CUEDC2 | Decreased STAT3 phosphorylation | ALP, RUNX2, OSX | Decreased cell differentiation | [65] |
| Prx1Cre; Leprfl/fl mice SSCs | JAK2/STAT3 | LepR | Decreased STAT3 phosphorylation | Blocked adipocyte differentiation; | [61] | |
| Human BMSCs | JAK1/STAT3 | RPN2 | Decreased STAT3 phosphorylation and nuclear location | OCN, OPN, RUNX2, BSP, ALP | Increased osteogenic differentiation and mineralized nodes | [67] |
| ΔTsc1 primary calvarial cells | STAT3/p63/Jagged/Notch | mTOR1 | Increased STAT3 phosphorylation | RUNX2 | Prevented osteoblast maturation and mineralization | [68] |
| MC3T3-E1 cells | JAK2/STAT3 | miR-135b | Decreased JAK2 STAT3 phosphorylation | ALP | Decreased cell viability, and mineralized nodes; increased cell apoptosis | [72] |
| Human MSCs | STAT3/miR-7-5p/CRY2 CLOCK, BMAL1/ P300 | Increased STAT3 phosphorylation | ALP, RUNX2, OCN, TYPE I COLLAGEN | Increased cell differentiation and mineralization | [74] | |
| MC3T3-E1 cells | STAT3 | miR-3074-5p, | Decreased STAT3 phosphorylation | XIAP, c-IAP2, SURVIVIN, | Promoted cell apoptosis | [73] |
| Human MSCs | JAK/STAT3 | miR‐224 | Decreased STAT3 phosphorylation | OCN, OPN, RUNX2, BSP, and ALP | Increased cell differentiation and mineralization | [77] |
| Human BMSCs | JAK/STAT3 | OSM | Increased STAT3 phosphorylation | ALP, RUNX2 | Increased cell differentiation and mineralized nodes | [109] |
| Vascular smooth muscle cells | STAT3/ Runx2 | IL-6/IL-6R | Increased STAT3 phosphorylation | RUNX2, ALP, OPN | Increased cell osteoblast-like differentiation and mineralization | [110] |
These cell-specific knockout or knockdown strategies showed that that STAT3 was necessary and essential for bone formation and development and had a somewhat more vital impact when the deficiency occurred in a more nascent linage. Thus, whether the deletion of STAT3 in mature osteoblasts using osteoblast-specific STAT3-knockout mice (OcnCre; STAT3fl/fl, homozygosis floxed STAT3 and expressing Cre under the Osteocalcin (Ocn) promoter) would also affect bone formation or cause skeletal deformities is still unknown.
Proteins regulating osteogenesis via STAT3 activation
To uncover the role of STAT3 in osteogenesis with or without specific stimulation, increasing numbers of in vivo and in vitro studies have been performed. Although most studies have shown that many proteins contribute to osteogenic differentiation and promote osteogenesis by activating STAT3, some proteins have been shown to stimulate osteogenesis by inhibiting the activation of STAT3. Otherwise, p-STAT3 could regulate downstream genes and signalling pathways, subsequently suppressing osteoblastic differentiation and osteogenesis. We reviewed the cell-specific effects and mechanisms with respect to the role of STAT3 in osteogenesis.
Cytokines
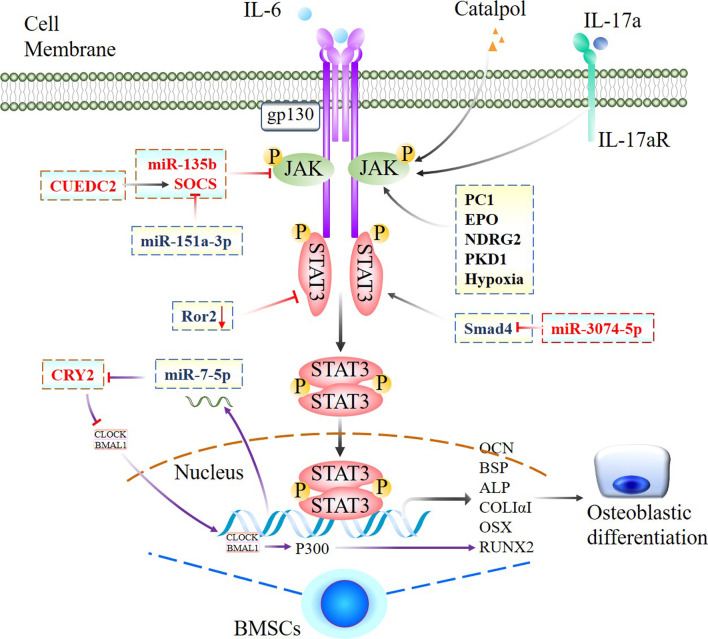
IL-6 family cytokines act on osteoblasts and induce the differentiation of osteoblasts or osteoclasts by binding to the gp130 receptor, which is involved in multiple signalling pathways, such as YXXQ-dependent STAT3 signalling and Y759-dependent signalling [55]. Compared with the gp130WT/WT mice, mutant gp130 knock-in (mice gp130F759/F759), which lack the Y759/SHP-2-dependent negative regulatory signal, exhibited an osteosclerotic phenotype with higher bone volume (BV), trabecular number (Tb.N), and trabecular thickness (Tb.Th) and lower trabecular separation (Tb.Sp). Conversely, the activities of alkaline phosphatase (ALP) and mineralization induced by IL-6 family cytokines were significantly decreased in mutant gp130 knock-in mice (gp130FXXQ/FXXQ), in which the YXXQ/STAT3-dependent signal was selectively disrupted. These results revealed that STAT3 signal activation by gp130 positively regulates osteoblastic differentiation to promote bone formation [55]. IL-17A, which is the most abundant cytokine in ankylosing spondylitis sera and synovial fluid, enhanced osteoblast activity by increasing phosphorylated JAK2 (p-JAK2), total JAK2, and phospho-STAT3 (Tyr705) signalling. The addition of exogenous IL-17A to primary bone-derived cells (BdCs) promoted the osteogenic stimulus-induced increase in ALP activity and mineralization. Blocking IL-17A attenuated ALP activity and mineralization in BdCs by inhibiting JAK2 phosphorylation and downregulating the expression osteoblast-associated genes [56] (Tables 1, 2; Fig. 2).
Fig. 2.
Jak2/STAT3 signal network positively regulates osteogenic differentiation. Proposed model of the role of JAK/STAT3 mediated network that positively regulating osteogenic differentiation of osteoblast precursors. A series of cytokines, molecules including some miRNAs interacted with JAK/STAT3 axis to regulate downstream effectors take account for osteogenic differentiation, all marked with event-specific colored line or arrows. Those delineated in a rectangle with dotted line indicate functional similarity. Ending with red bars indicate inhibition or downregulation, arrows indicate positive stimulation or upregulation
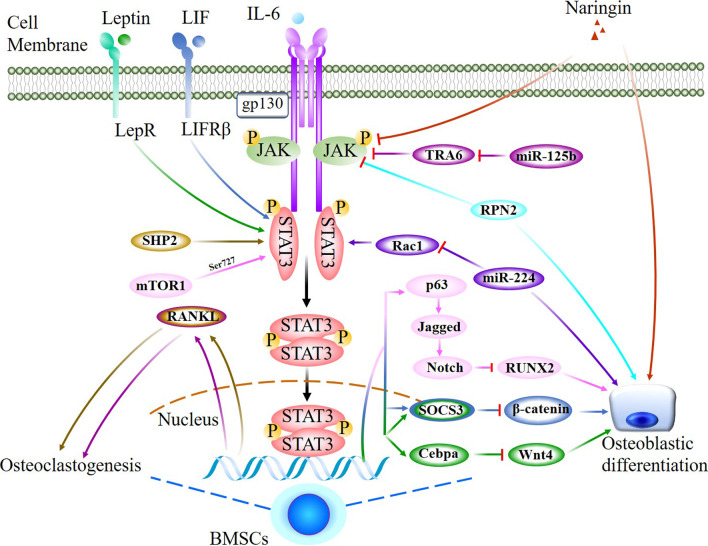
Leukaemia inhibitory factor (LIF) is a pleiotropic cytokine that belongs to the IL-6 family and has a suppressive effect on osteoblast differentiation [57]. By binding to the receptors gp130 and LIFRꞵ, LIF activates the JAK3/STAT3 signalling pathway, promotes the expression of SOCS3 and induces the interaction of SOCS3 and ꞵ-catenin, reducing ꞵ-catenin protein levels and suppressing osteoblastic differentiation [43]. Similarly, an in vitro study demonstrated that LIF was implicated in the high glucose (HG)-mediated inhibition of osteoblast differentiation in human osteosarcoma MG-63 cells by promoting STAT3/SOCS3 signalling, as the downregulation of osteogenic differentiation markers by LIF was restored by a STAT3 inhibitor [58] (Table 2; Fig. 3).
Fig. 3.
Jak2/STAT3 signal network negatively regulates osteogenic differentiation. JAK/STAT3 mediated signaling pathway and interlinked network that negatively regulation of osteogenesis via regulating activations of JAK and/or STAT3, a complicated and stimulator-specific role of STAT3 were indicated and marked with event-specific colored line or arrows. Ending with red bars indicate inhibition or downregulation, arrows indicate positive stimulation or upregulation
Transmembrane proteins
The ability of BMSCs to differentiate into osteoblasts requires Runt-related transcription factor 2 (Runx2) expression, which has been proven to be regulated by STAT3. P-STAT3 translocates to the nucleus, where it binds to the Runx2 promoter and enhances transcription, thereby triggering osteoblastic differentiation in BMSCs [59, 60]. In human osteoblastic cells, Polycystin-1 (PC1) is a transmembrane mechanosensor that senses extracellular mechanical cues and generating a response through cleavage of its C-terminal cytoplasmic tail (CT). The PC1-CT in turn triggers JAK2/STAT3 activation and promotes Runx2 transcription, inducing bone formation [60] (Table 2; Fig. 2).
