Abstract
Prevalence estimates of autism are essential for informing public policy, raising awareness, and developing research priorities. Using a systematic review, we synthesized estimates of the prevalence of autism worldwide. We examined factors accounting for variability in estimates and critically reviewed evidence relevant for hypotheses about biological or social determinants (viz., biological sex, sociodemographic status, ethnicity/race, and nativity) potentially modifying prevalence estimates of autism. We performed the search in November 2021 within Medline for studies estimating autism prevalence, published since our last systematic review in 2012. Data were extracted by two independent researchers. Since 2012, 99 estimates from 71 studies were published indicating a global autism prevalence that ranges within and across regions, with a median prevalence of 100/10,000 (range: 1.09/10,000 to 436.0/10,000). The median male‐to‐female ratio was 4.2. The median percentage of autism cases with co‐occurring intellectual disability was 33.0%. Estimates varied, likely reflecting complex and dynamic interactions between patterns of community awareness, service capacity, help seeking, and sociodemographic factors. A limitation of this review is that synthesizing methodological features precludes a quality appraisal of studies. Our findings reveal an increase in measured autism prevalence globally, reflecting the combined effects of multiple factors including the increase in community awareness and public health response globally, progress in case identification and definition, and an increase in community capacity. Hypotheses linking factors that increase the likelihood of developing autism with variations in prevalence will require research with large, representative samples and comparable autism diagnostic criteria and case‐finding methods in diverse world regions over time.
Lay Summary
We reviewed studies of the prevalence of autism worldwide, considering the impact of geographic, ethnic, and socioeconomic factors on prevalence estimates. Approximately 1/100 children are diagnosed with autism spectrum disorder around the world. Prevalence estimates increased over time and varied greatly within and across sociodemographic groups. These findings reflect changes in the definition of autism and differences in the methodology and contexts of prevalence studies.
Keywords: autism, methodology, prevalence, risk factors, social determinants, time trends
INTRODUCTION
Autism (or autism spectrum disorders, ASD) is defined on the basis of social and communication problems and repetitive and restrictive behaviors that can vary in individuals along a continuum of severity (Lord et al., 2018). A diagnosis of autism can be made as early as 18–24 months of age; it is around this age that characteristic symptoms can be distinguished from typical development and from other delays or other developmental conditions.
Advances in autism research have gone hand in hand with significant progress in international policy. In addition to the policy response resulting from the significant increase in awareness and advocacy worldwide, autism has also benefited from progress in complementary areas, including human rights, maternal and child health, and mental health (WHO 2013a,b, 2014, 2015, 2018; WHO & UNICEF, 2018). This progress has been grounded and motivated in large part by the United Nations Convention of the Rights of Persons with Disabilities (UNCRPD), which outlines key principles including respect for dignity, freedom of choice and independence, non‐discrimination, full participation and inclusion in society, and accepting people with disabilities as part of human diversity.
Global advocacy and mobilization have also called for a move from awareness toward capacity building in the area of autism (WHO, 2013a). In most world regions, autism awareness has advanced substantially but has not always led to a significant increase in available services. In recent years, the World Health Assembly adopted WHO's Comprehensive Mental Health Action Plan 2013–2020 (WHO, 2013b). The plan supports access to “high‐quality, culturally‐appropriate health and social care in a timely way.” The vision is that of a world in which mental health is valued and promoted, mental disorders are prevented, and persons affected by these disorders are able to exercise the full range of human rights and to access high quality, culturally appropriate health, and social care in a timely manner. The plan is guided by principles such as universal health coverage, evidence‐based practice, multisectoral approaches, a life‐course approach, and empowerment of persons with mental disorders.
Progress has also been made at country and regional level‐specific policies for autism that have been developed or refined in response to needs in the community (e.g., UK: Autism Act, 2009; France; Schendel & Thorsteinsson, 2018; Quebec, Canada: Zeidan et al., 2019; Parkin et al., 2018).
The considerable progress achieved in increasing autism awareness and public health response worldwide has gone hand in hand with epidemiological studies offering objective indicators of the impact of autism, including estimates of cases and their associated social and economic impacts. Specifically, epidemiological estimates can reflect the state of identification, services, and supports offered to the affected population and signal to policymakers strategies for improvement (Fombonne, 2019; Franz et al., 2017; Raina et al., 2017).
Increasingly, epidemiological studies have also been used, albeit indirectly, for inference about etiological factors linked with autism. For example, an increase in prevalence over time is taken as a reflection of change in exposure to environmental risk factors. Similarly, variation in prevalence by key sociodemographic factors (e.g., geographic, ethnic, social, or economic) is interpreted as reflecting true underlying variation in biological and/or environmental etiology (e.g., Hewitt et al., 2016). Competing hypotheses have instead explained such associations as reflecting health disparities, where stigma and/or systemic barriers lead— to marginalization of ethnic or socioeconomic subgroups, modify their access to services, and as a result lead to a variation in prevalence (e.g., Durkin et al., 2017; Elsabbagh, 2020).
The aim of the current systematic review is to update our previously published estimates of the global prevalence of autism. Based on ~70 estimates, the last systematic review of global prevalence conducted in 2012 found that the median prevalence of ASD was 62/10,000 children, with a consistently higher prevalence in boys (Elsabbagh et al., 2012). There was substantial variability in estimates within and across geographic regions and some estimates were very limited or completely absent from many world regions such as Eastern Europe and Africa. Moreover, estimates were mainly available for children, with very few studies including populations older than 18 years of age. In the current manuscript, we update the previous review by (1) comprehensively reviewing available epidemiological surveys published since 2012 in different world regions and (2) re‐examining possible methodological features that can lead to over‐ or under‐estimation of true population prevalence. Moreover, in this review, we (3) examined the extent to which the epidemiological evidence we identified has offered support for hypotheses linking epidemiological data to underlying etiology.
METHODS
Search strategy
MEDLINE was searched for relevant articles. The first search was conducted in December 2020 and a final search was updated in November 2021. The search strategy was defined by identifying two key terms from the research question related to “autism,” and “prevalence.” Search terms and their combinations are presented in Table 1. Results were limited to studies involving human participants, published within peer‐reviewed journals from 2012 onward. The search was limited from (and including) 2012. Articles returned from the search were screened independently by title and abstract by JZ. The full‐text of studies meeting inclusion criteria were then reviewed by JZ. AY independently screened all studies at the title/abstract stage and at the full‐text review stage. Reference lists of relevant reviews were searched manually by JZ to ensure the inclusion of any additional studies. PRISMA flowchart in Figure 1 summarizes the number of articles identified, screened, and included in the final review.
TABLE 1.
Search strategy terms and combinations
| Search | Terms |
|---|---|
| 1 | Autistic Disorder/ or Autis*.mp. or Autism Spectrum Disorder/ or Child Development Disorders, Pervasive/ or ASPERGER SYNDROME/ or asperger.mp. |
| 2 | PREVALENCE/ or prevalence.mp. or cross‐sectional studies.mp. or Cross‐Sectional Studies/ or genetic screening.mp. or Genetic Testing/ |
| 3 | (2012* or 2013* or 2014* or 2015* or 2016* or 2017* or 2018* or 2019* or 2020* or 2021* or 2022*).dt,ez. |
| 4 | S1 AND S2 AND S3 |
| 5 | Limit S4 to humans |
FIGURE 1.
