Abstract
Abstract
Rodent studies highlight enhancement of glucose tolerance and insulin sensitivity as potential clinically relevant effects of chronic beta2‐agonist treatment. However, the doses administered to rodents are not comparable with the therapeutic doses used for humans. Thus, we investigated the physiological effects of prolonged beta2‐agonist treatment at inhaled doses resembling those used in respiratory diseases on insulin‐stimulated whole‐body glucose disposal and putative mechanisms in skeletal muscle and adipose tissue of healthy men. Utilizing a randomized placebo‐controlled parallel‐group design, we assigned 21 healthy men to 4 weeks daily inhalation of terbutaline (TER; 4 mg × day−1, n = 13) or placebo (PLA, n = 8). Before and after treatments, we assessed subjects’ whole‐body insulin‐stimulated glucose disposal and body composition, and collected vastus lateralis muscle and abdominal adipose tissue biopsies. Glucose infusion rate increased by 27% (95% CI: 80 to 238 mg × min−1, P = 0.001) in TER, whereas no significant changes occurred in PLA (95% CI: −37 to 195 mg × min−1, P = 0.154). GLUT4 content in muscle or adipose tissue did not change, nor did hexokinase II content or markers of mitochondrial volume in muscle. Change in lean mass was associated with change in glucose infusion rate in TER (r = 0.59, P = 0.03). Beta2‐agonist treatment in close‐to‐therapeutic doses may augment whole‐body insulin‐stimulated glucose disposal in healthy young men and part of the change is likely to be explained by muscle hypertrophy. These findings highlight the therapeutic potential of beta2‐agonists for improving insulin sensitivity.
Key points
While studies in rodents have highlighted beta2‐agonists as a means to augment insulin sensitivity, these studies utilized beta2‐agonists at doses inapplicable to humans.
Herein we show that a 4‐week treatment period with daily therapeutic inhalation of beta2‐agonist increases insulin‐stimulated whole‐body glucose disposal in young healthy lean men.
This effect was associated with an increase of lean mass but not with changes in GLUT4 and hexokinase II or basal glycogen content in skeletal muscle nor GLUT4 content in abdominal adipose tissue.
These findings suggest that the enhanced insulin‐stimulated whole‐body glucose disposal induced by a period of beta2‐agonist treatment in humans, at least in part, is attributed to muscle hypertrophy.
Our observations extend findings in rodents and highlight the therapeutic potential of beta2‐agonists to enhance the capacity for glucose disposal and whole‐body insulin sensitivity, providing important knowledge with potential application in insulin resistance.
Keywords: adrenergic, adrenoceptor, clenbuterol, glucose transport, insulin sensitivity, muscle hypertrophy, SABA, salbutamol
Abstract figure legend In this study, we show that selective beta2‐agonist, terbutaline, in close‐to‐therapeutic doses, may increase insulin‐stimulated whole‐body glucose disposal with 4 weeks of treatment in humans, and that at least part of this increase is attributed to skeletal muscle hypertrophy. This finding highlights the therapeutic potential of beta2‐agonists in enhancing the capacity for glucose disposal and whole‐body insulin sensitivity in humans.

Introduction
Inhaled beta2‐agonists are first‐line drugs in obstructive respiratory diseases (Simpson et al. 2016; Tesse et al. 2018). While the airways are the main therapeutic target, inhaled beta2‐agonists have a high systemic bioavailability of around 20–30% and elicit several systemic effects (Ward et al. 2000; Dyreborg et al. 2016). In particular, beta2‐agonists exert effects in skeletal muscle and adipose tissue where beta2‐adrenergic receptors are abundant (Elfellah et al. 1989; Deng et al. 1996; Blondin et al. 2020). These effects include muscle growth (Hostrup et al. 2015; Jessen et al. 2018), slow‐to‐fast twitch muscle fibre type transition (Hostrup et al. 2018a ), lipolysis (Onslev et al. 2019), and alterations of numerous proteins involved in cellular metabolism (Hostrup et al. 2018a , b ). This has made the beta2‐adrenergic receptor an emerging area in pharmaceutical drug development, with ongoing pre‐clinical (Koziczak‐Holbro et al. 2019; Skagen et al. 2021) and clinical trials aiming to assess the applicability of selective beta2‐agonists in lifestyle diseases such as obesity and type 2 diabetes, as well as muscle atrophic conditions (patent no: WO2005072714A1, ClinicalTrials.gov Identifier: NCT03005717.23‐25).
Because chronic treatment with beta2‐agonists enhances insulin sensitivity and glucose tolerance in rodents (Torgan et al. 1993; Pan et al. 2001; Sato et al. 2014; Kalinovich et al. 2020), there has been speculation about the potential applicability of beta2‐agonists in treatment of insulin resistance (Pan et al. 2001; Kalinovich et al. 2020). However, the treatment regimens showing effects on insulin sensitivity in rodents are not comparable with the therapeutic doses used for humans. For example, daily intraperitoneal or oral doses of clenbuterol at 25 μg × kgbw −1 or 105 μg, respectively, for 4–5 days improve glucose tolerance in rodents (Kalinovich et al. 2020; Meister et al. 2022), but are also associated with adverse cardiac hypertrophy, even in doses as low as 10 μg × kgbw −1 (Burniston et al. 2006). Such doses exceed those considered safe for humans (Kamalakkannan et al. 2008; Koeberl et al. 2018) and larger mammals (Sleeper et al. 2002), and the adverse cardiac effects are one of the main reasons why clenbuterol is seldomly prescribed to humans and why it is not marketed in several countries (Spiller et al. 2013; Milano et al. 2018). But given the compelling findings related to glucose tolerance in rodents, it is relevant to assess the physiological effect of a commonly prescribed beta2‐agonist at clinically relevant doses on insulin‐stimulated glucose disposal in humans.
To our knowledge, only one study (Scheidegger et al. 1984) has investigated the effect of chronic beta2‐agonist treatment on insulin‐stimulated glucose uptake in humans. In that study, oral treatment with terbutaline (15 mg × day−1) for 1–2 weeks enhanced insulin‐stimulated whole‐body glucose disposal by 29% in young healthy lean men. However, the study utilized a non‐placebo controlled open‐label design (Scheidegger et al. 1984), and the subjects ingested 5 mg oral terbutaline ∼13 h before the hyperinsulinaemic‐euglycaemic clamp. Given the slow absorption of oral terbutaline, which reaches peak systemic concentrations after 10–18 h after repeated daily dosing (Borgström et al. 1989), this precludes inferences on whether the enhanced insulin‐stimulated whole‐body glucose disposal was due to adaptation from chronic treatment or due to an acute effect of terbutaline on glucose uptake and metabolism (Onslev et al. 2019). Indeed, Scheidegger et al. observed a ∼8% higher basal metabolic rate during the post‐treatment clamp with terbutaline compared to control, which suggests some residual effect of terbutaline on metabolism. Hence, data are needed to assess whether chronic treatment of beta2‐agonists at more clinically relevant inhaled doses, and with a sufficient wash‐out period before post‐intervention assessment, affects glucose disposal during insulin stimulation in humans.
