Abstract
Objectives
The TITAN study is a randomized, double‐blind, placebo‐controlled, multinational trial that evaluated apalutamide with androgen deprivation therapy in patients with metastatic castration‐sensitive prostate cancer. At the first interim analysis in the Japanese subpopulation (median follow‐up 25.7 months), there was an improvement in overall survival and radiological progression‐free survival with apalutamide versus placebo. Here, we report the final analysis results for the Japanese subpopulation.
Methods
Patients were randomized 1:1 to receive apalutamide 240 mg or placebo. After the first interim analysis, protocol treatment was unblinded, and crossover was allowed. Efficacy and safety were evaluated in the preplanned, event‐driven final analysis.
Results
Fifty‐one patients were Japanese (apalutamide n = 28; placebo n = 23). After a median follow‐up of 46.0 months, the median overall survival was not reached neither in the apalutamide nor the placebo group; the hazard ratio was 0.45, favoring apalutamide, which was consistent with the overall population. Hazard ratios for time to cytotoxic chemotherapy (0.39), time to pain progression (0.87), and time to chronic opioid use (0.82) also favored apalutamide and were comparable with those of the overall population. Time to prostate‐specific antigen progression and progression‐free survival 2, respectively, was favored in the apalutamide group (0.21 and 0.44). Apalutamide was associated with higher incidences of rash and fracture in the Japanese subpopulation compared with the overall population.
Conclusions
The efficacy of apalutamide with androgen deprivation therapy in Japanese patients was consistent with efficacy demonstrated in the overall population. No new safety concerns emerged with long‐term follow‐up.
Keywords: androgen deprivation therapy, apalutamide, Japan, metastatic castration‐sensitive prostate cancer
Abbreviations & Acronyms
- ADT
androgen deprivation therapy
- BSA
bone sparing agent
- CI
confidence interval
- HR
hazard ratio
- mCSPC
metastatic castration‐sensitive prostate cancer
- NE
not evaluable
- nmCRPC
non‐metastatic castration‐resistant prostate cancer
- OS
overall survival
- PC
prostate cancer
- PCWG2
prostate cancer clinical trials working group 2
- PFS2
progression‐free survival 2
- PSA
prostate‐specific antigen
- rPFS
radiographic progression‐free survival
- SAE
serious adverse event
- TEAE
treatment‐emergent adverse event
Introduction
PC was the second most frequently diagnosed cancer (14.1%) and the fifth leading cause of cancer mortality (6.8%) worldwide among men in 2020. 1 The prevalence of PC has increased in Asian countries, with the incidence rates in some countries, including Japan, being now comparable to Western countries. 2 In most patients, tumors are initially castration‐sensitive and respond to ADT. ADT combined with chemotherapy or new hormonal therapies has shown clinical benefit over ADT alone in randomized controlled trials 3 , 4 , 5 , 6 for patients with mCSPC. Internationally, treatment guidelines have uniformly recommended these ADT combinations for patients with mCSPC. 7 , 8 , 9
Apalutamide is a next‐generation, potent, selective, and orally bioavailable nonsteroidal antiandrogen. 10 It was first approved in the United States in 2018 and in Japan in 2019 to treat patients with nmCRPC according to results from the phase III SPARTAN study. 11 , 12 Apalutamide was also tested in patients with mCSPC in the phase III TITAN study. In this study, combination therapy with apalutamide and ADT showed improved OS and rPFS compared with placebo combined with ADT. 4 , 13 From these results, apalutamide was approved for the treatment of mCSPC in the United States in 2019 and in Japan in 2020.
Subgroup analyses of the TITAN study characterized the efficacy and safety of apalutamide in the Japanese subpopulation. 13 In the first preplanned analysis, which was the final analysis of rPFS and the first interim analysis of OS, the efficacy and safety of apalutamide in Japanese mCSPC patients were similar to results in the overall population. 13
The aim of this report is to present the final results of OS, secondary and other efficacy endpoints, and safety profile in the Japanese subpopulation in the TITAN study, with approximately 2 years of additional follow‐up.
Methods
Ethics
The TITAN study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. This study was registered at ClinicalTrials.gov (NCT02489318) and JAPIC CTI (JapicCTI‐163118). The study protocol and amendments were reviewed and approved by an Independent Ethics Committee or Institutional Review Board at each study site. Participants were informed of the risks and benefits and all provided written informed consent.
Study design
The TITAN study was a randomized, double‐blind, placebo‐controlled, multinational phase III trial conducted at 260 sites in 23 countries. 4 Recruitment started on 9 December 2015. The study was unblinded on 28 January 2019 and crossover from placebo to apalutamide was allowed. The cutoff date for this final analysis was 7 September 2020 after 405 OS events had occurred.
Patients and intervention
Patients with castration‐sensitive adenocarcinoma of the prostate with distant metastasis were eligible. The eligibility criteria were previously described in detail. 4
Eligible patients were stratified according to Gleason score at diagnosis (≤7 vs >7), prior docetaxel use (yes vs no), and geographic region (North America and Europe vs other countries). Patients were randomized at a 1:1 ratio to oral apalutamide 240 mg once daily + ADT (apalutamide group) or placebo + ADT (placebo group). One treatment cycle was 28 days, and treatment was continued until clinical progression or occurrence of unacceptable treatment‐related toxicity. If the subject had radiographic progression without clinical progression and alternate therapy was not initiated, treatment was continued at the discretion of the investigator. The study drug was continued in patients with increasing PSA values, unless clinical or radiographic progression was observed.