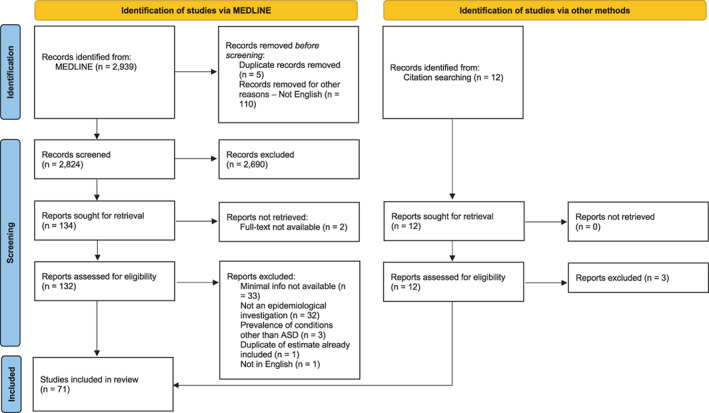
PRISMA flowchart
Eligibility criteria
Studies that were included had as a primary aim to estimate the prevalence of ASD since 2012. Studies were excluded when (1) their primary aim was to test screening tools with/without generating a prevalence estimate, (2) if they focused on other disorders, or when they focused on estimating ASD prevalence in other conditions, for example, intellectual disability, psychosis, and so forth. Studies were excluded if full‐text articles were not available. Studies were also excluded when minimal information necessary to establish prevalence (i.e., size of the population, age range covered, number of cases with ASD, and 95% confidence intervals [CI] for the estimate) was not reported and could not be ascertained based on published information about the study.
A data extraction form was designed and piloted on a selected number of included studies. For each of the studies included, the following variables were extracted: country, area, year of the study, size of the population for which prevalence was ascertained, number of cases with ASD, the diagnostic criteria used in case ascertainment, age range covered, gender ratio within the affected sample, prevalence estimate (number per 10,000), 95% CI for the estimate, proportion of ASD cases classified in the range of intellectual disability (ID), with an IQ < = 70 or a clinical diagnosis of ID. Data were extracted by JZ, AY, and AI. Any discrepancies were discussed and if necessary, were referred to another fourth independent reviewer (ME) for a final decision.
When different estimates were available within a single study, we separated the estimates and reported them in different rows of the summary tables. This is the case for estimates reported by country (e.g., in Europe, Delobel‐Ayoub et al., 2019), region (e.g., in China, Sun et al., 2019), years (e.g., in the United States, Christensen et al., 2019 and Taiwan, Lai et al., 2012), birth cohorts or age groups (e.g., in Australia, May et al., 2020; May et al., 2017).
Data analysis
The primary objective of the current review was to provide an update to prevalence estimates available from different world regions. The diversity in methodological approaches and study designs used to derive prevalence estimates precluded a meta‐analysis. Nevertheless, we calculated median estimates for each world region and presented these along with ranges. For secondary objectives, we used a narrative review, comprehensively synthesizing descriptive findings as they relate to questions of high social value and public relevance.
RESULTS
A total of 2951 records were screened through the Medline database (n = 2939) and hand searches (n = 12). After titles and abstracts were screened, and duplicates removed, 132 full‐text articles were assessed for eligibility, of which 71 were retained for inclusion in the review (Figure 1).
Prevalence estimates
Among the 71 studies included in the review (Figure 1), we identified 99 prevalence estimates in 34 countries, as illustrated in Figure 2. Table 2 summarizes the number of estimates, sample size, prevalence, sex ratio, and proportion of ASD cases with ID, by world region. Detailed estimates by country are reported in Supplementary Tables S1–S6.
FIGURE 2.
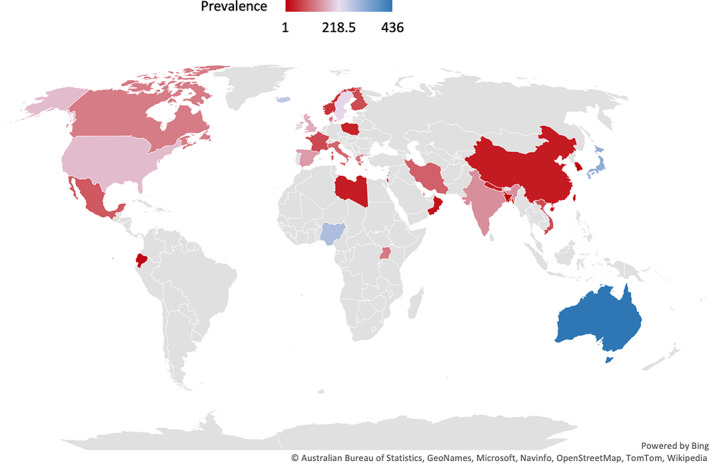
Autism prevalence per 10,000 from 2012 to 2021
TABLE 2.
Summary of autism prevalence estimates across world regions from 2012 to 2021
| Region | # estimates | Sample size | ASD prevalence (/10,000) | Gender ratio | Proportion of cases with ID (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQR | Range | Median | IQR | Range | Median | IQR | Range | Median | IQR | Range | Median | |||
| Americas | 30 | 200,737.5 | 465–8,129,270 | 54,960 | 82.3 | 11.1–250 | 132.3 | 0.7 | 2.7–5.3 | 4.3 | 13.2 | 24–47 | 33 | |
| Europe | 31 | 454,113.5 | 1796–2,431,649 | 32,342 | 63.5 | 24–268 | 100 | 1.3 | 0.8–5.4 | 4.1 | 28.7 | 0–47.5 | 20.9 | |
| Western Pacific | 22 | 4,236,842.3 | 3300–51,529,338 | 19,349 | 203.1 | 1.09–436 | 28.3 | 2.1 | 2.2–6.1 | 4.7 | 15.1 | 36.8–67 | 45.4 | |
| South‐East Asia | 8 | 9,442.3 | 3964–28,070 | 8143 | 34.0 | 7.5–140 | 23.2 | 1.0 | 1.15–3 | 1.75 | ‐ | ‐ | ‐ | |
| Eastern Mediterranean | 6 | 98,253.0 | 998–837,655 | 30,754 | 86.5 | 10–153 | 62.6 | 1.9 | 1.1–4.3 | 2.85 | 0 | 70.3–70.3 | 70.3 | |
| Africa | 2 | ‐ | ‐ | ‐ | ‐ | 120–290 | 205 | 0.1 | 1–1.2 | 1.1 | ‐ | ‐ | ‐ | |
| All | 99 | 323,890 | 465–51,529,338 | 31,000 | 119.25 | 1.09–436 | 100 | 1.66 | 0.8–6.1 | 4.2 | 21.8 | 0–70.3 | 33 |
Sample sizes were fairly large and ranged from 465 to ~50 million participants. Prevalence ranged from 1.09/10,000 to 436/10,000, with a median prevalence of 100/10,000. Similar to the last review, most studies were conducted in the United States and Northern Europe but there is a larger number of studies from previously under‐represented regions such as Africa and the Middle Eastern region (al‐Mamari et al., 2019; Alshaban et al., 2019; Chinawa et al., 2016). The populations surveyed were mostly children, but a few studies included adults aged 18 years and older (Kočovská et al., 2012; Poovathinal et al., 2016), and two focused specifically on adults (Brugha et al., 2016; Jariwala‐Parikh et al., 2019). Males consistently outnumbered females but the male‐to‐female ratio ranged from 0.8 to 6. Few or no estimates were available on the proportion of cases with intellectual disability in many world regions but ranged from 0% to 70% in Europe, Americas, Eastern Mediterranean, and the Western Pacific.
Although epidemiological studies have rarely used comparable variables to describe clinical presentations, frequently reported variables are age, sex, and to some extent, the proportion of cases with ID. As evident in Table 2, recent prevalence estimates, like earlier ones, are highly variable, likely due, in part, to the variability in methodological features, including sample characteristics and methods used for case finding and definition. These considerations are discussed next as they relate to case finding, definition, and evaluation.
