Abstract
Diazirines are an attractive class of potential molecular tags for magnetic resonance imaging owing to their biocompatibility and ease of incorporation into a large variety of molecules. As recently reported, 15N2-diazirine can be hyperpolarized by the SABRE-SHEATH method, sustaining both singlet and magnetization states, thus offering a path to long-lived polarization storage. Herein, we show the generality of this approach by illustrating that the diazirine tag alone is sufficient for achieving excellent signal enhancements with long-lasting polarization. Our investigations reveal the critical role of Lewis basic additives, including water, on achieving SABRE-promoted hyperpolarization. The application of this strategy to a 15N2-diazirine-containing choline derivative demonstrates the potential of 15N2-diazirines as molecular imaging tags for biomedical applications.
Keywords: diazirine, hyperpolarization, imaging agents, iridium, structure–activity relationships
Magnetic resonance imaging (MRI) is a powerful, non-invasive approach, based on nuclear magnetic resonance (NMR) spectroscopy, to visualize structure and function with high spatial and temporal resolution. Yet one of the most critical challenges of magnetic resonance (NMR and MRI),[1] is poor nuclear polarization at thermal equilibrium associated with low sensitivity, especially for nuclei of low natural abundance such as 13C and 15N. Hyperpolarization induces non-equilibrium polarization, increasing fractional magnetization of target nuclei, and therefore raises detectable signal by several orders of magnitude.[2] Hyperpolarized heteronuclei (e.g., 13C and 15N) often allow signal detection for extended time periods, due to their large relaxation time (T1) compared to 1H.[3] Among different hyperpolarization techniques,[4] Signal amplification by reversible exchange (SABRE) has been established as an experimentally simple and cost-effective method,[5] which uses para-hydrogen[6] as hyperpolarization source and iridium N-heterocyclic carbene complexes as catalysts.[7] More recently, it has been shown to hyperpolarize 15N nuclei efficiently in a process termed SABRE in shield enables alignment transfer to (SABRE-SHEATH).[8]
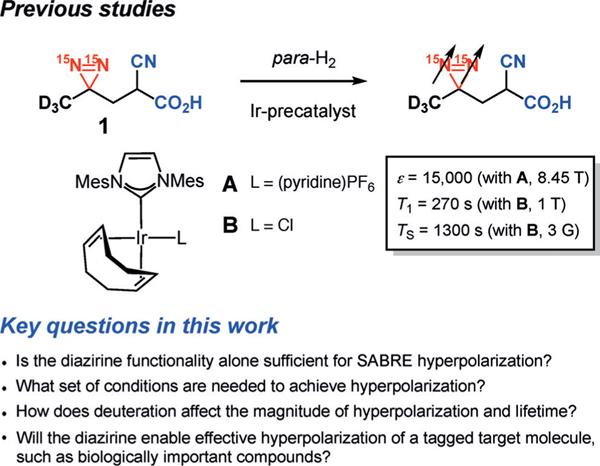
In the context of developing novel NMR and MRI strategies using hyperpolarized markers, we envisioned that 15N2-diazirine motifs could serve as unique hyperpolarizable tags. First, the structure of the double 15N-labeled diazirines gives access to observable 15N singlets, which support long-lived states. Furthermore, 15N2-diazirines have several attractive features, making them excellent candidates for imaging tags. For example, diazirines have been successfully incorporated into a large number of biologically relevant small molecules, metabolites, and biomolecules. They are small in size, therefore imparting minimal alteration to the original molecule’s properties,[9] and are known to be biocompatible and stable under either acidic or basic conditions. Indeed, diazirines have been extensively applied as photoaffinity labelling tags for biochemical investigations.[10] To explore the feasibility of a 15N2-diazirine as a potential imaging tag, we have investigated the hyperpolarization of 15N2-labeled diazirine 1 by SABRE-SHEATH (Figure 1).[11] These studies established that 15N2-diazirine 1 is capable of being hyperpolarized, achieving a 15000-fold signal enhancement (Ɛ) over thermal signal at the diazirine nitrogen atoms. Additionally, we found relaxation time constants of both 15N2 magnetization (T1) and singlet spin order (Ts) to be orders of magnitude larger than typical polarization decay time constants.[11]
Figure 1.