However, as a nutritional/adiposity sensor, Leptin receptor (LepR) acts on mesenchymal stromal cells in the bone marrow to increase adipogenesis and reduce osteogenesis by enhancing the activation of JAK2/STAT3 signalling. Two weeks after bone fracture, micro-CT analysis showed significantly increased trabecular BV, Tb.N, and Tb.Th and reduced Tb.Sp in osteoblast lineage-specific Lepr-knockout mice (Prx1-Cre; Leprfl/fl; homozygosis floxed STAT3 and expressing Cre under the Prx1 promoter). Femurs from osteoblast lineage-specific JAK2-activated mice (Prx1-Cre; JAK2V617F; JAK2 activated and expressing Cre under the Prx1 promoter) exhibited a significant reduction in femur length and trabecular BV. In vitro, a dramatic reduction in phospho-STAT3 (Tyr705) levels was observed in PDGFRα+CD45−Ter119−CD31− skeletal stem cells (SSCs) from Prx1-Cre; Leprfl/fl mice compared to control mice [61] (Tables 1, 2; Fig. 3).
Cytoplasmic proteins
N-myc downstream regulator gene 2 (NDRG2) is involved in cell proliferation, differentiation and hormone response. Chen et al. studied the role of NDRG2 and found that NDRG2 overexpression promoted bone morphogenetic protein 2 (BMP2)-induced osteoblastic differentiation by increasing the expression of Runx2, OPG, OSX and OCN through the JAK3/STAT3 signalling pathway [62]. The protein kinase D (PKD) family of serine/threonine kinases has been implicated in the regulation of bone biology. Heterozygous animals with attenuated PKD1 activity (PRKD1± mice) exhibit decreased bone mass and impaired osteoblast function during pubertal growth [63]. Furthermore, compared with littermate controls, preosteoblast-specific PKD1-knockout mice (OsxCre; PKD1fl/fl; homozygosis floxed PKD1 and expressing Cre under the Osx promoter) showed significantly lower bone mass and deterioration of the microarchitecture. Mechanistically, silencing endogenous PKD1 reduced osteoblast markers, such as Runx2, OSX, OPN and OCN, in a preosteoblast cell line (MC3T3-E1 cells), impaired osteoblast differentiation and decreased the phosphorylation of STAT3 (Ser727) and p38, whereas the overexpression of PKD1 upregulated the expression of osteoblastic markers and STAT3 and p38 phosphorylation [64]. JAK/STAT3 signalling is negatively regulated by SOCS3, as mentioned previously. CUE domain-containing 2 (CUEDC2), which is involved in ubiquitin-mediated protein degradation, binds to SOCS3 and reduces the ubiquitination of SOCS3. CUEDC2 has been shown to inhibit osteoblast differentiation and bone formation by inhibiting STAT3 activation through interactions with SOCS3. Knockdown of CUEDC2 increased the phosphorylation of STAT3 in the cytosol of MC3T3-E1 cells. Conversely, treatment with Stattic, an inhibitor of STAT3, significantly inhibited Ad-shCUEDC2-induced osteoblast differentiation in a dose-dependent manner [65]. The receptor tyrosine kinase-like orphan receptor 2 (Ror2) promoter region contains a GAS motif, which is thought to be a cis element for STATs. An experiment confirmed that Ror2 deficiency impaired osteogenesis in mouse BMSCs by deactivating STAT3, but whether Ror2 and STAT3 interact directly during mouse BMSC osteogenesis remains unclear [66] (Tables 1, 2; Fig. 2).
However, the highly conserved glycoprotein Ribophorin II (RPN2) is present in the rough endoplasmic reticulum membrane and participates in multiple biological reactions. RPN2 overexpression promoted hBMSCs osteogenic differentiation and markedly reduced the expression of JAK1 and p-STAT3 by inducing JAK1 ubiquitination. Silencing JAK1 could rescue the decreased expression of osteogenic genes caused by reducing RPN2, indicating an inhibitory effect of JAK1/STAT3 on osteogenic differentiation [67]. Mechanistic target of rapamycin complex 1 (mTOR1) regulates cell growth and metabolism by integrating both intracellular and extracellular signals. Preosteoblast-specific Tsc1-knockout mice (Osx-GFP::CreTG/+; Tsc1flox/flox (ΔTsc1); homozygosis floxed Tsc1 and expressing the GFP-Cre fusion protein under the Osx1 promoter) showed marked increases in bone mass in distal regions of the femur, and the BV/TV, Tb.N, Tb.Th increased by 240%, 140% and 170%, respectively, while the Tb.Sp decreased by 50%. In vitro, mTOR1 induced the phosphorylation of STAT3 at Ser727, and phosphorylated STAT3 bound to the p63 promoter in osteoblasts. P63 is a positive regulator of Jagged expression and Notch activity. mTORC1 inhibits osteoblast maturation by activating the STAT3/p63/Jagged/Notch pathway and downregulating Runx2 [68]. SHP2 is a ubiquitously expressed protein tyrosine phosphatase (PTPase) that plays an essential role in osteogenesis and bone mineral homeostasis. BV/TV, connectivity density, and Tb.N. were markedly reduced in the proximal tibiae of 10-week-old osteoblast-specific SHP2 knockout-mice (SHP2KOBglap mice; homozygosis floxed SHP2 and expressing Cre under the Bglap promoter) compared with age- and sex-matched SHP2CTRBglap littermate controls, while the structure model index, Tb.Sp. and bone marrow cavity were significantly increased. SHP2 deficiency promoted RANKL production in osteoblasts and osteocytes by promoting STAT3 Tyr705 phosphorylation, followed by local osteoclastogenesis and mineral resorption [52] (Tables 1, 2; Fig. 3).
Drugs that regulate osteogenesis via STAT3 activation
Following the development of osteoporosis treatments, the use of traditional Chinese medicine to treat osteoporosis has attracted extensive attention in recent years. A number of traditional Chinese medicines inhibit osteoporosis through the JAK2/STAT3 pathway. Catalpol is a natural iridoid glycoside that is mainly enriched in the dried root of Rehmanniae and possesses a variety of biological activities, such as anti-ischaemic, antioxidative and anti-inflammatory effects. Catalpol facilitated bone regeneration and vessel formation in a calvarial defect rat model with OVX-induced osteoporosis and promoted the osteogenic ability of BMSCs and BMSC-dependent angiogenesis through by activating the JAK2/STAT3 axis; these benefits could be partially abolished by knocking down STAT3 [69]. Erythropoietin (EPO) is a glycoprotein that can promote fracture healing. Recent studies focused on the mechanisms of this effect and showed that EPO treatment enhanced the levels of p-JAK2 and p-STAT3, ALP activity, OCN secretion, osteoprotegerin (OPG) and osteopontin (OPN) expression but inhibited RANKL expression in osteoblasts. These effects were abrogated by a JAK2/STAT3 inhibitor (AG490), which indicated that EPO significantly stimulated osteoblast proliferation and differentiation via the JAK2/STAT3 pathway [70]. Naringin, which is the main active ingredient of Chinese herbal medicines, facilitates BMSC proliferation and osteogenic differentiation by inhibiting the JAK2/STAT3 pathway and alleviates bone loss in an ovariectomy (OVX)-induced postmenopausal osteoporosis (PMOP) rat model [71]. Thus far, studies have focused on the role of STAT3 in bone development and bone homeostasis, particularly in response certain stimuli, and a potential treatment strategy or agent showed inconsistent results in terms of the activation of STAT3 and the effect on bone or osteoblast lineage cells, which indicates a nonspecific role of STAT3 in osteogenesis. However, most of these studies demonstrated a parallel association between STAT3 activation and osteogenesis (Table 2; Figs. 2, 3).
MicroRNAs that regulate osteogenesis via STAT3
MicroRNAs (miRNAs) have been indicated to play an important role in the posttranscriptional regulation of gene expression via translational repression of target mRNAs. An increasing number of miRNAs have been shown to regulate bone homeostasis, and some target STAT3 or its upstream activator JAK2.
JAK2 is an important activator of the STAT3 signalling pathway and has been confirmed as a downstream target gene of miR-135b. With increasing levels of miR-135b, JAK2, p-JAK2 and p-STAT3 levels decreased. MiR-135b inhibition in MC3T3-E1 cells promoted the expression of osteogenesis-related genes and osteoblastic differentiation but reduced apoptosis by upregulating JAK2 expression to activate the JAK2/STAT3 signalling pathway [72]. In apoptotic MC3T3-E1 cells under iron overload conditions, the expression of miR-3074-5p was upregulated. The results of the dual-luciferase reporter assay showed that overexpression of miR-3074-5p caused apoptosis by regulating its downstream target gene Smad4, which blocked the phosphorylation of AKT, ERK and STAT3 [73]. In addition, p-STAT3 also regulates miRNA levels. For example, p-STAT3 directly regulates miR-7-5p expression, which targets cryptochrome circadian regulator 2 (CRY2). Reduced expression of CRY2 stimulated the transcription of P300 by releasing the circadian locomotor output cycles kaput/brain and muscle ARNT-like 1 complex. P300 subsequently promoted the acetylation of histone 3 and the formation of a transcriptional complex with Runx2 to enhance osteogenesis [74] (Table 2; Fig. 2).