Case definition
Two main clinical references are most frequently used to determine autism “caseness”: the Diagnostic and Statistical Manual (DSM) of the American Psychiatric Association and the International Classification of Diseases (ICD) of the World Health Organization (WHO); the most recent editions are the ICD‐11 (First et al., 2015) and the DSM‐5 (American Psychiatric Association, 2013). The DSM‐5 is already in use whereas the ICD‐11 will come into use in 2022. A few epidemiological studies used the Chinese Classification of Mental Disorders (CCMD‐3; Chinese Society of Psychiatry, 2001), although the ICD has also been in use in China as well. However, the CCMD itself was originally developed based on the DSM and ICD (Pang et al., 2018; Wang et al., 2019), and therefore, a major impact on prevalence estimates is not expected.
Broadening of diagnostic boundaries likely explains at least some of the increase in measured prevalence over time. Until recently, different prevalence estimates were derived for distinct diagnostic categories within ASD or “Pervasive developmental disorders.” For example, the last systematic review in 2012, distinguished estimates for more narrowly defined Autistic Disorder from those for ASD, where the median was 17/10,000 for the former and 62/10,000 for the latter (Elsabbagh et al., 2012).
The most recent versions of diagnostic classifications have diverged from “sub‐types” of autism toward the “spectrum.” The DSM‐5 features a single diagnostic category (ASD), reflecting the variability of symptoms. Moreover, the ICD‐11 will not differentiate autism with and without intellectual disability. Available estimates, in Supplementary Tables S1 to S6, have used the older criteria or a combination of older and more recent criteria, reflecting the state of clinical practice when the data were collected.
Case finding
The largest epidemiological studies were conducted through national surveillance programs. The most well‐established is the Center for Disease Control (CDC) surveillance system implemented through the Autism and Developmental Disabilities Monitoring Network (Maenner, 2020), which has regularly published updated prevalence estimates of autism among 8‐year‐old children, and more recently among 4‐year‐olds, who live in up to 16 sites. Canada also established the National Autism Spectrum Disorder Surveillance System and reported prevalence among 5‐17‐year‐old children across six provinces and the Yukon territory (Ofner et al., 2018). The system identified children from health and social services or from educational records, wherein diagnosis was made by a licensed health professional. In one province, data were extracted from a broader surveillance system established for several health conditions; the Quebec Integrated Chronic Disease Surveillance System (Diallo et al., 2018).
In Europe, data from national health registries allowed estimation of ASD prevalence in France, Denmark, Finland, and Iceland (Delobel‐Ayoub et al., 2019; Schendel & Thorsteinsson, 2018; van Bakel et al., 2015). Such registries rely on data available across the lifespan through universal health care systems and are highly representative of local populations. Studies in other countries also used administrative databases available through governmental hospitals (al‐Mamari et al., 2019), insurance providers (Jariwala‐Parikh et al., 2019), or educational systems (Diallo et al., 2018; Pinborough‐Zimmerman et al., 2012).
Other prevalence estimates were obtained from longitudinal cohort studies, such as the Neurodevelopmental Disorders Epidemiological Research in Spain (Morales‐Hidalgo et al., 2018), and the Longitudinal Study of Australian Children (May et al., 2017; May et al., 2020; Randall et al., 2016). National health surveys were also used to estimate autism prevalence such as the US National Survey of Children's Health (Kogan et al., 2018) and the US National Health Interview Survey (Xu et al., 2019). Finally, population‐based epidemiological surveys relied on a multistage approach: the screening stage identified possible cases and the final stage determined the proportion of screened cases who have a confirmed diagnosis (Alshaban et al., 2019; Fombonne et al., 2016; Sun et al., 2019).
While surveillance systems, registries, and administrative data offers large sample sizes, prevalence may still be underestimated if there are many cases of autism in the community that do not have a diagnosis. These studies focus on populations with access to services rather than sampling from the population at large, leading to possible underestimation of prevalence. In contrast, population‐based surveys that rely on active case‐finding procedures usually yield higher prevalence estimates than studies using administrative data (Fombonne, 2009). However, the success of active screening procedures may be limited by population coverage, sample representativeness, and response rates from participants during different stages. Across study types, factors that influence prevalence estimates relate to the specific procedures used to evaluate cases, resulting in possible cases or confirmed cases of autism.
Case identification and evaluation
Different studies rely on varying procedures to identify probable cases and to confirm diagnosed cases. Studies using administrative databases report cases as they were identified in health or educational systems. These studies are limited by variability in clinical practice and high potential for inconsistency. For these reasons, the CDC established a more refined strategy for their surveillance systems. First, records are screened, and a social trigger allows the creation of a research record for a given child using multiple linked data sources (health and educational). In the second step (confirmation stage), the research record is reviewed by a panel of clinicians to ascertain defined cases. In case of disagreement, other clinicians may arbitrate.
Population‐based studies usually evaluate smaller samples and therefore use a multistage approach to screen the target population and then confirm the accuracy of their final caseness. When the screening phase is completed, cases identified as positive and, unless the screening tool is 100% sensitive, a sample screening negative go through the next step involving a more in‐depth diagnostic evaluation to confirm their case status, either on the full sample or on a randomly selected subsample. Unless the validity of the screening tool is established in a given population, samples of children screening negative should be evaluated to estimate the sensitivity of the screening procedure and adjust prevalence estimates accordingly (Alshaban et al., 2019).
Standard tools frequently used during the screening stage of epidemiological studies include original or translated versions of the M‐CHAT (Chaaya et al., 2016; Hoang et al., 2019), the SCQ (Narzisi et al., 2020; Rudra et al., 2017), the CAST (Morales‐Hidalgo et al., 2018; Sun et al., 2019), and the Autism Spectrum Rating Scale (ASRS; Zhou et al., 2020), the Social Responsiveness Scale (Constantino & Gruber, 2012; Fombonne et al., 2016) or the Autism Quotient (Brugha et al., 2016; Heys et al., 2018).
When participants are directly examined for diagnostic confirmation, various approaches are used ranging from an unstructured examination by a clinical expert to the use of batteries of standardized measures by trained research staff. Standardized diagnostic tools that have been used to confirm ASD include the ADOS (Fombonne et al., 2016; Sun et al., 2019), ADI‐R (Morales‐Hidalgo et al., 2018; Sun et al., 2019) and/or other assessments used to characterize the heterogenous profiles within the spectrum. Interestingly, the use of such tools is still not a guarantee for accurate case ascertainment. As discussed below, the utility of standardized assessment tools is contingent on the level of training and expertise of the people administering them. Moreover, anecdotal evidence suggests that clinicians in some communities may lean towards over‐applying a diagnostic label for autism because such labels facilitate access to services that are otherwise limited.
Choices made by different investigators regarding which tools to use for diagnosis depend on existing clinical and/or research capacity in the target community. Indeed, some epidemiological studies such as those conducted in India (Arora et al., 2018; Raina et al., 2017) have gone beyond raising awareness among policymakers about early identification and have also generated newly validated culturally appropriate screening and/or diagnostic tools, and supported training for clinical and/or research teams. As the number of these studies increases, there is also more awareness of the significant barriers simultaneously impacting research and clinical care in under‐represented world regions, including the high costs and high level of specialized autism expertise needed for rigorous and standardized methods for case identification and evaluation (Abubakar et al., 2016; Durkin et al., 2015; Elsabbagh et al., 2014).
For these reasons, epidemiological studies have responded to the lack of contextually appropriate tools using different approaches. One approach that offers comparable estimates across different world regions involves translations and/or cross‐cultural adaptations of screening and diagnostic tools from their original form in English to other languages and cultural settings; one such example is the Arabic version of the M‐CHAT (Chaaya et al., 2016) or the Arabic version of the SCQ (Aldosari et al., 2019). Other studies have used original validated screening or diagnostic tools purposefully developed for cross‐cultural use or for a specific country/region, albeit limiting comparability with other tools used in autism research (Arora et al., 2018; Kakooza‐Mwesige et al., 2014; Raina et al., 2017). Some studies have used unvalidated although useful tools for their context. For example, a number of studies done in a school setting used a Teacher Nomination Form, where teachers are asked to identify possible cases based on nontechnical descriptions of possible signs or symptoms (Narzisi et al., 2020).
