Abstract
Background:
Reducing transmission depends on the percentage of infected partners treated; if many are missed, impact on transmission will be low. Traditional partner services metrics evaluate the number of partners found and treated. We estimated the proportion of partners of syphilis patients not locatable for intervention.
Methods:
We reviewed records of early syphilis cases (primary, secondary, early latent) reported during 2015-2017 in seven jurisdictions (Florida, Louisiana, Michigan, North Carolina, Virginia, New York City, and San Francisco). Among interviewed syphilis patients, we determined the proportion who reported named partners (with locating information), reported unnamed partners (no locating information), and did not report partners. For patients with no reported partners, we estimated their range of unreported partners to be between one and the average number of partners for patients who reported partners.
Results:
Among 29,719 syphilis patients, 23,613 (80%) were interviewed and 18,581 (63%) reported 84,224 sex partners (average=4.5; 20,853 (25%) named and 63,371 (75%) unnamed). An estimated 11,138 to 54,521 partners were unreported. Thus, 74,509 to 117,892 (of 95,362 to 138,745) partners were not reached by partner services (78--85%). Among interviewed patients, 71% reported ≥1 unnamed partner or reported no partners; this proportion was higher for men who reported sex with men [MSM] (75%), compared to men who reported sex with women only (65%), and women (44%).
Conclusion:
Approximately 80% of sex partners were either unnamed or unreported. Partner services may be less successful at interrupting transmission in MSM networks where a higher proportion of partners are unnamed or unreported.
Short Summary:
Among partner services programs in seven US jurisdictions, most sex partners of early syphilis patients (80%) are unlocatable or unreported, limiting the potential for partners services to intervene on transmission.
Introduction
Health departments across the United States conduct partner services to assure syphilis treatment of sex partners to disrupt syphilis transmission. To accomplish these goals, disease intervention specialists (DIS) interview persons newly diagnosed with syphilis to elicit, find, test, and treat sex partners (1). Traditional measures to assess the effectiveness of partner services focus primarily on partners whom DIS find for testing and treatment. However, measuring the number of partners that DIS cannot locate can inform programs about the limits of partner services for preventing transmission in the community. Past estimates from both Oregon and Fulton County, Georgia indicate that between 75--80% of all reported partners cannot be found by DIS (2,3). In addition, a multi-jurisdictional analysis from 2003 found syphilis cases among men who have sex with men reported an average of three to ten partners, but DIS were only able to contact 0.4 to 1.5 partners per patient (4).
Partner services programs are unable to test and treat all sex partners of reported syphilis patients if 1) syphilis patients are not interviewed by DIS, 2) interviewed patients do not report all (or any) of their sex partners to DIS, or 3) interviewed patients report partners, but do not provide enough information for DIS to find the sex partner. The reasons syphilis patients underreport sex partners are likely multifaceted and include: fear of consequences due to naming partners, distressed emotional state at the time of the interview, desire for privacy, lack of motivation to report partners, shortage of time during interviews, memory issues, lack of a desire to admit to stigmatizing behaviors, and the perception that it is not important or valuable to participate in health department sponsored partner services (5,6). Furthermore, many patients may prefer to notify partners themselves, rather than have health department staff do it (7). Previous findings suggest that patients with a history of syphilis who have previously interacted with DIS are less willing to cooperate during future partner services encounters (8). Finally, some patients cannot contact all their sex partners because encounters were anonymous and increasingly facilitated by the proliferation of online dating websites and apps (6,9).
Partner services is a critical component of programmatic efforts to prevent STDs at the state and local level. The success of these programs at preventing transmission depends on the proportion of infected partners that are reached. To quantify the proportion of the syphilis transmission network that is not reached, we estimated the number of partners that were unreported and the number that were reported without names or adequate locating information in seven jurisdictions in the United States. We also assessed the characteristics of interviewed syphilis patients who did not provide locating information for any partners.
Methods
Providers and laboratories report cases of syphilis state or local health departments per state and local mandates (10). Dependent on case volume, staff resources, and public health priorities at any given time, DIS attempt to contact newly diagnosed syphilis patients to assure treatment, obtain information about all sexual partners that may have transmitted or acquired infection, and locate, test, and treat as many partners as possible. Jurisdictions decide which syphilis patients are prioritized for these partner services, usually based on local epidemiology and the likelihood of interrupting transmission (with primary and secondary syphilis being most likely) or preventing serious sequelae. Each jurisdiction maintains surveillance and field investigation information about syphilis patients and their partners in databases tailored to local needs and priorities.
For this analysis, we requested a de-identified dataset that included diagnosis and treatment dates, demographics, gender of sex partners, date of partner services interview, and any STD diagnosis history (as determined by either self-report or documented in a surveillance data system) for all cases of primary, secondary, and early latent syphilis reported in 2015 and 2016 from New York City, San Francisco, Florida, Louisiana, Michigan, North Carolina, and in 2016 and 2017 from Virginia. Each jurisdiction provided the total number of partners reported by each interviewed patient during their infectious period as determined by the stage of disease — the infectious period for primary syphilis was three months prior to the onset of symptoms; secondary was six months prior to the onset of symptoms; and early latent was twelve months prior to diagnosis. Reported partners were classified as “named” if the syphilis patient provided the DIS with enough locating information to initiate an investigation. Otherwise, we classified partners as “unnamed”. The type and amount of locating information necessary to initiate an investigation was determined locally and varied by jurisdiction. A linking identifier connected each partner in the partner dataset to the syphilis patient who named them.
