Abstract
Vericiguat is a soluble guanylate cyclase stimulator indicated to reduce the risk of cardiovascular death and heart failure (HF) hospitalization in adults with symptomatic chronic HF and ejection fraction less than 45%. Guidelines recommend short‐acting nitrates, such as sublingual nitroglycerin, for the treatment of acute angina pectoris in patients with chronic coronary syndromes (CCSs), common comorbidities in HF. We evaluated safety, tolerability, and the pharmacodynamic interaction between vericiguat and nitroglycerin, coadministered in patients with CCSs. In this phase Ib, double‐blind, randomized, multicenter study, 36 patients with CCSs received either vericiguat 2.5 mg (up‐titrated every 2 weeks to 5 mg and 10 mg) or placebo. Patients also received nitroglycerin (0.4 mg sublingual). In total, 31 patients completed the study (vericiguat + nitroglycerin, n = 21; placebo + nitroglycerin, n = 10). There was no increase in treatment‐emergent adverse events (TEAEs) with vericiguat + nitroglycerin vs. placebo + nitroglycerin; three patients discontinued due to TEAEs (vericiguat + nitroglycerin, n = 1; placebo + nitroglycerin, n = 2). Decreases in mean blood pressure (BP; 6–10 mmHg systolic BP (SBP); 4–6 mmHg diastolic BP (DBP)) were independent of vericiguat exposure and occurred to a similar extent at trough and peak concentrations with all vericiguat doses and placebo. Coadministration of vericiguat with nitroglycerin in patients with CCSs was well tolerated, and the combination is unlikely to cause significant adverse effects beyond those known for nitroglycerin.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Vericiguat is indicated to reduce the risk of cardiovascular death and heart failure (HF) hospitalization following a hospitalization for HF or need for outpatient intravenous diuretics in adults with symptomatic chronic HF and ejection fraction less than 45%.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ This study evaluated the coadministration of vericiguat and short‐acting sublingual nitroglycerin in patients with chronic coronary syndromes (CCSs) and the impact on the safety profile of vericiguat and the pharmacodynamic response. Coadministration was generally well tolerated.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
☑ The findings from this study supported the use of short‐acting sublingual nitroglycerin with vericiguat in the phase III VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) trial.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
☑ With the recent approval of vericiguat for use as a treatment for HF, this study adds information on the concomitant use of vericiguat with short‐acting sublingual nitroglycerin.
The magnitude of the global burden of heart failure (HF) is significant. As well as being a growing economic problem, 1 clinical outcomes for patients with chronic HF remain poor, despite the availability of HF therapies and the implementation of current guidelines. 2
Comorbidities are of great importance in HF, as they add to the complexity of patient management as well as potentially increase mortality risk. 3 Coronary artery disease (CAD) is a major risk factor for HF, 4 and recent guidelines categorize patients with CAD as having acute or chronic coronary syndromes (CCSs). 5 Angina pectoris is a common symptom in patients with HF and CCSs. 6 , 7 Short‐acting nitrates, such as nitroglycerin, are recommended for the acute treatment of angina in patients with CCSs, according to recent guidelines. 5 , 7
Vericiguat is an orally administered, direct soluble guanylate cyclase (sGC) stimulator indicated to reduce the risk of cardiovascular death and HF hospitalization following a hospitalization for HF or need for outpatient intravenous diuretics in adults with symptomatic chronic HF and ejection fraction less than 45%. 8 , 9 , 10 Nitric oxide (NO) availability and the functionality of sGC are impaired in HF; this results in a loss of production of the molecular messenger cyclic guanosine monophosphate (cGMP), which may contribute to the progression of cardiovascular disease. 11 , 12 Vericiguat enhances the NO–sGC–cGMP pathway by directly stimulating sGC through a binding site independent of NO and by sensitizing sGC to endogenous NO. 9 , 12 , 13 , 14 , 15 Given that vericiguat and nitroglycerin both act on the NO–sGC–cGMP pathway, and may be coadministered to patients with HF, it is important to understand their potential pharmacodynamic interactions.
In a previous phase I drug–drug interaction study in healthy male subjects (EudraCT number: 2014‐001235‐36; https://eudract.ema.europa.eu/; data on file), exposure to vericiguat (5 mg) was not affected by coadministration with nitroglycerin (0.2 mg sublingual), and the combination was generally well tolerated. In this article, we report the results from the phase Ib Vericiguat Nitroglycerin Clinical Interaction (VENICE) study (ClinicalTrials.gov: NCT02617550; EudraCT number: 2015‐001444‐11), which investigated the hemodynamic effects of vericiguat plus nitroglycerin in patients with CCSs and whether concomitant administration would be well tolerated without clinically significant adverse effects beyond those known for nitroglycerin.
METHODS
VENICE was a phase Ib, multicenter, randomized, placebo‐controlled, double‐blind, group comparison study that enrolled patients with CCSs with or without HF. The study was performed across six study centers in Germany. Participants gave written informed consent to participate before entering the study. The study was conducted in accordance with the currently accepted version of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Guideline. The protocol was approved by the Ethics Committee of all participating centers, the coordinating investigator (Ethikkommission der Aerztekammer Hamburg, Hamburg, Germany), and the competent authority (Bundesinstitut für Arzneimittel und Medizinprodukte (The Federal Institute for Drugs and Medical Devices) (BfArM), Bonn, Germany) before the study commenced.