However, recent studies have demonstrated that the activation of JAK2/STAT3 signalling is increased by some miRNAs regulation in osteoporosis samples; for example, miR-151a-3p and miR-125b. MiR-151a-3p upregulation significantly decreased the bone mineral density and biomechanical parameters of femurs in OVX rats by targeting SOCS5, and the JAK2/STAT3 pathway is a downstream target of the miR-151a-3p-SOCS5 axis [75]. In vitro studies suggested that miR-125b enhanced the RANKL/OPG ratio and suppressed the levels of BMP2 and Runx2 and cell viability. In a rat OVX model, miR-125b activated the JAK2/STAT3 pathway by altering the downstream target gene TRAF6, thereby reducing bone mineral density and bone biomechanical parameters [76]. Furthermore, miR‐224 may serve as a positive mediator of osteogenic differentiation by deactivating the JAK/STAT3 and Wnt/β-catenin pathways by targeting Rac1 [77] (Table 2; Fig. 3).
In addition to the abovementioned miRNAs that directly or indirectly target STAT3, other studies focusing on cartilage or nonskeletal tissue indicate a more complicated mechanism and network that STAT3 mediates during the development of a series of diseases. It is reasonable to propose that at least some of the miRNAs that affect STAT3 in other tissues are also involved in bone diseases.
Physiological environment
The duration of hypoxia is crucial for osteogenic differentiation of precursor cells. CoCl2-induced hypoxia promoted bone defect healing and upregulated HIF-1α, p-STAT3 and ALP protein expression in the bone defect area, and a STAT3 inhibitor reversed these effects. In vitro, inhibiting STAT3 signalling reduced the hypoxia-induced osteogenic differentiation of BMSCs. Moreover, hypoxia upregulated STAT3 phosphorylation and VEGF expression in MSCs. These results suggest that STAT3 activation is essential for hypoxia-induced osteogenic differentiation in precursor cells and bone defect healing [78] (Table 2; Fig. 2).
STAT3 signalling pathway and osteoclastogenesis
Bone resorption is dominated by osteoclasts, and these cells attach to the bone surface during the initial stage of bone remodelling in both physiological and pathological conditions. Osteoclastogenesis is a complex process that includes the regulation of various cytokines and the cell–cell fusion of osteoclast precursors. RANKL and macrophage-colony stimulating factor (M-CSF) are indispensable cytokines for osteoclast precursor differentiation and fusion into functional multinucleated giant cells [79]. When RANKL binds to its receptor RANK, osteoclast precursors known as tartrate-resistant acid phosphatase (TRAP)-mononuclear macrophages recruit tumour necrosis factor (TNF) receptor-associated factor 6 (TRAF6). Subsequently, the NF-κB and mitogen-activated protein kinase (MAPK) signalling pathways are activated and further promote the transcription factors nuclear factor of activated T cells (NFATc1) and c-Fos [80]. Thus, the expression of downstream osteoclastic marker genes such as tartrate-resistant acid phosphatase (TRAP), cathepsin K (CTSK), osteoclast-associated receptor (OSCAR), and matrix metalloproteinase-9 (MMP-9) are upregulated and result in osteoclast differentiation and maturation [81, 82]. An increasing number of studies have recently focused on the involvement of other signalling molecules in osteoclastogenesis, particularly in the regulation of osteoclast precursor differentiation and function, including JAK-STAT signalling.
Dual roles of osteoclast-derived STAT3 in osteoclastogenesis and osteogenesis
Yang et al. investigated the role of STAT3 in osteoclasts in vivo with conditional knockout mice. Analysis of the quantitative microarchitectural parameters of femora from 20-week-old male and female osteoclast-specific STAT3 knockout mice (STAT3Ctsk; homozygosis floxed STAT3 and expressing Cre under the Ctsk promoter) showed markedly higher bone mass than their STAT3fl/fl littermates. Far fewer TRAP-positive multinuclear osteoclasts surrounded the trabecular bone of STAT3Ctsk mice than in littermate mice, indicating that the increase in bone mass in STAT3Ctsk mice was mainly due to a direct decrease in bone resorption. The downregulation of osteoclast-specific marker genes, such as OSCAR and dendritic cell-specific transmembrane protein (Dc Stamp), in BMMs from STAT3Ctsk mice suggested that the deletion of STAT3 disrupted osteoclast differentiation, whereas the overexpression of NFATc1 in STAT3-deficient BMMs could rescue the impaired osteoclast differentiation. Mechanistically, STAT3 could directly bind to the promotor of NFATc1 to drive its transcription and osteoclast differentiation [83] (Table 1).
However, another study used mice with conditional knockout of STAT3 in osteoclasts and showed opposite results in terms of bone mass; 8-week-old STAT3-cKO female femurs exhibited significantly lower BMD than those of littermate control females, and there was a lower bone formation rate. Microcomputed tomography (μCT) analysis showed that both male and female STAT3-cKO mice had significantly decreased trabecular bone mass [84]. When STAT3 was knocked down in monocyte/macrophage-like cells (RAW264.7 cells), the expression of early oestrogen-induced gene 1 (EE1G1), NF-kB inhibitor epsilon (NFkBie), and NFATc1 and CTSK signalling were increased, suggesting that STAT3 may regulate osteoclast differentiation and activity through CTSK and its interaction with oestrogen signalling pathways [84].
These results indicate that knocking out STAT3 in osteoclast precursors not only impacts osteoclastic differentiation but also affects osteoblast function, possibly via feedback. In this context, the inconsistent results in bone mass in these two studies, as the authors stated, might be caused by the differences in the genetic backgrounds of the mice and the genotypes of the control mice. However, one major difference that should not be ignored is that the animals in the later study were much younger (8 weeks old) and still in a rapid growth period, during which osteoblast activity plays a more predominant role than that of osteoclasts, while in 20-week-old mice with stable bone remodelling, the function of osteoclasts was more activated than that in the young mice, which may lead to relatively different responses in osteoblasts and osteoclasts to STAT3-cKO; that is, there are more sensitive osteoblasts in younger mice but relatively more sensitive osteoclasts in adult mice to the osteoclast-specific deficiency of STAT3.
Proteins that regulate osteoclastogenesis via STAT3
Cytokines
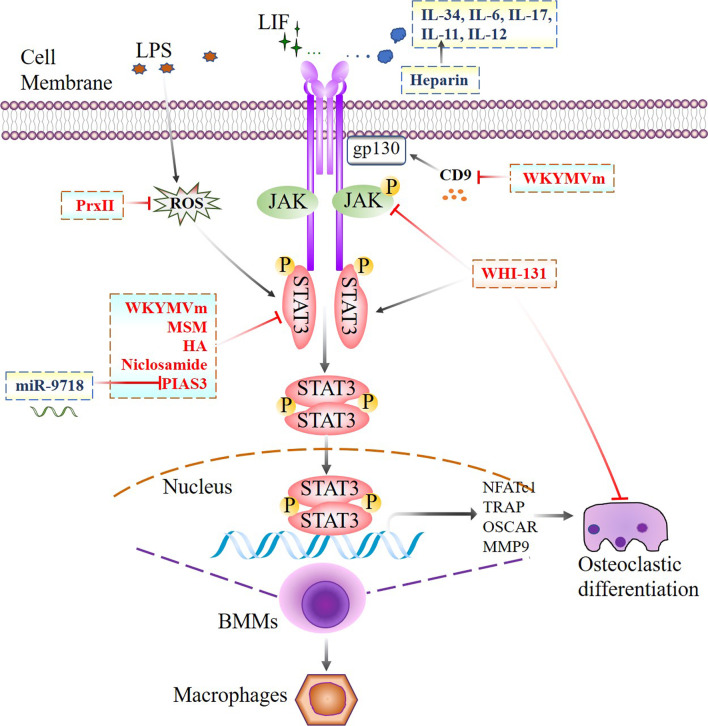
Previous studies proved that IL-6 induced osteoclastogenesis mainly due to the production of RANKL by osteoblastic cells, which in turn stimulated the differentiation of osteoclast precursors into osteoclasts, thus inducing their maturation and functions. Duplomb et al. demonstrated that IL-6 directly inhibited RANKL-induced osteoclastogenesis by inducing macrophage differentiation. A basal level of p-STAT3 on Ser727 was associated with osteoclastogenesis, and a decrease in Ser727 phosphorylation inhibited osteoclast differentiation. However, Tyr705 phosphorylation prevailed in response to IL-6 stimulation, while Ser727 phosphorylation caused the formation of macrophages instead of osteoclasts [85]. As a novel cytokine involved in bone-related diseases, IL-34 significantly maintained the survival of BMMs and increased the expression of osteoclast-related genes. In addition, higher p-STAT3 levels were observed in BMMs stimulated with IL-34 and RANKL than in those stimulated with RANKL alone, and inhibiting p-STAT3 with AG490 protected mothers against decapentaplegic homologue (Smad7) expression, suggesting that the IL-34/STAT3/Smad7 pathway is critical for BMM proliferation and osteoclastic differentiation [86] (Table 3; Fig. 4).
Table 3.