Given that skeletal muscle is the primary site for insulin‐stimulated glucose disposal (DeFronzo et al. 1981), the muscle hypertrophic actions of beta2‐agonists may be a mechanism underlying the greater capacity for glucose disposal and augmented insulin sensitivity observed in rodents (Torgan et al. 1993; Jacob et al. 1999; Castle et al. 2001; Pan et al. 2001; Sato et al. 2014). Although human data on the effect of beta2‐agonists on insulin sensitivity are lacking, studies show that a few weeks of treatment with beta2‐agonist at high inhaled or oral doses increases muscle mass in young healthy trained men (Holgate et al. 1980; Hostrup et al. 2015; Jessen et al. 2018). Furthermore, data in rodents suggest that beta2‐agonists may alter the regulation and expression of proteins involved in glucose transport and metabolism in both skeletal muscle (Pan et al. 2001) and adipocytes (Granneman et al. 2005), such as GLUT4 (Sato et al. 2014) and hexokinase II (Jones & Dohm, 1997). Thus, beta2‐agonists may potentially enhance the capacity for glucose disposal via other mechanisms than hypertrophy of skeletal muscle but this hypothesis warrants further investigation in humans.
Herein we investigated the effect of daily inhaled treatment with the selective beta2‐agonist terbutaline on insulin‐stimulated whole‐body glucose disposal, along with putative physiological mechanisms in skeletal muscle and adipose tissue. As a proof of concept approach, we examined these effects in healthy young men who were not insulin resistant or suffering from other chronic diseases. We hypothesized that daily inhalation of beta2‐agonist for 4 weeks would increase insulin‐stimulated whole‐body glucose disposal, which would be associated with an increase in lean mass.
Methods
Study design
The study was part of a larger study (Jessen et al. 2018) and was designed as a randomized, placebo‐controlled, double‐blinded parallel study (Fig. 1). The present study included a subgroup that underwent assessment of insulin‐stimulated whole‐body glucose disposal and body composition, and had muscle and adipose tissue biopsies taken. In this subgroup analysis, the main outcome measure was insulin‐stimulated whole‐body glucose disposal estimated by glucose infusion rate during the final 20 min of a 2 h hyperinsulinaemic‐euglycaemic clamp. The study adhered to the 2013 Helsinki Declaration and was approved by the ethics committee of Copenhagen, Denmark (H‐4‐2014‐002), and registered at Clinicaltrials.gov (NCT02557581). Subjects provided oral and written informed consent before inclusion in the study.
Figure 1. Flow chart of subject allocation in the study.
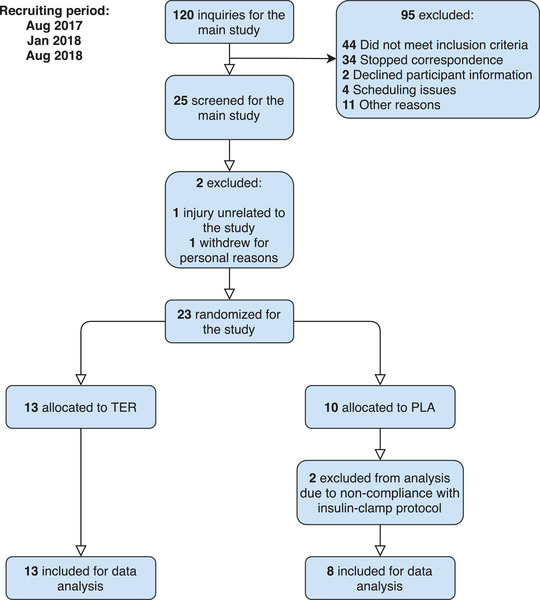
[Colour figure can be viewed at wileyonlinelibrary.com]
Subjects and eligibility criteria
Subjects were recruited via online posters and flyers. Before inclusion, subjects met for an assessment of eligibility criteria. The criteria were: healthy men, 18–36 years of age, non‐smoker, activity level 2–5 h × week−1, maximum oxygen uptake () 40–60 ml × kg−1 × min−1, lean mass 55–65 kg or lean mass index 14–22 kg × m−2, no use of beta2‐agonists or other prescription medicine, and no allergy towards terbutaline. Withdrawal criteria were unacceptable side effects, complications related to the study, or non‐compliance. Subjects’ body composition was assessed by dual‐energy X‐ray absorptiometry (Lunar DPX IQ, Version 4.7 E, Lunar Corporation, Madison, WI, USA) (DXA) followed by incremental cycling to exhaustion on a bike ergometer for assessment of by indirect calorimetry (Monark LC4, Monark Exercise AB, Vansbro, Sweden) as previously described (Jessen et al. 2018).
Randomization
Subjects were block‐randomized to either an experimental group receiving terbutaline (TER) or placebo (PLA), stratified for and lean mass. Subjects were assigned (1.3:1) in favour of active treatment as we expected a higher drop‐out rate due to side effects in TER and a larger within‐group variability due to individual differences in beta2‐agonist response.
Intervention
Subjects in TER were instructed to inhale eight doses of 0.5 mg × dose−1 terbutaline (4 mg × day−1), while subjects in PLA were instructed to inhale eight doses of placebo once daily for a 4‐week treatment period. During the intervention, inhalations were supervised daily via online monitoring (Skype or Facetime) by the investigators, hence ensuring 100% drug compliance. Subjects and investigators were blinded during the intervention and unblinding occurred after collection of all data. Terbutaline Turbuhalers (Bricanyl, Turbuhaler, 0.5 mg × dose−1, AstraZeneca, Cambridge, UK) were delivered by the regional pharmacy of Copenhagen, Denmark, while identical‐looking placebo Turbuhalers were kindly delivered by AstraZeneca (AstraZeneca, Cambridge, UK). The elimination half‐life of inhaled terbutaline is normally around 4–6 h (Krogh et al. 2017) and has a duration of action around 6 h (Sears & Lötvall, 2005).
Pre and post‐intervention assessment
Before and 48–72 h after the final day of the 4‐week treatment period (to ensure washout of the experimental drug), subjects reported to the laboratory for assessment of insulin‐stimulated whole‐body glucose disposal during a hyperinsulinaemic‐euglycaemic clamp in a fasting state, having abstained from vigorous physical activity and alcohol for 48 h and caffeine and nicotine for 24 h (Fig. 2). Before the clamp, subjects ingested four to five potassium tablets to prevent hypokalaemia (Kaleorid, 750 mg KCl, Karo Pharma, Sweden) and had a catheter placed in the antecubital vein for infusion of glucose and insulin. A second catheter was placed in the contralateral dorsal hand vein, which was arterialized with a heating pad to ensure an O2‐saturation > 92%. The clamp was initiated with a 1 min priming dose of 53.5 pmol × kgbw −1 insulin (100 IU × ml−1, Novo Nordisk, Copenhagen, Denmark) and continued for 120 min with a constant insulin infusion (8 pmol × kgbw −1 × min−1). Arterialized venous blood samples were collected before the clamp and every 5 min during the clamp for assessment of glucose concentration. Furthermore, blood samples were collected before and during the clamp for determination of plasma insulin. Glucose was infused during the clamp from a 20% glucose solution (Fresenius Kabi, 200 mg × ml−1) to maintain euglycaemia at ∼5 mm.
Figure 2. Experimental overview of the study.