Even when valid and reliable tools are used, the sensitivity of the screening methodology is rarely if ever 100% in autism surveys and some true cases that are not identified. This implies that, in general, available prevalence of autism is underestimated relative to true rates. Recent epidemiological studies in previously under‐represented world regions have also been limited by relatively low participation rates and poor specificity of the screening tools used in the context of these studies (Fombonne, 2019).
In relation to case confirmation, when no standardized tools were available, investigators confirmed cases based on clinical judgment (Ouellette‐Kuntz et al., 2014). A few studies unfortunately omitted the case confirmation stage altogether due to limited research or clinical capacity but acknowledged this as a methodological weakness limiting the value of the resulting estimates (Chaaya et al., 2016; Chinawa et al., 2016; Mohammadi et al., 2019).
Therefore, available studies highlight an unfortunate trade‐off between methodological rigor versus representativeness of samples in epidemiological estimates. The use of standardized tools increases the comparability of findings. In the context of screening, knowledge of the sensitivity and specificity of the tools used is useful in understanding and interpreting resulting estimates. Similarly, the use of standardized tools for diagnostic confirmation reduces reliance on clinical judgment so that cases identified are comparable within and across studies. However, higher costs associated with the use of standardized tools have made research samples less representative of the world's population. This is especially the case in low‐ and middle‐income countries where clinical and research capacity for autism is still developing.
Prevalence estimates and etiological factors
Several hypotheses have emerged linking variations in prevalence in different populations and the time trends of increasing prevalence to possible differences in underlying etiological factors. Moreover, there is accumulating evidence in the studies reviewed that the variation in prevalence reflects disparities linked to a broad category of social determinants including demographic, racial, and socioeconomic. Evidence pertaining to factors identified in the review are discussed next.
Biological sex
Male sex is one of the most well established etiological factors for autism, giving rise to the notion of a “female protective effect,” where females would require greater etiologic load to manifest the same degree of affectedness as males (Elsabbagh, 2020; Lord et al., 2018). This pattern is confirmed by reviewed estimates, where males outnumber females across studies. Paradoxically, the female protective effect also implies that when identified as having autism, females are more likely to exhibit a more severe phenotype. Consistently, most of the studies that reported IQ levels also found a higher proportion of autism cases with ID among girls than boys (Idring et al., 2012; Delobel‐Ayoub et al., 2019; van Bakel et al., 2015). Similarly, another set of studies found that males with ASD were overrepresented in the range of higher IQ (Baio et al., 2018; Brugha et al., 2016). These findings are consistent with previous observations in the US stating that the male‐to‐female ratio increased as the severity of ID decreased (Yeargin‐Allsopp et al., 2003).
Sociodemographic status
Besides methodological differences across studies, major challenge to the comparability of autism prevalence worldwide is that most studies are performed in Western societies. When available, studies in underrepresented regions tended to have small sample sizes and some omitted diagnostic confirmation (Chinawa et al., 2016; Kakooza‐Mwesige et al., 2014). Therefore, while there are currently no sufficient studies with a comparable methodology to address the question of regional variation, existing estimates show equivalent or greater intra‐ than interregional variability. Nevertheless, some studies used comparable methodology to examine variation of prevalence among neighboring geographical regions, for example, the CDC in the United States. The 2019 survey (Christensen et al., 2019) found a threefold variation of rate by site, where Missouri had the lowest rates (85, 81, and 96 per 10,000 for 2010, 2012, and 2014, respectively) and the highest were in New Jersey (197, 221, and 284 per 10,000, for the same years, respectively). Prevalence estimates were consistently higher in sites that reviewed education and health sources to identify cases compared with sites that relied solely on education records (Christensen et al., 2019; Maenner, 2020).
Another study from India estimated prevalence across five regions using a comparable methodology, showing wide differences in prevalence between regions. (Arora et al., 2018). While pooled estimates revealed no differences between rural and urban settings, another cross‐sectional study conducted in India showed higher autism prevalence in rural areas relative to urban and tribal areas (Raina et al., 2017). Conversely, an overall higher prevalence in urban versus rural populations was observed in Taiwan, but this gap decreased overtime (Lai et al., 2012). In Greece, neither the correlation between overall prevalence and percentage of rural residents nor that between prevalence and percentage of the population living in mountainous areas were statistically significant after excluding major cities, suggesting that local prevalence may not be associated with difficulty of access to the educational centers where children are evaluated (Thomaidis et al., 2020). Taken together, there is no consistent socio‐demographic factor that clearly accounts for the observed variation, but some findings may be related to regional differences in availability and/or accessibility of services.
Race/ethnicity and nativity
Racial disparities reflected in autism prevalence have been monitored in the United States over time and the pattern of change suggests a “catch up” in diagnosis in minorities who were initially underdiagnosed (Christensen et al., 2019; Maenner, 2020). For example, in 2016, overall prevalence estimates were almost identical for white, non‐Hispanic, black, and Asian/Pacific Islander children, but lower for Hispanic children, and the differences in prevalence between whites and blacks were only observed in two sites (Maenner, 2020). In 2010, however, non‐Hispanic white children were ~2.5 times more likely to be diagnosed with ASD than non‐Hispanic black children. On the other hand, available data from insurance (Medicaid) still suggests higher prevalence among white as compared with other ethnicities (Jariwala‐Parikh et al., 2019). Outside of the United States, a recent study found significantly lower rates of ASD among Arabs and Ultra‐Orthodox Jews relative to the general population (Raz et al., 2015). Racial differences also impact clinical presentation in the United States; a higher proportion of blacks diagnosed with autism were classified in the range of ID compared with Hispanic and white children (Baio et al., 2018).
Time trends
Time trends have been closely monitored in the United States where one of the latest CDC reports estimating prevalence in 2010, 2012, and 2014 found an increase over time in prevalence in New Jersey but estimates remained stable in Arizona and Missouri (Christensen et al., 2019). The study also found that the proportion of children with ASD and ID was also stable over time (Christensen et al., 2019). Another study of low‐income populations in the United States reported an increase of prevalence among adults diagnosed with autism between 2006 and 2008 (Jariwala‐Parikh et al., 2019).
Reported prevalence over time has also been consistently described in various other countries, including in South Korea from 2008 to 2015 (Hong et al., 2020) and in Taiwan every year in the period from 2004 to 2010 (Lai et al., 2012). Cohort effects have also been observed in France where prevalence was higher among children born in 2003 relative to those born in 1997 (van Bakel et al., 2015). Similarly in Australia, prevalence was higher for children born 4 years apart (1999/2000 vs. 2003/2004; May et al., 2017; May et al., 2020; Randall et al., 2016).
DISCUSSION
In recent years, the international community witnessed tremendous positive improvements in public awareness and public health response for autism. Among the benefits are the significant improvements in early identification of the condition, which in part, accounts for higher prevalence rates over time. In parallel, epidemiological estimates have been increasing worldwide, especially in previously under‐represented regions such as Africa and the Middle Eastern region (al‐Mamari et al., 2019; Alshaban et al., 2019; Chinawa et al., 2016). These studies continue to inform the public health response across the globe. Recent studies are continuing to demonstrate the relatively high prevalence and the health and economic impacts of autism.