To estimate the number of potentially exposed partners in each jurisdiction during the two-year period, we summed the number of named and unnamed partners reported by interviewed patients. Among interviewed patients who reported at least one partner, we calculated the median and average number of reported, named, and unnamed partners per patient. We then estimated a range for the number of unreported partners among patients who did not report any partners (including both patients who were interviewed and patients who were not interviewed). The minimum estimate was one per patient who did not report any partners. For the maximum estimate, we used the average number of partners reported by interviewed patients who reported a partner in the patient’s jurisdiction. We considered the total syphilis transmission network to be comprised of the reported partners (named and unnamed) and the estimated unreported partners (using both the minimum and maximum estimates). We conducted this analysis for the total patient population and by gender and gender of sex partners. We classified male patients that either named a male partner or reported sex with a male partner as MSM. All other male patients who reported sex with only women were classified as men who have sex with women (MSW). The remaining group of male patients for whom we had no information about the gender of their sex partners were classified as “unknown men.” We calculated unreported partner estimates for women, regardless of the gender of their sex partners. Because not all jurisdictions identified transgender patients in their data and numbers were small, we did not calculate unreported partner estimates specifically for this group. Transgender patients are, however, included in the total estimates.
Next, among patients who were interviewed in each of the seven jurisdictions, we determined the proportion who reported 1) only named partners, 2) both named and unnamed partners, 3) only unnamed partners, and 4) no partners (whether named or unnamed). We also estimated these proportions and 95% Clopper Pearson confidence intervals among interviewed patients by gender and gender of sex partners (MSM, MSW, and women), syphilis stage, and history of a syphilis diagnosis (yes versus no).
We conducted all analyses jointly and separately, by jurisdiction, using SAS version 9.4 (Cary, NC). This evaluation of public health program data received a non-research determination by the Centers for Disease Control and Prevention.
Results
A total of 29,719 primary, secondary, and early latent syphilis case patients were reported in the seven jurisdictions during the two-year analysis period (range per jurisdiction: 1,411 in Michigan to 9,433 in Florida), of whom 23,613 (80%) were interviewed (range per jurisdiction: 50% in San Francisco to 99.5% in Florida). Most interviewed patients had either secondary (37%) or early latent syphilis (47%). Not all jurisdictions regularly assign early latent syphilis patients for partner services interview, so the proportion of interviewed patients with early latent syphilis varied by jurisdiction (26% in San Francisco to 55% in Virginia). The median time between diagnosis and interview was 14 days (interquartile range (IQR) 6-28 days). The median age of interviewed patients was 31 years (IQR 25-41) [Table 1]. Interviewed patients were overwhelmingly male (N=20,603; 87%), most of whom were MSM (N=16,637; 81%). Nearly half of interviewed patients were living with HIV (48%, N=11,245) and 57% (N=13,460) had previously been diagnosed with an STD. Five sites were able to specify whether or not an interviewed patient had a previous syphilis diagnosis (Louisiana and Michigan could not); 27% (N=6413) of patients in these jurisdictions had a prior history of syphilis.
Table 1.
Demographic and clinical characteristics of interviewed primary, secondary & early latent syphilis patients in 7 US jurisdictions, 2015-2016
| All Sites | Florida | Louisiana | Michigan | North Carolina | Virginia (2016-2017) |
NYC | San Francisco | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total reported | N= 29,719 | N= 9,433 | N= 2,453 | N= 1,411 | N= 3,779 | N= 1,470 | N= 8,830 | N= 2,343 | ||||||||
| Total interviewed | N= 23,613 | N= 9,388 | N= 2,346 | N= 1,291 | N= 3,727 | N= 1,243 | N= 4,445 | N= 1,173 | ||||||||
| Age (years) | ||||||||||||||||
| Median (IQR) | 31 (25-41) | 33 (26-45) | 26 (22-34) | 28 (24-38) | 30 (24-41) | 29 (24-38) | 31 (26-37) | 38 (30-48) | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Gender | ||||||||||||||||