Phenotypes of osteoclastic lineage cells with modified STAT3 signaling pathway
| Origin | Signaling pathway | Regulating factors | Effect on STAT3 (phosphorylation sites) | Downstream target gene | Phenotypes | Reference |
|---|---|---|---|---|---|---|
| RAW264.7cells and mice BMMs | STAT3/ RANKL | MSM | Decreased STAT3 phosphorylation at Ser727 | NFATc1, TRAP, OSCAR, MMP9 | Decreased cell viability and TRAP-positive multinucleated cells | [81] |
| RAW264.7cells and mice BMMs | STAT3, CD9/gp130/STAT3 | WKYMVm | Decreased STAT3 phosphorylation at Ser727 | NFATc1, c-Fos, MMP9, CTSK | Decreased TRAP-positive multinucleated cells | [82] |
| ST2 cells and mice BMMs | STAT3 | HA | Decreased STAT3 phosphorylation | RANKL | Decreased TRAP-positive multinucleated cells | [97] |
| RAW264.7cells and mice BMMs | IL-34/STAT3/SMAD7 | IL-34 | Increased STAT3 phosphorylation | SMAD7 | Increased TRAP-positive multinucleated cells | [86] |
| Mice bone marrow cells | STAT3 | Heparin | Increased STAT3-DNA binding and transactivation at Ser727 | gp130, RANKL | Increased TRAP-positive multinucleated cells | [93] |
| RAW264.7cells and BMMs | STAT3 | PIAS3 | Decreased DNA binding activity of STAT3 | c-Fos, NFATc1, OSCAR, RANKL | Decreased TRAP-positive multinucleated cells | [90, 91] |
| Mice BMMs | STAT3 | Niclosamide | Decreased STAT3 phosphorylation at Ser727 | c-Fos, NFATc1, TRAP, OSCAR, av/b3 integrin, CTSK | Decreased TRAP-positive multinucleated cells | [95] |
| Mice BMMs | JAK3/STAT3 | WHI-131 | Increased STAT3 phosphorylation at Ser727 | c-Fos, NFATc1 | Decreased TRAP-positive multinucleated cells | [98] |
| Mice BMMs | STAT3 | LIF | Increased STAT3 phosphorylation | CTSK, NFATc1, TRAP, Atp6a3, c-Fos | Increased TRAP-positive multinucleated cells | [108] |
Fig. 4.
Jak2/STAT3 signal network regulates osteoclastic differentiation. JAK/STAT3 signaling pathway mediated network in regulating osteoclastic differentiation of BMMs with either some members of interleukins or other agents, marked with event-specific colored line or arrows. Those delineated in a rectangle with dotted line indicate functional similarity. Ending with red bars indicate inhibition or downregulation, arrows indicate positive stimulation or upregulation
Cytoplasmic proteins
Peroxiredoxin II (PrxII) is a prime candidate for cell surface receptor-initiated regulation of H2O2 signalling [87]. In vitro, it has been confirmed that lipopolysaccharide (LPS) phosphorylates STAT3 at Tyr705 or Ser727 via reactive oxygen species (ROS), IL-1β, IL-6, and NO production induces osteoclast formation, and this osteoclastogenesis could be inhibited by PrxII. In vivo, under LPS stimulation, PrxII−/− mice showed significantly reduced BV/TV, Tb.Th and Tb.N. PrxII inhibits ROS production and regulates LPS-induced osteoclast formation and bone loss via the STAT3 pathway [88]. PIAS3 binds to STAT3 and blocks DNA binding of STAT3, thereby inhibiting STAT3-mediated gene activation [89]. Transgenic mice overexpressing PIAS3 showed significant increases in BV/TV, Tb.N, Tb.Th and reductions in Tb.Sp. In osteoclast precursors, PIAS3 suppressed RANKL-induced osteoclastogenesis by inhibiting the expression of c-Fos, NFATc1 and OSCAR. In osteoblasts, PIAS3 inhibits the IL-6 cytokine family-gp130-STAT3 signalling, attenuating RANKL expression and inhibiting osteoclastogenesis [90, 91] (Table 3; Fig. 4).
Peptides that regulate osteoclastogenesis via STAT3
Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMVm) is a synthetic peptide selected from peptide libraries that negatively regulates RANKL- and LPS-induced osteoclastogenesis by directly inhibiting STAT3 phosphorylation at Ser727 and indirectly inhibiting CD9/gp130/STAT3 activity [82]. In vivo, WKYMVm partially rescued LPS-induced bone loss in mice (Table 3; Fig. 4).
Drugs that regulate osteoclastogenesis via STAT3
Methylsulfonylmethane (MSM) is a naturally occurring sulfur compound with excellent antioxidant and anti-inflammatory properties. STAT3 plays a key role in RANKL-induced osteoclast formation, and MSM attenuates RANKL-induced osteoclastogenesis by blocking the activity of NF-κB and the phosphorylation of STAT3 Ser727 [81]. Long-term heparin exposure is associated with a reduction in bone density [92]. Using an animal model of heparin-induced osteoporosis, Walton et al. found that heparin enhanced both IL-11-induced STAT3-DNA complex formation and transactivation independent of tyrosine or serine phosphorylation of STAT3, leading to the expression of RANKL and gp130, which stimulated osteoclastogenesis and bone loss [93]. Niclosamide (5-chloro-salicyl-(2-chloro-4-nitro) anilide) is a potent inhibitor of STAT3 [94]. In vivo, niclosamide significantly inhibited LPS-induced bone loss in a mouse model. In vitro, niclosamide inhibited RANKL-induced osteoclast differentiation by inhibiting serine-threonine protein kinase phosphorylation, inhibitor of nuclear factor-kappaB (IκB) activation, and STAT3 Ser727 [95]. Hyaluronic acid (HA) is a ubiquitously expressed glycosaminoglycan in the extracellular matrix [96]. HA degradation stimulates the osteoclastogenic potential of osteoclast-supporting cells (ST2 cells) by upregulating RANKL expression and enhancing the expression of vitamin D receptor (VDR) and STAT3 phosphorylation, while stimulating HA attenuated ST2 cell differentiation. These results showed that the accumulation of HA in bone marrow cells may affect RANKL-mediated osteoclast-supporting activity by regulating the VDR and STAT3 signalling pathways [97] (Table 3; Fig. 4).
The small molecule WHI-131, which increases RANKL-induced phosphorylation of STAT3 (Ser727) by inhibiting the expression of JAK3, negatively regulates osteoclast differentiation induced by IL-1, IL-6 or RANKL. Bone histomorphometric analyses showed that WHI-131 treatment alleviated LPS-induced bone loss and notably prevented bone destruction [98] (Table 3; Fig. 4).
Thus, despite a few discrepant data from different groups, most studies confirm a positive association between STAT3 activity and osteoclast function (Fig. 4; Table 3).
MicroRNAs that regulate osteoclastogenesis via STAT3
Liu et al. identified miR-9718 in primary mouse osteoclasts that promoted osteoclast differentiation by repressing PIAS3 at the posttranscriptional level. In vivo, silencing miR-9718 using a specific antagomir inhibited bone resorption and increased bone mass in OVX mice, indicating that miR-9718 played an important role in osteoclast differentiation by targeting PIAS3 both in vitro and in vivo [99] (Table 3; Fig. 4).
Periprosthetic osteolysis
The inflammatory response to wear debris is an important factor in the development of periprosthetic osteolysis. It was confirmed that polymethylmethacrylate and titanium significantly inhibited IL-6-induced STAT3 activation in osteoclast precursors, suggesting that the mechanism of wear debris in periprosthetic osteolysis may be through the inhibition of anti-osteoclastogenic cytokine signalling [100] (Table 3; Fig. 4).
Cell–cell interactions via STAT3 activation
Haematopoietic cells and osteoclasts
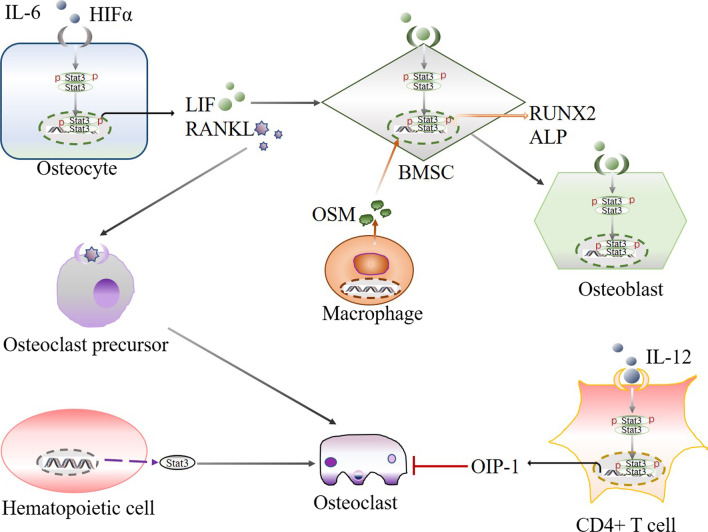
Interestingly, haematopoietic cell-specific knockout of STAT3 in transgenic mice not only induced hyperproliferation of the myeloid lineage but also led to reductions in BV and Tb.Th and Tb.N by 69.5%, 84.6% and 77.0%, respectively. In addition, increased numbers of Mac1 + cells and c-kit + cells were also identified, suggesting an increased number of osteoclast precursors in STAT3-deficient mice. These results showed that haematopoietic cell-derived STAT3 plays a negative role in regulating osteoclastogenesis [101] (Fig. 5).