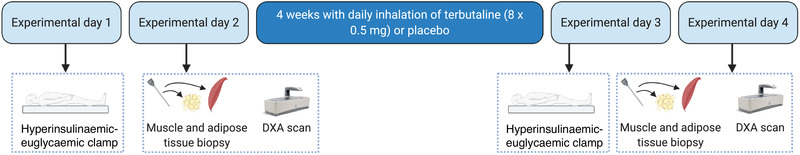
DXA: dual‐energy X‐ray absorptiometry. [Colour figure can be viewed at wileyonlinelibrary.com]
On a separate day in a fasting state before and after the intervention, body composition was measured during a whole‐body DXA scan using the mean of two separate scans. Adipose tissue compartments were assessed with automated software estimating adipose tissue deposition in the pelvic (gynoid) and abdominal area (android) as defined by standard region of interest settings. After the scan, resting vastus lateralis muscle and abdominal subcutaneous adipose biopsies were obtained under local anaesthesia (2 ml lidocaine without noradrenaline (epinephrine), Xylocaine 20 mg × ml−1, AstraZeneca, Cambridge, UK) using the Bergström biopsy needle procedure (Bergström, 1975). After sampling, muscle and adipose biopsies were cleaned to remove visible blood, connective tissue, and fat (muscle samples) and immediately frozen in liquid nitrogen and stored at −80°C until analysis. Muscle biopsies were assessed for content of GLUT4, hexokinase II, citrate synthase (CS), cytochrome c oxidase subunit 4 (COXIV), as well as for glycogen (only muscle). Adipose tissue biopsies were assessed for content of GLUT4. Subjects were instructed to replicate nutritional intake and activity level before study visits.
Immunoblotting
Protein content was determined in muscle and subcutaneous adipose lysates through SDS‐PAGE and western blot analysis. First, freeze‐dried and dissected muscle and adipose sample were homogenized for 2 × 30 s at 29 Hz (Qiagen Tissuelyser II, Retsch GmbH, Haan, Germany) in fresh cold buffer for the muscle samples (10% glycerol, 20 mm sodium pyrophosphate, 150 mm NaCl, 50 mm Hepes (pH 7.5), 1% NP‐40, 20 mm β‐glycerophosphate, 2 mm Na3VO4, 10 mm NaF‐poison, 2 mm PMSF, 1 mm EDTA (pH 8.0), 1 mm EGTA (pH 8.0), 10 μg × ml−1 aprotinin, 10 μg × ml−1 leupeptin, 3 mm benzamidine) and the adipose samples (10% glycerol, 20 mm sodium pyrophosphate, 150 mm NaCl, 50 mm Hepes, 1% NP‐40, 20 mm β‐glycerophosphate, 10 mm NaF‐poison, 1 mm EDTA, 1 mm EGTA, 20 μg × ml−1 aprotinin, 10 μg × ml−1 leupeptin, 2 mm Na3VO4, and 3 mm benzamidine, pH 7.5). Sodium dodecyl sulfate (SDS) was added to the adipose samples immediately after homogenization to obtain a final concentration of 2% SDS. After homogenization, both muscle and adipose samples were rotated end over end for 1 h at 4°C. Muscle and adipose samples were then centrifuged for 20 min at 13,000 rpm at 4°C. The supernatant in the muscle samples was collected for further analysis. Total protein concentration for each sample was determined in triplicate using a BSA standard kit (Thermo Scientific, Waltham, MA, USA) for muscle and adipose lysates. Samples were mixed with 6 × Laemmli buffer (7 ml of 0.5 m Tris‐base, 3 ml glycerol, 0.93 g DTT, 1 g SDS, and 1.2 mg Bromophenol Blue) to reach equal protein concentration before protein expression was determined by western blotting.
Equal amounts of protein were loaded in separate wells on 4–15% (hexokinase II, CS, COXIV) and 7.5% (GLUT4) pre‐cast Criterion gels (Bio‐Rad Laboratories, Hercules, CA, USA). All samples from the same subject were loaded on the same gel in adjacent wells and individual samples were normalized to the average intensity of two human standards in each gel, to allow between‐gel comparisons. Proteins were separated according to their molecular weight via SDS‐PAGE and proteins were semi‐dry blotted to a polyvinylidene difluoride membrane (MilliporeSigma, Burlington, MA, USA). The membranes were blocked in 2% skimmed milk in Tris‐buffered saline (TBS) including 0.1% Tween‐20 (TBST) before incubation overnight at 4°C in primary antibody (GLUT4; Thermo Fischer Scientific, PA1‐1065; hexokinase; Cell Signalling Technology, 2867, CS: Abcam, ab96600; COXIV: Cell Signalling Technology, 4844). Afterwards, membranes were washed in TBST and incubated in horseradish peroxidase‐conjugated secondary antibody at room temperature for 1 h. Membranes were then washed in TBST for 3 × 15 min. Membrane staining was visualized by incubation with a chemiluminescent horseradish peroxidase substrate (MilliporeSigma, Burlington, MA, USA) and recorded with a digital camera (ChemiDoc MP Imaging System, Bio‐Rad Laboratories). Densitometry quantification of the western blot band intensity was done using Image Lab version 4.0 (Bio‐Rad Laboratories) and determined as the total band intensity adjusted for background intensity. Representative blots are shown in Fig. 6. Due to imaging issues for GLUT4 determination in adipose tissue samples, six samples were excluded from analysis (TER, n = 8; PLA, n = 7).
Figure 6. Muscle and adipose tissue protein expression.
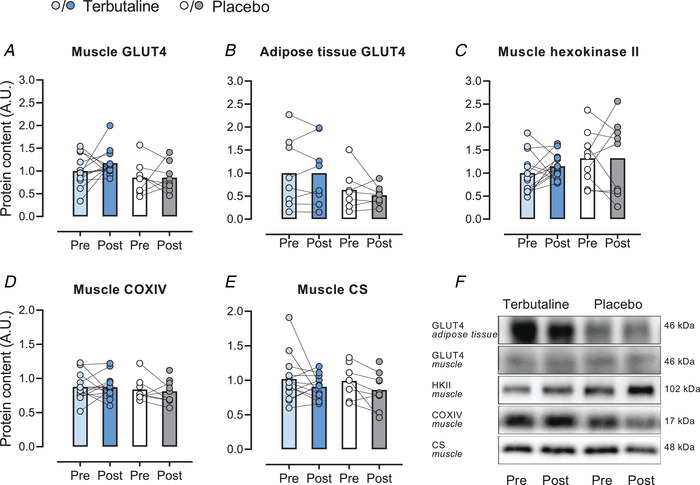
Content of glucose transporter 4 (GLUT4) in vastus lateralis muscle (A) and subcutaneous adipose tissue (B), content of hexokinase II in muscle (C), content of cytochrome c oxidase subunit 4 (COXIV) in muscle (D), content of citrate synthase (CS) in muscle (E), and representative western blots (F) of healthy lean subjects before (Pre) and after (Post) 4 weeks of terbutaline (inhalation, 4 mg × day−1; TER, n = 13) or placebo (PLA, n = 8). Data are presented as means with individual values. [Colour figure can be viewed at wileyonlinelibrary.com]
Muscle glycogen content
Approximately 2 mg dry weight muscle tissue was extracted in 1 n HCl and hydrolysed at 100°C for 3 h. Glycogen was determined by the hexokinase method as previously described (Lowry & Passonneau, 1972).