| Male | 20,603 | 87% | 8,322 | 89% | 1,680 | 72% | 1,121 | 87% | 3,222 | 87% | 1,043 | 84% | 4,110 | 93% | 1,105 | 94% |
| Female | 2,853 | 12% | 1,066 | 11% | 666 | 28% | 131 | 10% | 483 | 13% | 185 | 15% | 276 | 6% | 46 | 4% |
| Transgender | 118 | 1% | 0 | 0% | 0 | 0% | 0 | 0% | 22 | 1% | 15 | 1% | 59 | 1% | 22 | 2% |
| Unknown | 39 | 0% | 0 | 0% | 0 | 0% | 39 | 3% | 0 | 0% | 0 | 0% | 0 | 0% | 0 | 0% |
| If male, sex of sex partners 1 | ||||||||||||||||
| MSM | 16,637 | 81% | 6,824 | 82% | 1,032 | 62% | 990 | 88% | 2,515 | 78% | 842 | 81% | 3,379 | 82% | 1,055 | 96% |
| MSW Only | 2,721 | 13% | 1,092 | 13% | 491 | 29% | 106 | 10% | 510 | 16% | 172 | 16% | 312 | 8% | 38 | 3% |
| Unknown Men | 1,245 | 6% | 406 | 5% | 157 | 9% | 25 | 2% | 197 | 6% | 29 | 3% | 419 | 10% | 12 | 1% |
| Race/Ethnicity | ||||||||||||||||
| White, non-Hispanic | 6,510 | 28% | 3,015 | 32% | 493 | 21% | 468 | 36% | 976 | 26% | 271 | 22% | 700 | 16% | 587 | 50% |
| Black, non-Hispanic | 10,315 | 44% | 3,151 | 34% | 1,782 | 76% | 677 | 52% | 2,374 | 64% | 702 | 57% | 1,488 | 34% | 141 | 12% |
| Hispanic | 4,960 | 21% | 2,794 | 30% | 50 | 2% | 71 | 6% | 246 | 7% | 131 | 11% | 1,393 | 31% | 275 | 23% |
| Other Race, non-Hispanic | 1,412 | 6% | 217 | 2% | 21 | 1% | 30 | 2% | 129 | 4% | 27 | 2% | 829 | 19% | 159 | 14% |
| Unknown/refused/missing | 416 | 2% | 211 | 2% | 0 | 0% | 45 | 4% | 2 | 0% | 112 | 9% | 35 | 1% | 11 | 1% |
| Syphilis stage | ||||||||||||||||
| Primary | 3,844 | 16% | 1,270 | 14% | 397 | 17% | 260 | 20% | 704 | 19% | 166 | 13% | 645 | 15% | 402 | 34% |
| Secondary | 8,756 | 37% | 3,229 | 34% | 1,049 | 45% | 484 | 38% | 1,511 | 41% | 397 | 32% | 1,616 | 36% | 470 | 40% |
| Early latent | 11,013 | 47% | 4,889 | 52% | 900 | 38% | 547 | 42% | 1,512 | 41% | 680 | 55% | 2,184 | 49% | 301 | 26% |
| HIV-Infected 2 | ||||||||||||||||
| Yes | 11,245 | 48% | 4,785 | 51% | 708 | 30% | 543 | 42% | 1,648 | 44% | 496 | 40% | 2,506 | 56% | 559 | 48% |
| Previous STD diagnosis ever 3 | ||||||||||||||||
| Any STD4 | 13,486 | 57% | 4,674 | 50% | 1,278 | 55% | 505 | 39% | 2,571 | 69% | 660 | 53% | 2,955 | 67% | 843 | 72% |
| Syphilis (any stage) | 6,413 | 32% | 2,851 | 30% | NA | NA | NA | NA | 1,209 | 32% | 246 | 20% | 1,720 | 39% | 387 | 33% |
MSM=Men who reported sex with men; MSW Only=Men who reported only sex with women; Men who without a reported sex of sex partners and subsequently could not be classified as either “MSM” or “MSW Only” were classified as “Unknown Men”.
HIV infection status was verified by matching to HIV surveillance within each jurisdiction.
Previous STD diagnosis was determined by each jurisdiction and was defined by both self-report and/or matching to STD surveillance.
Any STD was defined by each jurisdiction. STDs include, but are not limited to, syphilis (any stage), chlamydia, and gonorrhea.
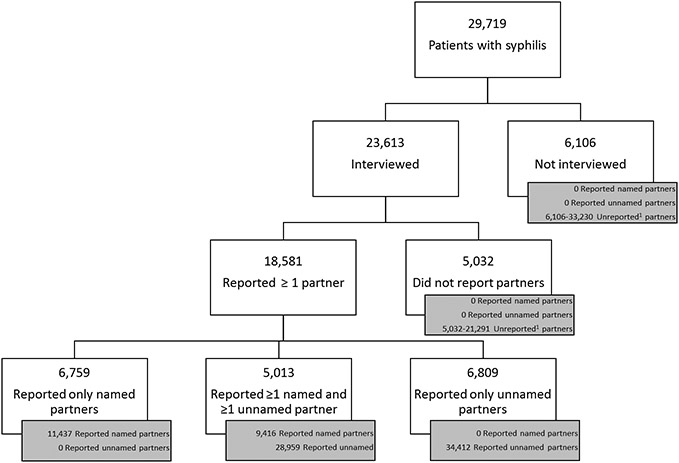
Of the 29,719 patients with syphilis, 18,581 (63%) reported 84,224 sex partners (average 4.5 partners per patient reporting at least one partner) [Figure 1]. Most reported partners were unnamed (75%, N=63,371) and could not be located. In total, 6,106 (21%) patients were not interviewed and 5,032 (17%) were interviewed but did not report any partners. If each of these patients had only one partner, they would have had 11,138 unreported partners; however, if they had the same number of partners as the average patient who disclosed sex partners to DIS in their jurisdiction (range 2.1 in Virginia to 11.2 in San Francisco, Table 2), then they would have had a total of 54,521 unreported partners. When the upper and lower limits are summed with the 63,371 unnamed partners that were reported to DIS, we estimated between 74,509 and 117,892 partners of syphilis patients in these jurisdictions could not be reached by partner services, representing 78% to 85% of all partners [74,509/(11,138 unreported+63,371 unnamed+20,853 named partners) to 117,892/(54,521 unreported+63,371 unnamed+20,853 named partners)]. Among interviewed patients who reported at least one partner overall, MSM reported the most partners per patient (5.8) as compared to MSW (2.3) and women (2.4) [Table 2]. Using the population-specific average within each jurisdiction, we estimated that unnamed and unreported partners represented between 80 to 85% of partners of MSM, 63 to 66% of partners of MSW, and 55 to 59% of partners of women [Supplemental Figures 1a-c].