After the first interim analysis and subsequent unblinding of the trial, patients were able to continue treatment in the apalutamide group or crossover from placebo to apalutamide by providing additional written informed consent.
Primary endpoints
The primary endpoints were rPFS and OS (dual‐primary endpoints). In this report, we conducted the final OS analysis, because the final rPFS analysis was reported previously. 13 OS was defined as the time from randomization to the date of death from any cause.
Secondary and other endpoints
For the secondary endpoints, we analyzed the time to cytotoxic chemotherapy, time to pain progression, time to chronic opioid use, and time to skeletal‐related event. 14 As other clinically relevant endpoints, we evaluated the following: time to PSA progression based on the PCWG2 criteria, 15 PFS2 (defined as time from random assignment to the first occurrence of investigator‐determined disease progression [PSA progression, progression on imaging, or clinical progression] on first subsequent therapy or death), and time to symptomatic local progression. In addition, time to castration resistance was assessed in an ad hoc analysis. Time to castration resistance was defined as time from random assignment to the date of radiographic disease progression, PSA progression based on PCWG2, or symptomatic skeletal event, whichever occurred first.
Safety
TEAEs were monitored and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.03. TEAEs were collected that occurred between from the first dose of study drug to 30 days after the last dose. According to previous studies, several TEAEs were defined as TEAEs of special interest, including skin rash (e.g. rash, stomatitis, and rash maculo‐papular), fracture (e.g. spinal compression fracture, rib fracture, and foot fracture), falls, and seizure. Hypothyroidism was excluded from the TEAEs of special interest. Because the events are low grade and manageable with thyroid supplementation. On the other hand, ischemic heart disease and ischemic cerebrovascular disorders were included based on data from the interim analysis of TITAN study and final analysis of the SPARTAN study, respectively.
Statistics
All randomized patients were included in the intent‐to‐treat population, and data were analyzed for demographics and efficacy. All patients who received at least one dose were defined as the safety population, and these data were analyzed for safety.
The patients’ demographics and safety data were summarized descriptively. Time‐to‐event endpoints were summarized using the Kaplan–Meier method, and median times were calculated. Stratified and unstratified Cox proportional‐hazard models were used to estimate the HRs and 95% CIs. The stratified log rank test was used to compare HRs between treatment groups using the previously mentioned stratification factors. However, in the Japanese subpopulation, only one patient had a Gleason score ≤7 at primary diagnosis, no patients had prior docetaxel use, and all patients were located outside of North America or Europe. 13 Therefore, the nonstratified log rank test was used for the Japanese subpopulation analysis.
Detailed statistical methods of the overall population were described in the previous reports. 14 The statistical testing for Japanese subpopulation were performed without adjustment for multiple comparison. Therefore, in this study, all P‐values for the Japanese subpopulation analysis were nominal. 13
Results
Patient demographics
A total of 1052 patients participated in the overall population and 51 patients (4.8%) were Japanese. After randomization, 28 patients (median age 73 years) were assigned to the apalutamide group and 23 patients were assigned to the placebo group (median age 72 years). All patients received at least one dose of study treatment. Twelve patients in the apalutamide group discontinued treatment until the final cut‐off. In the placebo group, 14 patients discontinued treatment and nine patients crossed over to apalutamide (Fig. 1). Median treatment durations were 43.7 (range 1.1–53.5) months in the apalutamide group and 18.9 (2.3–30.8) months in the placebo group. After crossover, patients received apalutamide for a median of 16.8 (range 5.7–17.7) months.
Fig. 1.
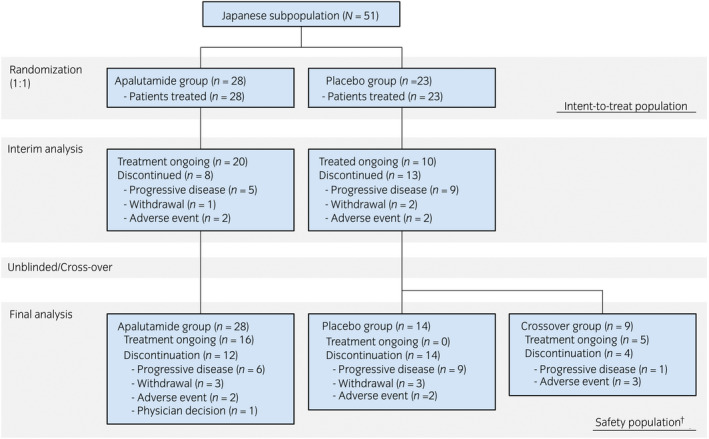
Patient flowchart. †The breakdown of 23 patients in the placebo group in this final safety analysis after crossover is the sum of 14 patients in the placebo group and nine patients in the crossover group.
The demographic and baseline characteristics of the Japanese intent‐to‐treat population were presented in the interim report 13 and are reproduced in addition to those of the overall population in Table S1. 4 In the Japanese subpopulation, most patients had Gleason scores of 8 or 9 (apalutamide 25 patients [89.3%]; placebo 17 patients [73.9%]) and M1 (apalutamide 27 patients [96.4%]; placebo 21 patients [91.3%]). No patients received previous docetaxel treatment. Overall, the demographics were similar in both groups.
Efficacy
The final results of efficacy endpoints are summarized in Table 1. The final rPFS analysis was reported previously. 13 In the Japanese subpopulation, median follow‐up period was 46.0 months (apalutamide 46.1 months; placebo 45.8 months). Death was observed in six patients (21.4%) in the apalutamide group and 10 patients (43.5%) in the placebo, and the median OS was not reached in either treatment group (Fig. 2). The HR for OS was 0.45 (95% CI 0.16–1.25) and favored apalutamide group.