Fig. 5.
STAT3 signal mediated cellular interaction within osteocyte, BMSC, osteoblast, osteoclast, osteoclast, precursor, macrophage, hematopoietic cell and CD4 + T cell through regulating osteogenesis- and osteoclastogenesis-related factors. Arrows indicate positive stimulation or upregulation
Immune cells & osteoclasts
Osteoclast formation is regulated by a variety of cell types and their products, such as immune cells that produce IL-12, which has been confirmed to be a potent inhibitor of osteoclast formation. Human osteoclast inhibitory peptide-1 (OIP-1/hSca) is a Ly-6 gene family member, and IL-12 stimulates OIP-1 expression through STAT-3 activation in CD4 + T cells. Chromatin immunoprecipitation (ChIP) assays confirmed STAT-3 binding to the OIP-1 promoter element in response to IL-12 stimulation [102].
In addition, IL-17 is produced by Th17 cells, which may be involved in the pathogenesis of bone loss. Oestrogen (E2) increased the differentiation of Th17 cells and upregulated STAT3 expression. IL-17 neutralizing antibody treatment of OVX mice significantly increased BV/TV, Tb.N. and connection density (Conn. Dn), and reduced Tb.Sp, trabecular pattern factor (Tb.Pf), the structure model index (SMI) and TRAP-positive cells [103] (Fig. 5).
Osteoblast lineage and osteoclasts
Osteoblasts can differentiate into osteocytes, which secrete M-CSF and RANKL in the bone matrix, regulating osteoclast formation.
Hypoxia‐inducible factor‐1α (HIF‐1α) is one of the major regulatory factors of the hypoxia response and plays an important role in inflammation, cell proliferation and bone metabolism [104]. Overexpression of HIF‐1α in osteocyte‐like MLO‐Y4 cells upregulated RANKL levels through the phosphoproteins JAK2 and STAT3 and facilitated the differentiation of RAW264.7 osteoclast precursors into osteoclasts [105].
IL-6 enhances osteocyte-mediated osteoclastic differentiation and osteoclastogenesis by activating the JAK2-STAT3 pathway, which is required for RANKL induction by IL-6/IL-6R [106]. IL-6-type cytokines also enhance osteoclast formation by activating the gp130 receptor subunit on stromal/osteoblastic cells, which leads to STAT3-mediated expression of RANKL [107].
LIF secretion by osteocytes can increase the tartrate-resistant acid phosphatase activity of BMM-derived osteoclasts and enhance alkaline phosphatase activity during the osteogenic differentiation of BMSCs. Mechanistically, LIF increases the phosphorylation of STAT3, which is associated with osteoclast and osteoblast formation [108] (Fig. 5).
Monocytes/macrophages and MSCs
It has been reported that monocytes induce STAT3 activation in human MSCs to promote osteoblast formation. Using in vitro cell cultures of human bone marrow-derived MSCs, it was shown that monocytes/macrophages effectively induced MSC differentiation into osteoblasts through cell contact, and the former produced oncostatin M to activate STAT3 in the latter. The phosphorylation of STAT3 in MSCs upregulated RUNX2 and ALP expression [108] (Fig. 5).
In contrast to studies that focused on single-cell functions regarding the role of STAT3 in osteogenesis and osteoclastogenesis, these data showed cellular interactions and provided valuable insight into STAT3-mediated events in bone, which should be further examined in future studies.
Conclusion
Although the data we reviewed are somewhat inconsistent in terms of the role of STAT3 in events involved in bone remodelling and osteoporosis, most evidence supports a positive effect of STAT3 on both osteogenesis and osteoclastogenesis in vivo, as cell-specific deficiency in either osteoblastic or osteoclastic linage cells impaired differentiation or even cell function. However, these complicated cellular interactions, either during the development of bone and related diseases, including osteoporosis, or under certain stimuli, make it difficult to draw firm conclusions with respect to the role of STAT3 in these processes; otherwise, we could state that the role of STAT3 play in these eventsis cell- and stimulation-specific. In this context, more studies should be performed to determine the cellular network in which STAT3 participates, thereby uncovering whether and how cell-specific alterations in STAT3 activity could affect the development of osteoporosis or whether systematically silencing or overactivating STAT3 could be a potential strategy for osteoporosis treatment. On the other hand, further investigation of the role of STAT3 in the development of subtypes other than oestrogen deficiency-induced osteoporosis is also important. Finally, STAT3-mediated cell–cell interactions involve a wide range of nonbone cells, which enlarges the window to show how these factors interact with bone cells during osteoporosis and should be examined in future studies.
Acknowledgements
Not applicable.
Abbreviations
- STAT3
Signal transducer and activator of transcription 3
- BMSCs
Bone marrow stromal cells
- BMM
Bone-marrow macrophages
- RANKL
Receptor activator of nuclear factor-kb ligand
- FDA
Food and Drug Administration
- SOCS
Suppressors of cytokine signaling
- PTPs
Protein tyrosine phosphatases
- SH2
Src homology 2
- Tyr705
Tyrosine 705
- Ser727
Serine 727
- IL
Interleukin
- gp130
Glycoprotein 130
- SHP-2
Src homology phosphatase-2
- BV
Bone volume
- Tb.N
Trabecular number
- Tb.Th
Trabecular thickness
- Tb.Sp
Trabecular separation
- Ct.Th
Cortical thickness
- Conn.Dn
Connection density
- Tb.Pf
Trabecular pattern factor
- SMI
Structure model index
- PIAS3
Protein inhibitor of activated STAT3
- OVX
Ovariectomy
- PC1
Polycystin-1
- EPO
Erythropoietin
- NDRG2
N-myc downstream regulator gene 2
- BMP2
Bone morphogenetic protein 2
- Ror2
Orphan receptor 2
- Runx2
Runt-related transcription factor 2
- OPG
Osteoprotegerin
- OSX
Osterix
- ALP
Alkaline phosphatase
- OCN
Osteocalcin
- PKD
Protein kinase D
- CUEDC2
CUE domain-containing 2
- LepR
Leptin receptor
- LIF
Leukemia inhibitory factor
- RPN2
Ribophorin II
- mTOR1
Mechanistic target of rapamycin complex 1
- miRNAs
MicroRNAs
- CRY2
Cryptochrome circadian regulator 2
- M-CSF
Macrophage-colony stimulating factor
- TRAP
Tartrate-resistant acid phosphatase
- TNF
Tumor necrosis factor
- TRAF6
TNF receptor-associated factor 6
- NFATc1
Nuclear factor of activated T cells
- μCT
Micro-computed tomography
- MAPK
Mitogen-activated protein kinases
- OSCAR
Osteoclast-associated receptor
- PrxII
Peroxiredoxin II
- LPS
Lipopolysaccharide
- ROS
Reactive oxygen species
- WKYMVm
Trp-Lys-Tyr-Met-Val-D-Met-NH2
- MSM
Methylsulfonylmethane
- HA
Hyaluronic acid
- IL-34
Interleukin-34
- PIAS3
Protein inhibitor of activated STAT3
- Niclosamide
5-Chloro-salicyl-(2-chloro-4-nitro) anilide
- EE1G1
Estrogen-induced gene 1
- NFkBie
NF-kB inhibitor epsilon
- RAS
Renin-angiotensin system
- ERK
Extracellular signal-regulated kinase
- PTPRD, PTPRT, PTPRK
PTP receptor-type D, T and K
- PTPN9
PTP: non-receptor type 9
- PTPN2
PTP non-receptor type 2
Author contributions
X-LH draft the manuscript and F-MT edited the manuscript to its final version. All authors read and approved the final manuscript.