15N2-diazirines as potential molecular tags for MRI by SABRE-SHEATH hyperpolarization.
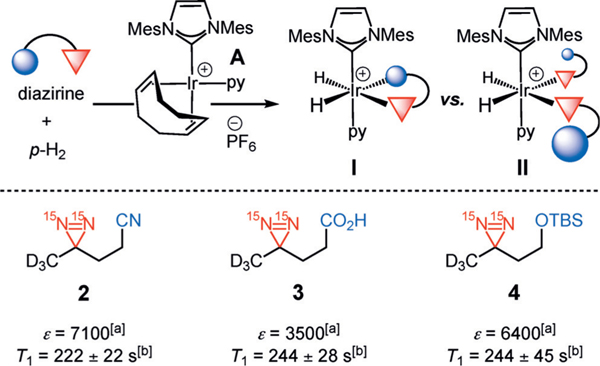
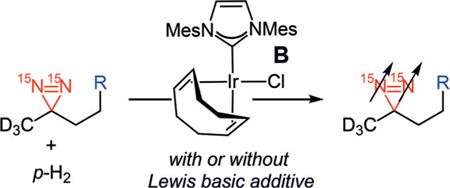
Herein, we report our investigations on the potential of diazirines to serve as molecular tags, which would provide enhanced signals with long lifetimes via SABRE-SHEATH. Several aspects had to first be assessed regarding diazirine hyperpolarization—a prerequisite for their ability to serve as general molecular tags. As the coordination of 1 to the iridium catalyst is essential for hyperpolarization by SABRE-SHEATH, we needed to clarify if either the nitrile or carboxylic acid group within 1 (Figure 1) were necessary for binding to iridium, thereby bringing the diazirine into the metalQs coordination sphere, or if the diazirine alone were sufficient. Additionally, we chose deuterated structure 1 in our previous studies with the hypothesis, based on related 13C-based hyperpolarization,[12] that deuteration proximal to the diazirine functionality in 1 would help to extend the T1 lifetime by reducing polarization loss. Yet the effects of deuteration within the molecule on the magnitude of hyperpolarization and its lifetime remained unclear. Finally, it would be desirable to establish the applicability of diazirines as molecular tags in biologically important molecules while retaining long-lasting hyperpolarization with large signal enhancements.
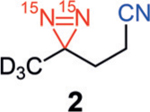
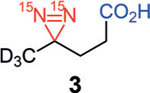
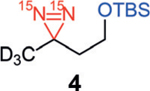
Our studies began with examining the structural features necessary for a diazirine-containing molecule to undergo SABRE-SHEATH hyperpolarization. To compare with the original structure of diazirine 1, a series of structural analogues 2–4 was prepared (Scheme 1). The absence of the Lewis basic carboxylic acid or nitrile group in 2–4 allowed us to probe the contribution of each functional group in successful diazirine hyperpolarization systematically. This would indicate whether the functional group might be involved in direct coordination to the catalyst (mode I) or if chelation were unnecessary, that is, the diazirine alone were sufficient to bind to Ir (mode II). Note that the hindered silyl ether of diazirine 4 was chosen to discourage, sterically and electronically, its coordination to the metal center. When subjected to standard SABRE-SHEATH conditions with pyridyl Ir precatalyst A (Scheme 1), cyanodiazirine 2, carboxylic acid-containing 3, and TBS-protected ether 4 are all efficiently hyperpolarized (Ɛ=7100, 3500, and 6400 at 8.45 T, respectively). Additionally, each substrate displays a T1 of 3–4 minutes at 1 T. The relatively small differences in signal enhancement and T1 among compounds 2–4 implies that while chelation might be possible in some cases (mode I), it is not required to achieve hyperpolarization. Therefore, coordination mode II is likely operative for diazirine hyperpolarization with complex A.[13]
Scheme 1.
Hyperpolarization of 15N2-diazirines 2–4. Standard conditions: diazirines 2–4 (12.5 mm) and Ir precatalyst A (125 μm) in [D4]MeOH. [a] Signal enhancement measured at 8.45 T and calculated by comparison to a reference of neat 15N-acetonitrile. [b] T1 measured at 1 T. Mes=2,4,6-trimethylphenyl; py=pyridyl; TBS=tert-butyldimethylsilyl.