Study population
Male and female patients with CCSs, between 30 and 80 years of age with clinically stable disease (defined as coronary artery stenosis in any of the three main coronary vessels > 50% documented by coronary angiography within the last 36 months prior to the first screening examination or history of myocardial infarction) for at least 3 months prior to the first screening examination, with stable existing chronic medication for at least 2 months before initiating treatment, were eligible for the study. Patients were excluded if they used phosphodiesterase type 5 (PDE5) inhibitors (from 14 days before screening to study end), other nitrate medications, or riociguat (both from 3 months before screening to study end). A full description of inclusion and exclusion criteria can be found in Table S1 .
During the last screening visit, all patients received a nitroglycerin 0.4‐mg priming dose to assess general tolerability. At the end of the last screening visit, eligible patients were sequentially assigned to a unique number in ascending order. Each number was randomly assigned to a treatment (active or placebo, ratio 2:1) according to computer‐generated randomization envelopes provided by the Bayer Randomization Management.
Study design
The study randomized patients 2:1 to vericiguat or placebo (see Figure 1 for the detailed study design). Patients received multiple oral doses of vericiguat, administered as 1.25‐mg or 5‐mg tablets, or matching placebo.
Figure 1.
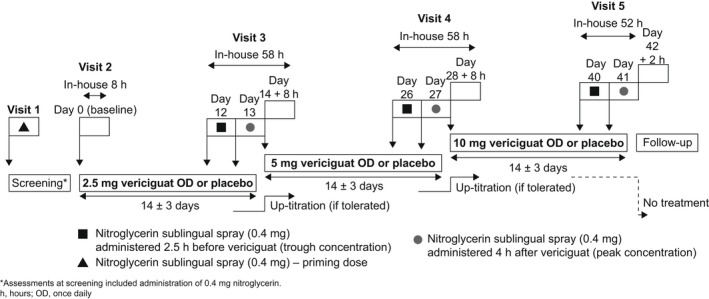
VENICE study design: vericiguat and nitroglycerin administration in patients with CCSs (chronic coronary syndromes).
The study consisted of three vericiguat treatment periods of 14 days each. Starting dose was 2.5 mg once daily, followed by 5 mg and 10 mg. Vericiguat was administered after breakfast (around 8:00 a.m.). Dose escalation only occurred if the previous dose was well tolerated.
Nitroglycerin sublingual spray (0.4 mg) was administered at screening (priming dose) and on the last 2 days of each treatment period. On the second to last day, nitroglycerin was administered 2.5 hours before vericiguat/placebo dosing (vericiguat trough concentration on Days 12, 16, and 40). On the last day, nitroglycerin was given 4 hours after vericiguat intake (vericiguat peak concentration on Days 13, 27, and 41).
Pharmacokinetics
Blood samples for vericiguat pharmacokinetic (PK) assessments were collected on Day 0 (1 hour and 4 hours after vericiguat intake) and on Days 12, 13, 14, 26, 27, 28, 40, and 41 (in the morning prior to vericiguat intake and 1–4 hours after vericiguat intake). Plasma concentrations of vericiguat also provided information about the patients’ adherence to the treatment regimen.
Vericiguat concentrations in plasma were determined using a validated bioanalytical assay. 16 The samples were analyzed using a liquid chromatography and tandem mass spectrometry assay, with a lower limit of quantification of 0.2 ng/mL. 16
Pharmacodynamics (hemodynamic profile)
The hemodynamic profiles were related to the nitroglycerin intake.
Blood pressure (BP) and heart rate (HR) were determined 30 minutes before the planned administration of nitroglycerin, measured after 15 minutes in supine position. Further measurements were taken after 10 minutes seated, then after 2 minutes standing, with three further BP and HR measurements (seated) taken within 10 minutes of dosing. Post dosing, BP and HR were measured in the seated position every 5 minutes until 30 minutes, and then at 30, 45, 60, 90, and 120 minutes. Immediately after the respective measurements in the seated position, BP and HR were measured again in the standing position; thereafter, subjects resumed the seated position. For details see Figure S1 .
Study objectives
The primary objective was to evaluate the safety and tolerability of the coadministration of vericiguat and nitroglycerin. The secondary objective was to evaluate the pharmacodynamic drug–drug interactions between vericiguat and nitroglycerin by monitoring the effects of their coadministration on BP and HR.
Study end points
The measurements of the hemodynamic profile started 45 minutes before nitroglycerin administration and were completed 122 minutes after nitroglycerin intake (Figure S1 ). Measurements after 2 minutes in the standing position were performed at given timepoints to test orthostatic tolerability.
Safety and tolerability assessments included the number of patients with adverse events (AEs) and serious AEs (SAEs), and the number of patients with clinically relevant findings in BP, HR, electrocardiogram, and safety laboratory parameters (including blood, urine, and thyroid‐stimulating hormone (TSH)).
Statistical analyses
A sample size of 24 patients (vericiguat + nitroglycerin, n = 16; placebo + nitroglycerin, n = 8) had 91% power to detect a difference of 10 mmHg nitroglycerin‐induced decrease in systolic BP (SBP), assuming a nitroglycerin‐induced decrease in SBP of 5 mmHg after placebo pretreatment. The common standard deviation was assumed to be 6.65 mmHg, as observed in a previous phase I study in healthy volunteers (EudraCT number: 2012‐000953‐30) 17 after multiple doses of vericiguat 10 mg once daily for all SBP measurements within a 4‐hour time window after dosing, including standardized hemodynamic challenge profile testing. This sample size estimation is applicable for a one‐way analysis of covariance, with significance level alpha = 0.05 and an unbalanced 1:2 randomization in favor of the vericiguat arm. No adjustment for multiplicity was performed.