Table 1.
Efficacy endpoints (intent‐to‐treat population)
| Japanese subpopulation (N = 51) | Overall population† (N = 1052) | |||||||
|---|---|---|---|---|---|---|---|---|
| Time to event (months), median (95% CI) | HR (95% CI) | P‐value‡ | Time to event (months), median (95% CI) | HR (95% CI) | P‐value§ | |||
|
Apalutamide (n = 28) |
Placebo (n = 23) |
Apalutamide (n = 525) |
Placebo (n = 527) |
|||||
| Primary endpoint | ||||||||
| OS | NE (NE–NE) | NE (33.1–NE) | 0.45 (0.16–1.25) | 0.1173 | NE (NE–NE) | 52.2 (41.9–NE) | 0.65 (0.53–0.79) | <0.0001 |
| Secondary endpoints | ||||||||
| Time to cytotoxic chemotherapy | NE (NE–NE) | NE (21.9–NE) | 0.39 (0.13–1.15) | 0.0765 | NE (NE–NE) | NE (NE–NE) | 0.47 (0.35–0.63) | <0.0001 |
| Time to pain progression | NE (34.0–NE) | NE (19.8–NE) | 0.87 (0.34–2.27) | 0.7790 | NE (NE–NE) | NE (51.3–NE) | 0.87 (0.70–1.08) | 0.1966 |
| Time to chronic opioid use | NE (NE–NE) | NE (35.4–NE) | 0.82 (0.24–2.85) | 0.7595 | NE (NE–NE) | NE (51.3–NE) | 0.79 (0.58–1.09) | 0.1563 |
| Time to skeletal‐related event | NE (NE–NE) | NE (NE–NE) | 2.39 (0.48–11.85) | 0.2707 | NE (NE–NE) | NE (51.8–NE) | 0.86 (0.62–1.19) | 0.3608 |
| Other clinically relevant endpoints | ||||||||
| Time to PSA progression | NE (NE–NE) | 16.6 (7.4–NE) | 0.21 (0.07–0.61) | 0.0016 | NE (NE–NE) | 12.9 (10.2–14.8) | 0.27 (0.22–0.33) | <0.0001 |
| PFS2 | NE (NE–NE) | 44.5 (26.7–NE) | 0.44 (0.17–1.13) | 0.0784 | NE (NE–NE) | 44.0 (38.9–NE) | 0.62 (0.51–0.75) | <0.0001 |
| Ad hoc analysis | ||||||||
| Time to castration resistance | NE (33.4–NE) | 14.5 (7.4–NE) | 0.28 (0.12–0.67) | 0.0025 | NE (NE–NE) | 11.4 (10.1–14.7) | 0.34 (0.29–0.41) | <0.0001 |
Fig. 2.
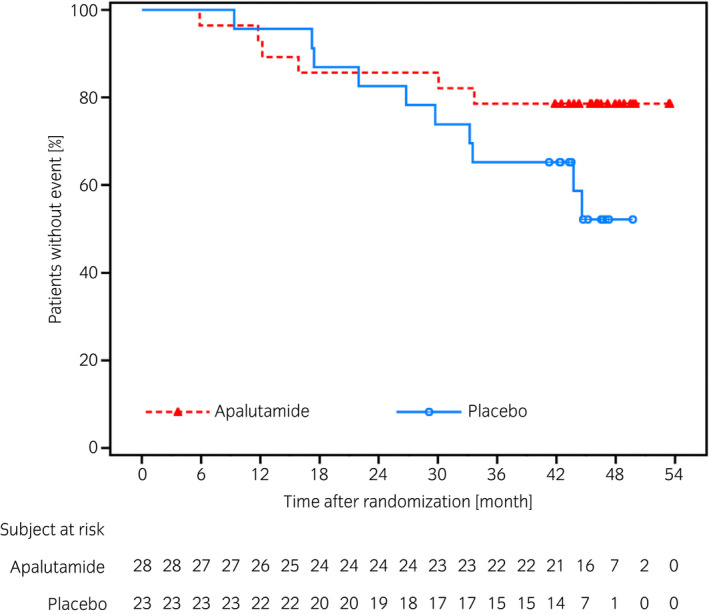
Kaplan–Meier plot of OS in the Japanese subpopulation.
The HRs for all secondary endpoints except time to skeletal‐related events were similar to those in the overall population (Table 1). HRs were 0.39 (95% CI 0.13–1.15) for time to cytotoxic chemotherapy, 0.87 (0.34–2.27) for time to pain progression, 0.82 (0.24–2.85) for time to chronic opioid use, and 2.39 (0.48–11.85) for time to skeletal‐related event. The median time to event for these endpoints was not reached in either group (Table 1).
The results of other clinically relevant endpoints showed the HR for time to PSA progression was 0.21 (95% CI 0.07–0.61), indicating a delay in the apalutamide group compared with placebo group (Fig. 3). Numbers of patients who experienced PSA progression were 5 (17.9%) in the apalutamide group and 13 (56.5%) in the placebo group. After study treatment, 7 of 12 patients (58.3%) who were alive in the apalutamide group and 13 of 18 patients (72.2%) who were alive in the placebo group initiated first subsequent therapy. The HR of PFS2 was 0.44 (95% CI 0.17–1.13) (Fig. 4). The median duration of the first subsequent therapy was 126 (range 48–375) days after apalutamide and 145 (61–637) days after placebo treatments. A detailed breakdown of the first subsequent therapies is shown in Table 2. Symptomatic local progression was not observed. Ad hoc analysis showed that the HR for time to castration resistance was 0.28 (95% CI 0.12–0.67, P = 0.0025), indicating a trend to delay in the apalutamide group (Fig. 5).