Funding
One-Hundred Innovative Talents Support Fundation of Hebei Province (JJK-2019-14); Nature Science Foundation of Hebei Province (H2019209550); Youth Talent Support Program of Hebei Province (JI-2016-10); Nation Nature Science Foundation of China (NSFC81874029); Basic Scientific Research Funds Program of Universities in Hebei Province (JYG2021005).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fu Y, Hu X, Gao Y, Li K, Fu Q, Liu Q, et al. LncRNA ROR/miR-145-5p axis modulates the osteoblasts proliferation and apoptosis in osteoporosis. Bioengineered. 2021;12(1):7714–7723. doi: 10.1080/21655979.2021.1982323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Q, Xia C, Shi B, Chen C, Yang C, Mao G, et al. Extracorporeal shock wave combined with teriparatide-loaded hydrogel injection promotes segmental bone defects healing in osteoporosis. Tissue Eng Regen Med. 2021;18(6):1021–1033. doi: 10.1007/s13770-021-00381-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zhang B, Chang X. Emerging roles of circular RNAs in osteoporosis. J Cell Mol Med. 2021;25(19):9089–9101. doi: 10.1111/jcmm.16906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Liu D, Zhou Z, Qin S. Role of long non-coding RNA H19 in the development of osteoporosis. Mol Med. 2021;27(1):122. doi: 10.1186/s10020-021-00386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J, Kweon HJ, Kwon KJ, Han SH. Incidence of osteoporosis and ambient air pollution in South Korea: a population-based retrospective cohort study. BMC Public Health. 2021;21(1):1794. doi: 10.1186/s12889-021-11866-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Yuan P, Xu G, Chen Z, Li Z, Ye H, et al. DUSP6 expression is associated with osteoporosis through the regulation of osteoclast differentiation via ERK2/Smad2 signaling. Cell Death Dis. 2021;12(9):825. doi: 10.1038/s41419-021-04110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Odén A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int. 2013;92(1):42–49. doi: 10.1007/s00223-012-9666-6. [DOI] [PubMed] [Google Scholar]
- 8.Qin XB, Wen K, Wu XX, Yao ZJ. MiR-183 regulates the differentiation of osteoblasts in the development of osteoporosis by targeting Smad4. Acta Histochem. 2021;123(7):151786. doi: 10.1016/j.acthis.2021.151786. [DOI] [PubMed] [Google Scholar]
- 9.Khan M, Cheung AM, Khan AA. Drug-related adverse events of osteoporosis therapy. Endocrinol Metab Clin N Am. 2017;46(1):181–192. doi: 10.1016/j.ecl.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Shih PC. Revisiting the development of small molecular inhibitors that directly target the signal transducer and activator of transcription 3 (STAT3) domains. Life Sci. 2020;242:117241. doi: 10.1016/j.lfs.2019.117241. [DOI] [PubMed] [Google Scholar]
- 11.Huang Q, Zhong Y, Dong H, Zheng Q, Shi S, Zhu K, et al. Revisiting signal transducer and activator of transcription 3 (STAT3) as an anticancer target and its inhibitor discovery: where are we and where should we go? Eur J Med Chem. 2020;187:111922. doi: 10.1016/j.ejmech.2019.111922. [DOI] [PubMed] [Google Scholar]
- 12.Chun KS, Jang JH, Kim DH. Perspectives regarding the intersections between STAT3 and oxidative metabolism in cancer. Cells. 2020;9(10):2202. doi: 10.3390/cells9102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Y, Liao S, Bennett S, Tang H, Song D, Wood D, et al. STAT3 and its targeting inhibitors in osteosarcoma. Cell Prolif. 2021;54(2):e12974. doi: 10.1111/cpr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims NA. The JAK1/STAT3/SOCS3 axis in bone development, physiology, and pathology. Exp Mol Med. 2020;52(8):1185–1197. doi: 10.1038/s12276-020-0445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.James R, Griffin JGL, Senior C, Love R. The role of the Radiographer in osteoporosis and fracture prevention services—a narrative review. Radiography (Lond) 2021;27(Suppl 1):S34–s38. doi: 10.1016/j.radi.2021.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Duque G, Troen BR. Understanding the mechanisms of senile osteoporosis: new facts for a major geriatric syndrome. J Am Geriatr Soc. 2008;56(5):935–941. doi: 10.1111/j.1532-5415.2008.01764.x. [DOI] [PubMed] [Google Scholar]
- 17.Mishra BH, Mishra PP, Mononen N, Hilvo M, Sievänen H, Juonala M, et al. Uncovering the shared lipidomic markers of subclinical osteoporosis-atherosclerosis comorbidity: the Young Finns Study. Bone. 2021;151:116030. doi: 10.1016/j.bone.2021.116030. [DOI] [PubMed] [Google Scholar]
- 18.Wu D, Cline-Smith A, Shashkova E, Perla A, Katyal A, Aurora R. T-cell mediated inflammation in postmenopausal osteoporosis. Front Immunol. 2021;12:687551. doi: 10.3389/fimmu.2021.687551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenkre JS, Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308–327. doi: 10.1177/0004563218759371. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Chen X, Lu L, Yu X. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020;52:88–98. doi: 10.1016/j.cytogfr.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Charoenngam N, Shirvani A, Holick MF. The ongoing D-lemma of vitamin D supplementation for nonskeletal health and bone health. Curr Opin Endocrinol Diabetes Obes. 2019;26(6):301–305. doi: 10.1097/MED.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 22.Rødbro LL, Bislev LS, Sikjær T, Rejnmark L. Bone metabolism, density, and geometry in postmenopausal women with vitamin D insufficiency: a cross-sectional comparison of the effects of elevated parathyroid levels. Osteoporos Int. 2018;29(10):2211–2218. doi: 10.1007/s00198-018-4602-x. [DOI] [PubMed] [Google Scholar]
- 23.Bravenboer N, Oostlander A, Evan Bodegraven AA. Bone loss in patients with inflammatory bowel disease: cause, detection and treatment. Curr Opin Gastroenterol. 2021;37(2):128–134. doi: 10.1097/MOG.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 24.Choi KH, Lee JH, Lee DG. Sex-related differences in bone metabolism in osteoporosis observational study. Medicine (Baltimore) 2021;100(21):e26153. doi: 10.1097/MD.0000000000026153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadji P, Colli E, Regidor PA. Bone health in estrogen-free contraception. Osteoporos Int. 2019;30(12):2391–2400. doi: 10.1007/s00198-019-05103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santoni M, Miccini F, Cimadamore A, Piva F, Massari F, Cheng L, et al. An update on investigational therapies that target STAT3 for the treatment of cancer. Expert Opin Investig Drugs. 2021;30(3):245–251. doi: 10.1080/13543784.2021.1891222. [DOI] [PubMed] [Google Scholar]
- 27.Chalikonda G, Lee H, Sheik A, Huh YS. Targeting key transcriptional factor STAT3 in colorectal cancer. Mol Cell Biochem. 2021;476(9):3219–3228. doi: 10.1007/s11010-021-04156-8. [DOI] [PubMed] [Google Scholar]
- 28.Domoszlai T, Martincuks A, Fahrenkamp D, Schmitz-Van de Leur H, Küster A, Müller-Newen G. Consequences of the disease-related L78R mutation for dimerization and activity of STAT3. J Cell Sci. 2014;127(Pt 9):1899–1910. doi: 10.1242/jcs.137422. [DOI] [PubMed] [Google Scholar]
- 29.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307(5707):269–273. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 30.Park IH, Li C. Characterization of molecular recognition of STAT3 SH2 domain inhibitors through molecular simulation. J Mol Recognit. 2011;24(2):254–265. doi: 10.1002/jmr.1047. [DOI] [PubMed] [Google Scholar]
- 31.Tošić I, Frank DA. STAT3 as a mediator of oncogenic cellular metabolism: pathogenic and therapeutic implications. Neoplasia. 2021;23(12):1167–1178. doi: 10.1016/j.neo.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Wu K, Fang L, Yang X, Zheng N, Du Z, et al. Discovery of N-substituted sulfamoylbenzamide derivatives as novel inhibitors of STAT3 signaling pathway based on Niclosamide. Eur J Med Chem. 2021;218:113362. doi: 10.1016/j.ejmech.2021.113362. [DOI] [PubMed] [Google Scholar]
- 33.Andrés RM, Hald A, Johansen C, Kragballe K, Iversen L. Studies of Jak/STAT3 expression and signalling in psoriasis identifies STAT3-Ser727 phosphorylation as a modulator of transcriptional activity. Exp Dermatol. 2013;22(5):323–328. doi: 10.1111/exd.12128. [DOI] [PubMed] [Google Scholar]
- 34.Yang J, Kunimoto H, Katayama B, Zhao H, Shiromizu T, Wang L, et al. Phospho-Ser727 triggers a multistep inactivation of STAT3 by rapid dissociation of pY705-SH2 through C-terminal tail modulation. Int Immunol. 2020;32(2):73–88. doi: 10.1093/intimm/dxz061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaguchi M, Oka M, Iwasaki T, Fukami Y, Nishigori C. Role and regulation of STAT3 phosphorylation at Ser727 in melanocytes and melanoma cells. J Invest Dermatol. 2012;132(7):1877–1885. doi: 10.1038/jid.2012.45. [DOI] [PubMed] [Google Scholar]
- 36.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: recent advances towards specific inhibition. Curr Opin Immunol. 2015;34:75–82. doi: 10.1016/j.coi.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Johnson DE, O'Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ernst M, Thiem S, Nguyen PM, Eissmann M, Putoczki TL. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin Immunol. 2014;26(1):29–37. doi: 10.1016/j.smim.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial–mesenchymal transition. Cells. 2020;9(1):217. doi: 10.3390/cells9010217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20(20):2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 41.Coppo P, Dusanter-Fourt I, Millot G, Nogueira MM, Dugray A, Bonnet ML, et al. Constitutive and specific activation of STAT3 by BCR-ABL in embryonic stem cells. Oncogene. 2003;22(26):4102–4110. doi: 10.1038/sj.onc.1206607. [DOI] [PubMed] [Google Scholar]