During the SABRE-SHEATH hyperpolarization of diazirines 2–4, no hyperpolarization was observed for any substrate when the neutral Ir-chloride precatalyst B was employed under identical conditions to those with complex A (Table 1, entries 1, 5, and 9). Interestingly, the addition of exogenous Lewis bases restored effective hyperpolarization with complex B. For example, in the presence of pyridine, diazirines 2–4 were hyperpolarized with T1 values of 2–4 min (entries 2, 6, and 10). Acetonitrile as an additive also enabled signal enhancements to be observed (entries 3, 7, and 11), although the volatility of this additive proved problematic in some instances (e.g., low signal enhancement and large T1 error with silyl ether-containing diazirine 4, entry 11). It is noteworthy that diazirine 2, bearing a cyano group, is insufficient on its own to allow for SABRE hyperpolarization but exogenous nitrile, even though in lower concentration, is able to reverse this deficiency. The addition of D2O as a Lewis base is also sufficient to bring about diazirine hyperpolarization (entries 4, 8, and 12); these conditions are similar to those originally reported for 1. Presumably the smaller steric presence of D2O compared to [D4]MeOH, the solvent, is the prime factor in facilitating SABRE-SHEATH in these instances even though the D2O concentration is significantly lower. Signal enhancements and T1 times are similar to those with pyridine or acetonitrile additives.
Table 1:
Hyperpolarization of diazirines 2–4 with Ir-chloride precatalyst B via the SABRE-SHEATH method.[a]
| ||||
|---|---|---|---|---|
|
| ||||
| Entry | Diazirine | Additive | Ɛ[e] | T1 [s] [f] |
|
| ||||
| 1 |
|
none | _[g] | n/a |
| 2[b] | pyridine | 3500 | 168 ± 18 | |
| 3[c] | MeCN | 3400 | 179 ± 16 | |
| 4[d] | D2O | 11200 | 176 ± 3 | |
| 5 |
|
none | _[g] | n/a |
| 6[b] | pyridine | 6000 | 215 ± 22 | |
| 7[c] | MeCN | 3200 | 172 ± 17 | |
| 8[d] | D2O | 1900 | 234 ± 28 | |
| 9 |
|
none | _[g] | n/a |
| 10[b] | pyridine | 16 000 | 141 ± 4 | |
| 11 [c] | MeCN | 1000 | 263 ± 83[h] | |
| 12[d] | D2O | 2500 | 164 ± 25 | |
Conditions for all experiments: diazirines 2–4 (12.5 mm) and Ir precatalyst B (125 μm) in [D4]MeOH. See the Supporting Information for more details on the hyperpolarization method.
Pyridine (1.00 mm, 8 equiv with respect to B).
Acetonitrile (1.00 mm).
D2O (925 mm).
Signal enhancement measured at 8.45 T and calculated by comparison to a reference of neat 15N-acetonitrile.
T1 measured at 1 T. See the Supporting Information for measurements at other fields.
No hyperpolarized signal observed.
The low signal enhancement and volatility of acetonitrile contribute to the larger T1 error. n/a=not applicable.
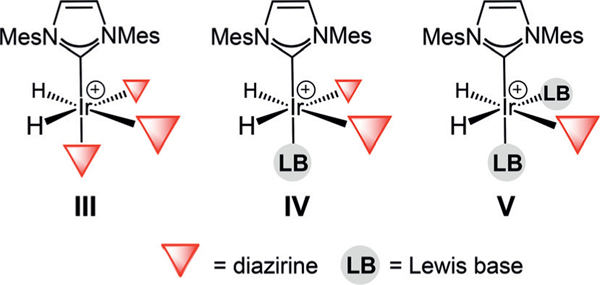
The fact that no signal enhancement was observed for any of the diazirines 2–4 with precatalyst B in the absence of a Lewis basic additive suggests that the diazirine moiety is either incapable of substituting the chloro ligand of B in the catalyst initiation step and/or of forming an active catalyst with three facially-coordinated diazirine ligands due to steric congestion (Figure 2, complex III). Attempts to substitute the chloride in complex B for a diazirine stoichiometrically, which would then lead to III upon para-hydrogen addition, failed to generate any observable reaction. We cannot rule out that III might be formed in small quantities in the presence of one of the Lewis basic additives; however, it is most likely that polarization transfer occurs from either complex IV or V.