Fig. 3.
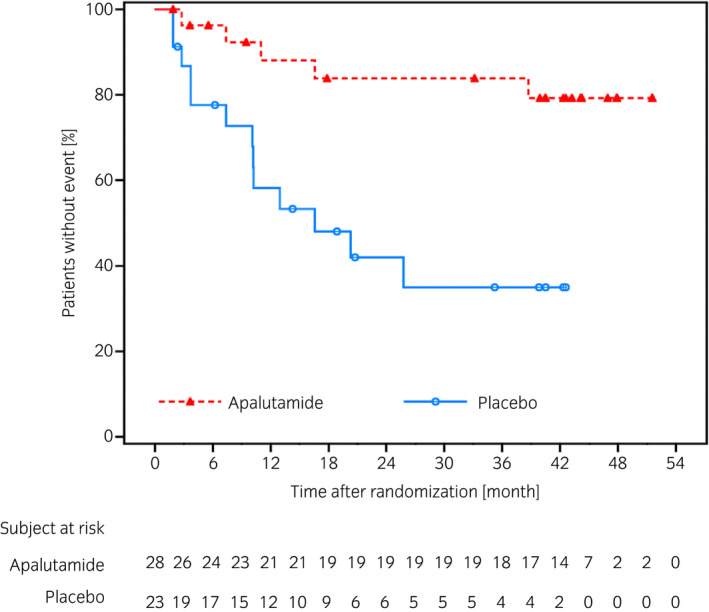
Kaplan–Meier plot of time to PSA progression in the Japanese subpopulation.
Fig. 4.
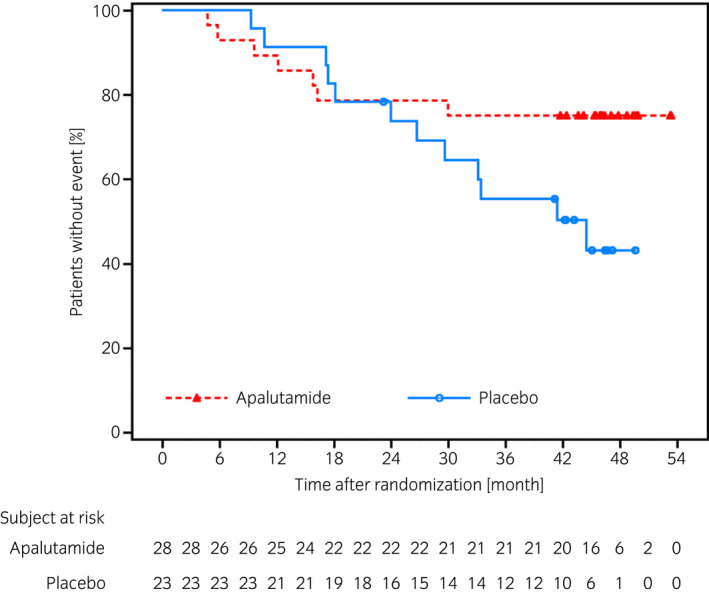
Kaplan–Meier plot of time to PFS2 in the Japanese subpopulation.
Table 2.
First subsequent systematic therapy for PC (intent‐to‐treat population)
| Japanese subpopulation (N = 51) | Overall population† (N = 1052) | |||
|---|---|---|---|---|
|
Apalutamide (n = 28) |
Placebo (n = 23) |
Apalutamide (n = 525) |
Placebo (n = 527) |
|
| Duration of first subsequent therapy (days), median (range) | 126 (48–375) | 145 (61–637) | 95 (1–403) | 119 (1–648) |
| Number of patients alive at treatment discontinuation, n | 12 | 18 | 247 | 345 |
| Number of patients with first subsequent systemic therapy for PC, n (%) | 7 (58.3) | 13 (72.2) | 120 (48.6) | 221 (64.1) |
| Hormonal | 6 (50.0) | 8 (44.4) | 58 (23.5) | 124 (35.9) |
| Bicalutamide | 4 (33.3) | 5 (27.8) | 16 (6.5) | 30 (8.7) |
| Abiraterone acetate plus prednisone | 2 (16.7) | 2 (11.1) | 27 (10.9) | 65 (18.8) |
| Enzalutamide | 0 | 1 (5.6) | 9 (3.6) | 24 (7.0) |
| Chemotherapy | 1 (8.3) | 4 (22.2) | 50 (20.2) | 88 (25.5) |
| Docetaxel | 1 (8.3) | 4 (22.2) | 42 (17.0) | 78 (22.6) |
| Other | 1 (8.3) | 2 (11.1) | 32 (13.0) | 63 (18.3) |
| Prednisolone | 1 (8.3) | 1 (5.6) | 6 (2.4) | 12 (3.5) |
| Radium‐223 | 0 | 1 (5.6) | 5 (2.0) | 5 (1.4) |
Data are from Chi et al. 14
Fig. 5.