For safety evaluations, the safety analysis set, defined as all patients who received at least one dose of study medication, was used. AEs were considered to be treatment‐emergent if they started or worsened after first dose of study medication up to 30 days after the end of treatment. Causality of the AE relationship to treatment was determined by the investigator under double‐blind conditions. AEs and SAEs were provided as listings. Summary statistics were provided for laboratory, vital signs, and electrocardiographic data.
All patients with at least one evaluable PK concentration were included in the PK analysis set. Peak and trough plasma concentrations were summarized per day and separated according to actual dose. The following statistics were calculated for each of the sampling points: arithmetic mean, standard deviation and coefficient of variation; geometric mean, geometric standard deviation and coefficient of variation; minimum, median, and maximum value; and the number of measurements. Means for any timepoint were only calculated if at least two‐thirds of the individual data were measured and were above the lower limit of quantification.
The pharmacodynamic data set included all patients with evaluable pharmacodynamic data and no major protocol deviations. Missing data were not replaced. Changes in seated SBP, diastolic BP (DBP), and HR after nitroglycerin administration (with pretreatment of vericiguat in the steady state) were analyzed assuming normally distributed data using analysis of covariance, including treatment, time, and treatment‐by‐time effects, with the baseline variable (SBP, DBP, or HR) as a covariate. The analysis was conducted separately for peak and trough levels for each dose of vericiguat. Point estimates (least squares mean) and exploratory 90% confidence intervals (CIs) for the differences “vericiguat − placebo” were calculated. The highest treatment effect was expected for the hemodynamic profiles after multiple dosing of the highest dose.
An interaction of vericiguat and nitroglycerin at the trough/peak concentration of vericiguat after multiple dosing of vericiguat 10 mg (on Day 40/Day 41) was assumed if the 90% CI for the difference “vericiguat − placebo” was completely above 0. If an interaction for vericiguat 10 mg was observed, the vericiguat 5 mg (on Day 26/Day 27) and vericiguat 2.5 mg (on Day 12/Day 13) doses were investigated stepwise analogously. This stepwise procedure was performed for trough and peak concentrations separately.
RESULTS
Patient disposition and baseline characteristics
Between November 18, 2015, and March 11, 2016, 52 patients with CCSs were enrolled and screened. Of the 52 individuals enrolled, 36 patients (33 males, three females) 46–79 years of age (mean 62.4 years) were randomized to treatment with either vericiguat + nitroglycerin (n = 24) or placebo + nitroglycerin (n = 12). Of those, 31 completed the study (vericiguat + nitroglycerin group, n = 21; placebo + nitroglycerin group, n = 10; Figure 2 ). Five patients did not complete the study: three discontinued owing to AEs (vericiguat + nitroglycerin group, n = 1; placebo + nitroglycerin group, n = 2), and two patients in the vericiguat + nitroglycerin group withdrew consent for personal reasons unrelated to study treatment. Follow‐up was 7–14 days for each patient.
Figure 2.
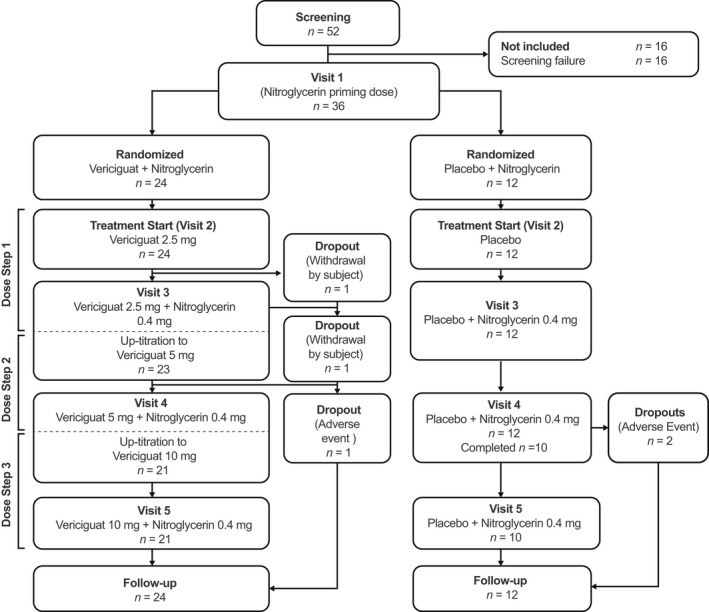
Patient disposition.
Baseline characteristics for the hemodynamic variables of interest were comparable between the two groups (Table 1 ), although SBP was slightly higher in the vericiguat + nitroglycerin group.
Table 1.
Baseline demographic characteristics
| Characteristic |
Vericiguat + nitroglycerin n = 24 |
Placebo + nitroglycerin n = 12 |
|---|---|---|
| Sex, n (%) | ||
| Male | 21 (87.5) | 12 (100.0) |
| Female | 3 (12.5) | 0 (0.0) |
| Race, n (%) | ||
| White | 24 (100.0) | 12 (100.0) |
| Age in years, mean (range) | 62.4 (47–79) | 62.5 (46–74) |
| Weight, kg | 89.6 (11.1) | 83.1 (13.1) |
| Height, cm | 174.5 (6.3) | 175.3 (4.2) |
| BMI, kg/m2 | 29.4 (3.4) | 27.0 (4.1) |
| SBP, mmHg a | 126.7 (17.6) | 119.8 (16.6) |
| DBP, mmHg a | 73.0 (10.5) | 71.4 (8.7) |
| Seated HR, bpm a | 68.5 (10.9) | 66.8 (10.2) |
| Medical history | ||
| Patients with cardiac disorders, n (%) | 24 (100.0) | 12 (100.0) |
| CAD (CCS) b | 23 (95.8) | 11 (91.7) |
| Chronic HF | 2 (8.3) | 2 (16.7) |
| Acute MI c | 9 (37.5) | 5 (41.7) |
| MI | 10 (41.7) | 7 (58.3) |
Data are mean (SD), unless indicated otherwise.