Figure 2.
Possible coordination modes for SABRE-promoted hyperpolarization of diazirines.
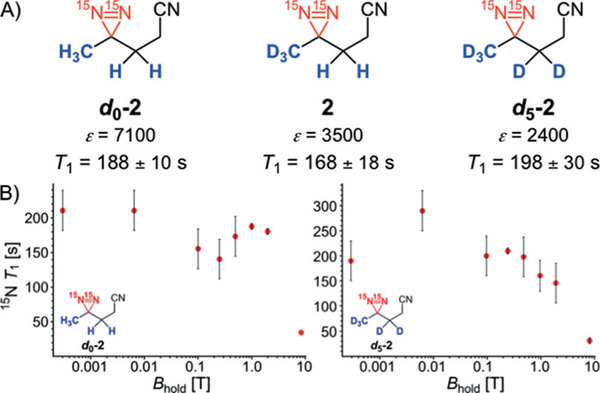
We prepared a series of isotopomers of diazirine 2 to determine the effect of deuteration at the α-positions of the diazirine motif on hyperpolarization and T1 relaxation times (Figure 3). We used Ir complex B in the presence of pyridine additive for a comparative study. Diazirines d0-2, 2, and d5-2 all provide comparable T1 values of approximately three minutes at 1 T (Figure 3A), showing that deuterium incorporation does not strongly affect T1 relaxation. The negligible effect of deuterium is consistent with small differences of T1 between d0-2 and d5-2(Figure 3B).[14]
Figure 3.
Effect of deuteration on T1-relaxation rates and enhancements Ɛ of 15N2-diazirine 2. A) Negligible effect of deuteration of diazirines 2 on T1-relaxation rates at 1 T (within the experimental error). B) T1-relaxation rate for 15N polarization of d0−2 and d5-2 as a function of the magnetic field. SABRE-SHEATH conditions: diazirine d0-2, 2, or d5-2 (12.5 mm) in [D4]MeOH, Ir precatalyst B (125 μm), and pyridine (1.00 mm).
The study of the field dependence of T1 relaxation rates is of special interest (Figure 3B) because for body-noise dominated hyperpolarized MRI, the signal-to-noise is roughly field independent whereas signal decay time constants are not.[15] Thus, it is important for future applications and choice of optimal MRI field strength to understand at what magnetic field we observe longest hyperpolarization lifetimes. Mechanistically, SABRE-SHEATH involves coherent polarization transfer from p-H2 to 15N at the region of Level Anti Crossings,[16] which is most efficient at about 0.6 μT for the characteristic JHH and JNH couplings in iridium-diazirine complexes. Commercial MRI operates between 1 T and 4 T. It is therefore noteworthy that T1 did not vary significantly over a large magnetic field range, falling significantly only at very high field strength (Figure 3B). The data indicate that magnetic fields of about 1 T and slightly below may be most advantageous for future applications, which is intriguing given the ongoing progress in low-field NMR[17] and low-field MRI.[18]
We have established the independence of the 15N2-diazirine motif for SABRE-SHEATH hyperpolarization and its ability to deliver long-lasting polarization. To demonstrate the applicability of 15N2-diazirines as molecular tags for biologically important molecules, we prepared 15N2-diazirine-tagged choline derivative 5 (Figure 4). It has been shown that choline analogues, in which a methyl group is replaced with an alkyl chain of up to five carbon atoms in length, incorporate efficiently into phospholipids.[19] Subjecting 5 to SABRE-SHEATH hyperpolarization with Ir precatalyst B in the presence of pyridine or D2O as the Lewis base leads to greater than 2000-fold signal enhancement with T1 of approximately three minutes. Compared to 15N-choline hyperpolarized by dynamic nuclear polarization (DNP),[20] the effectiveness observed on hyperpolarized 5 by SABRE-SHEATH is comparable yet SABRE-SHEATH is operationally a simpler and more economical protocol. Particularly encouraging is the effective enhancement and long-lasting polarization observed in the presence of D2O, presenting an important first step toward biomedical in vivo applications with this tag.