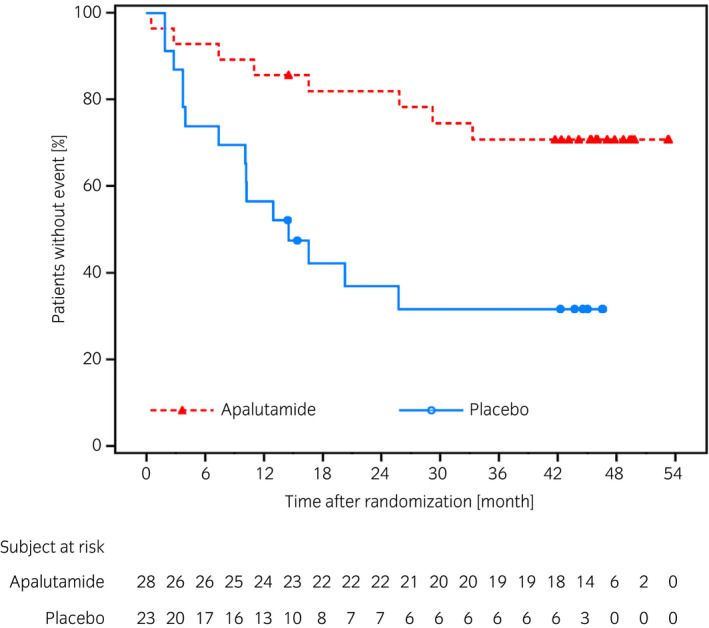
Kaplan–Meier plot of time to castration resistance in the Japanese subpopulation.
Safety
Most TEAEs in the Japanese subpopulation were similar to those reported in the overall population. The frequencies of TEAEs are summarized in Table 3. In the Japanese subpopulation, any TEAE was observed in 27 patients (96.4%) in the apalutamide group and 23 patients (100.0%) in the placebo group (Table 3).
Table 3.
Summary of TEAEs (safety population)
| Japanese subpopulation (N = 51) | Overall population† (N = 1051) | |||||
|---|---|---|---|---|---|---|
|
Apalutamide (n = 28) |
Placebo‡ (n = 23) |
Crossover (n = 9) |
Apalutamide (n = 524) |
Placebo‡ (n = 527) |
Crossover (n = 208) |
|
| Summary of TEAEs, n (%) | ||||||
| Any TEAE | 27 (96.4) | 23 (100.0) | 9 (100.0) | 510 (97.3) | 510 (96.8) | 174 (83.7) |
| Grade 3 or 4 | 16 (57.1) | 10 (43.5) | 4 (44.4) | 259 (49.4) | 220 (41.7) | 57 (27.4) |
| Any SAE | 9 (32.1) | 7 (30.4) | 3 (33.3) | 153 (29.2) | 115 (21.8) | 29 (13.9) |
| Grade 3 or 4 | 8 (28.6) | 6 (26.1) | 3 (33.3) | 124 (23.7) | 93 (17.6) | 27 (13.0) |
| Any TEAEs leading to discontinuation | 2 (7.1) | 1 (4.3) | 3 (33.3) | 62 (11.8) | 30 (5.7) | 16 (7.7) |
| Any TEAEs leading to death | 0 | 0 | 0 | 20 (3.8) | 17 (3.2) | 7 (3.4) |
| All deaths within 30 days of last dose | 1 (3.6) | 0 | 0 | 31 (5.9) | 35 (6.6) | 10 (4.8) |
| Death due to PC | 1 (3.6) | 0 | 0 | 11 (2.1) | 12 (2.3) | 3 (1.4) |
| Adverse events | 0 | 0 | 0 | 20 (3.8) | 23 (4.4) | 7 (3.4) |
Data are from Chi et al. 14
Including crossover patients.
Grade 3 or 4 TEAEs were observed in 16 patients (57.1%) in the apalutamide group and 10 patients (43.5%) in the placebo group. Two grade 4 TEAEs were observed in one patient (3.6%) in the apalutamide group (dyspnea and suicide attempt), and no grade 4 TEAEs was observed in the placebo group.
TEAEs required dose modification of the study drug were summarized in Table S2. Two patients (7.1%) in the apalutamide group and one patient (4.3%) in the placebo group experienced any TEAEs leading to a discontinuation of treatment. TEAEs leading to death were not reported.
After crossover, nine patients (100.0%) experienced at least one TEAE. Four patients (44.4%) experienced grade 3 or 4 TEAEs and three patients (33.3%) experienced any TEAEs leading to discontinuation of treatment.
The incidence of SAEs was nine patients (32.1%) in the apalutamide group, seven patients (30.4%) in the placebo group, and three patients (33.3%) in the crossover group (Table 3).
TEAEs of special interest were summarized in Table 4. Skin rash was the most commonly observed in 15 patients (53.6%) in the apalutamide group, three patients (13.0%) in the placebo group, and seven patients (77.8%) in the crossover group. Detailed information on the skin rash is shown in Table S3. Fracture was observed eight patients (28.6%) in the apalutamide group and three patients (13.0%) in the placebo group. The proportion of BSA use is shown in Table S4. Other TEAEs of special interest observed in the apalutamide group were fall (four patients [14.3%]), and ischemic heart disease (one patient [3.6%]); in the placebo group and crossover group were fall (three patients [13.0%], and one patient [11.1%], respectively). Seizure was not observed any groups in the Japanese subpopulation. In terms of severity grading, TEAEs of special interest of grade 4 or higher was not observed.
Table 4.