In response to the need for an up‐to‐date global estimate of ASD prevalence, our review revealed a median prevalence of 65/10,000 as opposed to 62/10,000 in the previous review. In line with previous evidence, recent studies continue to report an increase in measured prevalence over time either at a country level and/or for specific subgroups, for example, the United States (Christensen et al., 2019; Jariwala‐Parikh et al., 2019), South Korea (Hong et al., 2020), and Taiwan (Lai et al., 2012). Similarly, an increase in measured prevalence has been reported in later birth cohorts in France (van Bakel et al., 2015) and Australia (May et al., 2020; May et al., 2017; Randall et al., 2016).
Our findings also confirm that the substantial variability observed in the estimates can be, in part, accounted for by methodological and contextual differences among studies. First, surveillance systems, national registries, and other administrative databases offer larger and more representative samples relative to other study designs, but they are usually associated with lower sensitivity for case finding (Dodds et al., 2009), especially in areas with limited availability and/or access to service. In contrast, active case‐finding procedures in cohort studies or population‐based epidemiological surveys may result in more rigorous estimates, but their results are often confounded by multiple factors related to diverging strategies for case finding, evaluation, and confirmation used across studies. Second, methodological factors also interact with the broader community context where the level of awareness and capacity in health and education systems significantly impact autism identification, evaluation, and therefore prevalence estimates. Third, the evolving nature of the clinical definition of autism and its differentiation from overlapping conditions has continued to influence prevalence estimates.
Several epidemiological studies identified in our review attempted to use prevalence estimates to infer underlying etiological factors. Biological sex has been considered as a clear contributing factor based on the consistency in higher male‐to‐female ratio reported across study. Complicating inference about sex as a biological factor are hypotheses about differences in ascertainment that may well account for the very wide range in ratios (0.8 to 6.1). For example, some studies attributed sex differences to ‘boy‐centric’ aspects of diagnosis such as girls having more socially appropriate restricted interests than boys, or overall higher levels of social skills, or lower IQ (Chaaya et al., 2016; Frazier et al., 2014). Moreover, current assessment practices for autism are not optimized for girls relative to boys (Beggiato et al., 2017; Van Wijngaarden‐Cremers et al., 2014). In particular, some studies have shown that many girls with a positive ASD screening were false positives (Morales‐Hidalgo et al., 2018; Van Wijngaarden‐Cremers et al., 2014). Therefore, while male sex can reasonably be used as a biological indicator, the impact of ascertainment issues on prevalence estimates is unknown.
The evidence we reviewed also links autism prevalence with a range of social determinants, including geography and race/ethnicity. Other research focusing on the social determinants of autism has found a positive association between SES and autism prevalence, albeit not consistently. We could not find compelling evidence for direct causal associations between any of these factors and variation in autism prevalence. Instead, social determinants likely interact with biological factors in complex ways, exerting an influence on prevalence and outcomes by modifying patterns of help seeking and access to care. For example, the inconsistency in the association between SES and autism prevalence may reflect differences in availability and affordability of health services in countries like Sweden (Rai et al., 2012) and France (Delobel‐Ayoub et al., 2015), but not in other countries like the United States (Durkin et al., 2017). Similarly, previous evidence from in the United States has consistently revealed racial dipartites, but recent findings suggest that such disparities diminish after stratification by SES (Durkin et al., 2017). New findings from India also report more cases of autism among higher relative to lower SES groups, albeit without reaching statistical significance (Raina et al., 2017). Paradoxically, prevalence of other childhood disabilities, including ID, is consistently higher among lower SES groups (Spencer et al., 2015). As such, findings from autism epidemiology converge with other areas of research signaling the need for improved understanding and targeted policies to address health disparities.
Similarly, time trends in autism prevalence also reflect the combined effects of multiple factors rather than a single causal pathway. Factors known to account for the rise in prevalence include the increase in community awareness and public health response globally, changes in case definition that have broadened diagnostic boundaries over time, increased diagnosis of milder forms, and increase in the identification of autism in previously under‐diagnosed populations defined by sex, geography, race/ethnicity, or SES. To date, there is weak or conflicting evidence for the hypothesis that geographic variation or time trends in prevalence may reflect differences in exposure to environmental risk factors (Nevison, 2014) or increased patterns of migration (Keen et al., 2010).
Key limitations are common to our original 2012 systematic review are important to acknowledge. The review protocols were not pre‐registered and we did not conduct an appraisal of methodological quality. Only minimum criteria were used for including studies in the systematic review related to completeness of data relevant for the calculation of prevalence estimates. Instead of excluding data based on quality, we opted to report all data comprehensively and synthesize various methodological features that contribute to variation in prevalence. As this area expands in the future, more studies will hopefully adopt higher standards for study design and reporting, enabling more precision in estimates and more direct implications for shaping public policy.
Along the same lines, future studies can benefit from further refinement in hypotheses about the mechanisms underlying putative associations of various etiological factors with autism prevalence. More specifically, post hoc explanations linking autism prevalence with environmental exposure or with social determinant need to be replaced with well‐designed and justified studies with prespecified hypothesis. The complex interplay between various etiological factors and social determinants can be elucidated with strengthened measurement and robust data analyses. For example, hypothesis linking immigration status to an increase in prevalence could measure more directly possible exposure to environmental risk or trauma and can control for confounding factors like ethnicity and socioeconomic disadvantage.
CONCLUSION
An up‐to‐date review of autism epidemiology in the last decade demonstrates the impressive progress made in this area of research. Today, researchers and policymakers worldwide have access to a wealth of epidemiological evidence relevant for public awareness and policy response. Remaining gaps in evidence limit clear inference about underlying causal mechanisms that can be gleaned from epidemiological data. On the other hand, there is compelling evidence for health disparities affecting specific underserved groups and highlighting the need to strengthen awareness and policy response to address these disparities globally. The tremendous increase in the number of studies in previously under‐represented regions offers unique opportunities to advance scientific discovery of autism etiology while simultaneously expanding community capacity to address unmet needs, especially among underserved populations.
CONFLICT OF INTEREST
The authors declare that they have no known conflicts of interest.
ETHIC STATEMENT
Institutional ethics review was not sought because the current review relied on secondary use of group‐level data.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENT
The work was supported by Fonds de Recherche du Québec Santé (FRQS), and Canadian Institutes of Health Research (CIHR).
Zeidan, J. , Fombonne, E. , Scorah, J. , Ibrahim, A. , Durkin, M. S. , Saxena, S. , Yusuf, A. , Shih, A. , & Elsabbagh, M. (2022). Global prevalence of autism: A systematic review update. Autism Research, 15(5), 778–790. 10.1002/aur.2696
Funding informationFonds de Recherche du Québec Santé (FRQS), and Canadian Institutes of Health Research (CIHR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DATA AVAILABILITY STATEMENT
Data that support the findings of this study are available in the Supplementary Tables. Other materials used in the review are available upon request from the corresponding author, ME.