Figure 1. Number of reported named, reported unnamed, and estimated unreported partners from syphilis partner services in 7 US jurisdictions.
1. Unreported partners=For patients who did not report ≥1 named or unnamed partner, we estimated the number of unreported partners to be between 1 to the average number of partners reported by patients who did report ≥1 partner in the patient’s jurisdiction (range per jurisdiction 2.1 to 11.2; Table 2). We could not calculate the number of unreported partners for patients who reported ≥1 partner.
Table 2.
Number of reported, named and unnamed partners of persons reported to have syphilis by gender and gender of sex partners in 7 US jurisdictions, 2015-2016
| All Sites | Florida | Louisiana | Michigan | North Carolina |
Virginia (2016-17) |
New York City | San Francisco | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | ||
| Total 1 | Reported patients | 29,719 | 9,433 | 2,453 | 1,411 | 3,779 | 1,470 | 8,830 | 2,343 | ||||||||
| Interviewed patients | 23,613 | 79% | 9388 | 100% | 2,346 | 96% | 1,291 | 91% | 3,727 | 99% | 1,243 | 85% | 4,445 | 50% | 1,173 | 50% | |
| Patients who reported ≥1 partner | 18,581 | 63% | 7,624 | 81% | 1,818 | 74% | 1,143 | 81% | 3,225 | 85% | 853 | 58% | 2,848 | 32% | 1,070 | 46% | |
| Reported partners (per patient2) | 84,224 | (4.5) | 38,923 | (5.1) | 5,011 | (2.8) | 5,261 | (4.6) | 9,328 | (2.9) | 1,818 | (2.1) | 11,863 | (4.2) | 12,020 | (11.2) | |
| Named partners (per patient2) | 20,853 | (1.1) | 7,194 | (0.9) | 1,931 | (1.1) | 1,756 | (1.5) | 4,523 | (1.4) | 1,298 | (1.5) | 2,226 | (0.8) | 1,925 | (1.8) | |
| Unnamed partners (per patient2) | 63,371 | (3.4) | 31,729 | (4.2) | 3,080 | (1.7) | 3,505 | (3.1) | 4,805 | (1.5) | 520 | (0.6) | 9,637 | (3.4) | 10,095 | (9.4) | |
| MSM 3 | Reported patients | 18,771 | 6,850 | 1,033 | 1,068 | 2,529 | 887 | 4,361 | 2,043 | ||||||||
| Interviewed patients | 16,637 | 89% | 6,824 | 100% | 1,032 | 100% | 990 | 93% | 2,515 | 99% | 842 | 95% | 3,379 | 77% | 1,055 | 52% | |
| Patients who reported ≥1 partner | 13,363 | 71% | 5,585 | 82% | 790 | 76% | 874 | 82% | 2,286 | 90% | 546 | 62% | 2,307 | 53% | 975 | 48% | |
| Reported partners (per patient2) | 71,288 | (5.3) | 33,272 | (6.0) | 2,917 | (3.7) | 4,641 | (5.3) | 7,022 | (3.1) | 1,274 | (2.3) | 10,629 | (4.6) | 11,533 | (11.8) | |
| Named partners (per patient2) | 15,466 | (1.2) | 5,299 | (0.9) | 854 | (1.1) | 1,384 | (1.6) | 3,379 | (1.5) | 894 | (1.6) | 1,824 | (0.8) | 1,832 | (1.9) | |
| Unnamed partners (per patient2) | 55,822 | (4.2) | 27,973 | (5.0) | 2,063 | (2.6) | 3,257 | (3.7) | 3,643 | (1.6) | 380 | (0.7) | 8,805 | (3.8) | 9,701 | (9.9) | |
| MSW Only 3 | Reported patients | 2,781 | 1,095 | 491 | 107 | 517 | 175 | 339 | 57 | ||||||||
| Interviewed patients | 2,721 | 98% | 1,092 | 100% | 491 | 100% | 106 | 99% | 510 | 99% | 172 | 98% | 312 | 92% | 38 | 67% | |
| Patients who reported ≥1 partner | 2,413 | 87% | 1,022 | 93% | 427 | 87% | 106 | 99% | 470 | 91% | 129 | 74% | 225 | 66% | 34 | 60% | |
| Reported partners (per patient2) | 5,653 | (2.3) | 2,531 | (2.5) | 972 | (2.3) | 267 | (2.5) | 1,061 | (2.3) | 234 | (1.8) | 463 | (2.1) | 125 | (3.7) | |
| Named partners (per patient2) | 2,218 | (0.9) | 865 | (0.8) | 418 | (1.0) | 126 | (1.2) | 487 | (1.0) | 158 | (1.2) | 145 | (0.6) | 19 | (0.6) | |
| Unnamed partners (per patient2) | 3,435 | (1.4) | 1,666 | (1.6) | 554 | (1.3) | 141 | (1.3) | 574 | (1.2) | 76 | (0.6) | 318 | (1.4) | 106 | (3.1) | |
| Unknown Men 3 | Reported patients | 4,850 | 415 | 243 | 33 | 223 | 193 | 3,601 | 142 | ||||||||
| Interviewed patients | 1,245 | 26% | 406 | 98% | 157 | 65% | 25 | 76% | 197 | 88% | 29 | 15% | 419 | 12% | 12 | 8% | |
| Patients who reported ≥1 partner | 144 | 3% | 53 | 13% | 3 | 1% | 0 | 0% | 26 | 12% | 1 | 1% | 60 | 2% | 1 | 1% | |