Plasma glucose and insulin concentrations
Blood samples were collected in heparinized syringes during the hyperinsulinaemic‐euglycaemic clamp for determination of arterialized venous plasma glucose concentrations using a blood gas analyser (ABL 800 FLEX, Radiometer, Copenhagen, Denmark). Further arterialized venous blood samples were drawn in EDTA tubes and centrifuged at 4°C and 3000 rpm for 5 min, after which plasma was collected and stored at −80°C until analysis for insulin. Plasma insulin was determined using an enzyme immunoassay ELISA kit (Alpco; Salem, New Hampshire, USA), and plates were read on a Multiskan FC plate reader (Thermo Fischer Scientific) according to manufacturer's instructions.
Statistics
Statistical analyses were performed in SPSS version 26 (IBM, Armonk, US). Data were normally distributed based on Q‐Q plots and the Shapiro Wilk's test, and are presented as means ± SD, with effect sizes presented with 95% confidence intervals (CI) for physiological outcomes. To estimate within‐ and between‐group changes with the intervention, a linear mixed model was used with group and time as fixed factors, and subjects as a random factor and baseline value of lean mass as a time‐invariant covariate, as it may influence the effect of beta2‐agonists (Cheymol, 2000; Jessen et al. 2018; Hostrup et al. 2018b ). In case of repeated measures, the Benjamini‐Hochberg procedure was applied to adjust P values. Pearson's correlation coefficient was used to estimate associations for the primary outcome measure. Sample size was determined based on the predicted effect of terbutaline on lean mass (Jessen et al. 2018). The significance level was set at P ≤ 0.05.
Results
Subjects
Of the 67 subjects included in the main study (Jessen et al. 2018), 23 subjects (all from the non‐exercise intervention arm of Jessen et al. 2018) participated in this sub‐study consisting of the hyperinsulinaemic‐euglycaemic clamp and sampling of muscle and adipose tissue biopsies. Of these 23 subjects, 21 completed all measurements and were included for analysis (Fig. 1). Characteristics of the subjects who completed the sub‐study are presented in Table 1.
Table 1.
Characteristics of healthy lean men receiving either terbutaline (TER) or placebo (PLA)
| TER (n = 13) | PLA (n = 8) | Between‐group differences P value | |
|---|---|---|---|
| Age, years | 24.5 ± 2.4 | 23.8 ± 3.0 | 0.723 |
| Height, cm | 182 ± 6 | 184 ± 6 | 0.367 |
| Weight, kg | 76.3 ± 5.7 | 80.1 ± 5.7 | 0.180 |
| BMI, kg × m−2 | 23.1 ± 0.8 | 23.4 ± 1.4 | 0.241 |
| Fat% | 17.1 ± 3.3 | 17.8 ± 4.0 | 0.703 |
| Fat mass, kg | 12.7 ± 2.7 | 13.8 ± 3.7 | 0.471 |
| Lean mass, kg | 61.1 ± 4.3 | 63.8 ± 3.6 | 0.246 |
| Android fat mass, kg | 0.8 ± 0.3 | 0.9 ± 0.3 | 0.250 |
| Gynoid fat mass, kg | 2.0 ± 0.5 | 2.2 ± 0.5 | 0.539 |
| , ml × kg−1 × min−1 | 51.7 ± 4.9 | 50.9 ± 6.4 | 0.779 |
Data are presented as means ± SD. Fat%; body fat percentage; BMI: body mass index; : maximal oxygen uptake.
Hyperinsulinaemic‐euglycaemic clamp
During the hyperinsulinaemic‐euglycaemic clamp, arterialized venous plasma insulin concentrations increased from basal levels of 35 ± 11 pmol × l−1 to levels of 599 ± 139 pmol × l−1 after 30 min during the clamp and maintained a steady‐state with no within‐ or between‐group differences (Fig. 3A ). Euglycaemia was maintained throughout the clamp in both groups before and after the intervention with a CV < 6% and with no within‐ or between‐group differences (Fig. 4A–C ).
Figure 3. Plasma insulin concentrations.
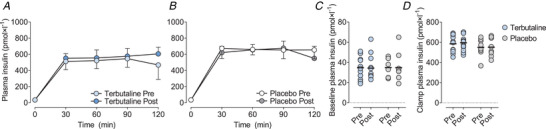
Mean plasma insulin concentrations during a 2‐h hyperinsulinaemic‐euglycaemic clamp in healthy lean subjects (A and B), individual baseline plasma insulin concentrations (C), and plasma insulin concentrations during the hyperinsulinaemic‐euglycaemic clamp (D) before (Pre) and after (Post) 4 weeks of either terbutaline (inhalation, 4 mg × day−1; TER, n = 13; A) or placebo (PLA, n = 8; B) treatment. Data are presented as means ± SD. Horizontal bars in C and D represent means. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4. Plasma glucose concentrations.
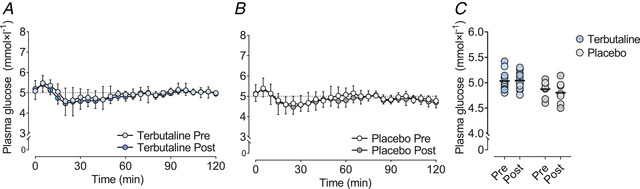
Mean plasma glucose concentrations (A and B) and individual plasma glucose concentrations during the last 20 min of a 2‐h hyperinsulinaemic‐euglycaemic clamp (C) in healthy lean subjects, before (Pre) and after (Post) 4 weeks of terbutaline (inhalation, 4 mg × day−1; TER, n = 13) or placebo (PLA, n = 8) treatment. Data are presented as means ± SD in panels A and B. Horizontal bars in C represent means. [Colour figure can be viewed at wileyonlinelibrary.com]
Glucose infusion rate during the final 20 min of the 2 h hyperinsulinaemic‐euglycaemic clamp increased by 27% with the intervention in TER (95% CI: 80 to 238 mg × min−1, P = 0.001) but was not significantly different (P = 0.153) from the change in PLA (95% CI: −37 to 195 mg × min−1 , P = 0.154) (Fig. 5). Post hoc analysis revealed that glucose infusion rate was higher (P < 0.001) 40–120 min into the clamp after the intervention than before in TER, whereas no within‐group changes were observed at specific sampling times in PLA (Fig. 5).
Figure 5. Insulin‐stimulated glucose disposal.
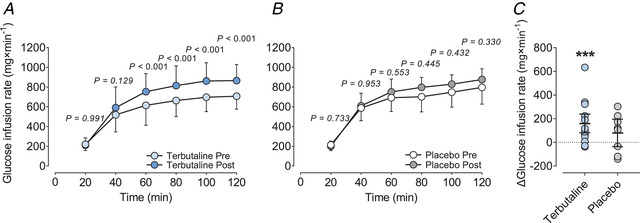
Glucose infusion rate (A and B) and individual change in glucose infusion rate during the last 20 min (C) of a 2‐h hyperinsulinaemic‐euglycaemic clamp in healthy lean subjects before (Pre) and after (Post) 4 weeks of terbutaline (inhalation, 4 mg × day−1; TER, n = 13) or placebo (PLA, n = 8) treatment. Within‐group P values are adjusted using the Benjamini‐Hochberg procedure. Data are presented as means ± SD in panels A and B, and as means (horizontal bars) ± 95% CI in panel C. ***Significant (P ≤ 0.001) within‐group change. [Colour figure can be viewed at wileyonlinelibrary.com]
Proteins regulating glucose transport
No within‐ or between‐group changes were observed with the intervention for muscle content of GLUT4, hexokinase II, CS, COXIV, and adipose tissue content of GLUT4 (Fig. 6).