REFERENCES
- Abubakar, A. , Ssewanyana, D. , de Vries, P. J. , & Newton, C. R. (2016). Autism spectrum disorders in sub‐Saharan Africa. The Lancet Psychiatry, 3(9), 800–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldosari, M. , Fombonne, E. , Aldhalaan, H. , Ouda, M. , Elhag, S. , Alshammari, H. , Ghazal, I. , Alsaleh, A. , Alqadoumi, T. , Thomson, R. , al Khasawneh, M. , Tolefat, M. , & Alshaban, F. (2019). Validation of the Arabic version of the social communication questionnaire. Autism, 23(7), 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al‐Mamari, W. , Idris, A. B. , Dakak, S. , al‐Shekaili, M. , al‐Harthi, Z. , Alnaamani, A. M. , Alhinai, F. I. , Jalees, S. , al Hatmi, M. , el‐Naggari, M. A. , & Islam, M. M. (2019). Revisiting the prevalence of autism Spectrum disorder among Omani children: A multicentre study. Sultan Qaboos University Medical Journal, 19(4), e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshaban, F. , Aldosari, M. , al‐Shammari, H. , el‐Hag, S. , Ghazal, I. , Tolefat, M. , Ali, M. , Kamal, M. , Abdel Aati, N. , Abeidah, M. , Saad, A. H. , Dekair, L. , al Khasawneh, M. , Ramsay, K. , & Fombonne, E. (2019). Prevalence and correlates of autism spectrum disorder in Qatar: A national study. Journal of Child Psychology and Psychiatry, 60, 1254–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). Diagnostic and statistical manual of mental disorders (DSM‐5®). American Psychiatric Pub. [Google Scholar]
- Arora, N. K. , Nair, M. , Gulati, S. , Deshmukh, V. , Mohapatra, A. , Mishra, D. , Patel, V. , Pandey, R. M. , Das, B. C. , Divan, G. , Murthy, G. V. S. , Sharma, T. D. , Sapra, S. , Aneja, S. , Juneja, M. , Reddy, S. K. , Suman, P. , Mukherjee, S. B. , Dasgupta, R. , … Vajaratkar, V. (2018). Neurodevelopmental disorders in children aged 2–9 years: Population‐based burden estimates across five regions in India. PLoS Medicine, 15(7), e1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio, J. , Wiggins, L. , Christensen, D. L. , Maenner, M. J. , Daniels, J. , Warren, Z. , Kurzius‐Spencer, M. , Zahorodny, W. , Robinson, C. , White, T. , Durkin, M. S. , Imm, P. , Nikolaou, L. , Yeargin‐Allsopp, M. , Lee, L. C. , Harrington, R. , Lopez, M. , Fitzgerald, R. T. , Hewitt, A. , … Dowling, N. F. (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveillance Summaries, 67(6), 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggiato, A. , Peyre, H. , Maruani, A. , Scheid, I. , Rastam, M. , Amsellem, F. , Gillberg, C. , Leboyer, M. , Bourgeron, T. , Gillberg, C. , & Delorme, R. (2017). Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Research, 10(4), 680–689. [DOI] [PubMed] [Google Scholar]
- Brugha, T. S. , Spiers, N. , Bankart, J. , Cooper, S.‐A. , McManus, S. , Scott, F. J. , Smith, J. , & Tyrer, F. (2016). Epidemiology of autism in adults across age groups and ability levels. The British Journal of Psychiatry, 209(6), 498–503. [DOI] [PubMed] [Google Scholar]
- Chaaya, M. , Saab, D. , Maalouf, F. T. , & Boustany, R.‐M. (2016). Prevalence of autism spectrum disorder in nurseries in Lebanon: A cross sectional study. Journal of Autism and Developmental Disorders, 46(2), 514–522. [DOI] [PubMed] [Google Scholar]
- Chinawa, J. M. , Manyike, P. C. , Aniwada, E. C. , Chinawa, A. T. , Obu, H. A. , Odetunde, O. I. , Nwokocha, A. R. C. , & Ibekwe, R. R. (2017). Prevalence and socioeconomic correlates of autism among children attending primary and secondary schools in south East Nigeria. African Health Sciences, 16(4), 936–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese Society of Psychiatry . (2001). The Chinese Classification and Diagnostic Criteria of Mental Disorders Version 3 (CCMD‐3). Shandong Publishing Company of Science and Technology, China. [Google Scholar]
- Christensen, D. L. , Maenner, M. J. , Bilder, D. , Constantino, J. N. , Daniels, J. , Durkin, M. S. , Fitzgerald, R. T. , Kurzius‐Spencer, M. , Pettygrove, S. D. , Robinson, C. , Shenouda, J. , White, T. , Zahorodny, W. , Pazol, K. , & Dietz, P. (2019). Prevalence and characteristics of autism spectrum disorder among children aged 4 years—Early autism and developmental disabilities monitoring network, seven sites, United States, 2010, 2012, and 2014. MMWR Surveillance Summaries, 68(2), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , & Gruber, C. P. (2012). Social responsiveness scale: SRS‐2. Western Psychological Services Torrance. [Google Scholar]
- Delobel‐Ayoub, M. , Ehlinger, V. , Klapouszczak, D. , Maffre, T. , Raynaud, J.‐P. , Delpierre, C. , & Arnaud, C. (2015). Socioeconomic disparities and prevalence of autism spectrum disorders and intellectual disability. PLoS One, 10(11), e0141964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobel‐Ayoub, M. , Saemundsen, E. , Gissler, M. , Ego, A. , Moilanen, I. , Ebeling, H. , Rafnsson, V. , Klapouszczak, D. , Thorsteinsson, E. , Arnaldsdóttir, K. M. , Roge, B. , Arnaud, C. , & Schendel, D. (2020). Prevalence of autism Spectrum disorder in 7–9‐year‐old children in Denmark, Finland, France and Iceland: A population‐based registries approach within the ASDEU project. Journal of Autism and Developmental Disorders, 50, 949–959. 10.1007/s10803-019-04328-y [DOI] [PubMed] [Google Scholar]
- Diallo, F. B. , Fombonne, É. , Kisely, S. , Rochette, L. , Vasiliadis, H.‐M. , Vanasse, A. , Noiseux, M. , Pelletier, É. , Renaud, J. , St‐Laurent, D. , & Lesage, A. (2018). Prevalence and correlates of autism spectrum disorders in Quebec: Prévalence et corrélats des troubles du spectre de l'autisme au Québec. The Canadian Journal of Psychiatry, 63(4), 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds, L. , Spencer, A. , Shea, S. , Fell, D. , Armson, B. , Allen, A. , & Bryson, S. (2009). Validity of autism diagnoses using administrative health data. Chronic Diseases in Canada, 29(3), 102–107. [PMC free article] [PubMed] [Google Scholar]
- Durkin, M. S. , Elsabbagh, M. , Barbaro, J. , Gladstone, M. , Happe, F. , Hoekstra, R. A. , Lee, L. C. , Rattazzi, A. , Stapel‐Wax, J. , Stone, W. L. , Tager‐Flusberg, H. , Thurm, A. , Tomlinson, M. , & Shih, A. (2015). Autism screening and diagnosis in low resource settings: Challenges and opportunities to enhance research and services worldwide. Autism Research, 8(5), 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin, M. S. , Maenner, M. J. , Baio, J. , Christensen, D. , Daniels, J. , Fitzgerald, R. , Imm, P. , Lee, L. C. , Schieve, L. A. , van Naarden Braun, K. , Wingate, M. S. , & Yeargin‐Allsopp, M. (2017). Autism spectrum disorder among US children (2002–2010): Socioeconomic, racial, and ethnic disparities. American Journal of Public Health, 107(11), 1818–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh, M. (2020). Linking risk factors and outcomes in autism spectrum disorder: Is there evidence for resilience? BMJ, 368, l6880. [DOI] [PubMed] [Google Scholar]