| Reported partners (per patient2) | 426 | (3.0) | 225 | (4.2) | 8 | (2.7) | 0 | 74 | (2.8) | 2 | (2.0) | 115 | (1.9) | 2 | (2.0) | ||
| Named partners (per patient2) | 0 | (0.0) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| Unnamed partners (per patient2) | 426 | (3.0) | 225 | (4.2) | 8 | (2.7) | 0 | 74 | (2.8) | 2 | (2.0) | 115 | (1.9) | 2 | (2.0) | ||
| Female | Reported patients | 3,069 | 1,073 | 686 | 141 | 488 | 198 | 424 | 59 | ||||||||
| Interviewed patients | 2,853 | 93% | 1,066 | 99% | 666 | 97% | 131 | 93% | 483 | 99% | 185 | 93% | 276 | 65% | 46 | 78% | |
| Patients who reported ≥1 partner | 2,539 | 83% | 964 | 90% | 598 | 87% | 124 | 88% | 425 | 87% | 168 | 85% | 219 | 52% | 41 | 69% | |
| Reported partners (per patient2) | 6,186 | (2.4) | 2,895 | (3.0) | 1,114 | (1.9) | 273 | (2.2) | 1,098 | (2.6) | 294 | (1.8) | 386 | (1.8) | 126 | (3.1) | |
| Named partners (per patient2) | 3,016 | (1.2) | 1,030 | (1.1) | 659 | (1.1) | 177 | (1.4) | 633 | (1.5) | 237 | (1.4) | 232 | (1.1) | 48 | (1.2) | |
| Unnamed partners (per patient2) | 3,170 | (1.2) | 1,865 | (1.9) | 455 | (0.8) | 96 | (0.8) | 465 | (1.1) | 57 | (0.3) | 154 | (0.7) | 78 | (1.9) | |
248 transgender patients not included in gender/risk breakdown, but are included in the total row
Per patient who reported at least 1 partner
MSM=Men who reported sex with men; MSW Only=Men who reported only sex with women; Men who without a reported sex of sex partners and subsequently could not be classified as either “MSM” or “MSW Only” were classified as “Unknown Men”.
The number of named partners per patient was similar by gender and gender of sex partners for interviewed patients who reported at least one partner: MSM (average=1.2; median=1.0), MSW (average=0.9; median=1.0), and women (average=1.2; median=1.0) [Table 2]. By jurisdiction, the average number of named partners per interviewed patient who reported at least one partner ranged from 0.8 in NYC to 1.8 in San Francisco. There was also high variability in the average number of unnamed partners per interviewed patient by jurisdiction (between 0.6 in Virginia and 9.4 in San Francisco).
Half of interviewed patients did not report a named partner (29% [N=6,809] reported only unnamed partners and 21% [N=5,032] did not report any partners). An additional 21% (N=5,016) of interviewed patients reported both named and unnamed partners, resulting in a total of 16,857 (71%) interviewed patients with at least one partner who DIS could not attempt to locate [Figure 2]. This proportion varied by gender and gender of sex partners, with 75% of MSM, 65% of MSW, and 44% of women with at least one unnamed or unreported partner. A higher proportion of interviewed women reported a named partner (73%) compared to MSM (48%) and MSW (58%). Furthermore, 6% (N=1245) of interviewed men could not be classified as either MSM or MSW; most of these men (88%; N=1101) did not report any partners [Supplemental Table 1]. We observed that for interviewed MSM, MSW, and women, patients with a history of syphilis were less likely to report a named partner than patients who had not previously been diagnosed with syphilis. Early latent syphilis patients were more likely to not report any partners than primary and secondary syphilis patients. However, there was no difference in the reporting of named partners by stage [Supplemental Table 1]. Although the exact proportion of interviewed patients with one or more named partners differed by jurisdiction (28% in NYC to 73% in Michigan), we observed that women were more likely to have at least one named partner than MSM or MSW in all jurisdictions.
Figure 2. Proportion of total, MSM, MSW, and female interviewed early syphilis patients1 who reported only named partners, reported named & unnamed partners, reported only unnamed partners, and did not report any partners in 7 US jurisdictions, 2015-2016.