Muscle glycogen content
Basal muscle glycogen content did not change with the intervention in either group, being 434 ± 73 and 412 ± 77 mmol × kgdw−1 before and after, respectively, the intervention in TER (P = 0.363) and 405 ± 48 and 350 ± 47 mmol × kgdw−1 in PLA (P = 0.089).
Body composition
Lean mass increased by 1.1 kg with the intervention in TER (95% CI: 0.6 to 1.6 kg, P < 0.001; Jessen et al. 2018), being higher (P = 0.001) than the change in PLA (95% CI: −0.9 to 0.3 kg, P = 0.366), while fat mass declined by 0.5 kg with the intervention in TER (95% CI: −1.0 to −0.1 kg, P = 0.013) but not different from the change in PLA (95% CI: −1.0 to 0.0 kg, P = 0.065). Gynoid fat mass declined by 0.1 kg with the intervention in TER (95% CI: −0.2 to 0.0 kg, P = 0.004) but was not different from the change in PLA (95% CI: −0.2 to 0.0 kg, P = 0.109). No within‐ or between‐group changes were observed with the intervention for android fat mass.
Predictors of change in insulin sensitivity
Change in lean mass was the only significant independent predictor of the change in glucose infusion rate (final 20 min of the clamp) in TER (Pearson's r = 0.59, 95% CI: 0.05 to 0.86; P = 0.03, Fig. 7) but was not different in PLA (Pearson's r = 0.44, 95% CI: −0.38 to 0.87; P = 0.28) (Table 2).
Figure 7. Lean mass and insulin‐stimulated glucose disposal.
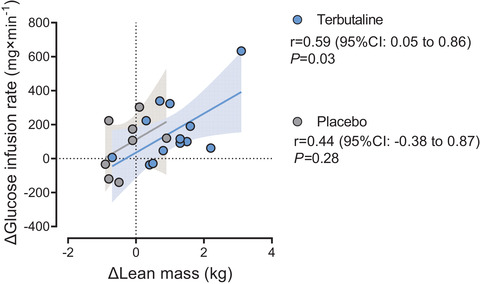
Individual relationship between change in lean mass and change in glucose infusion rate during the last 20 min of a 2‐h hyperinsulinaemic‐euglycaemic clamp in healthy lean subjects before and after 4 weeks of terbutaline (inhalation, 4 mg × day−1; TER, n = 13) or placebo (PLA, n = 8) treatment. Shaded area represents 95% CI. [Colour figure can be viewed at wileyonlinelibrary.com]
Table 2.
Predictors for the change in glucose infusion rate (GIR) during the last 20 min of the 2‐h hyperinsulinaemic‐euglycaemic clamp in healthy lean men receiving either terbutaline (TER) or placebo (PLA)
| TER (n = 13) | PLA (n = 8) | All (n = 21) | ||||
|---|---|---|---|---|---|---|
| Parameter | Pearson's r | P value | Pearson's r | P value | Pearson's r | P value |
| ΔLean mass | 0.59 | 0.04 * | 0.44 | 0.28 | 0.55 | 0.01 ** |
| ΔFat mass | 0.19 | 0.54 | 0.40 | 0.32 | 0.26 | 0.25 |
| ΔFat% | 0.05 | 0.86 | 0.30 | 0.47 | 0.09 | 0.67 |
| ΔAndroid fat mass | 0.07 | 0.81 | 0.40 | 0.32 | 0.22 | 0.33 |
| ΔGynoid fat mass | −0.19 | 0.51 | 0.46 | 0.25 | 0.10 | 0.66 |
| ΔGLUT4 (muscle) | 0.36 | 0.22 | 0.40 | 0.32 | 0.40 | 0.07 |
| ΔGLUT4 (adipose) | 0.18 | 0.66 | 0.22 | 0.62 | 0.23 | 0.40 |
| ΔHexokinase II | 0.08 | 0.78 | 0.58 | 0.13 | 0.30 | 0.17 |
| ΔCitrate synthase | 0.44 | 0.14 | 0.29 | 0.49 | 0.39 | 0.08 |
| ΔCOXIV | 0.43 | 0.15 | 0.30 | 0.47 | 0.39 | 0.08 |
| ΔGlycogen | 0.31 | 0.29 | 0.08 | 0.84 | 0.29 | 0.19 |
Significant (* P < 0.05, ** P < 0.01) correlation between change in glucose infusion rate (GIR). COXIV: cytochrome c oxidase subunit 4.
Discussion
The novel finding of the present study was that daily inhalation of the selective beta2‐agonist terbutaline for 4 weeks increased insulin‐stimulated whole‐body glucose disposal in healthy lean young men, which was associated with an increase in lean mass, while no changes were observed in GLUT4 and hexokinase II or basal glycogen content in skeletal muscle nor GLUT4 content in abdominal adipose tissue.
The observation that daily treatment with inhaled beta2‐agonist enhanced insulin‐stimulated whole‐body glucose disposal in lean young men extends findings in rodents highlighting a therapeutic potential of chronic beta2‐agonist treatment in enhancing the whole‐body insulin sensitivity (Torgan et al. 1993; Jacob et al. 1999; Castle et al. 2001; Pan et al. 2001; Sato et al. 2014). We administered inhaled doses (4 mg terbutaline) resembling those used for people with obstructive pulmonary disease. Even with such a dose, glucose infusion rate during the 2 h hyperinsulinaemic‐euglycaemic clamp was improved by 27% after the 4‐week treatment period with terbutaline, with 11 of the 13 subjects who received terbutaline experiencing an increase. This effect is on par with the 29% increase in glucose infusion rate observed by Scheidegger et al. (1984) in healthy men who ingested terbutaline tablets at doses of 15 mg × day−1 for 1–2 weeks. Aside from the more clinically relevant dosing regimen in the current study, we also introduced a long wash‐out at the end of the treatment period (48–72 h), in contrast to Scheidegger et al., to assert adaptive effects of prolonged treatment without any confounding acute effects on glucose uptake and metabolism (Onslev et al. 2019). While negligible residual levels of terbutaline may still have been present for some subjects in the present study (half‐life 4–6 h for inhaled terbutaline (Krogh et al. 2017)), such levels are unlikely to have had any meaningful physiological effect considering terbutaline's duration of action of 6 h (Sears & Lötvall, 2005). This is supported by the similar plasma glucose and insulin levels prior to the clamp, along with the observation of no changes in basal metabolic rate (Pre: 1.31 vs. Post: 1.37 kcal × min−1) and substrate utilization (Jessen et al. 2018) from before to after the intervention with terbutaline treatment. In Scheidegger et al. (1984), on the other hand, basal metabolic rate was around 8% higher during the hyperinsulaemic‐euglycaemic clamp after the treatment period, which possibly contributed to the greater glucose disposal in that study. Notwithstanding, the similar increase in insulin‐stimulated whole‐body glucose disposal with a period of terbutaline treatment in the two studies suggests that prolonged treatment with beta2‐agonist enhances the capacity for peripheral glucose disposal during insulin stimulation in humans and highlights a potential therapeutic application of beta2‐agonists outside of obstructive pulmonary disease treatment.