BMI, body mass index; bpm, beats per minute; CAD, coronary artery disease; CCS, chronic coronary syndrome; DBP, diastolic blood pressure; HF, heart failure; HR, heart rate; MedDRA, Medical Dictionary for Regulatory Activities; MI, myocardial infarction; SBP, systolic blood pressure; SD, standard deviation.
Baseline measurements taken on Day 0 (defined as the last predose measurement performed prior to the first administration of study medication).
According to protocol‐defined inclusion and exclusion criteria.
Acute MI: occurred > 6 months prior to the first screening examination (exclusion criterion 2). MI coded by MedDRA.
Prior and concomitant therapy
All patients took concomitant medication with a potential effect on the cardiovascular system (Table 2 ). Baseline antihypertensive drug use was balanced between the groups. Beta‐blocking agents were taken by 28 patients (77.8%) and angiotensin‐converting enzyme inhibitors by 24 patients (66.7%).
Table 2.
Concomitant medication with potential impact on the cardiovascular system
|
Vericiguat + nitroglycerin (n = 24) |
Placebo + nitroglycerin (n = 12) |
|
|---|---|---|
| Number of patients (%) with at least one concomitant medication | 24 (100.0) | 12 (100.0) |
| Blood and blood‐forming organs | 24 (100.0) | 12 (100.0) |
| Platelet aggregation inhibitors | 24 (100.0) | 11 (91.7) |
| Cardiovascular system | 24 (100.0) | 12 (100.0) |
| ACE inhibitors | 15 (62.5) | 9 (75.0) |
| Aldosterone receptor antagonists (MRAs) | 3 (12.5) | 0 (0.0) |
| α‐blocking and β‐blocking agents | 1 (4.2) | 1 (8.3) |
| β‐blockers | 19 (79.2) | 9 (75.0) |
| Angiotensin II antagonists | 5 (20.8) | 2 (16.7) |
| Angiotensin II antagonists and diuretics | 4 (16.7) | 0 (0.0) |
| Antiarrhythmics, Class IB | 1 (4.2) | 0 (0.0) |
| Dihydropyridine derivatives | 4 (16.7) | 2 (16.7) |
| Heparins or heparinoids for topical use | 1 (4.2) | 0 (0.0) |
| HMG‐CoA reductase inhibitors | 22 (91.7) | 12 (100.0) |
| Hydrazinophthalazine derivatives | 2 (8.3) | 0 (0.0) |
| Other cardiac preparations a | 4 (16.7) | 3 (25.0) |
| Other lipid‐modifying agents | 2 (8.3) | 0 (0.0) |
| Other potassium‐sparing agents | 0 (0.0) | 1 (8.3) |
| Products containing corticosteroids | 1 (4.2) | 0 (0.0) |
| Products containing local anesthetics | 1 (4.2) | 0 (0.0) |
| Sulfonamides | 3 (12.5) | 1 (8.3) |
| Thiazides | 0 (0.0) | 1 (8.3) |
ACE, angiotensin‐converting enzyme; HMG‐CoA, 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A; MRA, mineralocorticoid receptor antagonist.
Ibuprofen or ivabradine hydrochloride.
Safety and tolerability
Adverse events
All 36 patients were included in the safety analysis set, of whom 33 (91.7%) had at least one treatment‐emergent AE (TEAE): 12 of 36 patients (33.3%) after the nitroglycerin priming dose in the screening visit (including decreased BP and headache, among further TEAEs in single patients), 22 of 24 patients (91.7%) in the vericiguat + nitroglycerin group, and 11 of 12 patients (91.7%) in the placebo + nitroglycerin group in the treatment phase (Table 3 ).
Table 3.
Number of patients with TEAEs by treatment
| Number (%) of patients | ||
|---|---|---|
|
Vericiguat + nitroglycerin (n = 24) |
Placebo + nitroglycerin (n = 12) |
|
| Any TEAE, n (%) | 22 (91.7) | 11 (91.7) |
| Any vericiguat‐related TEAE a | 11 (45.8) | 5 (41.7) |
| Any nitroglycerin‐related TEAE a | 7 (29.2) | 5 (41.7) |
| Any TEAE related to study procedures a | 3 (12.5) | 1 (8.3) |
| TEAE‐related deaths, n (%) | 0 (0.0) | 0 (0.0) |
| Any SAEs, n (%) | 2 (8.3) | 1 (8.3) |
SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Relationship was assigned by the investigator (under double‐blind conditions) according to the most plausible cause.
SAEs occurred in three patients (Table 3 ): one patient in the placebo + nitroglycerin group had a myocardial infarction, one patient in the vericiguat + nitroglycerin group had a sinoatrial block of moderate intensity, and another reported acute painful constipation of moderate intensity leading to hospitalization. The latter patient had reported cephalgia and myalgia 2 days beforehand and abdominal pain and nausea 1 day before the event; the patient also had elevated alanine aminotransferase 1 week before the event.