Summary of TEAEs of special interest (safety population)
| Japanese subpopulation (N = 51) | Overall population† (N = 1051) | |||||
|---|---|---|---|---|---|---|
|
Apalutamide (n = 28) |
Placebo‡ (n = 23) |
Crossover (n = 9) |
Apalutamide (n = 524) |
Placebo‡ (n = 527) |
Crossover (n = 208) |
|
| Any TEAEs of special interest, n (%) | 18 (64.3) | 5 (21.7) | 8 (88.9) | 222 (42.4) | 99 (18.8) | 59 (28.4) |
| Skin rash | 15 (53.6) | 3 (13.0) | 7 (77.8) | 153 (29.2) | 49 (9.3) | 45 (21.6) |
| Rash | 10 (35.7) | 0 | 5 (55.6) | 106 (20.2) | 23 (4.4) | 26 (12.5) |
| Stomatitis | 3 (10.7) | 2 (8.7) | 0 | 7 (1.3) | 4 (0.8) | 1 (0.5) |
| Rash maculo‐papular | 3 (10.7) | 0 | 0 | 17 (3.2) | 5 (0.9) | 6 (2.9) |
| Erythema multiforme | 2 (7.1) | 0 | 1 (11.1) | 2 (0.4) | 0 | 1 (0.5) |
| Urticaria | 1 (3.6) | 1 (4.3) | 0 | 5 (1.0) | 5 (0.9) | 1 (0.5) |
| Blister | 1 (3.6) | 0 | 0 | 3 (0.6) | 0 | 0 |
| Dermatitis | 1 (3.6) | 0 | 0 | 9 (1.7) | 2 (0.4) | 3 (1.4) |
| Rash pruritic | 0 | 0 | 1 (11.1) | 6 (1.1) | 3 (0.6) | 2 (1.0) |
| Fracture | 8 (28.6) | 3 (13.0) | 0 | 54 (10.3) | 26 (4.9) | 5 (2.4) |
| Spinal compression fracture | 4 (14.3) | 2 (8.7) | 0 | 9 (1.7) | 2 (0.4) | 1 (0.5) |
| Rib fracture | 2 (7.1) | 3 (13.0) | 0 | 16 (3.1) | 14 (2.7) | 1 (0.5) |
| Foot fracture | 1 (3.6) | 0 | 0 | 3 (0.6) | 1 (0.2) | 1 (0.5) |
| Patella fracture | 1 (3.6) | 0 | 0 | 1 (0.2) | 0 | 0 |
| Wrist fracture | 1 (3.6) | 0 | 0 | 3 (0.6) | 0 | 0 |
| Fall | 4 (14.3) | 3 (13.0) | 1 (11.1) | 49 (9.4) | 37 (7.0) | 8 (3.8) |
| Ischemic heart disease | 1 (3.6) | 0 | 0 | 31 (5.9) | 11 (2.1) | 1 (0.5) |
| Angina pectoris | 1 (3.6) | 0 | 0 | 11 (2.1) | 6 (1.1) | 0 |
Data are from Chi et al. 14
Including cross‐over patients.
The grade 3 TEAEs of skin rash in the apalutamide group were erythema multiforme (two patients [7.1%]) and rash (two patients [7.1%]). One patient (11.1%) in the crossover group experienced erythema multiforme. In the placebo group, no grade 3 TEAEs were observed (Table S5). In the apalutamide group, two patients (7.1%) discontinued treatment because of skin rash (Table S2). However, most cases of skin rash were well manageable with dose reductions, treatment interruptions, or supportive medication.
Discussion
Apalutamide was approved to treat mCSPC in 2020 in Japan according to the first interim analysis results of the TITAN study, and the subgroup analyses showed comparable efficacy and safety in the Japanese subpopulation versus the overall population. 13 In the overall population, the final OS analysis was performed after an additional follow‐up and demonstrated the continuing long‐term benefit of apalutamide. Despite approximately 40% of patients in the placebo group having crossed over to apalutamide after unblinding, the final analysis of the overall population demonstrated a statistically significant improvement in OS, which was even greater when adjusted by Inverse Probability of Censoring Weighted method for crossover. There were also significant differences in time to PSA progression, time to castration resistance and PFS2 favoring apalutamide group. 14
The results of the current study in the Japanese subpopulation with a longer follow‐up period confirmed the results reported in the interim analysis, and are comparable to the results of the final analysis in the overall population. 14 The trends favoring OS in the apalutamide group over placebo group observed in the interim results became more evident in the Japanese subpopulation.
PFS2 is a clinically relevant endpoint for advanced PC treatment as there are a number of available and proven effective therapies after progression. Actually, PFS2 as an intermediate endpoint has been correlated with OS across solid tumors. 16 In the Japanese subpopulation, PFS2 was improved similar to the overall population. The difference in PFS2 in favor of apalutamide indicates that subsequent therapies in the placebo group did not make up for the benefits observed in the apalutamide group.
Additionally, the difference of the duration of the first subsequent therapy were not observed between the apalutamide group and placebo group, further consistent with the Japanese subpopulation and overall population (apalutamide 95.0 [1–403] days; placebo 119.0 [1–648] days). Taken together, these results support the efficacy of early treatment with apalutamide with initial ADT in mCSPC. Moreover, a preliminary report suggests that early treatment with apalutamide provides long‐term benefits compared with ADT monotherapy without an increased acquisition of androgen receptor aberrations. 17 In Japan, there is the ongoing CUARTET study, which evaluates the genomic alterations in mCSPC patients during apalutamide treatment using ctDNA and a custom 73 PC gene panel (Clinicaltrials.gov [NCT04601441], jRCT [jRCTs071200040]).