Consistent with the muscle hypertrophic actions of beta2‐agonists (Hostrup et al. 2020; Hostrup & Onslev, 2021), we observed a 1.1 kg lean mass accretion with terbutaline (Jessen et al. 2018) – an effect that mainly relates to an increased rate of myofibrillar protein synthesis (Koopman et al. 2010; Lee et al. 2015; Hostrup et al. 2018b ), and, in some instances, also a lowered proteolysis during treatment (Navegantes et al. 2000, 2001; Koopman et al. 2010). Skeletal muscle is the primary site for insulin‐stimulated glucose disposal (DeFronzo et al. 1981) and gains in lean mass correlate with increases in insulin sensitivity incurred from a period of resistance training (Miller et al. 1984). Therefore, the gain in lean mass observed in the present study may explain part of the increased whole‐body insulin‐stimulated glucose disposal after a period of terbutaline treatment. In support of this, we observed that change in lean mass correlated with change in glucose infusion rate (Fig. 7A ). This coincides with rodent studies showing that increases in insulin sensitivity and glucose tolerance with a period of beta2‐agonist treatment occur along with an increase in muscle mass (Torgan et al. 1993; Jacob et al. 1999; Castle et al. 2001; Pan et al. 2001). While the 1.1 kg increase in lean mass may seem small, it is clinically relevant and equivalent to the around 1.0–1.5 kg gained in lean mass by a period of full‐body resistance training of similar duration also shown to enhance insulin sensitivity in non‐type 2 diabetic men (Ismail et al. 2019).
Changes in the expression of proteins regulating glucose transport and metabolism in skeletal muscle and adipose tissue may also affect the potential for glucose disposal (Birnbaum, 1989; Charron et al. 1989). Indeed, the magnitude of increase in insulin‐stimulated glucose disposal exceeds the magnitude of lean mass gain in resistance training studies (Yaspelkis, 2006) and muscle GLUT4 content increases in response to resistance exercise training in parallel with an increase in insulin‐stimulated glucose disposal. Likewise, improved insulin sensitivity following aerobic training is associated with qualitative changes in muscle, such as mitochondrial biogenesis. Nevertheless, we observed no apparent effect of terbutaline treatment on muscle content of GLUT4 and hexokinase II, nor in markers of mitochondrial content (i.e. CS and COXIV content). In adipose tissue too, GLUT4 content did not change with terbutaline treatment and none of the changes in protein content contributed to explaining the changes observed in insulin‐stimulated glucose disposal. And although we cannot exclude the possibility that the period with terbutaline treatment altered insulin signalling and GLUT4 translocation, recent findings by Meister et al. (2022) suggest that beta2‐agonist treatment (with clenbuterol) augments the capacity of skeletal muscle for glucose uptake via mechanisms unrelated to GLUT4 protein levels and translocation in mice.
A strength of the present study was the strict control of drug intake, which was monitored daily, hence ensuring 100% compliance. It should, however, be noted that the study utilized an unbalanced design with subjects being enrolled in favour of active treatment as we expected a higher drop‐out rate due to side effects associated with terbutaline and a larger within‐group variability due to individual differences in beta2‐agonist response. Contrary to our expectations, the attrition rate was similar in the two groups resulting in more subjects in the terbutaline than in the placebo group, and the drop‐out of two subjects in the placebo group during the clamp‐trials reduced the power for between‐group comparisons. Thus, with the effect size and between‐group variation observed, at least 10 subjects should have completed the study in the placebo group for a significant between‐group interaction.
Taken together, the present study indicates that daily treatment with a selective beta2‐agonist, at close‐to‐therapeutic doses, augments insulin‐stimulated whole‐body glucose disposal, which may in part be attributed to muscle hypertrophy. These observations extend findings in rodents and highlight the therapeutic potential of beta2‐agonists to enhance the capacity for glucose disposal and whole‐body insulin sensitivity in humans.
Additional information
Competing interests
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Author contributions
M.H., J.B., V.B., and J.O. designed the study. S.J., J.O., K.E, T.B.S, and M.H. collected the data. T.B.S. and M.H. performed statistical analyses. All authors contributed to the drafting of the manuscript and approved the final version of the submitted manuscript. All authors are guarantors of this work and had full access to all the data in the study and take responsibility for the manuscript.
Funding
The study was funded by the Danish Ministry of Culture. The study funder was not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Translational perspective.
The relatively large effect observed on insulin‐stimulated whole‐body glucose disposal with only a few weeks daily treatment with selective beta2‐agonist is noteworthy as the subjects included were a homogeneous group of healthy lean young men with a high pre‐intervention insulin sensitivity (∼9.7 mg × kg body weight × min−1) well above insulin‐resistant individuals (<4.7 mg × kg body weight × min−1) (Bergman et al. 1985; Stern et al. 2005). Hence, the effect of beta2‐agonist may be more pronounced in insulin‐resistant subjects − particularly in persons with obesity. Although change in fat mass was not an independent predictor of change in insulin‐stimulated glucose disposal in the lean population investigated in this study, it is conceivable that beta2‐agonist treatment can improve insulin‐stimulated glucose uptake in populations with greater potential for reductions in fat mass as beta2‐adrenergic stimulation induces lipolysis in adipose tissue and accentuates whole‐body energy expenditure (Onslev et al. 2017; Jessen et al. 2020). For the same reason, the beta2‐adrenergic receptor has been highlighted as a potential target to induce leanness and improve insulin sensitivity (Hostrup & Onslev, 2021). Nevertheless, investigations on the applicability of beta2‐agonist treatment in such populations are warranted.
Supporting information
Statistical Summary Document
Peer Review History
Acknowledgements
The authors express their gratitude to Professor Henriette Pilegaard for the support during the protein extraction procedure of the abdominal adipose tissue biopsies. Figure 2 created with biorender.com.
Biographies
Søren Jessen is a postdoctoral researcher at the Department of Nutrition, Exercise and Sports (NEXS), University of Copenhagen, Denmark, whose research interests include muscle physiology and exercise performance. A special area of interest is the effects of pharmacological compounds on skeletal muscle metabolism, hypertrophy, and exercise performance in humans.

Morten Hostrup, PhD, is Associate Professor of human integrative physiology at NEXS. His research focuses on the health‐ and performance‐related benefits of exercise, performance optimization strategies and ergogenic substances. An extensive amount of his research has been devoted to beta2‐agonist pharmacology, with an emphasis on its effects in human skeletal muscle.

Edited by: Kim E. Barrett & Bettina Mittendorfer
Linked articles: This article is highlighted in a Perspective article by Dirks. To read this article, visit https://doi.org/10.1113/JP282992.
The peer review history is available in the Supporting Information section of this article (https://doi.org/10.1113/JP282421#support‐information‐section).
S. Jessen and T. Baasch‐Skytte contributed equally to the work.
Data availability statement
Data are available by request to the corresponding author.