The patient who experienced a sinoatrial block in the vericiguat + nitroglycerin group discontinued study participation; this was not considered to be related to treatment. Two patients in the placebo + nitroglycerin group discontinued study participation owing to TEAEs: one patient had orthostatic dysregulation, considered probably related to nitroglycerin, and one patient had postural dizziness that was, due to blinded assessment, believed to be related to vericiguat and nitroglycerin. Both TEAEs were related to the standing BP measurement procedure.
The percentage of patients with TEAEs within 24 hours after nitroglycerin administration was 66.7% for vericiguat + nitroglycerin as well as for placebo + nitroglycerin (Table 4 ).
Table 4.
TEAEs (≥2 patients) within 24 hours after nitroglycerin administration on 6 profile days a
| Primary system organ class b |
Vericiguat + nitroglycerin (n = 24) |
Placebo + nitroglycerin (n = 12) |
|---|---|---|
| Number of patients (%) with at least one TEAE | 16 (66.7) | 8 (66.7) |
| Hypotension | 7 (29.2) | 2 (16.7) |
| Headache | 4 (16.7) | 1 (8.3) |
| Nausea | 2 (8.3) | 2 (16.7) |
| Fatigue | 2 (8.3) | 1 (8.3) |
| Amylase increased | 1 (4.2) | 2 (16.7) |
| Lipase increased | 0 | 2 (16.7) |
MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment‐emergent adverse event.
Six profile days = 3 trough and 3 peak days for the vericiguat + nitroglycerin group (vericiguat 2.5 mg, 5 mg, and 10 mg) and placebo + nitroglycerin group.
Preferred term MedDRA version 19.0.
Laboratory parameters
In the observed safety laboratory parameters, clinically relevant deviations from the normal ranges were transient or related to the concomitant diseases of the patients and could not be attributed to the study drugs.
Other safety parameters
Symptomatic BP drops, with absolute values below 90 mmHg, were seen for one patient in the vericiguat + nitroglycerin group in the standing procedure and for two patients in the placebo + nitroglycerin group in the sitting period of the hemodynamic profile. TEAEs of low BP of mild intensity with no further clinical symptoms were reported in both groups. Nonsymptomatic decreases were seen slightly more often in the vericiguat + nitroglycerin group during administration of nitroglycerin on top of vericiguat peak levels. Low BP in the placebo + nitroglycerin group led to discontinuation due to orthostatic dizziness in one patient (minimum BP 62/34 mmHg, HR 43 beats per minute (bpm)) and postural dizziness (minimum BP 70/35 mmHg, HR 66 bpm) in another patient.
No effects of vericiguat or nitroglycerin on any measured electrocardiographic parameter were revealed.
Pharmacokinetics
Vericiguat plasma concentrations increased with up‐titration of the dose, and trough and peak concentrations were largely similar during profile Days 12 and 13 (2.5 mg), Days 26 and 27 (5 mg), and Days 40 and 41 (10 mg) (Table S2 ). These data also indicate that the patients had good adherence to the treatment regimen.
Pharmacodynamics
During up‐titration of vericiguat from 2.5 mg to 10 mg, mean baseline SBP values decreased from 126.7 ± 17.6 mmHg on Day 0 to 115.8 ± 11.7 mmHg on Day 41 (placebo + nitroglycerin group: 119.8 ± 16.6 mmHg on Day 0; 117.6 ± 12.4 mmHg on Day 41). This pattern was not observed in DBP or HR in either treatment group. However, a decrease in mean arterial pressure (MAP) was observed in the vericiguat + nitroglycerin group, from 90.9 ± 11.3 mmHg on Day 0 to 85.0 ± 9.3 mmHg on Day 41 (placebo + nitroglycerin group: 87.5 ± 10.1 mmHg on Day 0; 87.1 ± 8.1 mmHg on Day 41). There was high variability of the three secondary variables (SBP, DBP, and HR) throughout the course of the hemodynamic profiles.
Pharmacodynamic interaction between vericiguat and nitroglycerin
Mean SBP and DBP decreased by approximately 6–10 mmHg and 4–6 mmHg, respectively, 5 minutes after nitroglycerin administration, while HR increased by approximately 5–7 bpm (Figure 3 ). This effect was observed in all hemodynamic profiles, regardless of the vericiguat dose at the time of nitroglycerin administration or whether vericiguat or placebo was given in combination with nitroglycerin.
Figure 3.
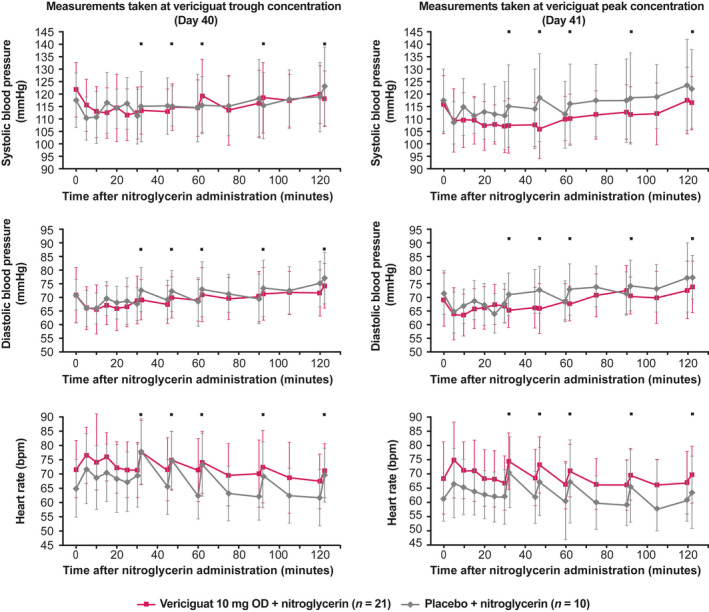
Hemodynamics after nitroglycerin administration with vericiguat 10 mg OD or placebo. Left panel, at trough concentration; right panel, at peak concentration. Data are presented as mean and standard deviation and represent measurements made when vericiguat had reached steady state. Measurements were taken in the seated position unless indicated otherwise. ⬛, measurements were taken in the standing position. bpm, beats per minute; OD, once daily. [Colour figure can be viewed at wileyonlinelibrary.com]
The changes in seated SBP, DBP, and HR observed after nitroglycerin administration were independent of vericiguat exposure and occurred to a similar extent at trough and peak concentrations with all vericiguat doses and placebo (Table 5 ). There were no significant differences between groups in the changes in seated SBP, DBP, or HR following nitroglycerin administration. A small increase in HR with vericiguat 2.5 mg (Day 13, peak concentration) was not considered clinically relevant (Table 5 ).