Figure 4.
Hyperpolarization of 15N2-diazirine-tagged choline derivative 5. SABRE-SHEATH conditions: 5 (12.5 mm) and Ir precatalyst B (125 μm) in [D4]MeOH with either pyridine (1.00 mm) or D2O (925 mm). Signal enhancement measured at 8.45 T and calculated by comparison to a reference of neat 15N-acetonitrile. T1 measured at 1 T. See the Supporting Information for more measurements at other fields. Ts=p-toluenesulfonyl.
In summary, we have established the independence of the 15N2− diazirine group from other functional groups for SABRE-SHEATH hyperpolarization and its ability to support long-lasting hyperpolarization for signal enhancement, marking its potential as a molecular tag for NMR and MRI. Furthermore, the studies on different SABRE-SHEATH hyperpolarization conditions reveal a critical contribution of Lewis basic additives for generating active Ir catalysts for polarization transfer. These studies also provide some structural information regarding the likely coordination sphere at Ir in SABRE-SHEATH with diazirines. Finally, successful hyperpolarization of a 15N2-diazirine-containing choline derivative demonstrates the applicability of 15N2-diazirine tags within biologically relevant molecules. Future studies are directed towards incorporating 15N2-diazirines into other biologically active molecules and their studies in molecular imaging.
Supplementary Material
Acknowledgements
We gratefully acknowledge the NSF (CHE-1363008 to W.S.W.), Camille and Henry Dreyfus Foundation (Teacher-Scholar Award to Q.W.), and Duke University for financial support of this work.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201704970.
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Dr. Kun Shen, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
Dr. Angus W. J. Logan, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
Dr. Johannes F. P. Colell, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
Junu Bae, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
Gerardo X. Ortiz, Jr., Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA)
Prof. Thomas Theis, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA)
Prof. Warren S. Warren, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA); Department of Physics, Duke University (USA); Department of Radiology, Duke University (USA).
Prof. Steven J. Malcolmson, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
Prof. Qiu Wang, Department of Chemistry, Duke University, French Family Science Center, 124 Science Drive, Durham, NC 27708 (USA).
References
- [1].a) Logothetis NK, Nature. 2008, 453, 869–878; [DOI] [PubMed] [Google Scholar]; b) Ardenkjaer-Larsen JH, Boebinger GS, Comment A, Duckett S, Edison AS, Engelke F, Griesinger C, Griffin RG, Hilty C, Maeda H, Parigi G, Prisner T, Ravera E, van Bentum J, Vega S, Webb A, Luchinat C, Schwalbe H, Frydman L, Angew. Chem. Int. Ed 2015, 54, 9162–9185; Angew. Chem. 2015, 127, 9292–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].a) Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K, Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR, Neoplasia 2011, 13, 81–97; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gottberg A, Stachura M, Kowalska M, Bissell ML, Arcisauskaite V, Blaum K, Helmke A, Johnston K, Kreim, Larsen FH, Neugart R, Neyens G, Ruiz RFG, Szunyogh D, Thulstrup PW, Yordanov DT, Hemmingsen L , ChemPhysChem 2014, 15, 3929–3932. [DOI] [PubMed] [Google Scholar]
- [3].a) Nelson SJ, Kurhanewicz, Vigneron DB, Larson PEZ, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA, Sci. Transl. Med 2013, 5, 198ra108; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Pileio G, Carravetta M, Hughes E, Levitt MH, J. Am. Chem. Soc 2008, 130, 12582–12583; [DOI] [PubMed] [Google Scholar]; c) Feng Y, Theis T, Liang, Wang Q, Zhou P, Warren WS, J. Am. Chem. Soc 2013, 135, 9632–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].a) Nikolaou P, Goodson BM, Chekmenev EY, Chem. Eur. J 2015, 21, 3156–3166; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Halse ME, TrA. Trends Anal. Chem 2016, 83, 76–83; [Google Scholar]; c) Kuhn LT, Hyperpolarization Methods in NMR Spectroscopy, Vol. 338, 1st ed., Springer, Berlin, 2013. [Google Scholar]