- 42.Cimica V, Chen HC, Iyer JK, Reich NC. Dynamics of the STAT3 transcription factor: nuclear import dependent on Ran and importin-β1. PLoS ONE. 2011;6(5):e20188. doi: 10.1371/journal.pone.0020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsushita K, Itoh S, Ikeda S, Yamamoto Y, Yamauchi Y, Hayashi M. LIF/STAT3/SOCS3 signaling pathway in murine bone marrow stromal cells suppresses osteoblast differentiation. J Cell Biochem. 2014;115(7):1262–1268. doi: 10.1002/jcb.24777. [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Morales LD, Jang IS, Cho YY, Kim DJ. Protein tyrosine phosphatases as potential regulators of STAT3 signaling. Int J Mol Sci. 2018;19(9):2708. doi: 10.3390/ijms19092708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim DJ, Tremblay ML, Digiovanni J. Protein tyrosine phosphatases, TC-PTP, SHP1, and SHP2, cooperate in rapid dephosphorylation of Stat3 in keratinocytes following UVB irradiation. PLoS ONE. 2010;5(4):e10290. doi: 10.1371/journal.pone.0010290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y, Wang J, Cao F, Jiang H, Li A, Li J, et al. SHP2 associates with nuclear localization of STAT3: significance in progression and prognosis of colorectal cancer. Sci Rep. 2017;7(1):17597. doi: 10.1038/s41598-017-17604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun H, Li Y, Quan X, Chen N, Jin X, Jin W, et al. PIAS3/SOCS1-STAT3 axis responses to oxidative stress in hepatocellular cancer cells. Am J Transl Res. 2021;13(11):12395–12409. [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Wang D, Xu J, Wang Y, Ma F, Li Z, et al. Stat3 activation is critical for pluripotency maintenance. J Cell Physiol. 2019;234(2):1044–1051. doi: 10.1002/jcp.27241. [DOI] [PubMed] [Google Scholar]
- 49.Khan MW, Saadalla A, Ewida AH, Al-Katranji K, Al-Saoudi G, Giaccone ZT, et al. The STAT3 inhibitor pyrimethamine displays anti-cancer and immune stimulatory effects in murine models of breast cancer. Cancer Immunol Immunother. 2018;67(1):13–23. doi: 10.1007/s00262-017-2057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grabner B, Schramek D, Mueller KM, Moll HP, Svinka J, Hoffmann T, et al. Disruption of STAT3 signalling promotes KRAS-induced lung tumorigenesis. Nat Commun. 2015;6:6285. doi: 10.1038/ncomms7285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guanizo AC, Fernando CD, Garama DJ, Gough DJ. STAT3: a multifaceted oncoprotein. Growth Factors. 2018;36(1–2):1–14. doi: 10.1080/08977194.2018.1473393. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Yang H, Huang J, Pei S, Wang L, Feng JQ, et al. Targeted Ptpn11 deletion in mice reveals the essential role of SHP2 in osteoblast differentiation and skeletal homeostasis. Bone Res. 2021;9(1):6. doi: 10.1038/s41413-020-00129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Cao X, Li P, Fan Y, Zhang L, Li W, et al. PSMC6 promotes osteoblast apoptosis through inhibiting PI3K/AKT signaling pathway activation in ovariectomy-induced osteoporosis mouse model. J Cell Physiol. 2020;235(7–8):5511–5524. doi: 10.1002/jcp.29261. [DOI] [PubMed] [Google Scholar]
- 54.Zhou S, Dai Q, Huang X, Jin A, Yang Y, Gong X, et al. STAT3 is critical for skeletal development and bone homeostasis by regulating osteogenesis. Nat Commun. 2021;12(1):6891. doi: 10.1038/s41467-021-27273-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Itoh S, Udagawa N, Takahashi N, Yoshitake F, Narita H, Ebisu S, et al. A critical role for interleukin-6 family-mediated Stat3 activation in osteoblast differentiation and bone formation. Bone. 2006;39(3):505–512. doi: 10.1016/j.bone.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 56.Jo S, Wang SE, Lee YL, Kang S, Lee B, Han J, et al. IL-17A induces osteoblast differentiation by activating JAK2/STAT3 in ankylosing spondylitis. Arthritis Res Ther. 2018;20(1):115. doi: 10.1186/s13075-018-1582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Falconi D, Aubin JE. LIF inhibits osteoblast differentiation at least in part by regulation of HAS2 and its product hyaluronan. J Bone Miner Res. 2007;22(8):1289–1300. doi: 10.1359/jbmr.070417. [DOI] [PubMed] [Google Scholar]
- 58.Liu C, Jiang D. High glucose-induced LIF suppresses osteoblast differentiation via regulating STAT3/SOCS3 signaling. Cytokine. 2017;91:132–139. doi: 10.1016/j.cyto.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 59.Komori T. Molecular mechanism of Runx2-dependent bone development. Mol Cells. 2020;43(2):168–175. doi: 10.14348/molcells.2019.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dalagiorgou G, Piperi C, Adamopoulos C, Georgopoulou U, Gargalionis AN, Spyropoulou A, et al. Mechanosensor polycystin-1 potentiates differentiation of human osteoblastic cells by upregulating Runx2 expression via induction of JAK2/STAT3 signaling axis. Cell Mol Life Sci. 2017;74(5):921–936. doi: 10.1007/s00018-016-2394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yue R, Zhou BO, Shimada IS, Zhao Z, Morrison SJ. Leptin receptor promotes adipogenesis and reduces osteogenesis by regulating mesenchymal stromal cells in adult bone marrow. Cell Stem Cell. 2016;18(6):782–796. doi: 10.1016/j.stem.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Chen S, Wang J, Cai C, Xie X. N-myc downstream-regulated gene 2 (NDRG2) promotes bone morphogenetic protein 2 (BMP2)-induced osteoblastic differentiation and calcification by Janus kinase 3 (JAK3)/signal transducer and activator of transcription 3 (STAT3) signaling pathway. Med Sci Monit. 2020;26:e918541. doi: 10.12659/MSM.918541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ford JJ, Yeh LC, Schmidgal EC, Thompson JF, Adamo ML, Lee JC. Protein kinase D1 is essential for bone acquisition during pubertal growth. Endocrinology. 2013;154(11):4182–4191. doi: 10.1210/en.2013-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li S, Xu W, Xing Z, Qian J, Chen L, Gu R, et al. A conditional knockout mouse model reveals a critical role of PKD1 in osteoblast differentiation and bone development. Sci Rep. 2017;7:40505. doi: 10.1038/srep40505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JW, Oh SH, Lee MN, Song JH, Jeong BC, Yang JW, et al. CUEDC2 controls osteoblast differentiation and bone formation via SOCS3-STAT3 pathway. Cell Death Dis. 2020;11(5):344. doi: 10.1038/s41419-020-2562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei L, Huang Z, Feng J, Huang Z, Tao Y, Hu X, et al. Loss of receptor tyrosine kinase-like orphan receptor 2 impairs the osteogenesis of mBMSCs by inhibiting signal transducer and activator of transcription 3. Stem Cell Res Ther. 2020;11(1):137. doi: 10.1186/s13287-020-01646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ni L, Yu J, Gui X, Lu Z, Wang X, Guo H, et al. Overexpression of RPN2 promotes osteogenic differentiation of hBMSCs through the JAK/STAT3 pathway. FEBS Open Bio. 2020;10(1):158–167. doi: 10.1002/2211-5463.12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang B, Wang Y, Wang W, Chen J, Lai P, Liu Z, et al. mTORC1 prevents preosteoblast differentiation through the notch signaling pathway. PLoS Genet. 2015;11(8):e1005426. doi: 10.1371/journal.pgen.1005426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L, Zhang RY, Xie J, Yang JY, Fang KH, Hong CX, et al. STAT3 activation by catalpol promotes osteogenesis-angiogenesis coupling, thus accelerating osteoporotic bone repair. Stem Cell Res Ther. 2021;12(1):108. doi: 10.1186/s13287-021-02178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo L, Luo T, Fang Y, Yang L, Wang L, Liu J, et al. Effects of erythropoietin on osteoblast proliferation and function. Clin Exp Med. 2014;14(1):69–76. doi: 10.1007/s10238-012-0220-7. [DOI] [PubMed] [Google Scholar]
- 71.Wang W, Mao J, Chen Y, Zuo J, Chen L, Li Y, et al. Naringin promotes osteogenesis and ameliorates osteoporosis development by targeting JAK2/STAT3 signalling. Clin Exp Pharmacol Physiol. 2022;49(1):113–121. doi: 10.1111/1440-1681.13591. [DOI] [PubMed] [Google Scholar]
- 72.Zhang XT, Sun M, Zhang L, Dai YK, Wang F. The potential function of miR-135b-mediated JAK2/STAT3 signaling pathway during osteoblast differentiation. Kaohsiung J Med Sci. 2020;36(9):673–681. doi: 10.1002/kjm2.12217. [DOI] [PubMed] [Google Scholar]
- 73.Feng Y, He PY, Kong WD, Cen WJ, Wang PL, Liu C, et al. Apoptosis-promoting properties of miR-3074-5p in MC3T3-E1 cells under iron overload conditions. Cell Mol Biol Lett. 2021;26(1):37. doi: 10.1186/s11658-021-00281-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang Z, Xu T, Li Y, Fei W, Yang G, Hong Y. Inhibition of CRY2 by STAT3/miRNA-7-5p promotes osteoblast differentiation through upregulation of CLOCK/BMAL1/P300 expression. Mol Ther Nucleic Acids. 2020;19:865–876. doi: 10.1016/j.omtn.2019.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fu Y, Xu Y, Chen S, Ouyang Y, Sun G. MiR-151a-3p promotes postmenopausal osteoporosis by targeting socs5 and activating JAK2/STAT3 signaling. Rejuvenation Res. 2020;23(4):313–323. doi: 10.1089/rej.2019.2239. [DOI] [PubMed] [Google Scholar]