References
- Bergman RN, Finegood DT & Ader M (1985). Assessment of insulin sensitivity in vivo. Endocr Rev 6, 45–86. [DOI] [PubMed] [Google Scholar]
- Bergström J (1975). Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35, 609–616. [PubMed] [Google Scholar]
- Birnbaum MJ (1989). Identification of a novel gene encoding an insulin‐responsive glucose transporter protein. Cell 57, 305–315. [DOI] [PubMed] [Google Scholar]
- Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, Phoenix S, Frisch F, Guerin B, Turcotte EE, Haman F, Richard D, Picard F, Rensen PCN, Scheele C & Carpentier AC (2020). Human brown adipocyte thermogenesis is driven by beta2‐AR stimulation. Cell Metab 32, 287–300.e7. [DOI] [PubMed] [Google Scholar]
- Borgström L, Liu CX & Walhagen A (1989). Pharmacokinetics of the enantiomers of terbutaline after repeated oral dosing with racemic terbutaline. Chirality 1, 174–177. [DOI] [PubMed] [Google Scholar]
- Burniston JG, Clark WA, Tan LB & Goldspink DF (2006). Dose‐dependent separation of the hypertrophic and myotoxic effects of the beta2‐adrenergic receptor agonist clenbuterol in rat striated muscles. Muscle Nerve 33, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle A, Yaspelkis BB 3rd, Kuo CH & Ivy JL (2001). Attenuation of insulin resistance by chronic beta2‐adrenergic agonist treatment possible muscle specific contributions. Life Sci 69, 599–611. [DOI] [PubMed] [Google Scholar]
- Charron MJ, Brosius FC 3rd, Alper SL & Lodish HF (1989). A glucose transport protein expressed predominately in insulin‐responsive tissues. Proc Natl Acad Sci U S A 86, 2535–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheymol G (2000). Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet 39, 215–231. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J & Felber JP (1981). The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, 1000–1007. [DOI] [PubMed] [Google Scholar]
- Deng C, Paoloni‐Giacobino A, Kuehne F, Boss O, Revelli JP, Moinat M, Cawthorne MA, Muzzin P & Giacobino JP (1996). Respective degree of expression of beta 1‐, beta 2‐ and beta 3‐adrenoceptors in human brown and white adipose tissues. Br J Pharmacol 118, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyreborg A, Krogh N, Backer V, Rzeppa S, Hemmersbach P & Hostrup M (2016). Pharmacokinetics of oral and inhaled terbutaline after exercise in trained men. Front Pharmacol 7, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfellah MS, Dalling R, Kantola IM & Reid JL (1989). Beta‐adrenoceptors and human skeletal muscle characterisation of receptor subtype and effect of age. Br J Clin Pharmacol 27, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG, Li P, Zhu Z & Lu Y (2005). Metabolic and cellular plasticity in white adipose tissue I: effects of beta3‐adrenergic receptor activation. Am J Physiol Endocrinol Metab 289, E608–E616. [DOI] [PubMed] [Google Scholar]
- Holgate ST, Stubbs WA, Wood PJ, McCaughey ES, Alberti kg & Tattersfield AE (1980). Airway and metabolic resistance to intravenous salbutamol: a study in normal man. Clin Sci (Lond) 59, 155–161. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Jacobson GA, Jessen S & Lemminger AK (2020). Anabolic and lipolytic actions of beta2 ‐agonists in humans and antidoping challenges. Drug Test Anal 12, 597–609. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Onslev J, Jessen S, Haase C, Habib S, Ortenblad N, Backer V & Bangsbo J (2015). Mechanisms underlying enhancements in muscle force and power output during maximal cycle ergometer exercise induced by chronic beta2‐adrenergic stimulation in men. J Appl Physiol (1985) 119, 475–486. [DOI] [PubMed] [Google Scholar]
- Hostrup M & Onslev J (2021). The beta2 ‐adrenergic receptor ‐ a re‐emerging target to combat obesity and induce leanness? J Physiol 600, 1209–1227. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Onslev J, Jacobson GA, Wilson R & Bangsbo J (2018a). Chronic beta2‐adrenoceptor agonist treatment alters muscle proteome and functional adaptations induced by high intensity training in young men. J Physiol 596, 231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostrup M, Reitelseder S, Jessen S, Kalsen A, Nyberg M, Egelund J, Kreiberg M, Kristensen CM, Thomassen M, Pilegaard H, Backer V, Jacobson GA, Holm L & Bangsbo J (2018b). Beta2‐adrenoceptor agonist salbutamol increases protein turnover rates and alters signalling in skeletal muscle after resistance exercise in young men. J Physiol 596, 4121–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail AD, Alkhayl FFA, Wilson J, Johnston L, Gill JMR & Gray SR (2019). The effect of short‐duration resistance training on insulin sensitivity and muscle adaptations in overweight men. Exp Physiol 104, 540–545. [DOI] [PubMed] [Google Scholar]
- Jacob S, Fogt DL, Dietze GJ & Henriksen EJ (1999). The beta2‐adrenergic modulator celiprolol reduces insulin resistance in obese Zucker rats. Life Sci 64, 2071–2079. [DOI] [PubMed] [Google Scholar]
- Jessen S, Onslev J, Lemminger A, Backer V, Bangsbo J & Hostrup M (2018). Hypertrophic effect of inhaled beta2‐agonist with and without concurrent exercise training: a randomized controlled trial. Scand J Med Sci Sports 28, 2114–2122. [DOI] [PubMed] [Google Scholar]
- Jessen S, Solheim SA, Jacobson GA, Eibye K, Bangsbo J, Nordsborg NB & Hostrup M (2020). Beta2‐adrenergic agonist clenbuterol increases energy expenditure and fat oxidation, and induces mTOR phosphorylation in skeletal muscle of young healthy men. Drug Test Anal 12, 610–618. [DOI] [PubMed] [Google Scholar]
- Jones JP & Dohm GL (1997). Regulation of glucose transporter GLUT‐4 and hexokinase II gene transcription by insulin and epinephrine. Am J Physiol Endocrinol Metab 273, E682–E687. [DOI] [PubMed] [Google Scholar]
- Kalinovich A, Dehvari N, Aslund A, van Beek S, Halleskog C, Olsen J, Forsberg E, Zacharewicz E, Schaart G, Rinde M, Sandstrom A, Berlin R, Ostenson CG, Hoeks J & Bengtsson T (2020). Treatment with a β‐2‐adrenoceptor agonist stimulates glucose uptake in skeletal muscle and improves glucose homeostasis, insulin resistance and hepatic steatosis in mice with diet‐induced obesity. Diabetologia 63, 1603–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalakkannan G, Petrilli CM, George I, LaManca J, McLaughlin BT, Shane E, Mancini DM & Maybaum S (2008). Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J Heart Lung Transpl 27, 457–461. [DOI] [PubMed] [Google Scholar]