Table 5.
Changes in seated SBP, DBP, and HR after nitroglycerin administration with vericiguat vs. placebo: statistical analysis
| Parameter (unit) | Vericiguat trough concentration | Vericiguat peak concentration | ||||
|---|---|---|---|---|---|---|
| Vericiguat dose (study day) | Difference “vericiguat‐placebo” (90% CI) | P value of t statistic | Vericiguat dose (study day) | Difference “vericiguat‐placebo” (90% CI) | P value of t statistic | |
| SBP (mmHg) | 2.5 mg (Day 12) | 2.47 (−2.23, 7.17) | 0.3802 | 2.5 mg (Day 13) | −0.15 (–5.10, 4.80) | 0.96 |
| 5 mg (Day 26) | 2.42 (−1.15, 6.00) | 0.2594 | 5 mg (Day 27) | 1.59 (−3.36, 6.53) | 0.59 | |
| 10 mg (Day 40) | –2.57 (−7.12, 1.98) | 0.3455 | 10 mg (Day 41) | −4.44 (−9.80, 0.91) | 0.17 | |
| DBP (mmHg) | 2.5 mg (Day 12) | 2.09 (−0.61, 4.78) | 0.1987 | 2.5 mg (Day 13) | 0.60 (−2.02, 3.22) | 0.70 |
| 5 mg (Day 26) | 1.89 (−0.39, 4.18) | 0.1699 | 5 mg (Day 27) | 3.20 (−0.10, 6.51) | 0.11 | |
| 10 mg (Day 40) | –0.89 (−3.85, 2.07) | 0.6124 | 10 mg (Day 41) | –0.70 (−3.73, 2.34) | 0.70 | |
| HR (bpm) | 2.5 mg (Day 12) | 1.33 (−1.07, 3.74) | 0.3548 | 2.5 mg (Day 13) | 2.31 (0.07, 4.54) | 0.09 |
| 5 mg (Day 26) | 2.35 (−0.39, 5.08) | 0.1565 | 5 mg (Day 27) | 0.52 (−1.58, 2.63) | 0.68 | |
| 10 mg (Day 40) | −0.31 (−2.67, 2.05) | 0.8233 | 10 mg (Day 41) | 1.31 (−0.28, 2.90) | 0.17 | |
The change in seated SBP, DBP, and HR after the nitroglycerin dose, with pretreatment of vericiguat in the steady state, was analyzed using an analysis of covariance model including treatment, time, and treatment‐by‐time effects, with the baseline variable (SBP, DBP, or HR) as a covariate. These analyses were restricted to measurements that were planned to be taken in the seated position and patients who received nitroglycerin. One patient was excluded from the Day 12 statistical analysis at vericiguat trough concentration because the administration of placebo and nitroglycerin was not done within the window specified in the protocol.
bpm, beats per minute; CI, confidence interval; DBP, diastolic blood pressure, HR, heart rate; SBP, systolic blood pressure.
DISCUSSION
The VENICE study evaluated the safety and pharmacodynamics of vericiguat coadministered with sublingual nitroglycerin in patients with CCSs. The combination was generally well tolerated, and no relevant differences in hemodynamic parameters between the vericiguat + nitroglycerin and the placebo + nitroglycerin groups, and between the treatment periods with and without nitroglycerin, were revealed.
During up‐titration of vericiguat from 2.5 mg to 10 mg, mean baseline SBP values decreased by ~ 10 mmHg—a trend that was not observed for DBP or HR. A BP reduction with placebo in clinical trials is not unknown and is caused by better and more intensive care of patients. When placebo‐corrected and baseline‐corrected, mean decreases in SBP during up‐titration were not more than 5 mmHg. However, there was a 5‐mmHg decrease in MAP during the treatment phase.