- 76.Wang G, Zhang L, Yan C, Wang F, Zhang Y. Overexpression of miR125b promotes osteoporosis through miR-125b-TRAF6 pathway in postmenopausal ovariectomized rats. Diabetes Metab Syndr Obes. 2021;14:671–682. doi: 10.2147/DMSO.S288338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai Q, Zheng P, Ma F, Zhang H, Li Z, Fu Q, et al. MicroRNA-224 enhances the osteoblastic differentiation of hMSCs via Rac1. Cell Biochem Funct. 2019;37(2):62–71. doi: 10.1002/cbf.3373. [DOI] [PubMed] [Google Scholar]
- 78.Yu X, Wan Q, Ye X, Cheng Y, Pathak JL, Li Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell Mol Biol Lett. 2019;24:64. doi: 10.1186/s11658-019-0191-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aoki K, Saito H, Itzstein C, Ishiguro M, Shibata T, Blanque R, et al. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J Clin Invest. 2006;116(6):1525–1534. doi: 10.1172/JCI22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boyce BF. Advances in the regulation of osteoclasts and osteoclast functions. J Dent Res. 2013;92(10):860–867. doi: 10.1177/0022034513500306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joung YH, Darvin P, Kang DY, Sp N, Byun HJ, Lee CH, et al. Methylsulfonylmethane inhibits RANKL-induced osteoclastogenesis in BMMs by suppressing NF-κB and STAT3 activities. PLoS ONE. 2016;11(7):e0159891. doi: 10.1371/journal.pone.0159891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu J, Li X, Chen Y, Han X, Li L, Yang Z, et al. The protective effect of WKYMVm peptide on inflammatory osteolysis through regulating NF-κB and CD9/gp130/STAT3 signalling pathway. J Cell Mol Med. 2020;24(2):1893–1905. doi: 10.1111/jcmm.14885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y, Chung MR, Zhou S, Gong X, Xu H, Hong Y, et al. STAT3 controls osteoclast differentiation and bone homeostasis by regulating NFATc1 transcription. J Biol Chem. 2019;294(42):15395–15407. doi: 10.1074/jbc.RA119.010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davidson RK, Himes ER, Takigawa S, Chen A, Horn MR, Meijome T, et al. The loss of STAT3 in mature osteoclasts has detrimental effects on bone structure. PLoS ONE. 2020;15(7):e0236891. doi: 10.1371/journal.pone.0236891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duplomb L, Baud'huin M, Charrier C, Berreur M, Trichet V, Blanchard F, et al. Interleukin-6 inhibits receptor activator of nuclear factor kappaB ligand-induced osteoclastogenesis by diverting cells into the macrophage lineage: key role of Serine727 phosphorylation of signal transducer and activator of transcription 3. Endocrinology. 2008;149(7):3688–3697. doi: 10.1210/en.2007-1719. [DOI] [PubMed] [Google Scholar]
- 86.Cheng X, Wan QL, Li ZB. AG490 suppresses interleukin-34-mediated osteoclastogenesis in mice bone marrow macrophages. Cell Biol Int. 2017;41(6):659–668. doi: 10.1002/cbin.10771. [DOI] [PubMed] [Google Scholar]
- 87.Lee TH, Kim SU, Yu SL, Kim SH, Park DS, Moon HB, et al. Peroxiredoxin II is essential for sustaining life span of erythrocytes in mice. Blood. 2003;101(12):5033–5038. doi: 10.1182/blood-2002-08-2548. [DOI] [PubMed] [Google Scholar]
- 88.Park H, Noh AL, Kang JH, Sim JS, Lee DS, Yim M. Peroxiredoxin II negatively regulates lipopolysaccharide-induced osteoclast formation and bone loss via JNK and STAT3. Antioxid Redox Signal. 2015;22(1):63–77. doi: 10.1089/ars.2013.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiao J, Zhang R, Li Z, Yin Y, Fang X, Ding X, et al. Nuclear Smad6 promotes gliomagenesis by negatively regulating PIAS3-mediated STAT3 inhibition. Nat Commun. 2018;9(1):2504. doi: 10.1038/s41467-018-04936-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hikata T, Takaishi H, Takito J, Hakozaki A, Furukawa M, Uchikawa S, et al. PIAS3 negatively regulates RANKL-mediated osteoclastogenesis directly in osteoclast precursors and indirectly via osteoblasts. Blood. 2009;113(10):2202–2212. doi: 10.1182/blood-2008-06-162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim K, Lee J, Kim JH, Jin HM, Zhou B, Lee SY, et al. Protein inhibitor of activated STAT 3 modulates osteoclastogenesis by down-regulation of NFATc1 and osteoclast-associated receptor. J Immunol. 2007;178(9):5588–5594. doi: 10.4049/jimmunol.178.9.5588. [DOI] [PubMed] [Google Scholar]
- 92.Li B, Lu D, Chen Y, Zhao M, Zuo L. Unfractionated heparin promotes osteoclast formation in vitro by inhibiting osteoprotegerin activity. Int J Mol Sci. 2016;17(4):613. doi: 10.3390/ijms17040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walton KJ, Duncan JM, Deschamps P, Shaughnessy SG. Heparin acts synergistically with interleukin-11 to induce STAT3 activation and in vitro osteoclast formation. Blood. 2002;100(7):2530–2536. doi: 10.1182/blood.V100.7.2530. [DOI] [PubMed] [Google Scholar]
- 94.You S, Li R, Park D, Xie M, Sica GL, Cao Y, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 2014;13(3):606–616. doi: 10.1158/1535-7163.MCT-13-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheon YH, Kim JY, Baek JM, Ahn SJ, So HS, Oh J. Niclosamide suppresses RANKL-induced osteoclastogenesis and prevents LPS-induced bone loss. Biochem Biophys Res Commun. 2016;470(2):343–349. doi: 10.1016/j.bbrc.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 96.Knudson CB. Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J Cell Biol. 1993;120(3):825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nakao Y, Hikiji H, Okinaga T, Takeuchi J, Habu M, Yoshiga D, et al. Accumulation of hyaluronic acid in stromal cells modulates osteoclast formation by regulation of receptor activator of nuclear factor kappa-B ligand expression. Biochem Biophys Res Commun. 2019;512(3):537–543. doi: 10.1016/j.bbrc.2019.03.137. [DOI] [PubMed] [Google Scholar]
- 98.Cheon YH, Kim JY, Baek JM, Ahn SJ, Jun HY, Erkhembaatar M, et al. WHI-131 promotes osteoblast differentiation and prevents osteoclast formation and resorption in mice. J Bone Miner Res. 2016;31(2):403–415. doi: 10.1002/jbmr.2612. [DOI] [PubMed] [Google Scholar]
- 99.Liu T, Qin A, Liao B, Shao H, Guo L, Xie G, et al. A novel microRNA regulates osteoclast differentiation via targeting protein inhibitor of activated STAT3 (PIAS3) Bone. 2014;67:156–165. doi: 10.1016/j.bone.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 100.Rakshit D, Ly K, Sengupta T, Nestor B, Sculco T, Ivashkiv L, et al. Wear debris inhibition of anti-osteoclastogenic signaling by interleukin-6 and interferon-gamma. Mech Insights Implic Periprosthetic Osteolysis. 2006;88(4):788–799. doi: 10.2106/JBJS.E.00711. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Z, Welte T, Troiano N, Maher S, Fu X, Bothwell AJB, et al. Osteoporosis with increased osteoclastogenesis in hematopoietic cell-specific STAT3-deficient mice. Biochem Biophys Res Commun. 2005;328(3):800–807. doi: 10.1016/j.bbrc.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 102.Shanmugarajan S, Kawanabe N, Koide M, Tsuruga E, Arroyo JE, Key LL, Jr, et al. IL-12 stimulates the osteoclast inhibitory peptide-1 (OIP-1/hSca) gene expression in CD4+ T cells. J Cell Biochem. 2009;107(1):104–111. doi: 10.1002/jcb.22104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tyagi AM, Srivastava K, Mansoori MN, Trivedi R, Chattopadhyay N, Singh D. Estrogen deficiency induces the differentiation of IL-17 secreting Th17 cells: a new candidate in the pathogenesis of osteoporosis. PLoS ONE. 2012;7(9):e44552. doi: 10.1371/journal.pone.0044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. Embo J. 2012;31(11):2448–2460. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhu J, Tang Y, Wu Q, Ji YC, Feng ZF, Kang FW. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J Cell Physiol. 2019;234(11):21182–21192. doi: 10.1002/jcp.28721. [DOI] [PubMed] [Google Scholar]
- 106.Wu Q, Zhou X, Huang D, Ji Y, Kang F. IL-6 enhances osteocyte-mediated osteoclastogenesis by promoting JAK2 and RANKL activity in vitro. Cell Physiol Biochem. 2017;41(4):1360–1369. doi: 10.1159/000465455. [DOI] [PubMed] [Google Scholar]
- 107.O'Brien CA, Lin SC, Bellido T, Manolagas SC. Expression levels of gp130 in bone marrow stromal cells determine the magnitude of osteoclastogenic signals generated by IL-6-type cytokines. J Cell Biochem. 2000;79(4):532–541. doi: 10.1002/1097-4644(20001215)79:4<532::AID-JCB20>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 108.Du J, Yang J, He Z, Cui J, Yang Y, Xu M, et al. Osteoblast and osteoclast activity affect bone remodeling upon regulation by mechanical loading-induced leukemia inhibitory factor expression in osteocytes. Front Mol Biosci. 2020;7:585056. doi: 10.3389/fmolb.2020.585056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nicolaidou V, Wong MM, Redpath AN, Ersek A, Baban DF, Williams LM, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE. 2012;7(7):e39871. doi: 10.1371/journal.pone.0039871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kurozumi A, Nakano K, Yamagata K, Okada Y, Nakayamada S, Tanaka Y. IL-6 and sIL-6R induces STAT3-dependent differentiation of human VSMCs into osteoblast-like cells through JMJD2B-mediated histone demethylation of RUNX2. Bone. 2019;124:53–61. doi: 10.1016/j.bone.2019.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.