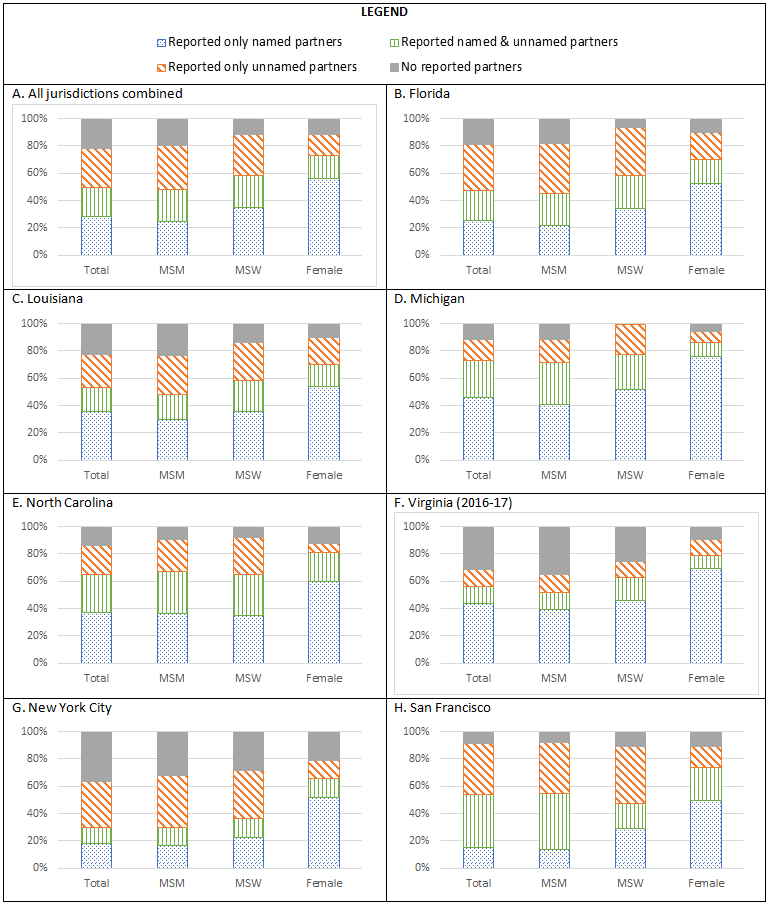
1. MSM=Men who reported sex with men; MSW Only=Men who reported only sex with women. Men who we could not classify as either MSM or MSW are included in the “Total” column, but not in any other column of each chart.
Discussion
The effectiveness of partner services programs at disrupting syphilis transmission is limited when patients do not provide locating information for most of their partners. Similar to previous assessments of partner services programs (2-4), we estimated that approximately 80% of sex partners were either unnamed or unreported and therefore unlocatable for partner services. Evaluations of partner services typically focus on the activities initiated after a patient is interviewed and partners are named (11-14). To understand the impact partner services has on disrupting transmission, it is important to understand what is not counted in traditional metrics. Partner services programs are less likely to interrupt transmission in the community when patients 1) are not interviewed and therefore cannot report partners, 2) are interviewed, but did not report any partners, and 3) are interviewed and only report unnamed partners; approximately 60% of patients under analysis fell into one of these three groups. An additional 17% of patients reported at least one unnamed partner for whom partner services could not be provided and an unknown proportion of patients who reported at least one partner could also have unreported partners at risk for transmitting syphilis. Consequently, even if DIS are successful at finding all named partners, they are only notifying and treating a fraction of potentially exposed partners.
To maximize limited resources, many health departments focus partner services activities on specific populations (12,15,16). In this analysis, approximately three-quarters of interviewed MSM had at least one unreported or unnamed partner. MSW and women also had unreported and unnamed partners, but the proportion was substantially lower than that of MSM. Many partner services programs increase their investigation efforts for women of child-bearing age to prevent congenital syphilis, one of the most severe outcomes associated with syphilis, (17) providing a possible explanation for differences in the number of unreported and unnamed partners across subpopulations. To stop transmission in MSM networks, health departments will most likely need to rely on existing, complementary strategies (e.g., screening, patient and provider education) and work to identify new approaches to reach the partners that cannot be found by traditional partner services methods. Because a high proportion of MSM with syphilis use the internet to meet partners (18,19), increasing the online presence of STD prevention services for MSM has shown some promise. Notification via online apps, e-mail, and text messaging can increase the number of partners found for both syphilis and HIV investigations (20,21), particularly for MSM (22,23). Furthermore, collaborations with online hook-up apps or social media sites to promote public health messaging about syphilis prevention may reach more partners of MSM (24,25).
The proportion of syphilis patients with unnamed partners has remained stable over the past three decades (2-4). However, the reasons for reporting unnamed partners may have changed. Recently reported reasons for not reporting partners include use of dating websites and apps (9) and distrust of public health officials (4). In our analysis, syphilis patients with a prior history of syphilis were less likely to report any partners, whether named or not, than patients with no history of syphilis. Previously diagnosed patients most likely were offered or engaged in partner services before. If these patients had a bad experience or did not understand the benefit of partner services, they may not want to participate fully in another interview (8,26). In this analysis, we could not differentiate partners who were truly anonymous from partners that patients did not want to name. It is possible that partners who were known but not named were later notified of their exposure by the syphilis patient, possibly due to education or encouragement provided by the DIS. Although a randomized control trial found partner notification reaches more partners when done by DIS than when left to patients (27), this study only enrolled 74 patients and was conducted in the 1980s. As such, further investigation is needed to understand the potential role of self-notification in the current context.