- [5].a) Bouchard LS, Burt SR, Anwar MS, Kovtunov KV, Koptyug IV, Pines A, Science. 2008, 319, 442–445; [DOI] [PubMed] [Google Scholar]; b) Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano, Williamson DC, Science 2009, 323, 1708–1711; [DOI] [PubMed] [Google Scholar]; c) Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Lopez-Serrano, Whitwood AC, J. Am. Chem. Soc 2009, 131, 13362–13368. [DOI] [PubMed] [Google Scholar]
- [6].a) Bowers CR in Encyclopedia of Nuclear Magnetic Resonance, Vol. 9 (Eds.: Grant DM, Harris RK), Wiley, Chichester, 2002, pp. 750–770; [Google Scholar]; b) Bowers CR, Weitekamp DP, Phys. Rev. Lett 1986, 57, 2645–2648; [DOI] [PubMed] [Google Scholar]; c) Bowers CR, Weitekamp DP, J. Am. Chem. Soc 1987, 109, 5541–5542; [Google Scholar]; d) Eisenschmid TC, Kirss RU, Deutsch PP, Hommeltoft SI, Eisenberg R, Bargon J, Lawler RG, Balch AL, J. Am. Chem. Soc 1987, 109, 8089–8091; [Google Scholar]; e) Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS, Magn. Reson. Med 2001, 46, 1–5. [DOI] [PubMed] [Google Scholar]
- [7].Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D, Mewis RE, J. Am. Chem. Soc 2011, 133, 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Theis T, Truong ML, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY, J. Am. Chem. Soc 2015, 137, 1404–1407; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Truong ML, Theis T, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS, Chekmenev EY, J. Phys. Chem. C 2015, 119, 8786–8797; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Shchepin RV, Barskiy DA, Coffey AM, Theis T, Shi F, Warren WS, Goodson BM, Chekmenev EY, ACS Sens. 2016, 1, 640–644; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Colell JFP, Logan AWJ, Zhou ZJ, Shchepin RV, Barskiy DA, Ortiz GX, Wang Q, Malcolmson SJ, Chekmenev EY, Warren WS, Theis T, J. Phys. Chem. C 2017, 121, 6626–6634; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Colell JFP, Emondts M, Logan AWJ, Shen, Bae J, Shchepin RV, Ortiz GX Jr., Spannring P, Wang Q, Malcolmson SJ, Chekmenev EY, Feiters MC, Rutjes F, Blumich B, Theis T, Warren WS, J. Am. Chem. Soc 2017, 139, 7761–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].a) Suchanek M, Radzikowska A, Thiele C, Nat. Methods 2005, 2, 261–267; [DOI] [PubMed] [Google Scholar]; b) MacKinnon AL, Garrison JL, Hegde RS, Taunton J, J. Am. Chem. Soc 2007, 129, 14560–14561; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) VilaPerelll M, Pratt MR, Tulin F, Muir TW, J. Am. Chem. Soc 2007, 129, 8068–8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].a) Hashimoto M, Hatanaka Y, Eur. J. Org. Chem 2008, 2513– 2523; [Google Scholar]; b) Das J, Chem. Rev 2011, 111, 4405–4417; [DOI] [PubMed] [Google Scholar]; c) Dubinsky L, Krom BP, Meijler MM, Bioorg. Med. Chem 2012, 20, 554–570; [DOI] [PubMed] [Google Scholar]; d) Sumranjit J, Chung SJ, Molecules 2013, 18, 10425–10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Theis T, Ortiz GX, Logan AWJ, Claytor KE, Feng Y, Huhn WP, Blum V, Malcolmson SJ, Chekmenev EY, Wang Q, Warren WS, Sci. Adv 2016, 2, e1501438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zacharias NM, Chan HR, Sailasuta N, Ross BD, Bhattacharya P, J. Am. Chem. Soc 2012, 134, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].a) Albini A, Kisch H, J. Organomet. Chem 1975, 94, 75–85; [Google Scholar]; b) Battaglia R, Kisch H, Kruger C, Liu LK, Z. Naturforsch. B 1980, 35, 719–723; [Google Scholar]; c) Holzmeier P, Gorner H, Knoch F, Kisch H, Chem. Ber 1989, 122, 1457–1463; [Google Scholar]; d) Holzmeier P, Kisch H, Kochi JK, J. Organomet. Chem 1990, 382, 129–141; [Google Scholar]; e) Cohen R, Rybtchinski B, Gandelman M, Rozenberg H, Martin JML, Milstein D, J. Am. Chem. Soc 2003, 125, 6532–6546. [DOI] [PubMed] [Google Scholar]
- [14].a) [See more details in the Supporting Information. T1 as a function of magnetic field is estimated by the signal intensity of the sample after a time delay of 60 s upon subjection to the SABRE-SHEATH procedure.]; b) A noticable difference was observed for enhancement levels of d0–2, 2, and d5–2, which might be contributed by the coupling between 15N and deuterium. Due to the relatively large error range in the current experimental data, it remains to be confirmed in further studies.