The HR for time to skeletal‐related events favored placebo in the Japanese subpopulation, although it favored apalutamide in the overall population. 14 In the interim analyses, the tendency was similar. 13 It is unclear whether this is clinically important because there was no evidence of increased bone fractures with apalutamide in the TITAN or SPARTAN studies in the overall population. 12 , 13 , 18 However, the Japanese subpopulation experienced more fractures than the overall population, irrespective of treatment. This trend was observed in the interim analyses, 13 but in this final analysis, fractures occurred twice as frequently in the apalutamide group compared with the placebo group. This difference may have been due to the difference of the treatment duration in each group (43.7 vs 18.9 months), and the proportion of patients receiving BSA in each group. On the other hand, prior or concurrent BSA treatment may reduce the risk of fracture. According to the previous report, ADT‐increased risk of any fracture which may have been associated with mortality in PC patients, 19 closer monitoring may be needed for skeletal‐related events in Japanese patients receiving apalutamide with ADT. Nonetheless, given the small number of patients and the few events that occurred, firm conclusions are not possible and further study is required.
The safety profiles in the final and interim analyses in the Japanese subpopulation were consistent, with no new safety concerns. Previously, skin rash has been significantly associated with higher apalutamide exposure. 20 However, the mechanism of apalutamide‐associated rash and the reason for higher incidence in the Japanese subpopulation compared with the overall population has not been clarified. In general, the grade of rash was not severe and was safely managed by dose modification without impeding efficacy. Overall, TEAEs and TEAEs of special interest were consistent with those previously described in the interim reports in the Japanese subpopulation or final analyses in the overall population.
This study had several limitations, including the small sample size and the nominal statistical test results. Some endpoints with a discrepancy compared with the overall population, e.g. incidence of rash and fracture, warrant further data collection in Japanese patients.
In conclusion, the final analysis of the efficacy and safety of apalutamide in Japanese patients with mCSPC in the TITAN study suggests that apalutamide in addition to ADT has favorable efficacy compared with ADT alone. There were no new safety concerns during the longer observation period, although Japanese patients tended to show higher incidences of rash and fracture compared with the overall population.
Author contributions
Hirotsugu Uemura: Conceptualization; Investigation; Methodology; Visualization; Writing – review & editing. Gaku Arai: Investigation; Visualization; Writing – review & editing. Hiroji Uemura: Investigation; Visualization; Writing – review & editing. Hiroyoshi Suzuki: Investigation; Visualization; Writing – review & editing. Junya Aoyama: Project administration; Visualization; Writing – review & editing. Tomoyoshi Hatayama: Data curation; Formal analysis; Visualization; Writing – review & editing. Miku Ito: Visualization; Writing – original draft. Florence Lefresne: Conceptualization; Data curation; Methodology; Project administration; Visualization; Writing – review & editing. Sharon McCarthy: Conceptualization; Data curation; Methodology; Project administration; Visualization; Writing – review & editing. Suneel Mundle: Conceptualization; Data curation; Methodology; Project administration; Visualization; Writing – review & editing. Jin He: Visualization; Writing – review & editing. Kim Chi: Conceptualization; Methodology; Visualization; Writing – review & editing.
Conflict of interest
Hirotsugu Uemura has received lecture fees from Pfizer Japan, Ono, Bayer Yakuhin, Bristol‐Myers Squibb, MSD; research fees/grants from Taiho, JPKK, Mebix, MSD, Astellas, Quintiles, Covance, Bayer Yakuhin, Parexel International, Pfizer Japan, ICON Japan, Ono, Daiichi Sankyo, AstraZeneca, EPS, PPDSNBL, Takeda, Chugai, IQVIA, Mediscience Planning, and Osaka Urology Research Foundation; and scholarship/encouragement donations from Asahi Kasei, Kissei, and Novartis. Gaku Arai has received lecture fees from JPKK. Hiroji Uemura has received lecture fees from JPKK, Bayer Yakuhin, and Takeda; scholarship/encouragement donations from Takeda; and travel fees from JPKK, Bayer Yakuhin, Takeda, Astellas Pharma, Sanofi, Daiichi Sankyo, and Kyowa Kirin. Hiroyoshi Suzuki has received lecture fees from Takeda, Astellas, AstraZeneca, JPKK, Sanofi, Bayer Yakuhin, MSD; research fee/grants from Takeda, Astellas, JPKK, AstraZeneca, Roche/Chugai; and scholarship/encouragement donations from Takeda, Astellas, Daiichi Sankyo, Kissei, Ono, Bayer Yakuhin, Sanofi, Nihon Shinyaku. Junya Aoyama, Tomoyoshi Hatayama, and Miku Ito are employees of JPKK. Florence Lefresne is an employee of Janssen Research and Development. Sharon McCarthy and Suneel Mundle are employees of Janssen R&D US. Jin He is an employee of Janssen Asia Pacific. Kim N Chi has received lecture fees and research fees/grants from Janssen R&D.
Approval of the research protocol by an Institutional Reviewer Board
The TITAN study was conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practices, and applicable regulatory requirements. The study protocol and amendments were reviewed and approved by an Independent Ethics Committee or Institutional Review Board at each study site. In the institution to which the first author of this manuscript affiliates was approved by the Institutional Review Board of Kindai University Hospital (1738).
Informed consent
All the participants provided written informed consent.
Registry and the Registration No. of the study/trial
ClinicalTrials.gov NCT02489318; Japic CTI JapicCTI‐163118.
Animal studies
N/A.
Supporting information
Table S1. Demographics and baseline characteristics (intent‐to‐treat population).