- Elsabbagh, M. , Divan, G. , Koh, Y. J. , Kim, Y. S. , Kauchali, S. , Marcín, C. , Montiel‐Nava, C. , Patel, V. , Paula, C. S. , Wang, C. , Yasamy, M. T. , & Fombonne, E. (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Research, 5(3), 160–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsabbagh, M. , Yusuf, A. , Prasanna, S. , Shikako‐Thomas, K. , Ruff, C. A. , & Fehlings, M. G. (2014). Community engagement and knowledge translation: Progress and challenge in autism research. Autism, 18(7), 771–781. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Reed, G. M. , Hyman, S. E. , & Saxena, S. (2015). The development of the ICD‐11 clinical descriptions and diagnostic guidelines for mental and behavioural disorders. World Psychiatry, 14(1), 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne, E. (2009). Epidemiology of pervasive developmental disorders. Pediatric Research, 65(6), 591–598. [DOI] [PubMed] [Google Scholar]
- Fombonne, É. (2019). Current issues in epidemiogical studies of autism. Revista Psicologia‐Teoria e Prática, 21(3), 405–417. [Google Scholar]
- Fombonne, E. , Marcin, C. , Manero, A. C. , Bruno, R. , Diaz, C. , Villalobos, M. , Ramsay, K. , & Nealy, B. (2016). Prevalence of autism spectrum disorders in Guanajuato, Mexico: The Leon survey. Journal of Autism and Developmental Disorders, 46(5), 1669–1685. [DOI] [PubMed] [Google Scholar]
- Franz, L. , Chambers, N. , von Isenburg, M. , & de Vries, P. J. (2017). Autism spectrum disorder in sub‐Saharan Africa: A comprehensive scoping review. Autism Research, 10(5), 723–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, T. W. , Georgiades, S. , Bishop, S. L. , & Hardan, A. Y. (2014). Behavioral and cognitive characteristics of females and males with autism in the Simons simplex collection. Journal of the American Academy of Child & Adolescent Psychiatry, 53(3), 329–340 e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt, A. , Hall‐Lande, J. , Hamre, K. , Esler, A. N. , Punyko, J. , Reichle, J. , & Gulaid, A. A. (2016). Autism spectrum disorder (ASD) prevalence in Somali and non‐Somali children. Journal of Autism and Developmental Disorders, 46(8), 2599–2608. [DOI] [PubMed] [Google Scholar]
- Heys, M. , Gibbons, F. , Haworth, E. , Medeiros, E. , Tumbahangphe, K. M. , Wickenden, M. , Shrestha, M. , Costello, A. , Manandhar, D. , & Pellicano, E. (2018). The estimated prevalence of autism in school‐aged children living in rural Nepal using a population‐based screening tool. Journal of Autism and Developmental Disorders, 48(10), 3483–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang, V. M. , le, T. V. , Chu, T. T. Q. , le, B. N. , Duong, M. D. , Thanh, N. M. , Tac Pham, V. , Minas, H. , & Bui, T. T. H. (2019). Prevalence of autism spectrum disorders and their relation to selected socio‐demographic factors among children aged 18‐30 months in northern Vietnam, 2017. International Journal of Mental Health Systems, 13, 29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, M. , Lee, S. M. , Park, S. , Yoon, S.‐J. , Kim, Y.‐E. , & Oh, I.‐H. (2020). Prevalence and economic burden of autism Spectrum disorder in South Korea using National Health Insurance Data from 2008 to 2015. Journal of Autism and Developmental Disorders, 50(1), 333–339. [DOI] [PubMed] [Google Scholar]
- Idring, S. , Rai, D. , Dal, H. , Dalman, C. , Sturm, H. , Zander, E. , Lee, B. K. , Serlachius, E. , & Magnusson, C. (2012). Autism spectrum disorders in the Stockholm youth cohort: Design, prevalence and validity. PLoS One, 7(7), e41280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwala‐Parikh, K. , Barnard, M. , Holmes, E. R. , West‐Strum, D. , Bentley, J. P. , Banahan, B. , & Khanna, R. (2019). Autism prevalence in the Medicaid program and healthcare utilization and costs among adult enrollees diagnosed with autism. Administration and Policy in Mental Health and Mental Health Services Research, 46(6), 768–776. [DOI] [PubMed] [Google Scholar]
- Kakooza‐Mwesige, A. , Ssebyala, K. , Karamagi, C. , Kiguli, S. , Smith, K. , Anderson, M. C. , Croen, L. A. , Trevathan, E. , Hansen, R. , Smith, D. , & Grether, J. K. (2014). Adaptation of the “ten questions” to screen for autism and other neurodevelopmental disorders in Uganda. Autism, 18(4), 447–457. [DOI] [PubMed] [Google Scholar]
- Keen, D. , Reid, F. , & Arnone, D. (2010). Autism, ethnicity and maternal immigration. The British Journal of Psychiatry, 196(4), 274–281. [DOI] [PubMed] [Google Scholar]
- Kočovská, E. , Biskupstø, R. , Carina Gillberg, I. , Ellefsen, A. , Kampmann, H. , Stórá, T. , Billstedt, E. , & Gillberg, C. (2012). The rising prevalence of autism: A prospective longitudinal study in The Faroe Islands. Journal of Autism and Developmental Disorders, 42(9), 1959–1966. [DOI] [PubMed] [Google Scholar]
- Kogan, M. D. , Vladutiu, C. J. , Schieve, L. A. , Ghandour, R. M. , Blumberg, S. J. , Zablotsky, B. , Perrin, J. M. , Shattuck, P. , Kuhlthau, K. A. , Harwood, R. L. , & Lu, M. C. (2018). The prevalence of parent‐reported autism spectrum disorder among US children. Pediatrics, 142(6), e20174161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, D.‐C. , Tseng, Y.‐C. , Hou, Y.‐M. , & Guo, H.‐R. (2012). Gender and geographic differences in the prevalence of autism spectrum disorders in children: Analysis of data from the national disability registry of Taiwan. Research in Developmental Disabilities, 33(3), 909–915. [DOI] [PubMed] [Google Scholar]
- Lord, C. , Elsabbagh, M. , Baird, G. , & Veenstra‐Vanderweele, J. (2018). Autism spectrum disorder. The Lancet, 392(10146), 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner, M. J. (2020). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2016. MMWR Surveillance summaries, 69, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, T. , Brignell, A. , & Williams, K. (2020). Autism Spectrum disorder prevalence in children aged 12–13 years from the longitudinal study of Australian children. Autism Research, 13, 821–827. [DOI] [PubMed] [Google Scholar]
- May, T. , Sciberras, E. , Brignell, A. , & Williams, K. (2017). Autism spectrum disorder: Updated prevalence and comparison of two birth cohorts in a nationally representative Australian sample. BMJ Open, 7(5), e015549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi, M.‐R. , Ahmadi, N. , Khaleghi, A. , Zarafshan, H. , Mostafavi, S.‐A. , Kamali, K. , Rahgozar, M. , Ahmadi, A. , Hooshyari, Z. , Alavi, S. , Shakiba, A. , Salmanian, M. , Molavi, P. , Sarraf, N. , Hojjat, S. K. , Mohammadzadeh, S. , Amiri, S. , Arman, S. , & Ghanizadeh, A. (2019). Prevalence of autism and its comorbidities and the relationship with maternal psychopathology: A national population‐based study. Archives of Iranian Medicine, 22(10), 546–553. [PubMed] [Google Scholar]
- Morales‐Hidalgo, P. , Roigé‐Castellví, J. , Hernández‐Martínez, C. , Voltas, N. , & Canals, J. (2018). Prevalence and characteristics of autism spectrum disorder among Spanish school‐age children. Journal of Autism and Developmental Disorders, 48(9), 3176–3190. [DOI] [PubMed] [Google Scholar]
- Narzisi, A. , Posada, M. , Barbieri, F. , Chericoni, N. , Ciuffolini, D. , Pinzino, M. , Romano, R. , Scattoni, M. L. , Tancredi, R. , Calderoni, S. , & Muratori, F. (2020). Prevalence of autism spectrum disorder in a large Italian catchment area: A school‐based population study within the ASDEU project. Epidemiology and Psychiatric Sciences, 29, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevison, C. D. (2014). A comparison of temporal trends in United States autism prevalence to trends in suspected environmental factors. Environmental Health, 13(1), 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofner, M. , Coles, A. , Decou, M. , Do, M. , Bienek, A. , Snider, J. , & Ugnat, A. (2018). Autism spectrum disorder among children and youth in Canada 2018. Public Health Agency of Canada Ottawa. [Google Scholar]