- Koeberl DD, Case LE, Smith EC, Walters C, Han SO, Li Y, Chen W, Hornik CP, Huffman KM, Kraus WE, Thurberg BL, Corcoran DL, Bali D, Bursac N & Kishnani PS (2018). Correction of biochemical abnormalities and improved muscle function in a phase I/II clinical trial of clenbuterol in Pompe disease. Mol Ther 26, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman R, Gehrig SM, Leger B, Trieu J, Walrand S, Murphy KT & Lynch GS (2010). Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β‐adrenoceptor stimulation in mice. J Physiol 588, 4811–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziczak‐Holbro M, Rigel DF, Dumotier B, Sykes DA, Tsao J, Nguyen NH, Bosch J, Jourdain M, Flotte L, Adachi Y, Kiffe M, Azria M, Fairhurst RA, Charlton SJ, Richardson BP, Lach‐Trifilieff E, Glass DJ, Ullrich T & Hatakeyama S (2019). Pharmacological characterization of a novel 5‐hydroxybenzothiazolone‐derived beta 2‐adrenoceptor agonist with functional selectivity for anabolic effects on skeletal muscle resulting in a wider cardiovascular safety window in preclinical studies. J Pharmacol Exp Ther 369, 188–199. [DOI] [PubMed] [Google Scholar]
- Krogh N, Rzeppa S, Dyreborg A, Dehnes Y, Hemmersbach P, Backer V & Hostrup M (2017). Terbutaline accumulates in blood and urine after daily therapeutic inhalation. Med Sci Sports Exerc 49, 1236–1243. [DOI] [PubMed] [Google Scholar]
- Lee P, Birzniece V, Umpleby AM, Poljak A & Ho KK (2015). Formoterol, a highly beta2‐selective agonist, induces gender‐dimorphic whole body leucine metabolism in humans. Metabolism 64, 506–512. [DOI] [PubMed] [Google Scholar]
- Lowry OH & Passonneau JV (1972). A Flexible System of Enzymatic Analysis. Academic Press, New York. [Google Scholar]
- Meister J, Bone DBJ, Knudsen JR, Barella LF, Velenosi TJ, Akhmedov D, Lee RJ, Cohen AH, Gavrilova O, Cui Y, Karsenty G, Chen M, Weinstein LS, Kleinert M, Berdeaux R, Jensen TE, Richter EA & Wess J (2022). Clenbuterol exerts antidiabetic activity through metabolic reprogramming of skeletal muscle cells. Nat Commun 13, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G, Chiappini S, Mattioli F, Martelli A & Schifano F (2018). beta‐2 agonists as misusing drugs? Assessment of both clenbuterol‐ and salbutamol‐related european medicines agency pharmacovigilance database reports. Basic Clin Pharmacol Toxicol 123, 182–187. [DOI] [PubMed] [Google Scholar]
- Miller WJ, Sherman WM & Ivy JL (1984). Effect of strength training on glucose tolerance and post‐glucose insulin response. Med Sci Sports Exerc 16, 539–543. [PubMed] [Google Scholar]
- Navegantes LC, Resano NM, Migliorini RH & Kettelhut IC (2000). Role of adrenoceptors and cAMP on the catecholamine‐induced inhibition of proteolysis in rat skeletal muscle. Am J Physiol Endocrinol Metab 279, E663–E668. [DOI] [PubMed] [Google Scholar]
- Navegantes LC, Resano NM, Migliorini RH & Kettelhut IC (2001). Catecholamines inhibit Ca2+‐dependent proteolysis in rat skeletal muscle through beta2‐adrenoceptors and cAMP. Am J Physiol Endocrinol Metab 281, E449–E454. [DOI] [PubMed] [Google Scholar]
- Onslev J, Jacobson G, Narkowicz C, Backer V, Kalsen A, Kreiberg M, Jessen S, Bangsbo J & Hostrup M (2017). Beta2‐adrenergic stimulation increases energy expenditure at rest, but not during submaximal exercise in active overweight men. Eur J Appl Physiol 117, 1907–1915. [DOI] [PubMed] [Google Scholar]
- Onslev J, Jensen J, Bangsbo J, Wojtaszewski J & Hostrup M (2019). Beta2‐agonist induces net leg glucose uptake and free fatty acid release at rest but not during exercise in young men. J Clin Endocrinol Metab 104, 647–657. [DOI] [PubMed] [Google Scholar]
- Pan SJ, Hancock J, Ding Z, Fogt D, Lee M & Ivy JL (2001). Effects of clenbuterol on insulin resistance in conscious obese Zucker rats. Am J Physiol Endocrinol Metab 280, E554–E561. [DOI] [PubMed] [Google Scholar]
- Sato M, Dehvari N, Oberg AI, Dallner OS, Sandstrom AL, Olsen JM, Csikasz RI, Summers RJ, Hutchinson DS & Bengtsson T (2014). Improving type 2 diabetes through a distinct adrenergic signaling pathway involving mTORC2 that mediates glucose uptake in skeletal muscle. Diabetes 63, 4115–4129. [DOI] [PubMed] [Google Scholar]
- Scheidegger K, Robbins DC & Danforth E Jr (1984). Effects of chronic beta receptor stimulation on glucose metabolism. Diabetes 33, 1144–1149. [DOI] [PubMed] [Google Scholar]
- Sears MR & Lötvall J (2005). Past, present and future–beta2‐adrenoceptor agonists in asthma management. Respir Med 99, 152–170. [DOI] [PubMed] [Google Scholar]
- Simpson AJ, Bood JR, Anderson SD, Romer LM, Dahlen B, Dahlén S‐E & Kippelen P (2016). A standard, single dose of inhaled terbutaline attenuates hyperpnea‐induced bronchoconstriction and mast cell activation in athletes. J Appl Physiol 120, 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skagen C, Nyman TA, Peng X‐R, O'Mahony G, Kase ET, Rustan AC & Thoresen GH (2021). Chronic treatment with terbutaline increases glucose and oleic acid oxidation and protein synthesis in cultured human myotubes. Curr Rese Pharmacol Drug Discov 2, 100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeper MM, Kearns CF & McKeever KH (2002). Chronic clenbuterol administration negatively alters cardiac function. Med Sci Sports Exerc 34, 643–650. [DOI] [PubMed] [Google Scholar]
- Spiller HA, James KJ, Scholzen S & Borys DJ (2013). A descriptive study of adverse events from clenbuterol misuse and abuse for weight loss and bodybuilding. Subst Abus 34, 306–312. [DOI] [PubMed] [Google Scholar]
- Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C & Stern MP (2005). Identification of individuals with insulin resistance using routine clinical measurements. Diabetes 54, 333–339. [DOI] [PubMed] [Google Scholar]
- Tesse R, Borrelli G, Mongelli G, Mastrorilli V & Cardinale F (2018). Treating pediatric asthma according guidelines. Front Pediatr 6, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgan CE, Brozinick JT Jr, Banks EA, Cortez MY, Wilcox RE & Ivy JL (1993). Exercise training and clenbuterol reduce insulin resistance of obese Zucker rats. Am J Physiol Endocrinol Metab 264, E373–E379. [DOI] [PubMed] [Google Scholar]
- Ward JK, Dow J, Dallow N, Eynott P, Milleri S & Ventresca GP (2000). Enantiomeric disposition of inhaled, intravenous and oral racemic‐salbutamol in man—no evidence of enantioselective lung metabolism. Br J Clin Pharmacol 49, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaspelkis BB 3rd (2006). Resistance training improves insulin signaling and action in skeletal muscle. Exerc Sport Sci Rev 34, 42–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical Summary Document
Peer Review History
Data Availability Statement
Data are available by request to the corresponding author.