Table S2. Summary of TEAEs leading to dose modification (safety population).
Table S3. Summary of skin rash onset, treatment, and outcome (safety population).
Table S4. Summary of usage of prior or concurrent BSA (safety population).
Table S5. TEAEs observed with a frequency of at least 10% in any group (safety population).
Acknowledgments
The authors thank the study patients, without whom this study would never have been accomplished, and all the investigators and study coordinators for their contributions. They also thank ASCA Corporation for medical writing assistance, Ryo Yano, PhD (CMIC Ashfield Co., Ltd.) for publication support, Keiichiro Imanaka, MD, PhD and Koji Fujii, PhD, MBA (Janssen Pharmaceutical K.K. [JPKK]) for medical advice for Japanese patients in the TITAN study. The study was funded by Janssen Research & Development, LLC and Janssen Pharmaceutical K.K.
References
- 1. Sung H, Ferlay J, Siegel RL et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021; 71: 209–49. [DOI] [PubMed] [Google Scholar]
- 2. Kimura T, Egawa S. Epidemiology of prostate cancer in Asian countries. Int. J. Urol. 2018; 25: 524–31. [DOI] [PubMed] [Google Scholar]
- 3. Sweeney CJ, Chen YH, Carducci M et al. Chemohormonal therapy in metastatic hormone‐sensitive prostate cancer. N. Engl. J. Med. 2015; 373: 737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chi KN, Agarwal N, Bjartell A et al. Apalutamide for metastatic, castration‐sensitive prostate cancer. N. Engl. J. Med. 2019; 381: 13–24. [DOI] [PubMed] [Google Scholar]
- 5. Fizazi K, Tran N, Fein L et al. Abiraterone plus prednisone in metastatic, castration‐sensitive prostate cancer. N. Engl. J. Med. 2017; 377: 352–60. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong AJ, Szmulewitz RZ, Petrylak DP et al. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone‐sensitive prostate cancer. J. Clin. Oncol. 2019; 37: 2974–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parker C, Castro E, Fizazi K et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 2020; 31: 1119–34. [DOI] [PubMed] [Google Scholar]
- 8. Kakehi Y, Sugimoto M, Taoka R. Committee for establishment of the evidenced‐based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced‐based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition). Int. J. Urol. 2017; 24: 648–66. [DOI] [PubMed] [Google Scholar]
- 9. Mohler JL, Antonarakis ES, Armstrong AJ et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2019; 17: 479–505. [DOI] [PubMed] [Google Scholar]
- 10. Clegg NJ, Wongvipat J, Joseph JD et al. ARN‐509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 2012; 72: 1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith MR, Saad F, Chowdhury S et al. Apalutamide treatment and metastasis‐free survival in prostate cancer. N. Engl. J. Med 2018; 378: 1408–18. [DOI] [PubMed] [Google Scholar]
- 12. Uemura H, Satoh T, Tsumura H et al. Efficacy and safety of apalutamide in Japanese patients with nonmetastatic castration‐resistant prostate cancer: a subgroup analysis of a randomized, double‐blind, placebo‐controlled, phase‐3 study. Prostate Int. 2020; 8: 190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uemura H, Arai G, Uemura H et al. Apalutamide for metastatic, castration‐sensitive prostate cancer in the Japanese population: a subgroup analysis of the randomized, double‐blind, placebo‐controlled phase 3 TITAN study. Int. J. Urol. 2021; 28: 280–7. [DOI] [PubMed] [Google Scholar]
- 14. Chi KN, Chowdhury S, Bjartell A et al. Apalutamide in patients with metastatic castration‐sensitive prostate cancer: final survival analysis of the randomized, double‐blind, phase III TITAN study. J. Clin. Oncol. 2021; 39: 2294–303. [DOI] [PubMed] [Google Scholar]
- 15. Scher HI, Halabi S, Tannock I et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008; 26: 1148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chowdhury S, Mainwaring P, Zhang L et al. Systematic review and meta‐analysis of correlation of progression‐free survival‐2 and overall survival in solid tumors. Front. Oncol. 2020; 10: 1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chi KN, Thomas S, Agarwal N et al. Androgen receptor (AR) aberrations in patients (Pts) with metastatic castration‐sensitive prostate cancer (mCSPC) treated with apalutamide (APA) plus androgen deprivation therapy (ADT) in TITAN. Ann. Oncol. 2019; 30: v347–8. Abstract Book of the 44th ESMO Congress (ESMO 2019) 27 September–1 October 2019, Barcelona, Spain. [Google Scholar]
- 18. Smith MR, Saad F, Chowdhury S et al. Apalutamide and overall survival in prostate cancer. Eur. Urol. 2021; 79: 150–8. [DOI] [PubMed] [Google Scholar]
- 19. Wang A, Obertová Z, Brown C et al. Risk of fracture in men with prostate cancer on androgen deprivation therapy: a population‐based cohort study in New Zealand. BMC Cancer 2015; 15: 837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perez‐Ruixo C, Ackaert O, Ouellet D et al. Efficacy and safety exposure‐response relationships of apalutamide in patients with nonmetastatic castration‐resistant prostate cancer. Clin. Cancer Res. 2020; 26: 4460–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and baseline characteristics (intent‐to‐treat population).
Table S2. Summary of TEAEs leading to dose modification (safety population).
Table S3. Summary of skin rash onset, treatment, and outcome (safety population).
Table S4. Summary of usage of prior or concurrent BSA (safety population).
Table S5. TEAEs observed with a frequency of at least 10% in any group (safety population).