The higher baseline SBP observed in the vericiguat + nitroglycerin group vs. the placebo + nitroglycerin group might have confounded the observed SBP decrease in the vericiguat + nitroglycerin arm towards a larger change compared with the placebo + nitroglycerin arm. Given the high variability of the SBP, DBP, and HR throughout the hemodynamic profiles, the clinical relevance of this observation remains unclear. In the phase III VICTORIA (Vericiguat Global Study in Subjects with Heart Failure with Reduced Ejection Fraction) study (ClinicalTrials.gov number, NCT02861534), in patients with symptomatic chronic HF (and left ventricular ejection fraction < 45%) who had a previous worsening HF event, SBP decreased slightly in both the vericiguat and placebo groups over the first 16 weeks, marginally more in the vericiguat group than in the placebo group, but returned to baseline thereafter. 9
In this study, the nonsymptomatic decreases in BP observed in both groups, more frequently in the vericiguat + nitroglycerin group during administration of nitroglycerin on top of vericiguat peak levels, may be attributed to the additional BP‐lowering effect of vericiguat, especially during time of peak concentration. Indeed, in previous studies in patients with worsening chronic HF with reduced ejection fraction (SOCRATES‐REDUCED (The Soluble Guanylate Cyclase Stimulator in Heart Failure with Reduced Ejection Fraction Study), NCT01951625; VICTORIA, NCT02861534) and HF with preserved ejection fraction (SOCRATES‐PRESERVED (The Soluble Guanylate Cyclase Stimulator in Heart Failure with Perserved Ejection Fraction Study), NCT01951638; and VITALITY (Evaluate the Efficacy and Safety of the Oral sGC Stimulator Vericiguat to Improve Physical Functioning in Daily Living Activities of Patients With Heart Failure and Preserved Ejection Fraction), NCT03547583), adverse effects observed with vericiguat were mostly related to its pharmacological mode of action, i.e., relaxation of smooth muscles, leading to hemodynamic changes such as hypotension and syncope. 9 , 18 , 19 , 20 However, although symptomatic hypotension and syncope were more common in patients receiving vericiguat than in those receiving placebo in the VICTORIA study, the difference in frequency between groups was not significant. 9
Distribution of TEAEs was similar in both treatment groups and most were classified as mild. In 12 patients, TEAEs (decreased BP and headache, among further TEAEs in single patients) occurred when nitroglycerin was administered alone during the screening phase, which correlates with existing knowledge that headache, dizziness, and hypotension are commonly reported in patients receiving nitroglycerin. 21 , 22 TEAEs considered related to vericiguat in the vericiguat + nitroglycerin group could mostly be explained by its vasodilative mode of action. The incidence of SAEs was very low and none were considered related to treatment with vericiguat or nitroglycerin.
The coadministration of vericiguat and nitroglycerin was well tolerated in a phase I study in healthy volunteers (EudraCT number: 2014‐001235‐36; https://eudract.ema.europa.eu/). Although a minor additive effect on lowering SBP was observed with the combination, there were no clinically significant TEAEs beyond those known for nitroglycerin.
Additive hemodynamic effects resulting in hypotension have been demonstrated with concurrent administration of riociguat (another sGC stimulator) with nitrates or NO donors, as well as with PDE5 or nonspecific PDE inhibitors, and, thus, their combined use is contraindicated. 23 It is not currently known why the combination of vericiguat with short‐acting nitrates does not result in similar hypotensive effects to those observed with riociguat. Use of both short‐acting and long‐acting nitrates in combination with PDE5 inhibitors, α‐adrenergic blockers, and calcium channel blockers should be avoided due to drug–drug interactions. 7
Concentrations of vericiguat in plasma increased with up‐titration of the dose. Overall, the pharmacokinetics of vericiguat in this patient population were in line with prior knowledge for vericiguat, based on findings from phase IIb studies.
The present study suggests that vericiguat in combination with nitroglycerin may exert therapeutic effects without clinically relevant changes in BP and HR in patients with CCSs, and these data supported the use of short‐acting nitrates in the VICTORIA study. With regard to the effects of the combination in a broader, more severely ill population, the phase III VICTORIA study included patients up to 6 months post discharge and spanned a range of worsening HF to allow a more representative HF patient population to be analyzed. 13 Short‐acting nitrates, such as sublingual nitroglycerin spray, were permitted as concomitant medications for symptom relief in VICTORIA.
The limitations of this study primarily relate to the small size of the study population. There were 31 patients who completed the study, with slight imbalances in some baseline characteristics noted between the groups. Both interpatient and intrapatient variability may have contributed to the overall differences. Although this study was small, the statistical design and analysis methods were optimized to address the hypothesis and the specific study population. To minimize bias, double‐blind follow‐up was performed, including assessment of TEAEs by the investigator under blinded conditions.
CONCLUSIONS
Overall, the combination of vericiguat with nitroglycerin administered to patients with CCSs was found to be generally well tolerated. There were no statistically significant differences in SBP, DBP, and HR between the vericiguat + nitroglycerin and placebo + nitroglycerin groups, and changes observed after nitroglycerin administration were independent of vericiguat exposure. As therapeutic doses of nitroglycerin can reduce SBP, DBP, and MAP, 22 the results here are fully aligned with the assumption that the effects of vericiguat and nitroglycerin on BP and HR are not more than additive, and the clear trends observed are related to the known effects of nitroglycerin alone.
Funding
Funding for this research was provided by Bayer AG, Berlin, Germany and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, United States.
Conflict of interest
M.B., T.K., M.G., and C.B are employees of Bayer and may own stock in the company. N.B. is an employee of Chrestos Concept GmbH & Co. KG, which received funding for this analysis from Bayer AG. N.W. has received speaker honoraria and travel expenses from Bayer and MSD. H‐D.D. has received institutional payment as an investigator and personal honoraria for advisory boards from Bayer. All other authors declared no competing interests for this work.
Author contributions
All authors wrote the manuscript, designed and performed the research, and analyzed the data.