For partners who are known to the patient, but remain unnamed, programs may need to explore methods to assure partner notification. Potential strategies that have shown some promise in increasing the number or named partners in certain settings include 1) emphasizing the benefits of partner services at preventing disease within the patient’s own community or sexual network (28), 2) educating providers about the partner services process and level of confidentiality to help prepare patients for their encounters with DIS (6), and 3) utilizing experienced DIS who are successful at eliciting named partners from a high proportion of patients as training and mentoring resources. By helping patients understand the importance of partner services in preventing ongoing transmission in the community, the hope is that more patients tell DIS how to contact their partners. Patients who prefer to notify partners themselves can be educated about self-notification approaches by DIS if interviewed, but unless partner testing and treatment can be verified it will be difficult to know if patients have successfully notified their partners and subsequently measure the impact of partner services programs. Because of their experience finding people and building trust with the public, DIS expertise is often sought for a variety of public health investigations, including most recently, COVID-19 contact tracing investigations (29). The strategies discussed here to elicit named partners could also help improve COVID-19 contact tracing outcomes.
Quantifying unnamed and unreported partnerships based on partner services data almost certainly includes error. It is possible patients do not provide a precise number of unnamed partners. Because programs do not maintain extensive records of unnamed partners, DIS may not accurately capture the number in local data systems. Our maximum unreported partner estimate could be biased if people who did not report partners reported more (or fewer) partners than those who did report partners. Furthermore, because we could not classify MSM or MSW status for many of the men who were not interviewed, our population-specific estimates could be skewed. Although many partners went unnamed or unreported in these seven jurisdictions, some of these partners are represented in surveillance data as either de novo syphilis patients or partners named by other patients. Some of these partners do not have syphilis.
Partner services metrics are difficult to compare across jurisdictions. Local differences in syphilis epidemiology, funding, and staff capacity can result in variability in the patients who are prioritized for DIS interview. Furthermore, jurisdictions utilize data systems that have been tailored to local needs and, as a result, differences in data capture and interpretation are likely. Despite these differences, in every jurisdiction a higher proportion of interviewed female patients named at least one partner compared to MSM and MSW.
Partner services programs cannot find partners for testing and treatment if they do not have the information needed to find them. We found that MSM reported a large number of partners to DIS, suggesting a level of comfort when discussing sexual practices with DIS. However, many MSM do not provide locating information for most of their partners. Consequently, the ability of partner services programs to interrupt syphilis transmission among MSM is limited. Focusing traditional time-intensive partner services on the highest priority patients (women of reproductive age who could deliver babies with congenital syphilis) (17) may make the best use of limited investigational resources. Given the higher rates of syphilis among MSM compared to other groups (30), it may be more efficient to find their infected partners by increasing screening.
Supplementary Material
Acknowledgement:
We would like to thank the Disease Investigation Specialists in each jurisdiction who work to prevent the transmission of syphilis through partner services. All information presented in this manuscript is a result of their tireless work. We would also like to acknowledge the valuable input of Felicia Lewis, Bruce Furness, and Dan Newman in the development and refinement of this analysis.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Recommendations for partner services programs for HIV infection, syphilis, gonorrhea, and chlamydial infection. MMWR Recomm Rep. 2008;57(RR-9). [PubMed] [Google Scholar]
- 2.Andrus JK, Fleming DW, Harger DR, Chin MY, Bennett DV, Horan JM, et al. Partner notification: Can it control epidemic syphilis? Ann Intern Med. 1990;112(7):539–43. [DOI] [PubMed] [Google Scholar]
- 3.Samoff E, Koumans EH, Katkowsky S, Shouse RL, Markowitz LE, Allen ML, et al. Contact-tracing outcomes among male syphilis patients in Fulton County, Georgia, 2003. Sex Transm Dis. 2007;34(7):456–60. [DOI] [PubMed] [Google Scholar]
- 4.Hogben M, Paffel J, Broussard D, Wolf W, Kenney K, Rubin S, et al. Syphilis partner notification with men who have sex with men: A review and commentary. Sex Transm Dis. 2005;32(10 SUPPL.):43–7. [DOI] [PubMed] [Google Scholar]
- 5.Brewer D. Case-finding effectiveness of partner notification and cluster investigation for STD/HIV, unabridged technical report. 2004;78–83. Available from: http://www.interscientific.net/stdpnrep.pdf [DOI] [PubMed] [Google Scholar]
- 6.Mimiaga MJ, Reisner SL, Tetu AM, Bonafide KE, Cranston K, Bertrand T, et al. Partner notification after STD and HIV exposures and infections: Knowledge, attitudes, and experiences of Massachusetts men who have sex with men. Public Health Rep. 2009;124(1):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apoola A, Radcliffe KW, Das S, Robshaw V, Gilleran G, Kumari BS, et al. Preferences for partner notification method: Variation in responses between respondents as index patients and contacts. Int J STD AIDS. 2007;18(7):493–4. [DOI] [PubMed] [Google Scholar]