- Ouellette‐Kuntz, H. , Coo, H. , Lam, M. , Breitenbach, M. M. , Hennessey, P. E. , Jackman, P. D. , Lewis, M. E. S. , Dewey, D. , Bernier, F. P. , & Chung, A. M. (2014). The changing prevalence of autism in three regions of Canada. Journal of Autism and Developmental Disorders, 44(1), 120–136. [DOI] [PubMed] [Google Scholar]
- Pang, Y. , Lee, C. M. , Wright, M. , Shen, J. , Shen, B. , & Bo, J. (2018). Challenges of case identification and diagnosis of autism spectrum disorders in China: A critical review of procedures, assessment, and diagnostic criteria. Research in Autism Spectrum Disorders, 53, 53–66. [Google Scholar]
- Parkin, E. , Bate, A. , Long, R. , Gheera, M. , Powell, A. , & Bellis, A. (2018). Autism – overview of UK policy and services. In Commons Briefing papers CBP‐7172. London: The House of Commons Library. [Google Scholar]
- Pinborough‐Zimmerman, J. , Bakian, A. V. , Fombonne, E. , Bilder, D. , Taylor, J. , & McMahon, W. M. (2012). Changes in the administrative prevalence of autism spectrum disorders: Contribution of special education and health from 2002–2008. Journal of Autism and Developmental Disorders, 42(4), 521–530. [DOI] [PubMed] [Google Scholar]
- Poovathinal, S. A. , Anitha, A. , Thomas, R. , Kaniamattam, M. , Melempatt, N. , Anilkumar, A. , & Meena, M. (2016). Prevalence of autism spectrum disorders in a semiurban community in South India. Annals of Epidemiology, 26(9), 663–665 e668. [DOI] [PubMed] [Google Scholar]
- Rai, D. , Lewis, G. , Lundberg, M. , Araya, R. , Svensson, A. , Dalman, C. , Carpenter, P. , & Magnusson, C. (2012). Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population‐based study. Journal of the American Academy of Child & Adolescent Psychiatry, 51(5), 467–476.e6. [DOI] [PubMed] [Google Scholar]
- Raina, S. K. , Chander, V. , Bhardwaj, A. K. , Kumar, D. , Sharma, S. , Kashyap, V. , Singh, M. , & Bhardwaj, A. (2017). Prevalence of autism spectrum disorder among rural, urban, and tribal children (1–10 years of age). Journal of Neurosciences in Rural Practice, 8(03), 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall, M. , Sciberras, E. , Brignell, A. , Ihsen, E. , Efron, D. , Dissanayake, C. , & Williams, K. (2016). Autism spectrum disorder: Presentation and prevalence in a nationally representative Australian sample. The Australian and New Zealand Journal of Psychiatry, 50(3), 243–253. [DOI] [PubMed] [Google Scholar]
- Raz, R. , Weisskopf, M. G. , Davidovitch, M. , Pinto, O. , & Levine, H. (2015). Differences in autism spectrum disorders incidence by sub‐populations in Israel 1992–2009: A total population study. Journal of Autism and Developmental Disorders, 45(4), 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra, A. , Belmonte, M. K. , Soni, P. K. , Banerjee, S. , Mukerji, S. , & Chakrabarti, B. (2017). Prevalence of autism spectrum disorder and autistic symptoms in a school‐based cohort of children in Kolkata, India. Autism Research, 10(10), 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel, D. E. , & Thorsteinsson, E. (2018). Cumulative incidence of autism into adulthood for birth cohorts in Denmark, 1980‐2012. JAMA, 320(17), 1811–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, N. J. , Blackburn, C. M. , & Read, J. M. (2015). Disabling chronic conditions in childhood and socioeconomic disadvantage: A systematic review and meta‐analyses of observational studies. BMJ Open, 5(9), e007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, X. , Allison, C. , Wei, L. , Matthews, F. E. , Auyeung, B. , Wu, Y. Y. , Griffiths, S. , Zhang, J. , Baron‐Cohen, S. , & Brayne, C. (2019). Autism prevalence in China is comparable to Western prevalence. Molecular Autism, 10(1), 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaidis, L. , Mavroeidi, N. , Richardson, C. , Choleva, A. , Damianos, G. , Bolias, K. , & Tsolia, M. (2020). Autism Spectrum disorders in Greece: Nationwide prevalence in 10–11 year‐old children and regional disparities. Journal of Clinical Medicine, 9(7), 2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bakel, M. M. E. , Delobel‐Ayoub, M. , Cans, C. , Assouline, B. , Jouk, P.‐S. , Raynaud, J.‐P. , & Arnaud, C. (2015). Low but increasing prevalence of autism spectrum disorders in a French area from register‐based data. Journal of Autism and Developmental Disorders, 45(10), 3255–3261. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden‐Cremers, P. J. , van Eeten, E. , Groen, W. B. , Van Deurzen, P. A. , Oosterling, I. J. , & Van der Gaag, R. J. (2014). Gender and age differences in the core triad of impairments in autism spectrum disorders: A systematic review and meta‐analysis. Journal of Autism and Developmental Disorders, 44(3), 627–635. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Cao, F. , & Boyland, J. T. (2019). Addressing autism spectrum disorders in China. New Directions for Child and Adolescent Development, 2019(163), 137–162. [DOI] [PubMed] [Google Scholar]
- WHO . (2013a). Meeting report: Autism spectrum disorders and other developmental disorders: From raising awareness to building capacity. World Health Organization. [Google Scholar]
- WHO . (2013b). Mental health action plan 2013–2020.
- WHO . (2014). Comprehensive and coordinated efforts for the management of autism spectrum disorders. WHO Press. [Google Scholar]
- WHO . (2015). Every woman every child. Global strategy for women's, children's and adolescents' health (2016–2030). WHO Press. [Google Scholar]
- WHO . (2018). WHO mental health gap action programme (mhGAP) 2008. WHO Press. [Google Scholar]
- WHO , & UNICEF . (2018). World Bank Group. Nurturing care for early childhood development: a framework for helping children survive and thrive to transform health and human potential. WHO Press. [Google Scholar]
- Xu, G. , Strathearn, L. , Liu, B. , O'Brien, M. , Kopelman, T. G. , Zhu, J. , Snetselaar, L. G. , & Bao, W. (2019). Prevalence and treatment patterns of autism spectrum disorder in the United States, 2016. JAMA Pediatrics, 173(2), 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeargin‐Allsopp, M. , Rice, C. , Karapurkar, T. , Doernberg, N. , Boyle, C. , & Murphy, C. (2003). Prevalence of autism in a US metropolitan area. JAMA, 289(1), 49–55. [DOI] [PubMed] [Google Scholar]
- Zeidan, J. , Shikako‐Thomas, K. , Ehsan, A. , Maioni, A. , & Elsabbagh, M. (2019). Progress and gaps in Quebec's autism policy: A comprehensive review and thematic analysis. Canadian Journal of Public Health, 110(4), 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, H. , Xu, X. , Yan, W. , Zou, X. , Wu, L. , Luo, X. , Li, T. , Huang, Y. , Guan, H. , Chen, X. , Mao, M. , Xia, K. , Zhang, L. , Li, E. , Ge, X. , Zhang, L. , Li, C. , Zhang, X. , Zhou, Y. , … LATENT‐NHC Study Team . (2020). Prevalence of autism spectrum disorder in China: A nationwide multi‐center population‐based study among children aged 6 to 12 years. Neuroscience Bulletin, 36, 961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information
Data Availability Statement
Data that support the findings of this study are available in the Supplementary Tables. Other materials used in the review are available upon request from the corresponding author, ME.