Supporting information
Supplementary Material
Acknowledgments
The authors would like to thank all the patients and their families who were involved in this study. Thanks also to all the investigators involved, including Sven Schmiedl and David Peters of the Philipp Klee‐Institute of Clinical Pharmacology, HELIOS Klinikum Wuppertal, Wuppertal, Germany. Boris Weimann of Chrestos Concept GmbH & Co. KG, Essen, Germany, provided support with the statistical analysis. Part of this analysis was presented at the 2019 European Society of Cardiology Heart Failure congress. Medical writing support, including assisting authors with the development of the outline and initial draft and incorporation of comments, was provided by Laila Guzadhur, PhD, and Caroline Sills, MSci, and editorial support, including fact checking, referencing, figure preparation, formatting, proofreading, and submission was provided by Annabel Ola, MSc, of Scion, London, supported by Bayer and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, United States, according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15‐0288). The Sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Data availability statement
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA (European Federation of Pharmaceutical Industries and Associations/Pharmaceutical Research and Manufacturers of America) “Principles for responsible clinical trial data sharing.” This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient‐level clinical trial data, study‐level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient‐level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study Sponsors section of the portal. Data access will be granted to anonymized patient‐level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
- 1. Cook, C. , Cole, G. , Asaria, P. , Jabbour, R. & Francis, D.P. The annual global economic burden of heart failure. Int. J. Cardiol. 171, 368–376 (2014). [DOI] [PubMed] [Google Scholar]
- 2. Butler, J. et al. Clinical course of patients with worsening heart failure with reduced ejection fraction. J. Am. Coll. Cardiol. 73, 935–944 (2019). [DOI] [PubMed] [Google Scholar]
- 3. RuDusky, B.M. Heart failure and comorbidities. JACC Heart Fail. 3, 1003 (2015). [DOI] [PubMed] [Google Scholar]
- 4. Velagaleti, R.S. & Vasan, R.S. Heart failure in the twenty‐first century: is it a coronary artery disease or hypertension problem? Cardiol. Clin. 25, 487–495 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knuuti, J. et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 41, 407–477 (2020). [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975 (2016). [DOI] [PubMed] [Google Scholar]
- 7. Montalescot, G. et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34, 2949–3003 (2013). [DOI] [PubMed] [Google Scholar]
- 8. US Food and Drug Administration . NDA Approval Letter. <https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2021/214377Orig1s000ltr.pdf> (2021). Accessed April 12, 2021. [Google Scholar]
- 9. Armstrong, P.W. et al. Vericiguat in patients with heart failure and reduced ejection fraction. N. Engl. J. Med. 382, 1883–1893 (2020). [DOI] [PubMed] [Google Scholar]
- 10. Verquvo [highlights of prescribing information]. (Merck, Whitehouse Station, NJ, 2021). <https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf>. Accessed February 8, 2021. [Google Scholar]
- 11. Sandner, P. From molecules to patients: exploring the therapeutic role of soluble guanylate cyclase stimulators. Biol. Chem. 399, 679–690 (2018). [DOI] [PubMed] [Google Scholar]
- 12. Sandner, P. , Zimmer, D.P. , Milne, G.T. , Follmann, M. , Hobbs, A. & Stasch, J.P. Soluble guanylate cyclase stimulators and activators. In Reactive Oxygen Species. Handbook of Experimental Pharmacology, vol. 264 (eds. Schmidt, H.H.H.W. , Ghezzi, P. & Cuadrado, A. ), January 29, 2019 edn. (Springer, Berlin, Heidelberg, 2019). [Google Scholar]
- 13. Armstrong, P.W. et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 6, 96–104 (2018). [DOI] [PubMed] [Google Scholar]
- 14. Follmann, M. et al. Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J. Med. Chem. 60, 5146–5161 (2017). [DOI] [PubMed] [Google Scholar]
- 15. Stasch, J.P. , Schlossmann, J. & Hocher, B. Renal effects of soluble guanylate cyclase stimulators and activators: a review of the preclinical evidence. Curr. Opin. Pharmacol. 21, 95–104 (2015). [DOI] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . Vericiguat (VERQUVO) Integrated Review ‐ Center for Drug Evaluation and Research. <https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214377Orig1s000IntegratedR.pdf> (2020). Accessed March 17, 2022. [Google Scholar]
- 17. Boettcher, M.‐F. et al. Safety, pharmacodynamic and pharmacokinetic characterization of vericiguat: key results from six phase I studies in healthy subjects. Eur. J. Heart Fail. 21, 293 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gheorghiade, M. et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES‐REDUCED randomized trial. JAMA 314, 2251–2262 (2015). [DOI] [PubMed] [Google Scholar]
- 19. Pieske, B. et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES‐PRESERVED) study. Eur. Heart J. 38, 1119–1127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong, P.W. et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: The VITALITY‐HFpEF randomized clinical trial. JAMA 324, 1512–1521 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Divakaran, S. & Loscalzo, J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J. Am. Coll. Cardiol. 70, 2393–2410 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. G. Pohl‐Boskamp GmbH & Co. KG . Nitrolingual® pumpspray. Full prescribing information. 1‐16 (G. Pohl‐Boskamp GmbH & Co. KG, 25551, Hohenlockstedt, Germany, 2018). [Google Scholar]
- 23. Khaybullina, D. , Patel, A. & Zerilli, T. Riociguat (adempas): a novel agent for the treatment of pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. P T 39, 749–758 (2014). [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Availability of the data underlying this publication will be determined later according to Bayer’s commitment to the EFPIA/PhRMA (European Federation of Pharmaceutical Industries and Associations/Pharmaceutical Research and Manufacturers of America) “Principles for responsible clinical trial data sharing.” This pertains to scope, timepoint, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient‐level clinical trial data, study‐level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States (US) and European Union (EU) as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the EU and US regulatory agencies on or after January 01, 2014. Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient‐level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the Study Sponsors section of the portal. Data access will be granted to anonymized patient‐level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.