- 8.Kerani R, Thibault C, Spellman D, Golden M, Barbee L. Partner services fatigue: does the number of previous STIs and partner services interviews predict PS interview completion and provision of identifiable partners? In: National HIV Prevention Conference. Atlanta, GA; 2019. [Google Scholar]
- 9.Nguyen TQ, Kohn RP, Ng RC, Philip SS, Cohen SE. Historical and Current Trends in the Epidemiology of Early Syphilis in San Francisco, 1955 to 2016. Sex Transm Dis. 2018;45(9):S55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chorba TL, Berkelman RL, Safford SK, Gibbs NP, Hull HF. Mandatory Reporting of Infectious Diseases by Clinicians. JAMA J Am Med Assoc. 1989;262(21):3018–26. [PubMed] [Google Scholar]
- 11.Rowlinson E, Goings S, Minnerly S, Surita K, Pogosjans S. Differences in Partner Services Outcomes for Men Who Have Sex with Men Diagnosed with Primary and Secondary Syphilis by HIV Serostatus. Sex Transm Dis. 2018;45(3):152–7. [DOI] [PubMed] [Google Scholar]
- 12.Samoff E, Cope AB, Maxwell J, Thomas F, Mobley VL. The Number of Interviews Needed to Yield New Syphilis and Human Immunodeficiency Virus Cases among Partners of People Diagnosed with Syphilis, North Carolina, 2015. Sex Transm Dis. 2017;44(8):451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avoundjian T, Stewart J, Peyton D, Lewis C, Johnson K, Glick SN, et al. Integrating Human Immunodeficiency Virus Testing into Syphilis Partner Services in Mississippi to Improve Human Immunodeficiency Virus Case Finding. Sex Transm Dis. 2019;46(4):240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogben M, Collins D, Hoots B, O’Connor K. Partner services in sexually transmitted disease prevention programs: A review. Sex Transm Dis. 2016;43(2):S53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoots BE, Lewis FMT, Anschuetz G, Schillinger JA, Blank S, Foskey T, et al. Would targeting increase efficiency of syphilis partner services programs? - Data from New York City, Philadelphia, Texas, and Virginia. Sex Transm Dis. 2014;41(6):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcus JL, Katz MH, Katz KA, Bernstein KT, Wolf W, Klausner JD. Prediction model to maximize impact of syphilis partner notification-San Francisco, 2004-2008. Sex Transm Dis. 2010;37(2):109–14. [DOI] [PubMed] [Google Scholar]
- 17.Technical Assistance Note # 7: Disease investigation and intervention for pregnant women and other women ofreproductive age with syphilis [Internet]. STD PCHD: Strengthening STD Prevention and Control for Health Departments. 2019. [cited 2020 Jan 9]. Available from: [Google Scholar]
- 18.Liau A, Millett G, Marks G. Meta-analytic examination of online sex-seeking and sexual risk behavior among men who have sex with men. Sex Transm Dis. 2006;33(9):576–84. [DOI] [PubMed] [Google Scholar]
- 19.Saberi P, Neilands TB, Lally MA, Hosek SG, Hightow-Weidman L. The Association between Use of Online Social Networks to Find Sex Partners and Sexually Transmitted Infection Diagnosis among Young Men Who Have Sex with Men and Transgender Women Living with HIV. J Int Assoc Provid AIDS Care. 2019;18:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hightow-Weidman L, Beagle S, Pike E, Kuruc J, Leone P, Mobley V, et al. No one’s at home and they won’t pick up the phone": Using the internet and text messaging to enhance partner services in North Carolina. Sex Transm Dis. 2014;41(2):143–8. [DOI] [PubMed] [Google Scholar]
- 21.Udeagu CCN, Bocour A, Shah S, Ramos Y, Gutierrez R, Shepard CW. Bringing HIV partner services into the age of social media and mobile connectivity. Sex Transm Dis. 2014;41(10):631–6. [DOI] [PubMed] [Google Scholar]
- 22.Mobley V, Cope A, Dzialowy N, Maxwell J, Foust E, Samoff E. A comparison of syphilis partner notification outcomes by reported use of internet-based apps to meet sex partners in North Carolina, 2013-2016. Sex Transm Dis. 2018;45(12):823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennise M, Inscho R, Herpin K, Owens J, Bedard BA, Weimer AC, et al. Using smartphone apps in STD interviews to find sexual partners. Public Health Rep. 2015;130(3):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tributino A, Montgomery MC, Bertrand T, Marak T, Almonte A, Van Den Berg J, et al. Partner notification outcomes after integration of an on-site disease intervention specialist at a sexually transmitted disease clinic. PLoS One. 2018;13(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustanski B, Lyons T, Garcia SC. Internet use and sexual health of young men who have sex with men: A mixed-methods study. Arch Sex Behav. 2011;40(2):289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cope AB, Mobley VL, Samoff E, et al. The changing role of disease intervention specialists in modern public health programs. Public Health Rep 2018; 134:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landis SE, Schoenbach VJ, Weber DJ, Mittal M, Krishan B, Lewis K, et al. Results of a Randomized Trial of Partner Notification in Cases of HIV Infection in North Carolina. N Engl J Med. 1992;326(2):101–6. [DOI] [PubMed] [Google Scholar]
- 28.Hogben M. Partner Notification for Sexually Transmitted Diseases. Clin Infect Dis. 2007;44(Supplement_3):S160–74. [DOI] [PubMed] [Google Scholar]
- 29.Health Departments: Interim Guidance on Developing a COVID-19 Case Investigation & Contact Tracing Plan. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/downloads/case-investigation-contact-tracing.pdf. Accessed July 13, 2020. [Google Scholar]
- 30.2018 Sexually Transmitted Diseases Surveillance. Centers for Disease Control and Prevention. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf. Accessed July 13, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.