- [15].a) Parra-Robles J, Cross AR, Santyr GE, Med. Phys 2005, 32, 221–229; [DOI] [PubMed] [Google Scholar]; b) Coffey AM, Truong ML, Chekmenev EY, J. Magn. Reson 2013, 237, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].a) Barskiy DA, Pravdivtsev AN, Ivanov KL, Kovtunov KV, Koptyug IV, Phys. Chem. Chem. Phys 2016, 18, 89–93; [DOI] [PubMed] [Google Scholar]; b) Ivanov KL, Pravdivtsev AN, Yurkovskaya AV, Vieth HM, Kaptein R, Prog. Nucl. Magn. Reson. Spectrosc 2014, 81, 1–36. [DOI] [PubMed] [Google Scholar]
- [17].a) Theis T, Ganssle P, Kervern G, Knappe S, Kitching J, Ledbetter MP, Budker D, Pines A, Nat. Phys 2011, 7, 571–575; [Google Scholar]; b) Theis T, Ledbetter MP, Kervern G, Blanchard JW, Ganssle PJ, Butler MC, Shin HD, Budker D, Pines A, J. Am. Chem. Soc 2012, 134, 3987–3990; [DOI] [PubMed] [Google Scholar]; c) Suefke M, Lehmkuhl S, Liebisch A, Blumich B, Appelt S, Nat. Phys 2017, 13, 568–572; [Google Scholar]; d) Suefke M, Liebisch A, Blumich B, Appelt S, Nat. Phys 2015, 11, 767–771; [Google Scholar]; e) Colell J, Turschmann P, Gloggler S, Schleker P, Theis T, Ledbetter M, Budker D, Pines A, Blumich B, Appelt S, Phys. Rev. Lett 2013, 110, 137602. [DOI] [PubMed] [Google Scholar]
- [18].a) Sarracanie M, LaPierre CD, Salameh N, Waddington DEJ, Witzel, Rosen MS, Sci. Rep 2015, 5, 15177; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barskiy DA, Salnikov OG, Shchepin RV, Feldman MA, Coffey AM, Kovtunov KV, Koptyug IV, Chekmenev EY, J. Phys. Chem. C 2016, 120, 29098–29106; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vogel MW, Giorni A, Vegh V, Pellicer-Guridi R, Reutens DC, PLoS One 2016, 11, e0157040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].a) Bieber LL, Newburgh RW, J. Lipid Res 1963, 4, 397–401; [PubMed] [Google Scholar]; b) Jao CY, Roth M, Welti R, Salic A, Proc. Natl. Acad. Sci. USA 2009, 106, 15332–15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].a) Gabellieri C, Reynolds S, Lavie A, Payne GS, Leach MO, Eykyn TR, J. Am. Chem. Soc 2008, 130, 4598–4599; [DOI] [PubMed] [Google Scholar]; b) Sarkar R, Comment A, Vasos PR, Jannin S, Gruetter R, Bodenhausen G, Hall H, Kirik D, Denisov VP, J. Am. Chem. Soc 2009, 131, 16014–16015; [DOI] [PubMed] [Google Scholar]; c) Cudalbu C, Comment A, Kurdzesau F, van Heeswijk RB, Uffmann K, Jannin S, Denisov V, Kirik D, Gruetter R, Phys. Chem. Chem. Phys 2010, 12, 5818–5823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.